IDH Mutations and Intraoperative 5-ALA Fluorescence in Gliomas: A Systematic Literature Review with Novel Exploratory Hypotheses on the Modulatory Effect of Vorasidenib
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Bias Considerations
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Intraoperative Fluorescence and Its Association with Tumor Grade and IDH Mutation
3.3. Surgical and Oncological Outcomes
3.4. Comprehensive Findings from the Literature Review
4. Discussion
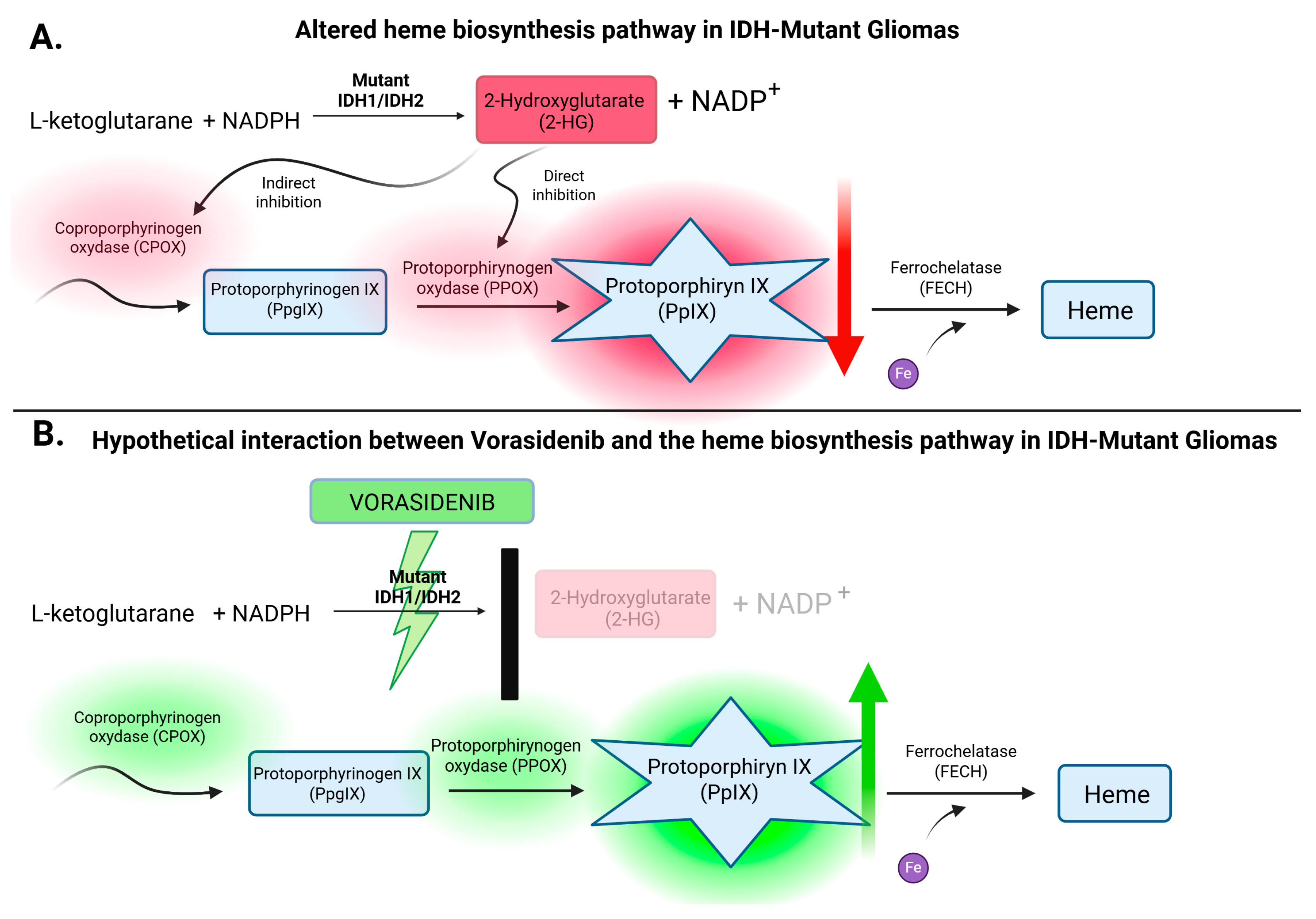
4.1. Vorasidenib: A Targeted IDH Inhibitor Redefining Glioma Metabolism
4.2. IDH Mutations and Vorasidenib: Understanding the Paradox of Favorable Genetics and Targeted Therapy
4.3. Implications for Future Research: Exploring the Potential of Vorasidenib to Enhance 5-ALA-Guided Fluorescence
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| IDH | isocitrate dehydrogenase |
| PFS | progression-free survival |
| OS | overall survival |
| GTR | gross total resection |
| WHO | World Health Organization |
| PGB | porphobilinogen |
| CPOX | coproporphyrinogen oxidase |
| PPOX | protoporphyrinogen oxidase |
| PpIX | protoporphyrin IX |
| FECH | ferrochelatase |
| 2-HG | 2-hydroxyglutarate |
| FDA the U.S. | Food and Drug Administration |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| GBM | Glioblastoma |
| LGGs | low-grade gliomas |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| CE | contrast enhancement |
| LOOCV | leave-one-out cross-validation |
| EOR | extent of resection |
| HO-1 | heme oxygenase-1 |
| TCA | tricarboxylic acid cycle |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Jiang, S.; Zanazzi, G.J.; Hassanpour, S. Predicting prognosis and IDH mutation status for patients with lower-grade gliomas using whole slide images. Sci. Rep. 2021, 11, 16849. Available online: https://www.nature.com/articles/s41598-021-95948-x (accessed on 17 September 