Impact of Body Composition Changes on Treatment-Related Toxicities and Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Patients Receiving Trastuzumab Deruxtecan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Breast Cancer Patients and Body Composition Analysis by CT Scan
2.3. Toxicities
2.4. Treatment Discontinuation and Dose Reduction
2.5. Progression-Free Survival and Overall Survival
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
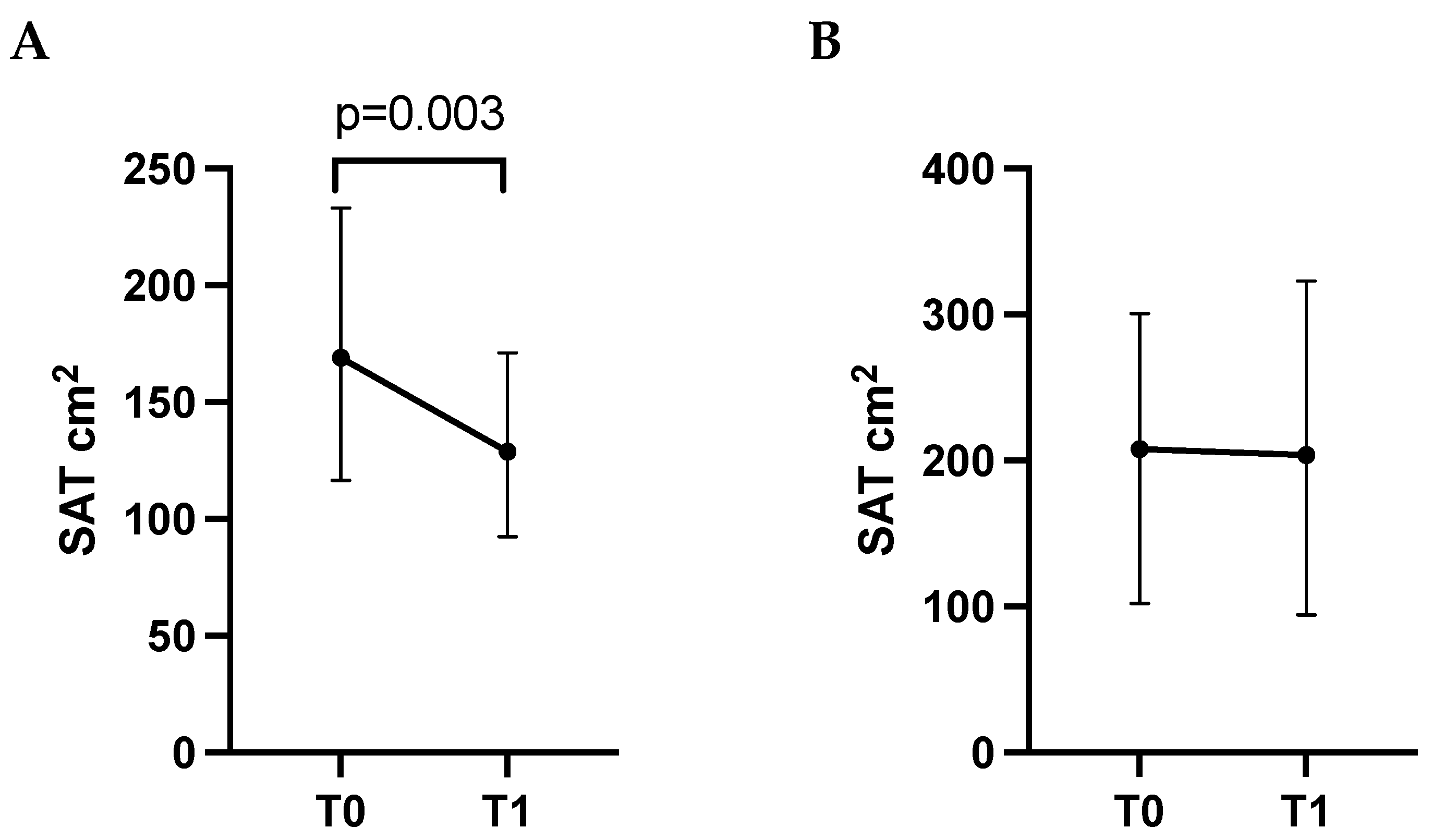
3.2. Changes in Body Composition Parameters from Baseline (T0) to First Follow-Up Visit (T1)
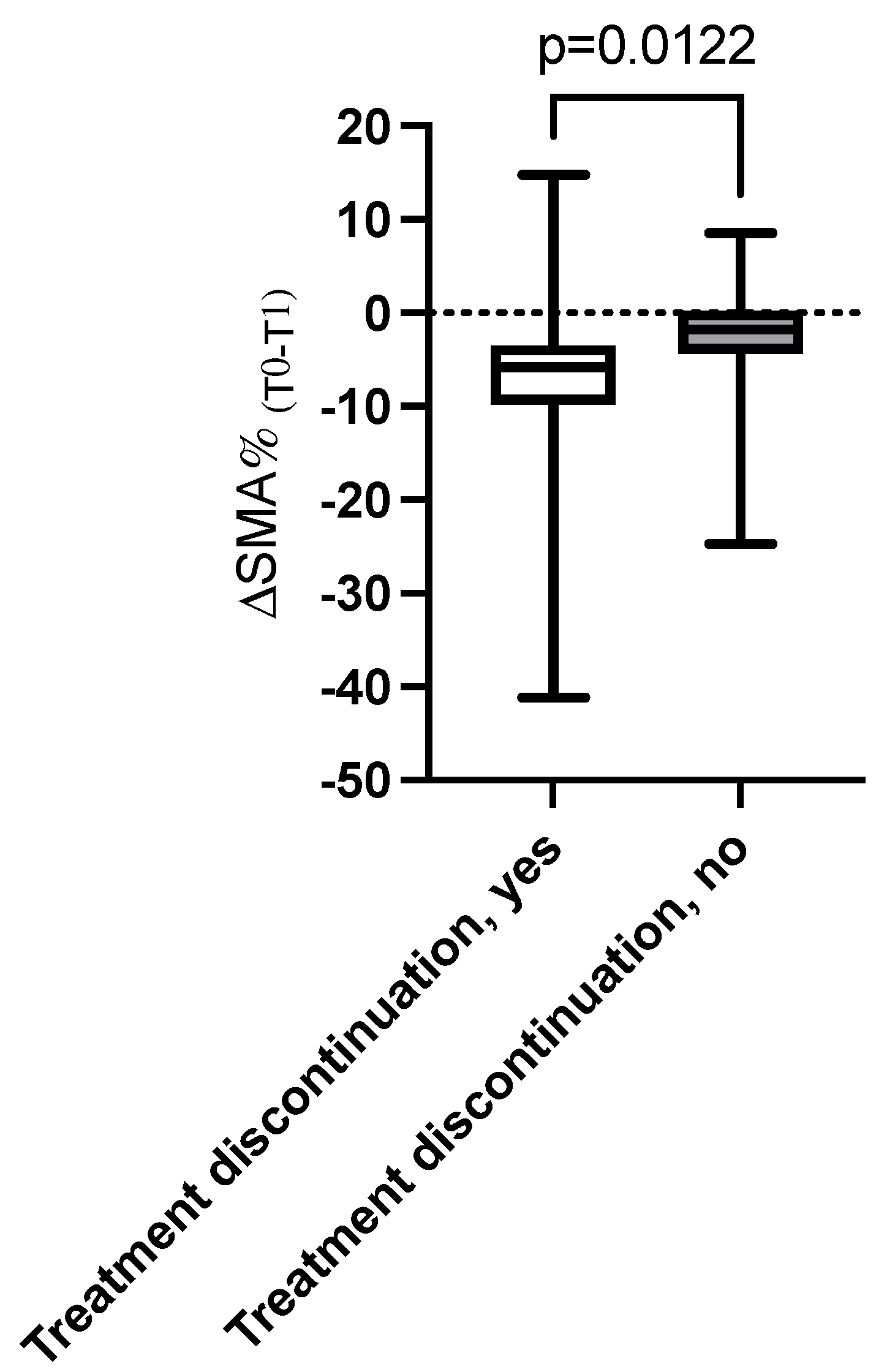
3.3. Association Between Changes over Time in Body Composition Parameters and Toxicities, Dose Reduction, and Treatment Discontinuation
3.4. Association Between Changes in Body Composition and Progression-Free Survival and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Breast Cancer. Available online: https://www.who.Int/News-Room/Fact-Sheets/Detail/Breast-Cancer (accessed on 20 July 2025).[Green Version]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’AVanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef]
- Jazieh, K.; Bell, R.; Agarwal, N.; Abraham, J. Novel targeted therapies for metastatic breast cancer. Ann. Transl. Med. 2020, 8, 907. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- André, F.; Park, Y.H.; Kim, S.-B.; Takano, T.; Im, S.-A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gregori, J.G.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.-A.; Iwata, H.; Curigliano, G.; Kim, S.-B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: Long-term survival analysis of the DESTINY-Breast03 trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- Dowling, G.P.; Daly, G.R.; Keelan, S.; Boland, F.; Toomey, S.; Hill, A.D.; Hennessy, B.T. Efficacy and Safety of Trastuzumab Deruxtecan in Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2023, 23, 847–855.e2. [Google Scholar] [CrossRef]
