Simple Summary
High-grade gliomas are aggressive brain tumors associated with poor outcomes. Mitochondria play an essential role in glioma growth and resistance to therapy, making them a promising target for novel therapeutic strategies. This review summarizes the mitochondrial dysfunctions that drive glioma progression, highlights clinical trials and therapies aimed at mitochondrial targeting, and discusses emerging approaches such as caseinolytic protease P (ClpP) and disruption of mitochondrial–epigenetic interactions that may improve outcomes for patients with high-grade glioma.
Abstract
High-grade gliomas are aggressive primary brain tumors and often fatal. They are characterized by rapid growth, treatment resistance, and significant heterogeneity both within and between tumors. A growing body of evidence highlights the mitochondria, dynamic organelles essential for energy production, apoptosis regulation, and metabolic rewiring, as a critical driver in glioma progression and treatment resistance. As a result, these insights have sparked growing interest in mitochondrial-directed therapies. This review highlights the distinct metabolic features and mitochondrial processes of glioma, outlining the rationale for targeting mitochondrial function. We discuss recent advances in mitochondrial-targeted therapies, with a focus on caseinolytic protease P (ClpP) agonism as a breakthrough in the treatment of diffuse midline glioma (DMG). Moreover, we discuss the pathogenic link between mitochondrial metabolism and epigenetic regulation, and the potential therapeutic benefit of disrupting this interaction.
1. Introduction
High-grade gliomas are malignant and incurable brain tumors, leading to significant morbidity and mortality in both adults and children [1]. The World Health Organization (WHO) classifies high-grade gliomas as either grade 3 or grade 4tumors, based on histopathological criteria [2]. Among these, glioblastoma and diffuse midline glioma (DMG) represent some of the most aggressive and lethal diseases.
Glioblastoma accounts for the majority of malignant gliomas and is diagnosed in approximately 3.27 per 100,000 people/year [1]. For the past two decades, the standard of care for newly diagnosed glioblastoma has remained initial maximal safe resection followed by radiation with concomitant and adjuvant temozolomide (TMZ)—an alkylating agent that induces DNA damage [3]. Despite this aggressive treatment, patients have a median survival of only around 14–20 months after initial diagnosis. Glioblastoma almost always develops resistance and recurs within 7 to 9 months, with rapid progression leading to an incredibly dismal prognosis [4].
Diffuse midline glioma (DMG) is a type of high-grade brain tumor that primarily affects children and young adults. It is the most lethal childhood cancer, with a median overall survival of 9–11 months and a 2-year survival of less than 10%, despite six decades of intense conventional and experimental therapies that have been unable to significantly improve patient outcomes [5,6]. DMG is now a broader term that includes tumors with similar genetic characteristics, particularly histone mutations such as H3K27M, located along the midline structures of the brain [2]. Critically, these mutations lead to hypomethylation of lysine 27 of histone H3 (H3K27), which is believed to drive an epigenetic phenotype that promotes aberrant expression of oncogenes resulting in rapid tumor growth. Over 80% of DMG cases exhibit the missense mutation H3K27M that leads to this hypomethylation [2,7].
Although extensive research efforts have provided exciting insight into the biochemical mechanisms that drive high-grade gliomas, clinical outcomes have improved only modestly, and both glioblastoma and DMG remain universally fatal.
Poor prognosis of these tumors can be attributed to rapid progression and resistance to chemotherapy and radiation. Growing evidence implicates the mitochondria as a key driver of these therapeutic challenges. In glioma, as well as breast, colorectal, lung, pancreatic and several other tumors, mitochondrial dysfunction has been identified as a contributor to tumor growth and therapy resistance, establishing the mitochondria as a common cancer driver [8,9,10,11,12]. These insights have led us and others to rationalize targeting the mitochondria as a promising therapeutic strategy for glioma [13].
Here, we begin by highlighting the distinct metabolic properties of glioma and focus on the mitochondria as a critical driver of metabolic rewiring. We discuss how aberrant mitochondrial quality control contributes to treatment resistance and glioma progression and highlight the mitochondria as an attractive therapeutic target. Finally, we provide a summary of mitochondrial targeting efforts, including a therapeutic breakthrough through ClpP agonism, and touch on crosstalk between mitochondrial metabolism and epigenetics that may be exploitable for glioma treatment.
2. Distinct Metabolic Properties of Glioma
Gliomas undergo extensive metabolic reprogramming to support their aggressive growth, acquiring distinct metabolic properties. Mitochondria are as central regulators of this reprogramming, with their functional state influencing both energy production and nutrient uptake. Critical metabolic processes, including fatty acid β-oxidation and the tricarboxylic acid (TCA) cycle take place within the mitochondrial matrix, highlighting the organelle’s essential role in glioma metabolic adaption [14,15].
2.1. The Warburg Effect
The first observation of reprogramming of cancer metabolism came from a landmark study in cancer biology by Warburg et al., which was later termed the Warburg effect— that cancer cells upregulate “aerobic glycolysis” by taking up high amounts of glucose to produce lactate regardless of the presence of oxygen [16]. The Warburg effect is well-described as a hallmark in glioma and a key part of the fine-tuned metabolic reprogramming that drives the survival and proliferation of glioma cells. This process seems counterintuitive and energetically inefficient, generating only a net of 2 ATP molecules per glucose compared to up to 38 ATP molecules produced during oxidative phosphorylation (OXPHOS) [17].
So, why would cancer cells prefer this energetically inefficient process? Discussion has centered on four main arguments. First, the Warburg effect reprograms cancer cells to improve access to a limited resource (glucose) for energy production to meet their needs for rapid proliferation. Secondly, it promotes energy flux into several biosynthetic pathways to produce vital cell building blocks, such as nucleotides, amino acids, and lipids. Thirdly, it acidifies the tumor microenvironment (TME) to disrupt tissue architecture and cause immune suppression. Lastly, it allows for cell signaling alterations due to changes in reactive oxygen species (ROS) and chromatin modulation [18]. While the benefit of the Warburg effect remains a highly disputed topic, the literature has supported a general explanation: the Warburg effect enables cancer cells to uptake and incorporate nutrients into the biomass (nucleotides, amino acids, and lipids) needed to produce a new cell [19].
The origin of extensive metabolic reprograming is driven, at least in part, by the intrinsic hypoxic properties of many primary brain tumors. Malignant gliomas cells are known to accumulate hypoxia-inducible factors (HIFs), acting as hypoxia sensors that orchestrate a coordinated response to increase their pro-survival and pro-invasive phenotype [20,21].
Metabolic rewiring in glioma is also evidenced by the upregulation in glucose uptake, addiction to glutamine, increased fatty acid synthesis and beta-oxidation, and the alterations of the TCA cycle [17,22,23,24,25].
2.2. Increased Reliance on Glucose and Glutamine
To support the need for glycolytic activity, glioma cells highly upregulate the expression of glucose-transporters, GLUT1 and GLUT3, enhancing glycolytic flux into the cell [22]. Astrocytic end-feet envelop capillaries in the blood–brain barrier, allowing malignant cells to tightly upregulate glucose influx [26]. Several studies have reported that increased expression of GLUT1 and GLUT3 is linked to worse prognosis and higher-grade tumors [22,27,28]. GLUT3 has been linked to tumor cell invasion in glioblastoma [28]. With increased glucose uptake, a pool of metabolites can then be shuttled to increase intracellular lipid synthesis, amino acid, and nucleotide stores to meet the cell’s mitotic needs [17].
In addition to glucose, glioma cells strongly rely on glutamine for fuel for several reasons. Glutamine serves as a critical anaplerotic substrate, replenishing TCA cycle intermediates in the mitochondria through its conversion to glutamate and subsequently to α-ketoglutarate. Glutamine metabolism also contributes to redox homeostasis by supporting the production of glutathione, a key antioxidant that helps glioma cells resist oxidative stress [23,29]. These metabolic adaptations are often driven by oncogenic signaling, including c-Myc-mediated upregulation of glutaminase and mutations in genes such as EGFR, which further enhance glutamine utilization to support tumor growth [30].
2.3. Upregulated Beta-Oxidation and Fatty Acid Synthesis
Fatty acid synthesis is generally upregulated in glioma, with fatty acid synthase expression correlating with higher tumor grades. Various studies have attributed this upregulation of fatty acid synthesis to the activity of sterol response element binding protein (SREBP), a transcription factor [24,30,31,32]. Fatty acids have various uses for tumor cells, such as oncogenic signaling molecules, or geared toward building new lipid bilayers and vacuolar membranes to meet the cell’s needs [25].
Fatty acid oxidation (FAO) is also upregulated in glioma, particularly in glioblastoma, providing a critical alternative energy source. Several studies have linked increased FAO to therapy resistance and survival in glioblastoma [25,33,34]. FAO is required for the respiration and proliferation of glioblastoma cells, and FAO enzymes have demonstrated potential as prognostic markers [34]. Enhanced FAO in glioblastoma was also shown to be directly dependent upon the TME, leading to a metabolic plasticity that allows glioma cells to adapt and proliferate [33].
2.4. Altered TCA Cycle Function
Glioma cells display highly altered TCA cycle function and maintain the TCA cycle under hypoxic conditions through replenishing α-ketoglutarate, by taking up glutamine and aspartate from their environment [35]. In high-grade gliomas, intermediates of the TCA cycle are shuttled into various biosynthetic pathways to fuel tumor growth. For example, citrate can be diverted into fatty acid synthesis while α-ketoglutarate can be shuttled into amino acid synthesis [35,36].
Isocitrate dehydrogenase (IDH) enzymes are key players in the TCA cycle, facilitating the conversion of isocitrate into α-ketoglutarate while using NADP+ or NAD+ as cofactors. While the IDH2 enzyme resides in the mitochondria and is more directly involved in the TCA cycle, IDH1 resides in the cytoplasm and is involved in redox homeostasis [37]. In glioblastoma, the expression of wild-type IDH1 is increased about four times relative to healthy brain tissue [38]. This makes IDH1 the major source of NADPH, a compound known to fuel antioxidant defenses and protect tumor cells from oxidative damage. Knockdown of IDH1 depletes NADPH levels and sensitizes glioblastoma cells to radiation, leading to cellular senescence [37,38].
Although IDH mutations are uncommon in gliomas diagnosed de novo as high-grade, they are present in approximately 70–80% of low-grade gliomas. Importantly, most IDH-mutant, low-grade gliomas eventually undergo malignant transformation into high-grade tumors. As a result, IDH mutation remains a defining metabolic alteration in a biologically distinct subset of high-grade gliomas [39,40,41].