2025). [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881–1888. Available online: https://jamanetwork.com/journals/jama/fullarticle/1386639 (accessed on 17 September 2025). [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. Available online: https://jamanetwork.com/journals/jamaoncology/fullarticle/2528564 (accessed on 17 September 2025). [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. Available online: https://thejns.org/view/journals/j-neurosurg/115/1/article-p3.xml (accessed on 17 September 2025). [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011, 114, 595–603. Available online: https://thejns.org/view/journals/j-neurosurg/114/3/article-p595.xml (accessed on 17 September 2025). [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. Available online: https://link.springer.com/article/10.1007/s11060-019-03098-y (accessed on 17 September 2025). [CrossRef]
- Rybaczek, M.; Chaurasia, B. Advantage and challenges in the use of 5-Aminolevulinic acid (5-ALA) in neurosurgery. Neurosurg. Rev. 2024, 47, 1–3. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. Available online: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(06)70665-9/abstract (accessed on 17 September 2025). [CrossRef]
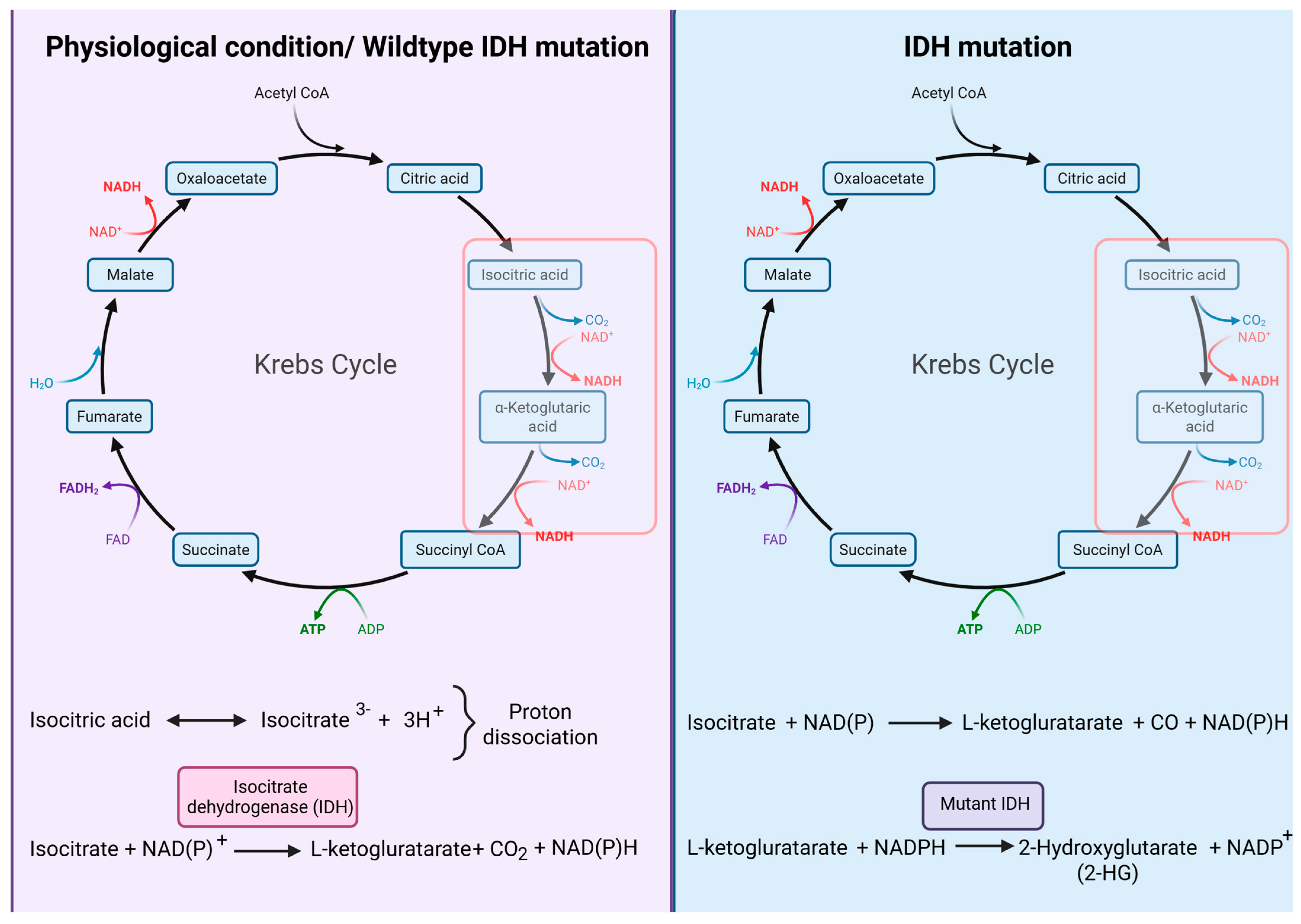
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744, Erratum in Nature 2010, 465, 966. Available online: https://www.nature.com/articles/nature08617 (accessed on 17 September 2025). [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Vorasidenib for Grade 2 Astrocytoma or Oligodendroglioma with a Susceptible IDH1 or IDH2 Mutation. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-or-oligodendroglioma-susceptible-idh1-or-idh2-mutation (accessed on 22 May 2025).
- Mellinghoff, I.K.; Bent, M.J.V.D.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2304194 (accessed on 17 September 2025). [CrossRef]