- Lucas, A.T.; Price, L.S.L.; Schorzman, A.N.; Storrie, M.; Piscitelli, J.A.; Razo, J.; Zamboni, W.C. Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies 2018, 7, 10. [Google Scholar] [CrossRef]
- Caan, B.J.; Feliciano, E.M.C.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Weiss, J.; Skrzypski, M.; Lam, J.M.; Karasaki, T.; Zambrana, F.; Kidd, A.C.; Frankell, A.M.; Watkins, T.B.K.; Martínez-Ruiz, C.; et al. Body composition and lung cancer-associated cachexia in TRACERx. Nat. Med. 2023, 29, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.; Seng, K.Y.; Ong, E.M.; Wang, L.Z.; Oscar, H.; Cordero, M.T.; Copones, R.; Fan, L.; Tan, S.H.; Goh, B.C.; et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res. Treat. 2014, 144, 143–152. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Vega, M.C.M.D.; Laviano, A.; Pimentel, G.D. Sarcopenia and chemotherapy-mediated toxicity. Einstein-Sao Paulo 2016, 14, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Bentahila, R.; Giraud, P.; Decazes, P.; Kreps, S.; Nay, P.; Chatain, A.; Fabiano, E.; Durdux, C. The impact of sarcopenia on survival and treatment tolerance in patients with head and neck cancer treated with chemoradiotherapy. Cancer Med. 2022, 12, 4170–4183. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Molfino, A.; Fanelli, F.R. Muscle depletion and the prediction of chemotherapy toxicity. Intern. Emerg. Med. 2013, 8, 373–375. [Google Scholar] [CrossRef][Green Version]
- Molfino, A.; Imbimbo, G.; Muscaritoli, M. Metabolic and histomorphological changes of adipose tissue in cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 235–242. [Google Scholar] [CrossRef]