Glioma cells exhibit extensive metabolic rewiring to support tumor growth, as exemplified by the Warburg effect and other altered metabolic pathways. Dysfunctional mitochondria play a central role in regulating these processes and have emerged as a promising focus of research [14]. Targeting mitochondrial-related metabolism has revealed novel therapeutic vulnerabilities. For instance, inhibiting glutaminolysis in mitochondrial-dependent glioblastoma cells disrupted TCA cycle function and energy production, leading to impaired tumor cell survival [42]. In addition to these metabolic dependencies, dysregulation of mitochondrial quality control has also been implicated in glioma progression.
3. Aberrant Mitochondrial Quality Control in Glioma
Glioma cells exhibit aberrant mitochondrial processes that contribute to tumor progression and therapy resistance. As summarized in Figure 1, processes such as mitochondrial transfer, mitophagy, mitochondrial fusion and fission, are highly altered in glioma in order to control mitochondrial quality.
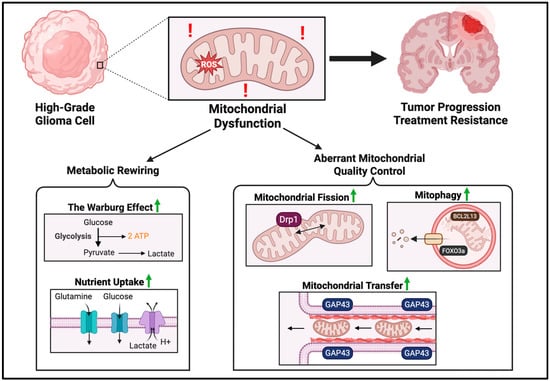
Figure 1.
Mitochondrial dysfunction drives glioma progression and treatment resistance. Mitochondrial dysfunction involves both metabolic rewiring and aberrant quality control. Metabolic rewiring is characterized by the Warburg effect, a reliance on glycolysis even in the presence of oxygen. To meet metabolic demands, glioma cells upregulate glucose and glutamine influx, while removing excess lactate. Mitochondrial quality is controlled in several ways: mitochondrial transfer from adjacent cells via tunneling actin nanotubules, upregulation of mitochondrial fission, and general upregulation of mitophagy levels. Red exclamation symbols are used to represent mitochondrial dysfunction. Green arrows are used to represent upregulation. The image was created with BioRender.com (accessed on 25 August 2025).
3.1. Mitochondrial Transfer
One alteration of mitochondrial processes in glioma progression is through a process known as mitochondrial transfer, to import mitochondria from and to adjacent cells via tunneling actin nanotubes. Mesenchymal stem cells were shown to transfer mitochondria to recipient glioblastoma cells, inducing metabolic and functional changes [43]. This transfer leads to a key metabolic shift from glucose to glutamine utilization in glioma stem cells (GSCs), accompanied by a higher orotate turnover and increased nucleotide synthesis, contributing to increased TMZ resistance in glioblastoma cells [43]. Watson et al. similarly reported a high prevalence of mitochondrial transfer from adjacent healthy astrocytes, increasing mitochondrial respiration and upregulating metabolic pathways linked to proliferation and tumorigenicity. This mitochondria transfer is facilitated by growth-associated protein 43 (GAP43), involved in neuron axon regeneration and astrocyte reactivity [44]. Further studies have also suggested a role of mitochondrial transfer in creating an immunosuppressive TME. Cancer cells can transfer mitochondria containing mutated mtDNA to tumor-infiltrating lymphocytes, reducing their energy production and impairing their antitumor activity [45]. Mitochondrial transfer appears to be an important process in glioma progression and remains a highly unexplored yet potentially promising area of research.
3.2. Upregulation of Mitophagy
The cellular process of mitophagy, the cellular breakdown and recycling of damaged mitochondria, is generally upregulated in glioma cells as a protective mechanism to deal with cellular stressors. Prognostic models using a mitophagy-related gene signature have shown remarkable accuracy in predicting overall survival, demonstrating the clinical relevance of mitophagy in glioma patients [46,47]. In particular, the BCL2L13 protein has been shown to promote mitochondrial fission-dependent protective mitophagy in glioblastoma cells [48]. Moreover, the induction of mitophagy by FOXO3a has been reported to promote cell survival by protecting glioma cells from temozolomide-induced cytotoxicity [49]. In high-grade glioma cells, mitochondrial respiratory cristae are stabilized and remodeled through mitophagy, enhancing OXPHOS and ultimately promoting aggressive disease progression [50]. While this evidence is strong, it has also been reported that cannabidiol treatment induces high mitophagy levels to kill glioma cells [51]. Thus, the role of mitophagy in tumor progression remains to be elucidated and may function as a double-edged sword.
3.3. Mitochondrial Dynamics
Glioma mitochondria also have altered mitochondrial dynamics—including fission and fusion—which are reported to be critical in glioma progression [52]. Mitochondrial fusion can allow gene products to be transferred between mitochondria for optimized function, especially under metabolic and environmental stresses. Mitochondrial fission is essential for mitochondrial division and quality control [14]. In glioma stem cells, which are responsible for tumor initiation and maintenance, mitochondrial fission is upregulated, along with its key regulator, dynamin-related protein 1 (Drp1), promoting increased cell migration and invasiveness [53]. Drp1 has also been shown to cause hypoxia-induced glioblastoma cell migration [54]. Increased mitochondrial fission and decreased mitochondrial fusion seem to contribute to tumorigenicity in glioma, with Drp1 emerging as a potential therapeutic target. Shifting of mitochondrial dynamics maintains mitochondrial quality and likely reflects the changing needs of the tumor cells throughout tumor progression.
Aberrant mitochondrial quality control and extensive metabolic reprogramming in glioma highlight the mitochondria as critical in tumor progression and treatment resistance. Notably, emerging literature suggests that targeting the mitochondria in glioma may help overcome longstanding therapeutic challenges [14,15,55,56].
4. Therapeutic Challenges in Glioma and the Promise of Mitochondrial Targeting
The challenges to treating glioma are multifold, resulting in difficulties in improving the standard of care. As previously discussed, glioma cells are dynamic and can be fueled through multiple metabolic pathways. The disease is also rapidly evolving, supported by self-renewing GSCs, protected by the blood–brain barrier (BBB) and the blood–tumor barrier (BTB), which are difficult to penetrate, and located within an immunosuppressive tumor microenvironment. Complicating therapy further, high-grade gliomas are characterized by incredibly high level of heterogeneity [57].
4.1. High Inter- and Intratumor Heterogeneity
A major challenge to treatment of high-grade gliomas, particularly glioblastoma, is the high intra- and intertumoral heterogeneity [58]. Characterizing intratumor heterogeneity through anatomical features, the Ivy Glioblastoma Atlas Project has mapped five key components within tumors: leading edge, tumor-infiltrating, cellular tumor, microvascular proliferation, and pseudo palisading cells around necrosis. These regions can be further categorized into specialized niches: perivascular (microvascular proliferation), hypoxia–necrotic (cellular tumor, pseudo palisading cells around necrosis), and invasive (leading edge and tumor-infiltrating) [59]. With each having varied metabolic dependencies, targeted therapies often only affect a certain subset of glioma cells, while residual niches may survive and cause recurrence. Thus, there is an unmet need for therapies that more universally target malignant glioma cells.
Intertumor heterogeneity also presents as a major challenge to treating glioma. Various glioblastoma subtypes between different tumors can be mapped along neurodevelopmental and metabolic axes, creating 4 general classifications: proliferative/progenitor, neuronal, glycolytic/plurimetabolic and mitochondrial [60].
4.2. Targeting the Mitochondria in Subtypes of High-Grade Gliomas
In the past, clinical trials for mitochondrial inhibitors have generally lacked distinction between different metabolic subtypes in high-grade gliomas. Future clinical trials may benefit from a more precise approach by targeting tumors with a higher mitochondrial dependency. Emerging literature has corroborated the efficacy of a precision medicine approach, utilizing mitochondrial-targeting therapies directed at high-grade gliomas that exhibit higher OXPHOS dependence. It was reported that mitochondrial, but not glycolytic/plurimetabolic glioblastoma, exhibited remarkable vulnerability to inhibitors of OXPHOS. Interestingly, the mitochondrial subtype showed higher frequency of CDK4/MDM2 amplification, and all NRAS-mutated glioblastomas mapped exclusively to this subtype, suggesting potential clinical markers. With over 20% of glioblastomas fueled by mitochondrial hyperactivity, this targeted strategy seems promising [60]. In addition, the mitochondrial energy output through OXPHOS has been identified as an essential factor for the rapid cellular proliferation of diffuse midline glioma (DMG) [61]. Thus, DMGs are also believed to be particularly vulnerable to the mitochondrial-targeting strategy, as discussed in detail in a later section.
Notably, while this strategy may be most effective in high-grade gliomas that are especially reliant on the mitochondria, there is evidence that mitochondrial targeting still has a broader effect across diverse glioma niches and subtypes. Given the high metabolic heterogeneity within a tumor, there is evidence that even glycolytic glioma cells can respond to mitochondrial targeting. Though these cells rely on glycolysis for energy production during early stages, lactic acid buildup progressively reduces glycolysis levels and significantly increases OXPHOS processes (approximately 2–4 times) throughout disease progression to generate adequate energy, rendering them vulnerable to OXPHOS inhibitors [62,63]. It is also reported that self-renewing GSCs, which are thought to initiate disease recurrence and treatment resistance, strongly rely on OXPHOS more than differentiated cells [64]. Additionally, it has been demonstrated that glioma cells do, in fact, contain intact energy-producing mitochondria with normal overall OXPHOS levels even while glycolysis remains upregulated [65,66]. Thus, a mitochondrial targeting strategy may indeed have a more wide-ranging efficacy across glioma subtypes and intertumoral niches.
With this strong rationale for mitochondrial targeting, several compounds have been developed to impair mitochondrial functioning in glioma, attempting to exploit vulnerabilities into clinically meaningful outcomes.
5. Efforts in Targeting the Mitochondria in Glioma
Mitochondrial targeting encompasses a large umbrella of drugs that inhibit various mitochondrial processes.
5.1. Targeting the Mitochondria Through OXPHOS Inhibitors
Targeting of the Electron Transport Chain (ETC) through OXPHOS inhibitors has emerged as an effective therapeutic strategy. The ETC, located in the inner mitochondrial membrane is made up of five complexes. Complexes I–IV are involved in the transfer of electrons from electron carriers (NADH and FADH2) to oxygen via oxidation, coupled with the pumping of protons into the intermembrane space. Complex V, known as ATP synthase, then utilizes this electrochemical gradient to phosphorylate ADP into ATP—known as oxidative phosphorylation [67,68]. The ETC is also involved in the production of reactive oxygen species (ROS), which function as somewhat of a double-edged sword in the cell. While moderate levels of ROS are generally associated with genomic instability and tumorigenicity, highly dysregulated ETC function can to a large buildup of ROS resulting in apoptosis [69,70]. In general, OXPHOS inhibitors work by interfering with the ETC to deplete energy stores and increase ROS accumulation.