- Mellinghoff, I.K.; Lu, M.; Wen, P.Y.; Taylor, J.W.; Maher, E.A.; Arrillaga-Romany, I.; Peters, K.B.; Ellingson, B.M.; Rosenblum, M.K.; Chun, S.; et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: A randomized, perioperative phase 1 trial. Nat. Med. 2023, 29, 615–622. Available online: https://www.nature.com/articles/s41591-022-02141-2 (accessed on 17 September 2025). [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Bruno, F.; La Cava, P.; Panico, F.; Rudà, R.; Melcarne, A.; Garbossa, D.; Cofano, F. Double fluorescence-guided surgery with 5-ALA and fluorescein sodium in grade 2 and grade 3 adult-type diffuse gliomas: Retrospective analysis of 112 cases. Brain Spine. 2025, 5, 104277. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Müther, M.; Jaber, M.; Johnson, T.D.; Orringer, D.A.; Stummer, W. A Data-Driven Approach to Predicting 5-Aminolevulinic Acid–Induced Fluorescence and World Health Organization Grade in Newly Diagnosed Diffuse Gliomas. Neurosurgery 2022, 90, 800–806. Available online: https://journals.lww.com/neurosurgery/fulltext/2022/06000/a_data_driven_approach_to_predicting.19.aspx (accessed on 17 September 2025). [CrossRef]
- Kim, J.K.; Jung, T.Y.; Jung, S.; Kim, I.Y.; Jang, W.Y.; Moon, K.S.; Kim, S.K.; Kim, J.H.; Lee, K.H. Relationship between tumor cell infiltration 5-aminolevulinic acid fluorescence signals after resection of MR-enhancing lesions its prognostic significance in glioblastoma. Clin. Transl. Oncol. 2021, 23, 459–467. [Google Scholar] [CrossRef]
- Hickmann, A.K.; Nadji-Ohl, M.; Hopf, N.J. Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: Retrospective analysis of surgical and neurological outcome in 58 patients. J. Neuro-Oncol. 2015, 122, 151–160. [Google Scholar] [CrossRef]