- Ligorio, F.; Zambelli, L.; Fucà, G.; Lobefaro, R.; Santamaria, M.; Zattarin, E.; de Braud, F.; Vernieri, C. Prognostic impact of body mass index (BMI) in HER2+ breast cancer treated with anti-HER2 therapies: From preclinical rationale to clinical implications. Ther. Adv. Med. Oncol. 2022, 14, 17588359221079123. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Thompson, B.; Stellmaker, R.; Koelmeyer, L. Body composition and chemotherapy toxicities in breast cancer: A systematic review of the literature. J. Cancer Surviv. 2024, 19, 914–929. [Google Scholar] [CrossRef]
- Molfino, A.; Carletti, R.; Imbimbo, G.; Amabile, M.I.; Belli, R.; di Gioia, C.R.; Belloni, E.; Spinelli, F.; Rizzo, V.; Catalano, C.; et al. Histomorphological and inflammatory changes of white adipose tissue in gastrointestinal cancer patients with and without cachexia. J. Cachex-Sarcopenia Muscle 2021, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.R.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 2016, 54, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; Tahir, I.; Hu, B.; Dietrich, A.-S.W.; Tonnesen, P.E.; Sharp, G.C.; Tillman, G.; Roeland, E.J.; Nipp, R.D.; Comander, A.; et al. Association of Sarcopenia With Toxicity-Related Discontinuation of Adjuvant Endocrine Therapy in Women With Early-Stage Hormone Receptor-Positive Breast Cancer. Int. J. Radiat. Oncol. 2023, 118, 94–103. [Google Scholar] [CrossRef]
- Rugo, H.; Bianchini, G.; Cortes, J.; Henning, J.-W.; Untch, M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 2022, 7, 100553. [Google Scholar] [CrossRef]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef]
- Sandhya, L.; Sreenivasan, N.D.; Goenka, L.; Dubashi, B.; Kayal, S.; Solaiappan, M.; Govindarajalou, R.; KT, H.; Ganesan, P. Randomized Double-Blind Placebo-Controlled Study of Olanzapine for Chemotherapy-Related Anorexia in Patients With Locally Advanced or Metastatic Gastric, Hepatopancreaticobiliary, and Lung Cancer. J. Clin. Oncol. 2023, 41, 2617–2627. [Google Scholar] [CrossRef]