5.1.1. Inhibitors of Complex I
Complex I (NADH ubiquinone–oxidoreductase) inhibitors have shown promise in preclinical and clinical trials for glioma. Metformin, a biguanide used as an anti-diabetic drug, is known to inhibit Complex I and impair OXPHOS [71,72]. Metformin has been investigated with mefloquine, memantine and TMZ in glioblastoma patients after radiation treatment in a phase II study that suggests effectiveness (median survival of 21 months with a 2-year OS of 43%) and a safety profile of the combined treatments [73]. However, more recently, a phase 2 trial (KNOG-1501 study) demonstrated that while the metformin plus temozolomide regimen was well tolerated, it did not confer a clinical benefit in patients with recurrent glioblastoma [74]. Still, various clinical trials are ongoing seeking to repurpose metformin to treat glioma, and the practicality of the therapy remains to be elucidated [75,76,77].
IACS-010759 is an incredibly selective and potent small-molecule complex I inhibitor, which was advanced into two dose-escalation phase I trials in patients with relapsed/refractory acute myeloid leukemia (NCT02882321, n = 17) and advanced solid tumors (NCT03291938, n = 23) [78]. Despite preclinical evidence of efficacy, only modest antitumor activity was observed at tolerated doses [78]. Intolerable neurotoxicity precluded adequate dosing and prevented the trial from continuing.
IM156, another biguanide and Complex I inhibitor, showed more promise [79]. Preclinical evidence in glioblastoma demonstrated a potent antitumor effect. In a first-in-human phase I study in patients with advanced solid tumors, IM156 was tolerated and was the first OXPHOS inhibitor to establish a recommended phase II dose (RP2D) for further clinical development in cancer. Adverse events were manageable, and stable disease for around 30% of patients with advanced solid tumors was the best reported response [79].
5.1.2. Inhibitors of Complex II
While complex II inhibition has not been investigated in clinical trials for glioma, several complex II inhibitors have demonstrated preclinical efficacy. The atypical adamantyl retinoid, ST1926, alleviated mitochondria-regulated bioenergetics in glioma cells via reducing ATP production and promoting ROS production [80]. The Complex I and II inhibitor and Vitamin E derivative, α-tocopheryl succinate, was reported to potentiate the response to etoposide, a topoisomerase inhibitor, in multidrug-resistant glioblastoma cells [81]. Gracillin, a selective Complex II inhibitor, exhibited potent energy depletion and induced apoptosis in a broad spectrum of cancer cell lines [82]. However, low BBB permeability of gracillin seems to limit its practicality in glioma treatment [82].
5.1.3. Inhibitors of Complex III
Targeting Complex III has also demonstrated preclinical efficacy in gliomas, including drugs such as Mahanine, Atovaquone, Antimycin A, and Licochalcone A. Mahanine, a plant-derived Complex III inhibitor, leads to cell cycle arrest and DNA damage response in glioblastoma cell lines. The accumulation of ROS resulted in Chk1/Chk2 upregulation and activation, which are essential factors for G0/G1 arrest [83,84]. Atovaquone demonstrated cytotoxicity against glioblastoma cell lines as well as provided a confirmed target for atovaquone brain concentrations in in vitro cell viability studies. Due to limitations in the bioavailability of atovaquone, enhanced amorphous solid dispersion was utilized as a proof-of-concept to deliver therapeutically effective brain levels of atovaquone for the treatment of glioblastoma [85]. Antimycin A demonstrated efficacy in treating glioblastoma cell lines when combined with the antimetabolite, 3-bromopyruvate, to critically increase ROS levels and induce cell death [86]. Licochalcone A, a natural chalconoid from licorice root, induced massive caspase-dependent death in GSCs through mitochondrial fragmentation and reduced ATP production [87]. Overall, targeting Complex III and the resulting ROS elevation may prove a promising therapeutic strategy though yet to be proven in a clinical setting.
5.1.4. Inhibitors of Complex IV
Complex IV inhibitors, such as arsenic trioxide (ATO), have shown promising anti-glioma effects through induction of autophagy and apoptosis in GSCs. In both in vitro and in vivo glioblastoma models, arsenic trioxide induced cell death through downregulation of survivin [88]. Several clinical trials have evaluated the efficacy of arsenic trioxide. A phase II clinical trial reported that arsenic trioxide did not improve overall survival in glioblastoma patients compared to historical data [89]. In contrast, a phase I clinical trial (NCT00045565) reported that arsenic trioxide with standard radiation is well tolerated in patients with newly diagnosed glioblastoma. Even without TMZ or adjuvant therapy, the overall survival of all patients (17.7 months) and especially patients who received biweekly arsenic trioxide (22.8 months) was promising [90]. It is important to note that while being a Complex IV inhibitor, arsenic trioxide has several off-target effects that contribute to its antitumor efficacy [91]. The practicality of this therapy in glioma still remains to be elucidated.
5.1.5. Inhibitors of Complex V
Targeting ATP Synthase (Complex V) has emerged as an exciting therapeutic vulnerability, corroborated by strong preclinical evidence. Gboxin, a small-molecule OXPHOS inhibitor, specifically targeted mouse and human glioblastoma cells but not that of mouse embryonic fibroblasts or neonatal astrocytes [92]. Due to limitations in bioavailability, a biomimetic Gboxin nanomedicine was developed that demonstrated good biocompatibility, improved pharmacokinetic profile, efficient BBB permeability and homotypic dual tumor cell and mitochondria targeting [93]. This established preclinical efficacy led to an ongoing Phase I clinical trial to evaluate S-Gboxin, in addition to standard TMZ/radiation therapy for glioblastoma and diffuse midline glioma (NCT06806228). Other Complex V inhibitors, such as bedaquiline and leucinostatin, have demonstrated ability to target ATP synthase, though their efficacy in glioma is unclear [94,95].
A summary of clinical trials in glioma that employ OXPHOS inhibitors is provided in Table 1.

Table 1.
Clinical Trials employing OXPHOS inhibitors to treat High-Grade Glioma.
5.2. Additional Mitochondrial Targeted Strategies in Glioma
While several OXPHOS inhibitors targeting the mitochondrial respiratory complexes have advanced to clinical trials for glioma, other mitochondrial-targeting strategies including inhibition of β-oxidation and disruption of mitochondrial matrix functions have demonstrated preclinical efficacy. Few of these approaches have advanced to the clinical trial stage.
5.2.1. Inhibitors of Beta-Oxidation
Targeting fatty acid oxidation has emerged as a promising therapeutic vulnerability. Etomoxir is a carnitine palmitoyltransferase I inhibitor that prevents ß-oxidation of fatty acids to reduce energy levels. Etoximir was shown to reduce energy production and cellular proliferation in glioma cells [34]. Interestingly, in patient-derived glioblastoma tumor spheres, etomoxir led to a lethal energy reduction, which was exacerbated in combination with TMZ [98]. Etomoxir also effectively reduced invasiveness and improved survival in xenograft glioblastoma mice models [98]. An apparent limitation, however, is that etomoxir was found to have cross reactivity with other proteins, leading to further targets beyond solely fatty acid oxidation [99]. To our knowledge, etomoxir has not yet been translated into clinical trials.
5.2.2. Compounds Targeting Mitochondrial Matrix
Devimistat or CPI-613 selectively targets the TCA cycle enzymes, pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, in order to compromise mitochondrial processes. This interference initiates apoptosis in glioblastoma cell lines and animal models, especially when combined with a BH3-mimetic [55,100,101]. CPI-613 achieved promising outcomes in phase I clinical trials in pancreatic cancer, with treatment being well-tolerated [102].
Gamitrinib, also known as geldanamycin, is a mitochondrial matrix inhibitor that was reported to inhibit of proliferation and induce apoptosis in several glioma cell lines, patient-derived organoids, and xenograft models [103]. This antitumor effect is also further potentiated when combined with a BH3-mimetic. Gamitrinib is currently undergoing assessment in a phase I clinical trial (NCT04827810) involving patients with advanced malignancies, including glioblastoma [104].
5.3. Effects of Mitochondrial Targeting on Cancer Signaling Networks
Mitochondrial targeting also activates several relevant signaling cascades. Many mitochondrial inhibitors lead to accumulation of ROS, which can overwhelm antioxidant defenses and disrupt key survival pathways, ultimately inducing cytotoxicity in glioma cells [86,105]. For example, the phytochemicals, celastrol and triptolide, significantly reduced mitochondrial membrane potential and increased ROS levels, thereby activating the ROS/JNK pathway and triggering autophagy and apoptosis in glioma cells [106,107]. Additionally, ROS accumulation has been shown to inhibit the PI3K/Akt/mTOR pathway, a critical regulator of cancer cell survival and therapy resistance [108]. Together, these findings underscore the therapeutic potential of elevating mitochondrial ROS to disrupt oncogenic signaling and suppress glioma progression.
Interestingly, it has also been shown that targeting mitochondrial function and OXPHOS can destabilize hypoxia-inducible factor 1-alpha (HIF-1α), a critical regulator of glioma progression under hypoxic conditions [109]. Pharmacological inhibition of OXPHOS with metformin or rotenone resulted in decreased HIF-1α expression levels in cancer cells, as well as decreased carbonic anhydrase IX and vascular endothelial growth factor levels, key drivers of invasiveness and angiogenesis [109].
Mitochondrial-targeting drugs can also disrupt mitochondrial membrane potential and cause structural damage. One key effect is outer membrane permeabilization (MOMP), often mediated by BH3-only BCL-2 proteins. This permeabilization facilitates the release of cytochrome c into the cytosol, leading to activation of caspases and initiation of the intrinsic apoptotic pathway in glioma cells [110,111]. These signaling cascades highlight the broad cellular impact of mitochondrial-targeting strategies, which aim to exploit redox balance and disrupt oncogenic pathways to induce potent antitumor responses.
6. Breakthrough in Targeting Mitochondrial Function: Imipridones Induce Mitochondrial Dysfunction in DMG via Hyperactivation of Caseinolytic Protease P
A new generation of mitochondrial-targeting compounds called imipridones has recently offered a glimmer of hope, particularly in the treatment of diffuse midline glioma (DMG). Imipridones are highlighted here, as they have demonstrated strong clinical potential in a disease that has seen limited therapeutic progress over the past several decades.
6.1. Imipridones, a New Drug Class with Potent Antitumor Effects
The first generation of imipridones, ONC201, was originally discovered as TNF-Related Apoptosis Inducing Ligand (TRAIL)-inducing compound 10, in a small-molecule screen of colorectal cancer cell lines [112]. It was reported to activate the integrated stress response, an adaptational signaling pathway that allows cells to shut down protein synthesis through ATF4/CHOP. This cascade ultimately causes cell death through endoplasmic reticulum stress and upregulation of TRAIL receptor DR5 [113].