- Ohba, S.; Murayama, K.; Kuwahara, K.; Pareira, E.S.; Nakae, S.; Nishiyama, Y.; Adachi, K.; Yamada, S.; Sasaki, H.; Yamamoto, N.; et al. The Correlation of Fluorescence of Protoporphyrinogen IX and Status of Isocitrate Dehydrogenase in Gliomas. Neurosurgery 2020, 87, 408–417. Available online: https://journals.lww.com/neurosurgery/abstract/2020/08000/the_correlation_of_fluorescence_of.29.aspx (accessed on 17 September 2025). [CrossRef]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Greco Crasto, S.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glio-blastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
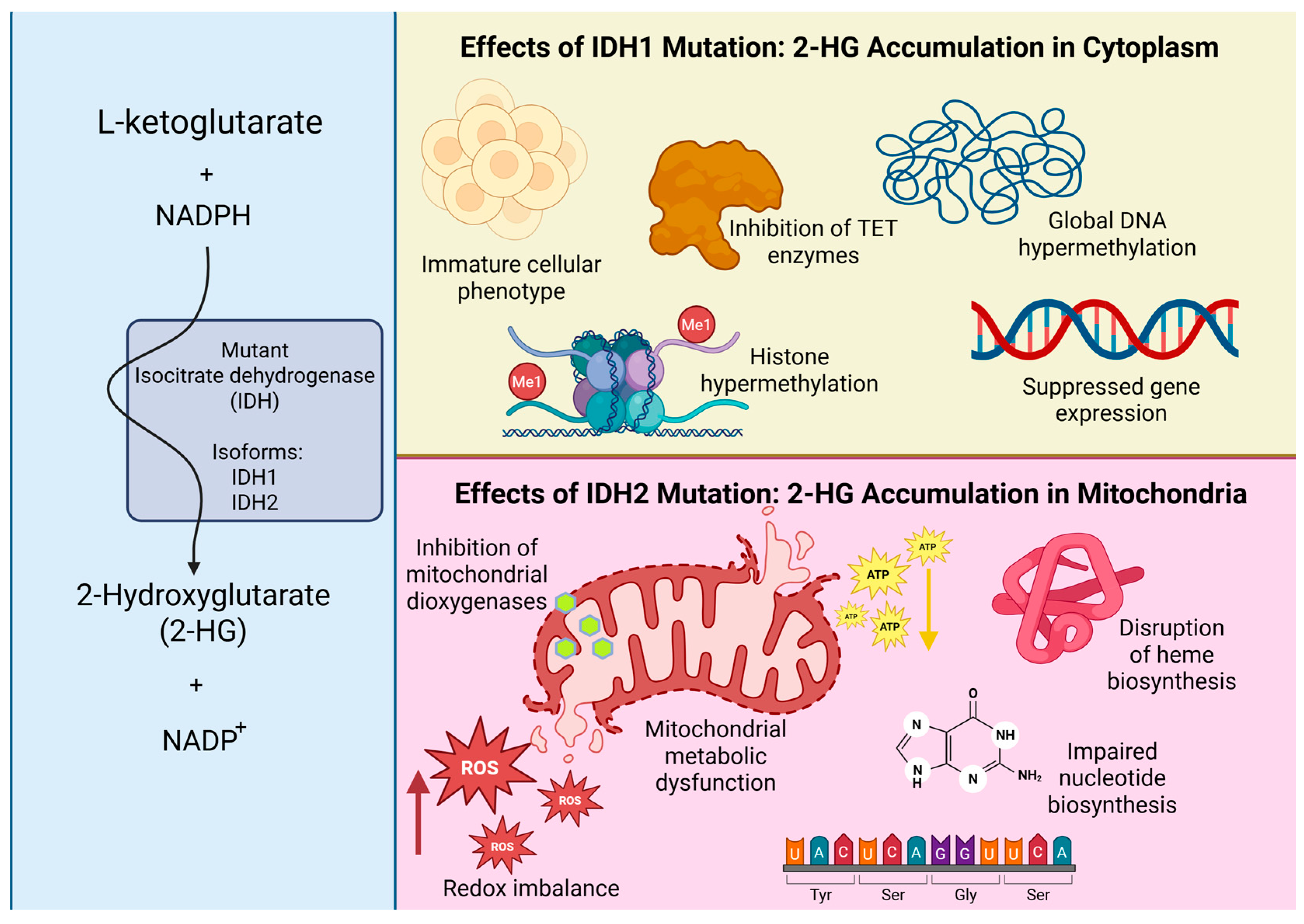
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.A.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.S.; et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef]
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An Inhibitor of Mutant IDH1 Delays Growth and Promotes Differentiation of Glioma Cells. Science 2013, 340, 626–630. Available online: https://www.science.org/doi/10.1126/science.1236062 (accessed on 17 September 2025). [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Furth, N.; Cohen, N.; Spitzer, A.; Salame, T.M.; Dassa, B.; Mehlman, T.; Brandis, A.; Moussaieff, A.; Friedmann-Morvinski, D.; Castro, M.G.; et al. Oncogenic IDH1mut drives robust loss of histone acetylation and increases chromatin heterogeneity. Proc. Natl. Acad. Sci. USA 2025, 122, e2403862122. Available online: https://www.pnas.org/doi/10.1073/pnas.2403862122 (accessed on 17 September 2025). [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa0808710 (accessed on 17 September 2025). [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0076988 (accessed on 17 September 2025). [CrossRef]
- Valdés, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative fluorescence using 5-aminolevulinic acid–induced protoporphyrin IX as a surgical adjunct in low-grade glioma surgery. J. Neurosurg. 2015, 123, 771–780. Available online: https://thejns.org/view/journals/j-neurosurg/123/3/article-p771.xml (accessed on 17 September 2025). [CrossRef]

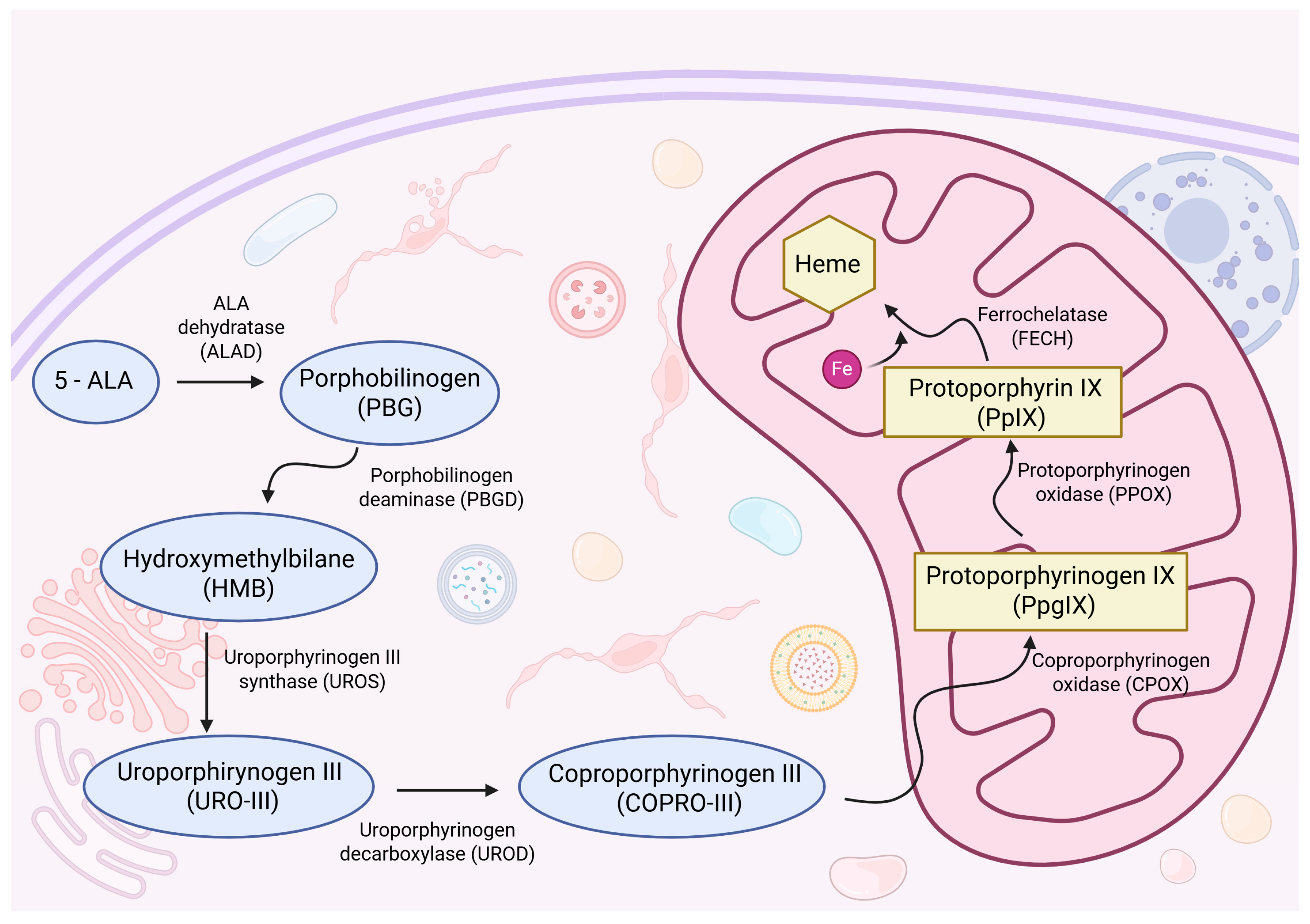
| Bianconi et al., 2025 [14] | Specchia et al., 2021 [15] | Muther et al., 2022 [16] | Kim et al., 2021 [17] | Hickmann et al., 2015 [18] | Ohba et al., 2020 [19] | Zeppa et al., 2022 [20] | |
|---|---|---|---|---|---|---|---|
| Study methodology | Retrospective single-center cohort study | Retrospective study | Retrospective single-center cohort study | Retrospective single-center study | Retrospective controlled cohort study | Retrospective observational study | Retrospective single-center cohort study |
| Study group | 112 patients: 69 IDH-mutant astrocytomas (28 WHO G2, 41 WHO G3). 43 IDH-mutant oligodendrogliomas (24 WHO G2, 19 WHO G3) | 44 GBM patients (16 IDH-mutant, 28 IDH-wildtype) | 179 patients: 113 WHO G2, 66 WHO G3 | Final study cohort: 25 patients GBM IDH-wildtype | 5-ALA group: 58 Control group: 65 All patients with recurrent gliomas | 104 patients, (68 underwent initial surgery, 36 were reoperated) | 99 GBM patients (WHO G4) |
| 5-ALA fluorescence rate - WHO G4 | Not included | IDH-mutant Grade 0: 8/16 (50%) Grade 1: 7/16 (43.75%) Grade 2: 1/16 (6.25%) IDH-wildtype Grade 0: 1/28 (3.6%) Grade 1: 4/28 (14.3%) Grade 2: 23/28 (82.1%) | Not included | Pink (n = 14) 50%, blue (n=122) 28.7% | Fluorescence in 36/38 (94.7%) | Fluorescence in 57/59 (96.6%) | 100% |
| 5-ALA fluorescence rate - WHO G3 | 43.3% (26/60) | --- | 75–85% | Not included | 17/25 (68.0%) | 20/26 (76.9%) | --- |
| 5-ALA fluorescence rate - WHO G2 | 25.0% (15/60) | --- | 16–20% | Not included | Not reported | 5/19 (26.3%) | --- |
| Association with IDH mutations | No significant correlation between IDH mutation status and fluorescence (p = 0.94) | Grade 2 in 66.7% of IDH-wildtype, IDH-mutant: mostly Grade 0/1. Not statistically significant (p = 0.25) | Fluorescence did not significantly correlate with IDH mutation status (p = 0.814) | Study focused exclusively on IDH-wildtype GBM | 100% of non-fluorescent tumors were IDH-mutant, vs. 48.9% of fluorescent tumors (p = 0.005) | IDH mutations were present in 35.6% of tumors (37/104), fluorescence was significantly lower in IDH-mutant tumors compared to IDH-wildtype across all WHO grades (51.4% vs. 94.0%, p < 0.01) | all cases IDH–wildtype |