Using a Bayesian machine learning approach, ONC201 was originally identified as a dopamine receptor D2 (DRD2) antagonist, with in silico modeling confirming ONC201 binds to the active site of DRD2 [114,115]. As DRD2 was previously reported as expressed across brain tumor types, and is linked to its metabolic plasticity, this antagonism was promising [116,117]. In several studies, the antagonism of DRD2 by imipridones was reported to inhibit cell proliferation, induce cell cycle G1 arrest, reduce cell invasion, and cause apoptosis [118]. Despite this, recent evidence has suggested that DRD2 antagonism cannot fully explain the antitumor effect of imipridones. Cancer cells with limited expression of DRD2 still remain sensitive to ONC201, suggesting alternative mechanisms of action [119,120]. Transient knockdown of DRD2 in colorectal cancer cells minimally affected response to ONC201, further indicating DRD2 antagonism may not be the cause of imipridone cytotoxicity [121].
6.2. ClpP Identified as a Target of Imipridones
Recent studies have attributed the antitumor effect of imipridones to agonism of the caseinolytic protease P (ClpP), a serine protease located specifically in the mitochondrial matrix that regulates protein integrity and several key mitochondrial functions. ClpP forms a heterodimer with its ClpX chaperone, creating a complex termed ClpXP. When functioning normally, ClpXP performs protein quality control in the mitochondria by degrading denatured or misfolded protein to maintain the integrity of the respiratory chain and sustain OXPHOS. Mechanistically, ClpP agonists displace ClpX, opening the pore of the ClpP protease and strongly increasing its protease activity leading to mitochondrial degradation [122].
ClpP was confirmed as a target of imipridones through crystallography and biochemical studies, and ClpP agonism was shown to induce selective lethality in cancer cells [119,120]. In silico binding of ONC201 to ClpP also confirmed ClpP as a target [115]. Furthermore, CRISPR-mediated ClpP knockdown in DMG cell lines abolished ONC201 efficacy, supporting ClpP as a critical mediator of its antitumor activity [123]. As ClpP is overexpressed across diffuse gliomas at an mRNA and protein level, the therapeutic potential of imipridones in glioma remains incredibly promising [124,125,126].
Moreover, investigators have recently developed a new class of more potent and selective imipridones, known as TR compounds [120]. These compounds were reported to be solely ClpP agonists without DRD2 antagonist function. Several preclinical studies so far have reported potent efficacy of TR-107 and related analogs in breast, colorectal, and brain tumor models [127,128,129]. Imipridone treatment and ClpP agonism hyperactivated mitochondrial proteolysis, leading to the uncontrolled degradation of essential mitochondrial proteins, disruption of OXPHOS, catastrophic metabolic collapse, and ultimately cell death [115,119,122]. In glioblastoma, combining TRAIL-secreting neural stem cells with TR-107 synergistically activated apoptotic caspases, overcame TRAIL resistance, suppressed tumor growth, and significantly extended survival in mouse xenograft models [129]. While imipridone therapy has been tested in several clinical trials across a variety of cancers, it has demonstrated specific clinical utility in H3K27-altered diffuse midline glioma.
6.3. Clinical Efficacy of Imipridones for Diffuse Midline Glioma
Several clinical trials have evaluated the safety and efficacy of imipridones, including ONC201 and its more potent analog ONC206, in diffuse gliomas. A first-in-human clinical trial of ONC201 (NCT02525692) for diffuse gliomas was opened in August 2015 and was later expanded to include H3K27-altered DMG patients after an exceptional responder experienced a near-complete objective response including regression of the primary thalamic region [130,131]. Various clinical trials have opened in recent years to evaluate the efficacy of imipridones in DMG. A phase I dose escalation and an expansion trial (NCT03416530 and NCT03134131) of ONC201 reported aggregate results, with a median overall survival of patients with non-recurrent H3K27M DMG (n = 35) of 21.7 months from diagnosis and 9.3 months at recurrence [132]. The trials reported no dose-limiting toxicities, with a treatment dose that was overall well tolerated [132]. These incredibly promising results have led to several ongoing Phase 2 and 3 trials, and ONC201 is expected to be submitted for accelerated FDA approval by the end of the year.
6.4. Evaluating the Response to Imipridone Therapy
To monitor treatment response, recent studies have searched for prognostic indicators and biomarkers that can evaluate H3K27M DMG’s response to imipridones. The H3K27M variant allele fraction (VAF) was measured in cell-free tumor DNA samples in DMG patients treated with ONC201. Decreased VAF levels correlated with increased progression-free survival, nearly doubling time to progression [133]. Interestingly, H3K27M VAF spikes preceded progression in a majority of cases [133]. As such, H3K27M VAF may serve as a potential prognostic indicator for imipridone therapy in DMG to predict progression.
Moreover, demonstrating the biochemical effects of imipridone therapy, it was reported that mice bearing orthotopic DMG xenografts treated with ONC206 accumulate gamma-aminobutyric acid (GABA) levels within a week of treatment [134]. Acting in an autocrine manner, it was further shown that GABA can mitigate imipridone-induced oxidative stress to avoid apoptosis. GABA seemed to be a unique compensatory adaptation to imipridones. The emergence of this GABA-mediated cytoprotection suggests that glioma cells may adapt to mitochondrial stress, underscoring the potential need for combination strategies [134].
A summary of clinical trials that employ imipridones in high-grade glioma is provided in Table 2.

Table 2.
Clinical Trials employing Imipridones to treat High-Grade Glioma.
7. Mitochondrial Targeting Can Alter the Epigenetic Landscape in Gliomas
Glioma exhibits strong pathogenic crosstalk between mitochondrial function and epigenetic regulation, which can be exploited through mitochondrial-targeting strategies. This interplay is mediated by key mitochondrial metabolites that serve as substrates or cofactors for epigenetic enzymes, as illustrated in Figure 2.
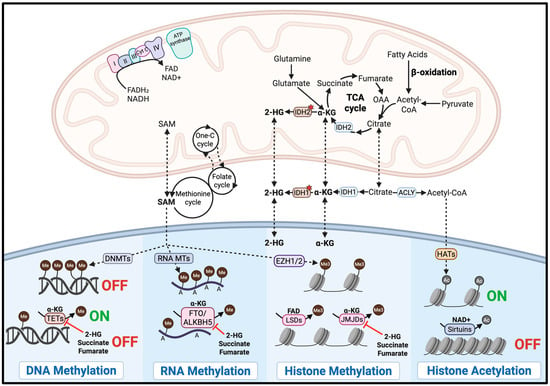
Figure 2.
Mitochondrial-Epigenetic Crosstalk in Glioma. Mitochondrial metabolites are linked to the activity of epigenetic enzymes. The red stars indicate mutant IDH. The image was created with BioRender.com accessed on 2 September 2025.
7.1. Mitochondrial-Epigenetic Crosstalk in Glioma
Mitochondria play a critical role in regulating epigenetic modifications by supplying key metabolites required for chromatin remodeling. For instance, histone acetylation through histone acetyltransferases (HATs) depends on acetyl-CoA, a metabolite primarily generated in the mitochondria, to promote gene activation. In glioblastoma, fluctuations in acetyl-CoA levels have been shown to regulate H3K27 acetylation at specific genomic loci, with ATP citrate lyase (ACLY)-dependent acetyl-CoA production driving oncogenic gene expression programs involved in cell adhesion and migration [136]. Conversely, histone deacetylation is mediated by histone deacetylases, including the NAD+-dependent sirtuin family, linking their activity directly to the mitochondrial metabolic state [137]. Therefore, targeting mitochondrial function can alter the availability of acetyl-CoA and NAD+, leading to epigenetic reprogramming that may suppress oncogenic pathways in glioma.
Furthermore, DNA, RNA, and histone methylation is regulated by DNA methyltransferases (DNMTs), RNA methyltransferases (RNA MTs), and histone methyltransferases, including enhancer of zeste homolog 1 and 2 (EZH1 and EZH2). All of these methyltransferases require S-adenosylmethionine (SAM) as the methyl group donor [138,139]. While SAM is primarily synthesized in the cytosol, its production depends on mitochondrial metabolism, which supplies one-carbon units via the folate and methionine cycles [137]. In glioma stem cells, methionine depletion and resulting reduction in SAM levels led to global DNA demethylation and altered histone methylation, ultimately decreasing the expression of stemness-related genes, impairing tumor growth and inducing cell death [140]. Thus, targeting mitochondrial function may reduce SAM availability, disrupt epigenetic regulation, and exert antitumor effects through epigenetic reprogramming.
DNA demethylation is mediated by ten-eleven translocation (TET) dioxygenases. RNA demethylation is performed by the fat mass and obesity-associated protein (FTO) and the AlkB homolog 5 protein (ALKBH5) [141,142]. Histone demethylation is carried out by JmjC-domain demethylases (JMJDs) and lysine demethylases (LSDs). TETs, FTO, ALKBH5, and JMJDs require α-ketoglutarate (α-KG) as a cofactor, a key intermediate of the TCA cycle in the mitochondria, whereas LSDs depend on FAD, another mitochondrial metabolite [137,138,142,143]. Therefore, targeting the mitochondria may reduce intracellular levels of α-KG or FAD, decreasing demethylase activity and thereby disrupting pathogenic gene expression programs in glioma.
Notably, oncometabolites such as 2-hydroxyglutarate (2-HG), succinate, and fumarate can competitively inhibit α-KG-dependent demethylases. In high-grade gliomas with the mitochondrial IDH2 mutation, mutant IDH2 aberrantly produce 2-HG, which inhibits TETs, FTO, ALKBH5, and JMJDs. This inhibition promotes widespread DNA hypermethylation and transcriptional silencing [144]. Targeting mitochondrial function may lower 2-HG levels and help reverse the epigenetic alterations driving oncogenesis in IDH-mutant gliomas.
Together, these metabolite–enzyme interactions underscore the critical role of mitochondrial metabolism in shaping the epigenetic landscape of glioma. By influencing the availability of key cofactors and substrates, mitochondria help regulate DNA, RNA, and histone modifications that control gene expression. These insights suggest that targeting mitochondrial function could potentially reverse pathogenic epigenetic alterations and suppress tumor growth. However, the epigenetic consequences of mitochondrial-targeted therapies in glioma remain largely unexplored, with limited direct evidence available to date.
7.2. Evidence for Epigenetic Modulation via Mitochondrial Targeting
The drug class of mitochondrial-targeting imipridones have demonstrated a remarkable ability to reverse the pathogenic phenotype of H3K27-altered DMG, which is characterized by global histone hypomethylation and a widespread reduction in H3K27me3, driving aggressive tumor growth. ONC201 can reverse the epigenetic state of DMG by increasing genomic H3K27me3 in H3K27M-DMG patient tumors. The early reversal of low H3K27me3 with ONC201 treatment was incredibly promising [135]. The investigators also observed a variety of favorable epigenetic alterations after treatment with ONC201, resulting from decreased chromatin accessibility at promoters and enhancers. By simultaneously suppressing key energy-producing metabolic pathways and reversing pathogenic reduction in H3K27me3, the treatment led to epigenetic downregulation of neuroglial differentiation and cell cycle genes in H3K27M-mutant DMG cells [135]. These epigenetic changes resulting from ClpP agonism suggest that targeting the mitochondria may also lead to epigenetic modulation, contributing to the therapeutic effect.
This connection has also demonstrated potential in other high-grade gliomas. A mitochondrial-targeting nanotherapy has been reported to exert potent antitumor effects in high-grade IDH2-mutated glioma by disrupting the mitochondrial–epigenetic axis. The hypericin-conjugated gold nanoparticles accumulate in glioma mitochondria and, upon red-light activation, induce oxidative stress and mitochondrial dysfunction, leading to a reduction in mutant IDH2 levels. In addition, degradation of the histone methyltransferases EZH1 and EZH2 led to decreased histone trimethylation, potentially reactivating tumor suppressor genes. Efficacy of this dual-targeting effect was demonstrated in xenograft mice models [145]. While epigenetic modulation via targeting the mitochondria in glioma remains highly unexplored, it holds potential in enhancing the therapeutic efficacy of mitochondrial targeting.
8. Conclusions
It is evident that the mitochondria are central orchestrators of glioma development, performing a wide array of dynamic functions that support rapid tumor growth. Through extensive metabolic reprogramming, glioma mitochondria supply the energy and biosynthetic intermediates required for glioma progression and resistance, establishing them as an attractive therapeutic target. Several preclinical and clinical efforts have aimed to exploit this vulnerability, though success has been mixed. A major clinical barrier has been toxicity, as demonstrated in the phase I trial of IACS-010759, which failed to reach the recommended phase II dosing due to neurotoxicity [78]. These challenges stemming from narrow therapeutic windows and systematic effects highlight the need for cautious advancement.
A precision medicine approach may represent the next logical step, leveraging mitochondrial-targeted therapies in gliomas that exhibit heightened mitochondrial dependence. Deeper understanding of glioma-specific mitochondrial biology will be essential to identify predictive biomarkers and design more selective targeting strategies [146]. Specific features such as altered mitochondrial transfer, mitochondrial dynamics, or dysregulated mitophagy, offer emerging avenues for intervention.
For example, since mitochondrial transfer has been implicated in glioma immune evasion, its inhibition may enhance responses to immunotherapy [45]. Similarly, as both mitochondrial transfer and BNIP3-mediated mitophagy are linked to chemoresistance, targeting these processes in combination with temozolomide may improve treatment efficacy [43,49]. More broadly, disrupting mitochondrial quality control could impair glioma cells’ ability to adapt metabolically and withstand radiation or chemotherapy. Given the strong association between mitochondrial dysfunction and therapy resistance, targeting mitochondrial metabolic pathways may also delay resistance and potentiate the standard-of-care, such as temozolomide and radiation [12].
Recently, the discovery of imipridones and induction of mitochondrial degeneration through ClpP agonism has sparked hope in the mitochondrial-targeting strategy, resulting in promising clinical trials for the treatment of DMG. The ability of imipridones to initiate mitochondrial collapse, trigger integrated stress response, and reverse epigenetic aberrations positions them as powerful, multifaceted agents, particularly in the context of DMG. Interestingly, combining ClpP agonists with epigenetic therapies, such as HDAC or EZH1/2 inhibitors, has also been shown to enhance antitumor efficacy in high-grade gliomas [147,148]. This new generation of imipridones hold promise in translating mitochondrial targeting into clinically meaningful outcomes.
Realizing the full therapeutic potential of mitochondrial targeting hinges on overcoming drug delivery challenges particularly across the blood–brain barrier (BBB) and into the mitochondrial compartment. Promising approaches include lipid-based nanocarriers, such as liposomes and solid lipid nanoparticles, engineered to cross the BBB and functionalized with mitochondrial-targeting moieties like triphenylphosphonium. Inorganic nanoparticles, including gold and mesoporous silica particles, can enhance intracellular delivery and be conjugated with mitochondrial-targeting ligands. Peptide-based systems, such as mitochondrial-penetrating peptides or mitochondrial targeting sequences, offer high specificity for mitochondrial membranes but often require integration with nanocarriers for effective blood–brain penetration. Collectively, advances in these platforms may help address the critical challenges of systemic toxicity while improving therapeutic specificity [149].
We acknowledge several limitations of this review. Firstly, our discussion of mitochondrial processes such as mitophagy and dynamics has been simplified to provide an overview of the current understanding. These processes are likely far more complex and context-dependent than represented here. Secondly, our discussion of drugs targeting mitochondrial respiratory complexes, including agents like metformin and arsenic trioxide, has been simplified. While these compounds exhibit mitochondrial activity, they often exert some antitumor effects through additional, non-mitochondrial mechanisms. Lastly, therapeutic strategies aimed at modulating epigenetics through mitochondrial targeting remain largely experimental. The current evidence base is limited, and further research is needed to validate the efficacy and mechanistic underpinnings of these approaches in glioma.
Looking ahead, the future of mitochondrial-targeted therapies in glioma will hinge on advancing our molecular understanding of glioma-specific mitochondrial vulnerabilities and on continued innovation in targeted drug delivery systems. As our insight into mitochondrial function in glioma deepens, so too does the potential to translate these insights into more precise, less toxic, and ultimately transformative therapies for patients with this devastating disease.
Author Contributions
Conceptualization and writing—original draft preparation, S.S. and J.W.; review and editing, S.S., Q.L., J.J. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Intramural Research Program of the NIH, National Cancer Institute (ZIA BC011840).
Acknowledgments
This work was supported by the National Cancer Institute’s Intramural Research Program. The authors would also like to thank all the patients and families for their participation in the glioma clinical trials reviewed in this article and for their support of our clinical research.
Conflicts of Interest
The authors declare no conflicts of interest. The contributions of the NIH author(s) were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered works of the United States Government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Hess, K.R.; Gleason, M.J.; Jaeckle, K.A.; Kyritsis, A.P.; Prados, M.D.; Levin, V.A.; Yung, W.K. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J. Clin. Oncol. 1999, 17, 2572–2578. [Google Scholar] [CrossRef]
- Jackson, S.; Patay, Z.; Howarth, R.; Pai Panandiker, A.S.; Onar-Thomas, A.; Gajjar, A.; Broniscer, A. Clinico-radiologic characteristics of long-term survivors of diffuse intrinsic pontine glioma. J. Neurooncol. 2013, 114, 339–344. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Veldhuijzen van Zanten, S.E.M.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Chan, K.M.; Fang, D.; Gan, H.; Hashizume, R.; Yu, C.; Schroeder, M.; Gupta, N.; Mueller, S.; James, C.D.; Jenkins, R.; et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013, 27, 985–990. [Google Scholar] [CrossRef]
- Kannan, A.; Wells, R.B.; Sivakumar, S.; Komatsu, S.; Singh, K.P.; Samten, B.; Philley, J.V.; Sauter, E.R.; Ikebe, M.; Idell, S.; et al. Mitochondrial Reprogramming Regulates Breast Cancer Progression. Clin. Cancer Res. 2016, 22, 3348–3360. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, A.; Wang, J.; Zhang, Z.; Tao, L. Mitochondrial metabolic reprogramming in colorectal cancer: Mechanisms of resistance and future clinical interventions. Cell Death Discov. 2025, 11, 375. [Google Scholar] [CrossRef]
- Chuang, C.H.; Dorsch, M.; Dujardin, P.; Silas, S.; Ueffing, K.; Holken, J.M.; Yang, D.; Winslow, M.M.; Gruner, B.M. Altered Mitochondria Functionality Defines a Metastatic Cell State in Lung Cancer and Creates an Exploitable Vulnerability. Cancer Res. 2021, 81, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Zhu, M.; Su, Q.; Zhu, Z.; Yang, T.; Chen, Y.; Peng, X.; Zhang, Y. Targeting mitochondrial dysfunctions in pancreatic cancer evokes new therapeutic opportunities. Crit. Rev. Oncol. Hematol. 2022, 180, 103858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Liu, X.; Deng, H. Multi-Omics Analyses Reveal Mitochondrial Dysfunction Contributing to Temozolomide Resistance in Glioblastoma Cells. Biomolecules 2023, 13, 1408. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Shang, C.; Hong, Y. The Role and Applied Value of Mitochondria in Glioma-Related Research. CNS Neurosci. Ther. 2024, 30, e70121. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Liu, Y.; Cheng, C. The promise of mitochondria in the treatment of glioblastoma: A brief review. Discov. Oncol. 2025, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Posener, K.; Negelein, E. On the metabolism of carcinoma cells. Biochem. Z. 1924, 152, 309–344. [Google Scholar] [CrossRef]