| Impact on surgical resection | Gross total resection (GTR) achieved in 71% (80/112); fluorescence was not significantly correlated with extent of resection | GTR rate not directly assessed | GTR rate not directly assessed | No correlation between residual fluorescence and tumor infiltration | Complete resection (EOR ≥ 98%) was achieved in: 58.8% of fluorescent cases, 50% of non-fluorescent cases (not significant, p = 0.622) | Not reported | ≥90% extent of resection: 87.5% (5-ALA), 77.3% (SF), 80% (combined); no significant difference (p = 0.783) |
| Fluorescence technology/ surgical equipment | Leica M530 OHX microscope with FL400 (5-ALA) and FL560 (FS) filters | Pentero Zeiss Microscope with a proper UV 400 nm filter | Zeiss Meditech Pentero, Zeiss Pentero 900, both equipped with BLUE400 module | Zeiss BLUE400 filter system | Blue light operating microscope with BLUE400 module | Pentero surgical microscope (Carl Zeiss Meditec, Germany) with the BLUE400 module | Operating microscopes with dedicated fluorescence filters (5-ALA and SF); specific device not reported. |
| Long-term outcomes | 5-ALA positivity associated with ~2.5-fold higher risk of shorter OS (p = 0.009) and PFS (p = 0.004); FS positivity with ~2.7-fold higher risk of shorter OS (p = 0.014), PFS not significant | Not reported | Not reported | Median PFS: 12.5 ± 2.1 months Median OS: 21.1 ± 3.5 months | PFS: 5-ALA group: 10.7 months Control: 10.6 months (not significant) OS: 5-ALA group: 17.6 months Control: 14.6 months (p = 0.025) | Not reported | Median OS: 20.0 months (5-ALA), 12.3 months (SF), 18.1 months (combined); overall cohort median OS 14.9 months; no significant differences |