- Strickland, M.; Stoll, E.A. Metabolic Reprogramming in Glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Cortes Ballen, A.I.; Amosu, M.; Ravinder, S.; Chan, J.; Derin, E.; Slika, H.; Tyler, B. Metabolic Reprogramming in Glioblastoma Multiforme: A Review of Pathways and Therapeutic Targets. Cells 2024, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Belisario, D.C.; Kopecka, J.; Pasino, M.; Akman, M.; De Smaele, E.; Donadelli, M.; Riganti, C. Hypoxia Dictates Metabolic Rewiring of Tumors: Implications for Chemoresistance. Cells 2020, 9, 2598. [Google Scholar] [CrossRef]
- Komaki, S.; Sugita, Y.; Furuta, T.; Yamada, K.; Moritsubo, M.; Abe, H.; Akiba, J.; Miyagi, N.; Nakamura, H.; Miyoshi, H.; et al. Expression of GLUT1 in Pseudopalisaded and Perivascular Tumor Cells Is an Independent Prognostic Factor for Patients With Glioblastomas. J. Neuropathol. Exp. Neurol. 2019, 78, 389–397. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Lewis, C.A.; Brault, C.; Peck, B.; Bensaad, K.; Griffiths, B.; Mitter, R.; Chakravarty, P.; East, P.; Dankworth, B.; Alibhai, D.; et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 2015, 34, 5128–5140. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Y.; Yang, Y.; Jin, M.; Ma, A.; Wang, X.; Zhao, Q.; Zhang, X.; Zheng, J.; Zheng, X. Lipid metabolism: The potential therapeutic targets in glioblastoma. Cell Death Discov. 2025, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Kacem, K.; Lacombe, P.; Seylaz, J.; Bonvento, G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 1998, 23, 1–10. [Google Scholar] [CrossRef]
- Boado, R.J.; Black, K.L.; Pardridge, W.M. Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Brain Res. Mol. Brain Res. 1994, 27, 51–57. [Google Scholar] [CrossRef]
- Libby, C.J.; Gc, S.; Benavides, G.A.; Fisher, J.L.; Williford, S.E.; Zhang, S.; Tran, A.N.; Gordon, E.R.; Jones, A.B.; Tuy, K.; et al. A role for GLUT3 in glioblastoma cell invasion that is not recapitulated by GLUT1. Cell Adh Migr. 2021, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Obara-Michlewska, M.; Szeliga, M. Targeting Glutamine Addiction in Gliomas. Cancers 2020, 12, 310. [Google Scholar] [CrossRef]
- El Khayari, A.; Bouchmaa, N.; Taib, B.; Wei, Z.; Zeng, A.; El Fatimy, R. Metabolic Rewiring in Glioblastoma Cancer: EGFR, IDH and Beyond. Front. Oncol. 2022, 12, 901951. [Google Scholar] [CrossRef]
- Ferrarese, R.; Izzo, A.; Andrieux, G.; Lagies, S.; Bartmuss, J.P.; Masilamani, A.P.; Wasilenko, A.; Osti, D.; Faletti, S.; Schulzki, R.; et al. ZBTB18 inhibits SREBP-dependent lipid synthesis by halting CTBPs and LSD1 activity in glioblastoma. Life Sci. Alliance 2023, 6, e202201400. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef]
- Kant, S.; Kesarwani, P.; Prabhu, A.; Graham, S.F.; Buelow, K.L.; Nakano, I.; Chinnaiyan, P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis. 2020, 11, 253. [Google Scholar] [CrossRef]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, X.; Gao, P.; Han, X.; Zhao, P.; Xie, F.; Liu, M. Advancing glioblastoma treatment by targeting metabolism. Neoplasia 2024, 51, 100985. [Google Scholar] [CrossRef]
- Miska, J.; Chandel, N.S. Targeting fatty acid metabolism in glioblastoma. J. Clin. Investig. 2023, 133, e163448. [Google Scholar] [CrossRef]
- Alzial, G.; Renoult, O.; Paris, F.; Gratas, C.; Clavreul, A.; Pecqueur, C. Wild-type isocitrate dehydrogenase under the spotlight in glioblastoma. Oncogene 2022, 41, 613–621. [Google Scholar] [CrossRef]
- Wahl, D.R.; Dresser, J.; Wilder-Romans, K.; Parsels, J.D.; Zhao, S.G.; Davis, M.; Zhao, L.; Kachman, M.; Wernisch, S.; Burant, C.F.; et al. Glioblastoma Therapy Can Be Augmented by Targeting IDH1-Mediated NADPH Biosynthesis. Cancer Res. 2017, 77, 960–970. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Pearson, D.M.; Kocialkowski, S.; Backlund, L.M.; Chan, R.; Jones, D.T.; Collins, V.P. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009, 11, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Park, I.; Lee, S.; Sun, C.H.; Koh, Y.; Park, S.H.; Kim, J.E.; Yun, H.; Lee, S.H. Genomic dynamics associated with malignant transformation in IDH1 mutated gliomas. Oncotarget 2015, 6, 43653–43666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miki, K.; Yagi, M.; Hatae, R.; Otsuji, R.; Miyazaki, T.; Goto, K.; Setoyama, D.; Fujioka, Y.; Sangatsuda, Y.; Kuga, D.; et al. Glutaminolysis is associated with mitochondrial pathway activation and can be therapeutically targeted in glioblastoma. Cancer Metab. 2024, 12, 35. [Google Scholar] [CrossRef]
- Nakhle, J.; Khattar, K.; Ozkan, T.; Boughlita, A.; Abba Moussa, D.; Darlix, A.; Lorcy, F.; Rigau, V.; Bauchet, L.; Gerbal-Chaloin, S.; et al. Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring. Cancer Res. Commun. 2023, 3, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Bayik, D.; Storevik, S.; Moreino, S.S.; Sprowls, S.A.; Han, J.; Augustsson, M.T.; Lauko, A.; Sravya, P.; Rosland, G.V.; et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat. Cancer 2023, 4, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Kawase, K.; Nishi, T.; Watanabe, T.; Takenaga, K.; Inozume, T.; Ishino, T.; Aki, S.; Lin, J.; Kawashima, S.; et al. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature 2025, 638, 225–236. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, G. Identification of mitophagy-related genes impacting patient survival in glioma. Discov. Oncol. 2025, 16, 140. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, X.; Huang, J.; Zhuo, Z.; Chen, H.; Zeng, R.; Wu, H.; Guo, K.; Yang, Q.; Ye, H.; et al. Development and validation of a novel mitophagy-related gene prognostic signature for glioblastoma multiforme. BMC Cancer 2022, 22, 644. [Google Scholar] [CrossRef]
- Wang, J.; Chen, A.; Xue, Z.; Liu, J.; He, Y.; Liu, G.; Zhao, Z.; Li, W.; Zhang, Q.; Chen, A.; et al. BCL2L13 promotes mitophagy through DNM1L-mediated mitochondrial fission in glioblastoma. Cell Death Dis. 2023, 14, 585. [Google Scholar] [CrossRef]
- He, C.; Lu, S.; Wang, X.Z.; Wang, C.C.; Wang, L.; Liang, S.P.; Luo, T.F.; Wang, Z.C.; Piao, M.H.; Chi, G.F.; et al. FOXO3a protects glioma cells against temozolomide-induced DNA double strand breaks via promotion of BNIP3-mediated mitophagy. Acta Pharmacol. Sin. 2021, 42, 1324–1337. [Google Scholar] [CrossRef]
- Li, X.; Tie, J.; Sun, Y.; Gong, C.; Deng, S.; Chen, X.; Li, S.; Wang, Y.; Wang, Z.; Wu, F.; et al. Targeting DNM1L/DRP1-FIS1 axis inhibits high-grade glioma progression by impeding mitochondrial respiratory cristae remodeling. J. Exp. Clin. Cancer Res. 2024, 43, 273. [Google Scholar] [CrossRef]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Eugenio-Perez, D.; Briones-Herrera, A.; Martinez-Klimova, E.; Pedraza-Chaverri, J. Divide et Impera: Drp1-mediated Mitochondrial Fission in Glioma Malignancy. Yale J. Biol. Med. 2019, 92, 423–433. [Google Scholar]
- Wan, Y.Y.; Zhang, J.F.; Yang, Z.J.; Jiang, L.P.; Wei, Y.F.; Lai, Q.N.; Wang, J.B.; Xin, H.B.; Han, X.J. Involvement of Drp1 in hypoxia-induced migration of human glioblastoma U251 cells. Oncol. Rep. 2014, 32, 619–626. [Google Scholar] [CrossRef]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Di Nunno, V.; Ghelardini, A.; Tosoni, A.; Bartolini, S.; Asioli, S.; Ratti, S.; Di Stefano, A.L.; Franceschi, E. Targeting Mitochondria in Glioma: New Hopes for a Cure. Biomedicines 2024, 12, 2730. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Duraj, T.; Garcia-Romero, N.; Carrion-Navarro, J.; Madurga, R.; Mendivil, A.O.; Prat-Acin, R.; Garcia-Canamaque, L.; Ayuso-Sacido, A. Beyond the Warburg Effect: Oxidative and Glycolytic Phenotypes Coexist within the Metabolic Heterogeneity of Glioblastoma. Cells 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, R.B.; Shah, N.; Miller, J.; Dalley, R.; Nomura, S.R.; Yoon, J.G.; Smith, K.A.; Lankerovich, M.; Bertagnolli, D.; Bickley, K.; et al. An anatomic transcriptional atlas of human glioblastoma. Science 2018, 360, 660–663. [Google Scholar] [CrossRef]
- Garofano, L.; Migliozzi, S.; Oh, Y.T.; D’Angelo, F.; Najac, R.D.; Ko, A.; Frangaj, B.; Caruso, F.P.; Yu, K.; Yuan, J.; et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat. Cancer 2021, 2, 141–156. [Google Scholar] [CrossRef]
- Shen, H.; Mudassar, F.; Ma, S.; Wang, X.; Nguyen, S.; Bal, N.; Huynh, Q.-S.; Wang, D.; Chang, C.; Ing, P.; et al. Inhibition of mitochondrial bioenergetics and hypoxia to radiosensitize diffuse intrinsic pontine glioma. Neuro-Oncology 2024, 27, 1061–1075. [Google Scholar] [CrossRef]
- Duan, K.; Liu, Z.J.; Hu, S.Q.; Huo, H.Y.; Xu, Z.R.; Ruan, J.F.; Sun, Y.; Dai, L.P.; Yan, C.B.; Xiong, W.; et al. Lactic acid induces lactate transport and glycolysis/OXPHOS interconversion in glioblastoma. Biochem. Biophys. Res. Commun. 2018, 503, 888–894. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, X. Lactic acidosis switches cancer cells from dependence on glycolysis to OXPHOS and renders them highly sensitive to OXPHOS inhibitors. Biochem. Biophys. Res. Commun. 2023, 671, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, E.; Lagadec, C.; Vergnes, L.; Matsutani, T.; Masui, K.; Poulou, M.; Popescu, R.; Della Donna, L.; Evers, P.; Dekmezian, C.; et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16062–16067. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, Y.; Wu, S. The Warburg effect: Evolving interpretations of an established concept. Free Radic. Biol. Med. 2015, 79, 253–263. [Google Scholar] [CrossRef]
- Brisudova, P.; Stojanovic, D.; Novak, J.; Nahacka, Z.; Oliveira, G.L.; Vanatko, O.; Dvorakova, S.; Endaya, B.; Truksa, J.; Kubiskova, M.; et al. Functional mitochondrial respiration is essential for glioblastoma tumour growth. Oncogene 2025, 44, 2588–2603. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.P.; Oliveira, P.J.; Urbano, A.M. Mitochondria: An overview of their origin, genome, architecture, and dynamics. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167803. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caffo, M.; Minutoli, L.; Marini, H.; Abbritti, R.V.; Squadrito, F.; Trichilo, V.; Valenti, A.; Barresi, V.; Altavilla, D.; et al. ROS and Brain Gliomas: An Overview of Potential and Innovative Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 984. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; ME, G.G.; Perez-Gomez, J.M.; Montero-Hidalgo, A.J.; Martin-Colom, J.; Doval-Rosa, C.; Blanco-Acevedo, C.; Torres, E.; Toledano-Delgado, A.; Sanchez-Sanchez, R.; et al. Metformin and simvastatin exert additive antitumour effects in glioblastoma via senescence-state: Clinical and translational evidence. EBioMedicine 2023, 90, 104484. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Mansour, H.M.; Aguilar, T.M.; Lucke-Wold, B. Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. Int. J. Mol. Sci. 2024, 25, 5694. [Google Scholar] [CrossRef]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2019, 125, 424–433. [Google Scholar] [CrossRef]
- Yoon, W.S.; Chang, J.H.; Kim, J.H.; Kim, Y.J.; Jung, T.Y.; Yoo, H.; Kim, S.H.; Ko, Y.C.; Nam, D.H.; Kim, T.M.; et al. Efficacy and safety of metformin plus low-dose temozolomide in patients with recurrent or refractory glioblastoma: A randomized, prospective, multicenter, double-blind, controlled, phase 2 trial (KNOG-1501 study). Discov. Oncol. 2023, 14, 90. [Google Scholar] [CrossRef]
- Shenouda, G.; Souhami, L.; Petrecca, K.; Owen, S.; Panet-Raymond, V.; Guiot, M.C.; Corredor, A.G.; Abdulkarim, B. A Phase 2 Trial of Neoadjuvant Temozolomide Followed by Hypofractionated Accelerated Radiation Therapy with Concurrent and Adjuvant Temozolomide for Patients with Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 487–494. [Google Scholar] [CrossRef]
- Khurshed, M.; Molenaar, R.J.; van Linde, M.E.; Mathot, R.A.; Struys, E.A.; van Wezel, T.; van Noorden, C.J.F.; Klumpen, H.J.; Bovee, J.; Wilmink, J.W. A Phase Ib Clinical Trial of Metformin and Chloroquine in Patients with IDH1-Mutated Solid Tumors. Cancers 2021, 13, 2474. [Google Scholar] [CrossRef]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J. Neurooncol. 2021, 153, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Daver, N.; Mahendra, M.; Zhang, J.; Kamiya-Matsuoka, C.; Meric-Bernstam, F.; Kantarjian, H.M.; Ravandi, F.; Collins, M.E.; Francesco, M.E.D.; et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: Phase I trials. Nat. Med. 2023, 29, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Beom, S.H.; Moon, Y.W.; Kim, T.W.; Shin, Y.G.; Yim, D.S.; Kim, G.M.; Kim, H.S.; Kim, S.Y.; Cheong, J.H.; et al. First-in-human study of IM156, a novel potent biguanide oxidative phosphorylation (OXPHOS) inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 1001–1010. [Google Scholar] [CrossRef]
- De, L.; Yuan, T.; Yong, Z. ST1926 inhibits glioma progression through regulating mitochondrial complex II. Biomed. Pharmacother. 2020, 128, 110291. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, E.; Youk, H.J.; Kim, S.H.; Lee, H.J.; Park, Y.G.; Lim, S.J. Potentiation by alpha-tocopheryl succinate of the etoposide response in multidrug resistance protein 1-expressing glioblastoma cells. Cancer Lett. 2005, 217, 181–190. [Google Scholar] [CrossRef]
- Min, H.Y.; Jang, H.J.; Park, K.H.; Hyun, S.Y.; Park, S.J.; Kim, J.H.; Son, J.; Kang, S.S.; Lee, H.Y. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019, 10, 810. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Bag, A.K.; Tripathi, R.; Samanta, S.K.; Pal, B.C.; Shaha, C.; Mandal, C. Mahanine, a novel mitochondrial complex-III inhibitor induces G0/G1 arrest through redox alteration-mediated DNA damage response and regresses glioblastoma multiforme. Am. J. Cancer Res. 2014, 4, 629–647. [Google Scholar]
- Chen, M.; Yin, X.; Lu, C.; Chen, X.; Ba, H.; Cai, J.; Sun, J. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem. Biol. Interact. 2019, 299, 1–7. [Google Scholar] [CrossRef]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020, 10, 17872. [Google Scholar] [CrossRef] [PubMed]
- Petricciuolo, M.; Davidescu, M.; Fettucciari, K.; Gatticchi, L.; Brancorsini, S.; Roberti, R.; Corazzi, L.; Macchioni, L. The efficacy of the anticancer 3-bromopyruvate is potentiated by antimycin and menadione by unbalancing mitochondrial ROS production and disposal in U118 glioblastoma cells. Heliyon 2020, 6, e05741. [Google Scholar] [CrossRef]
- Kuramoto, K.; Suzuki, S.; Sakaki, H.; Takeda, H.; Sanomachi, T.; Seino, S.; Narita, Y.; Kayama, T.; Kitanaka, C.; Okada, M. Licochalcone A specifically induces cell death in glioma stem cells via mitochondrial dysfunction. FEBS Open Bio 2017, 7, 835–844. [Google Scholar] [CrossRef]
- Chiu, H.W.; Ho, Y.S.; Wang, Y.J. Arsenic trioxide induces autophagy and apoptosis in human glioma cells in vitro and in vivo through downregulation of survivin. J. Mol. Med. 2011, 89, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Grimm, S.; Chandler, J.; Mehta, M.; Marymont, M.; Levy, R.; Muro, K.; Helenowski, I.; McCarthy, K.; Fountas, L.; et al. A phase II trial of arsenic trioxide and temozolomide in combination with radiation therapy for patients with malignant gliomas. J. Neurooncol. 2017, 133, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Ye, X.; Olson, J.J.; Mikkelsen, T.; Bangiyev, L.; Lesser, G.J.; Batchelor, T.; Nabors, B.; Desideri, S.; Walbert, T.; et al. Phase I and pharmacodynamic study of arsenic trioxide plus radiotherapy in patients with newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2024, 6, vdae089. [Google Scholar] [CrossRef]
- Lu, J.; Chew, E.H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef]
- Shi, Y.; Lim, S.K.; Liang, Q.; Iyer, S.V.; Wang, H.Y.; Wang, Z.; Xie, X.; Sun, D.; Chen, Y.J.; Tabar, V.; et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature 2019, 567, 341–346. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, Y.; Wang, Y.; Zhang, D.; Yang, H.; Wang, X.; Zheng, M.; Shi, B. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat. Commun. 2023, 14, 4557. [Google Scholar] [CrossRef]
- Fiorillo, M.; Scatena, C.; Naccarato, A.G.; Sotgia, F.; Lisanti, M.P. Bedaquiline, an FDA-approved drug, inhibits mitochondrial ATP production and metastasis in vivo, by targeting the gamma subunit (ATP5F1C) of the ATP synthase. Cell Death Differ. 2021, 28, 2797–2817. [Google Scholar] [CrossRef] [PubMed]
- Rimle, L.; Pliatsika, D.; Arnold, N.; Kurth, S.; Kaiser, M.; Maser, P.; Kemmler, M.; Adams, M.; Riedl, R.; von Ballmoos, C. Dissecting Structural Requirements of Leucinostatin A Derivatives for Antiprotozoal Activity and Mammalian Toxicity. J. Med. Chem. 2025, 68, 4237–4258. [Google Scholar] [CrossRef]
- Grimm, S.A.; Marymont, M.; Chandler, J.P.; Muro, K.; Newman, S.B.; Levy, R.M.; Jovanovic, B.; McCarthy, K.; Raizer, J.J. Phase I study of arsenic trioxide and temozolomide in combination with radiation therapy in patients with malignant gliomas. J. Neuro-Oncol. 2012, 110, 237–243. [Google Scholar] [CrossRef]
- Cohen, K.J.; Gibbs, I.C.; Fisher, P.G.; Hayashi, R.J.; Macy, M.E.; Gore, L. A phase I trial of arsenic trioxide chemoradiotherapy for infiltrating astrocytomas of childhood. Neuro-Oncology 2013, 15, 783–787. [Google Scholar] [CrossRef][Green Version]
- Shim, J.K.; Choi, S.; Yoon, S.J.; Choi, R.J.; Park, J.; Lee, E.H.; Cho, H.J.; Lee, S.; Teo, W.Y.; Moon, J.H.; et al. Etomoxir, a carnitine palmitoyltransferase 1 inhibitor, combined with temozolomide reduces stemness and invasiveness in patient-derived glioblastoma tumorspheres. Cancer Cell Int. 2022, 22, 309. [Google Scholar] [CrossRef]
- Choi, J.; Smith, D.M.; Lee, Y.J.; Cai, D.; Hossain, M.J.; O’Connor, T.J.; Deme, P.; Haughey, N.J.; Scafidi, S.; Riddle, R.C.; et al. Etomoxir repurposed as a promiscuous fatty acid mimetic chemoproteomic probe. iScience 2024, 27, 110642. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.D.; Schauble, A.; Gupta, S.; Kennedy, A.D.; Keppler, B.R.; Bingham, P.M.; Zachar, Z. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Torrini, C.; Shang, E.; Shu, C.; Mun, J.Y.; Gao, Q.; Humala, N.; Akman, H.O.; Zhang, G.; Westhoff, M.A.; et al. OGDH and Bcl-xL loss causes synthetic lethality in glioblastoma. JCI Insight 2024, 9, e172565. [Google Scholar] [CrossRef]
- Alistar, A.; Morris, B.B.; Desnoyer, R.; Klepin, H.D.; Hosseinzadeh, K.; Clark, C.; Cameron, A.; Leyendecker, J.; D’Agostino, R., Jr.; Topaloglu, U.; et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017, 18, 770–778. [Google Scholar] [CrossRef]
- Wei, S.; Yin, D.; Yu, S.; Lin, X.; Savani, M.R.; Du, K.; Ku, Y.; Wu, D.; Li, S.; Liu, H.; et al. Antitumor Activity of a Mitochondrial-Targeted HSP90 Inhibitor in Gliomas. Clin. Cancer Res. 2022, 28, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Elliott, G.T.; Olszanski, A.J.; Altieri, D.C. Feasibility and safety of targeting mitochondria for cancer therapy—Preclinical characterization of gamitrinib, a first-in-class, mitochondriaL-targeted small molecule Hsp90 inhibitor. Cancer Biol. Ther. 2022, 23, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, S.; Li, Y.; Liu, Z.; Mi, L.; Cai, Y.; Wang, X.; Chen, L.; Ran, H.; Xiao, D.; et al. Suppression of mitochondrial ROS by prohibitin drives glioblastoma progression and therapeutic resistance. Nat. Commun. 2021, 12, 3720. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Song, Y.; Gao, W. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 184. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Gao, W. Triptolide Induces Glioma Cell Autophagy and Apoptosis via Upregulating the ROS/JNK and Downregulating the Akt/mTOR Signaling Pathways. Front. Oncol. 2019, 9, 387. [Google Scholar] [CrossRef]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- van Gisbergen, M.W.; Offermans, K.; Voets, A.M.; Lieuwes, N.G.; Biemans, R.; Hoffmann, R.F.; Dubois, L.J.; Lambin, P. Mitochondrial Dysfunction Inhibits Hypoxia-Induced HIF-1alpha Stabilization and Expression of Its Downstream Targets. Front. Oncol. 2020, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rives, S.A.; Casique-Aguirre, D.; German-Castelan, L.; Velasco-Velazquez, M.A.; Gonzalez-Arenas, A. Apoptotic Signaling Pathways in Glioblastoma and Therapeutic Implications. Biomed. Res. Int. 2017, 2017, 7403747. [Google Scholar] [CrossRef]