| Study limitations | Lack of quantitative fluorescence assessment, no standardized grading of fluorescence intensity | No quantitative fluorescence assessment, lack of outcome data | Lack of outcome data | Small sample size, lack of quantitative fluorescence assessments | Subjective fluorescence assessment, no histological confirmation of residual fluorescent areas | Relatively small sample size, especially for subgroup analyses. Lack of outcome data | Limited generalizability (IDH-wildtype GBM only), small subgroup sizes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybaczek, M.; Jadeszko, M.; Lebejko, A.; Sawicka, M.; Mariak, Z.; Łysoń, T.; Car, H.; Wielgat, P. IDH Mutations and Intraoperative 5-ALA Fluorescence in Gliomas: A Systematic Literature Review with Novel Exploratory Hypotheses on the Modulatory Effect of Vorasidenib. Cancers 2025, 17, 3075. https://doi.org/10.3390/cancers17183075
Rybaczek M, Jadeszko M, Lebejko A, Sawicka M, Mariak Z, Łysoń T, Car H, Wielgat P. IDH Mutations and Intraoperative 5-ALA Fluorescence in Gliomas: A Systematic Literature Review with Novel Exploratory Hypotheses on the Modulatory Effect of Vorasidenib. Cancers. 2025; 17(18):3075. https://doi.org/10.3390/cancers17183075
Chicago/Turabian StyleRybaczek, Magdalena, Marek Jadeszko, Aleksander Lebejko, Magdalena Sawicka, Zenon Mariak, Tomasz Łysoń, Halina Car, and Przemysław Wielgat. 2025. "IDH Mutations and Intraoperative 5-ALA Fluorescence in Gliomas: A Systematic Literature Review with Novel Exploratory Hypotheses on the Modulatory Effect of Vorasidenib" Cancers 17, no. 18: 3075. https://doi.org/10.3390/cancers17183075
APA StyleRybaczek, M., Jadeszko, M., Lebejko, A., Sawicka, M., Mariak, Z., Łysoń, T., Car, H., & Wielgat, P. (2025). IDH Mutations and Intraoperative 5-ALA Fluorescence in Gliomas: A Systematic Literature Review with Novel Exploratory Hypotheses on the Modulatory Effect of Vorasidenib. Cancers, 17(18), 3075. https://doi.org/10.3390/cancers17183075