- Rana, R.; Huirem, R.S.; Kant, R.; Chauhan, K.; Sharma, S.; Yashavarddhan, M.H.; Chhabra, S.S.; Acharya, R.; Kalra, S.K.; Gupta, A.; et al. Cytochrome C as a potential clinical marker for diagnosis and treatment of glioma. Front. Oncol. 2022, 12, 960787. [Google Scholar] [CrossRef]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5, 171ra117. [Google Scholar] [CrossRef]
- Ishizawa, J.; Kojima, K.; Chachad, D.; Ruvolo, P.; Ruvolo, V.; Jacamo, R.O.; Borthakur, G.; Mu, H.; Zeng, Z.; Tabe, Y.; et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal. 2016, 9, ra17. [Google Scholar] [CrossRef] [PubMed]
- Madhukar, N.S.; Khade, P.K.; Huang, L.; Gayvert, K.; Galletti, G.; Stogniew, M.; Allen, J.E.; Giannakakou, P.; Elemento, O. A Bayesian machine learning approach for drug target identification using diverse data types. Nat. Commun. 2019, 10, 5221. [Google Scholar] [CrossRef]
- Duchatel, R.J.; Mannan, A.; Woldu, A.S.; Hawtrey, T.; Hindley, P.A.; Douglas, A.M.; Jackson, E.R.; Findlay, I.J.; Germon, Z.P.; Staudt, D.; et al. Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma. Neurooncol. Adv. 2021, 3, vdab169. [Google Scholar] [CrossRef]
- Chi, A.S.; Tarapore, R.S.; Hall, M.D.; Shonka, N.; Gardner, S.; Umemura, Y.; Sumrall, A.; Khatib, Z.; Mueller, S.; Kline, C.; et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J. Neurooncol. 2019, 145, 97–105. [Google Scholar] [CrossRef]
- Caragher, S.P.; Shireman, J.M.; Huang, M.; Miska, J.; Atashi, F.; Baisiwala, S.; Hong Park, C.; Saathoff, M.R.; Warnke, L.; Xiao, T.; et al. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J. Neurosci. 2019, 39, 1982–1993. [Google Scholar] [CrossRef]
- Staley, A.; Tucker, K.; Yin, Y.; Zhang, X.; Fan, Y.; Zhang, Y.; Fang, Z.; Sun, W.; Suo, H.; Zhao, X.; et al. Highly potent dopamine receptor D2 antagonist ONC206 demonstrates anti-tumorigenic activity in endometrial cancer. Am. J. Cancer Res. 2021, 11, 5374–5387. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Zarabi, S.F.; Davis, R.E.; Halgas, O.; Nii, T.; Jitkova, Y.; Zhao, R.; St-Germain, J.; Heese, L.E.; Egan, G.; et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell 2019, 35, 721–737.e9. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.R.; Aponte-Collazo, L.J.; Fennell, E.M.J.; Graves, A.C.; Hale, A.E.; Dicheva, N.; Herring, L.E.; Gilbert, T.S.K.; East, M.P.; McDonald, I.M.; et al. Mitochondrial Protease ClpP is a Target for the Anticancer Compounds ONC201 and Related Analogues. ACS Chem. Biol. 2019, 14, 1020–1029. [Google Scholar] [CrossRef]
- Kline, C.L.B.; Ralff, M.D.; Lulla, A.R.; Wagner, J.M.; Abbosh, P.H.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia 2018, 20, 80–91. [Google Scholar] [CrossRef]
- Nouri, K.; Feng, Y.; Schimmer, A.D. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020, 11, 841. [Google Scholar] [CrossRef]
- Przystal, J.M.; Cianciolo Cosentino, C.; Yadavilli, S.; Zhang, J.; Laternser, S.; Bonner, E.R.; Prasad, R.; Dawood, A.A.; Lobeto, N.; Chin Chong, W.; et al. Imipridones affect tumor bioenergetics and promote cell lineage differentiation in diffuse midline gliomas. Neuro Oncol. 2022, 24, 1438–1451. [Google Scholar] [CrossRef]
- Jackson, E.R.; Persson, M.L.; Fish, C.J.; Findlay, I.J.; Mueller, S.; Nazarian, J.; Hulleman, E.; van der Lugt, J.; Duchatel, R.J.; Dun, M.D. A review of current therapeutics targeting the mitochondrial protease ClpP in diffuse midline glioma, H3 K27-altered. Neuro Oncol. 2024, 26, S136–S154. [Google Scholar] [CrossRef]
- McLeod, C.; Gout, A.M.; Zhou, X.; Thrasher, A.; Rahbarinia, D.; Brady, S.W.; Macias, M.; Birch, K.; Finkelstein, D.; Sunny, J.; et al. St. Jude Cloud: A Pediatric Cancer Genomic Data-Sharing Ecosystem. Cancer Discov. 2021, 11, 1082–1099. [Google Scholar] [CrossRef]
- Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J.; et al. Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 2020, 183, 1962–1985.e31. [Google Scholar] [CrossRef] [PubMed]
- Fennell, E.M.J.; Aponte-Collazo, L.J.; Wynn, J.D.; Drizyte-Miller, K.; Leung, E.; Greer, Y.E.; Graves, P.R.; Iwanowicz, A.A.; Ashamalla, H.; Holmuhamedov, E.; et al. Characterization of TR-107, a novel chemical activator of the human mitochondrial protease ClpP. Pharmacol. Res. Perspect. 2022, 10, e00993. [Google Scholar] [CrossRef] [PubMed]
- Giarrizzo, M.; LaComb, J.F.; Patel, H.R.; Reddy, R.G.; Haley, J.D.; Graves, L.M.; Iwanowicz, E.J.; Bialkowska, A.B. TR-107, an Agonist of Caseinolytic Peptidase Proteolytic Subunit, Disrupts Mitochondrial Metabolism and Inhibits the Growth of Human Colorectal Cancer Cells. Mol. Cancer Ther. 2024, 23, 1761–1778. [Google Scholar] [CrossRef]
- Thang, M.; Mellows, C.; Kass, L.E.; Daglish, S.; Fennell, E.M.J.; Mann, B.E.; Mercer-Smith, A.R.; Valdivia, A.; Graves, L.M.; Hingtgen, S.D. Combining the constitutive TRAIL-secreting induced neural stem cell therapy with the novel anti-cancer drug TR-107 in glioblastoma. Mol. Ther. Oncol. 2024, 32, 200834. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin. Cancer Res. 2017, 23, 4163–4169. [Google Scholar] [CrossRef]
- Arrillaga-Romany, I.; Chi, A.S.; Allen, J.E.; Oster, W.; Wen, P.Y.; Batchelor, T.T. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget 2017, 8, 79298–79304. [Google Scholar] [CrossRef]
- Gardner, S.L.; Tarapore, R.S.; Allen, J.; McGovern, S.L.; Zaky, W.; Odia, Y.; Daghistani, D.; Diaz, Z.; Hall, M.D.; Khatib, Z.; et al. Phase I dose escalation and expansion trial of single agent ONC201 in pediatric diffuse midline gliomas following radiotherapy. Neurooncol. Adv. 2022, 4, vdac143. [Google Scholar] [CrossRef]
- Cantor, E.; Wierzbicki, K.; Tarapore, R.S.; Ravi, K.; Thomas, C.; Cartaxo, R.; Nand Yadav, V.; Ravindran, R.; Bruzek, A.K.; Wadden, J.; et al. Serial H3K27M cell-free tumor DNA (cf-tDNA) tracking predicts ONC201 treatment response and progression in diffuse midline glioma. Neuro Oncol. 2022, 24, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Batsios, G.; Udutha, S.; Taglang, C.; Gillespie, A.M.; Lau, B.; Ji, S.; Phoenix, T.; Mueller, S.; Venneti, S.; Koschmann, C.; et al. GABA production induced by imipridones is a targetable and imageable metabolic alteration in diffuse midline gliomas. Neuro-Oncology 2024, 26, viii291. [Google Scholar] [CrossRef]
- Venneti, S.; Kawakibi, A.R.; Ji, S.; Waszak, S.M.; Sweha, S.R.; Mota, M.; Pun, M.; Deogharkar, A.; Chung, C.; Tarapore, R.S.; et al. Clinical Efficacy of ONC201 in H3K27M-Mutant Diffuse Midline Gliomas Is Driven by Disruption of Integrated Metabolic and Epigenetic Pathways. Cancer Discov. 2023, 13, 2370–2393. [Google Scholar] [CrossRef]
- Lee, J.V.; Berry, C.T.; Kim, K.; Sen, P.; Kim, T.; Carrer, A.; Trefely, S.; Zhao, S.; Fernandez, S.; Barney, L.E.; et al. Acetyl-CoA promotes glioblastoma cell adhesion and migration through Ca(2+)-NFAT signaling. Genes Dev. 2018, 32, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Bassal, M.A. The Interplay between Dysregulated Metabolism and Epigenetics in Cancer. Biomolecules 2023, 13, 944. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Yokogami, K.; Kikuchi, T.; Watanabe, T.; Nakatake, Y.; Yamashita, S.; Mizuguchi, A.; Takeshima, H. Methionine regulates self-renewal, pluripotency, and cell death of GIC through cholesterol-rRNA axis. BMC Cancer 2022, 22, 1351. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Matilainen, O.; Quiros, P.M.; Auwerx, J. Mitochondria and Epigenetics—Crosstalk in Homeostasis and Stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.; Li, X.S.; Woon, E.C.; Yang, M.; et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kaundal, B.; Karmakar, S.; Roy Choudhury, S. Mitochondria-targeting nano therapy altering IDH2-mediated EZH2/EZH1 interaction as precise epigenetic regulation in glioblastoma. Biomater. Sci. 2022, 10, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dang, C.V. Time to hit pause on mitochondria-targeting cancer therapies. Nat. Med. 2023, 29, 29–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Huntington, K.E.; Seyhan, A.A.; Tapinos, N.; Lulla, R.R.; Monje, M.; Wong, E.T.; Chen, C.C.; El-Deiry, W.S. Reduced EZH1/2 expression in imipridone-treated cells correlates with synergy following combinations with EZH1/2 or HDAC inhibitors in diffuse glioma and other tumors. Am. J. Cancer Res. 2025, 15, 1307–1320. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shang, E.; Schiffgens, S.; Torrini, C.; Shu, C.; Akman, H.O.; Prabhu, V.V.; Allen, J.E.; Westhoff, M.A.; Karpel-Massler, G.; et al. Induction of Synthetic Lethality by Activation of Mitochondrial ClpP and Inhibition of HDAC1/2 in Glioblastoma. Clin. Cancer Res. 2022, 28, 1881–1895. [Google Scholar] [CrossRef]
- Singh, D. A sojourn on mitochondria targeted drug delivery systems for cancer: Strategies, clinical and future prospects. Mitochondrion 2024, 74, 101826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).