- Spei, M.-E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Zamboni, W.C.; Charlab, R.; Burckart, G.J.; Stewart, C.F. Effect of Obesity on the Pharmacokinetics and Pharmacodynamics of Anticancer Agents. J. Clin. Pharmacol. 2023, 63, S85–S102. [Google Scholar] [CrossRef] [PubMed]
- Kudiarasu, C.; Lopez, P.; Galvão, D.A.; Newton, R.U.; Taaffe, D.R.; Mansell, L.; Fleay, B.; Saunders, C.; Fox-Harding, C.; Singh, F. What are the most effective exercise, physical activity and dietary interventions to improve body composition in women diagnosed with or at high-risk of breast cancer? A systematic review and network meta-analysis. Cancer 2023, 129, 3697–3712. [Google Scholar] [CrossRef] [PubMed]
- Peretz, S.Y.; Kessner, R.; Bar, Y.; Sonnenblick, A.; Lerner, S.; Deutsch-Lukatsky, A.; Popuri, K.; Beg, M.F.; Shachar, S.S. Body composition metrics as a determinant of trastuzumab deruxtecan related toxicity and response. Npj Breast Cancer 2025, 11, 1–6. [Google Scholar] [CrossRef]

| Variable | n = 35 |
|---|---|
| Female, n (%) | 34 (97.1%) |
| Age, years | 57.1 ± 11.5 |
| BMI, kg/m2 | 24.0 ± 3.52 |
| Postmenopausal Status, n (%) | 22 (64.7) |
| Smoking habit, n (%) | 10 (28.6) |
| Alcohol consumption, n (%) | 3 (8.6) |
| Metastases site | |
| Lymph Node, n (%) | 25 (71.4) |
| Bone, n (%) | 17 (48.5) |
| Liver, n (%) | 14 (40) |
| Lung, n (%) | 9 (25.7) |
| Brain, n (%) | 6 (17.1) |
| Main comorbidities | |
| Hypertension, n (%) | 8 (22.8) |
| Thyroid disorders, n (%) | 3 (8.6) |
| Autoimmune disease, n (%) | 3 (8.6) |
| Dyslipidemia, n (%) | 2 (5.7) |
| Type 2 diabetes mellitus, n (%) | 2 (5.7) |
| Body Composition Parameter | Baseline (T0) | Follow-Up (T1) | Median ΔT0–T1% | p-Value |
|---|---|---|---|---|
| TAT (cm2) | 280.4 (147.0; 350.2) | 220.7 (143.8; 349.0) | −10.9 | 0.070 |
| SAT (cm2) | 171.0 (117.5; 245.5) | 135.7 (97.1; 204.9) | −14.1 | 0.023 |
| VAT (cm2) | 95.1 (37.4; 119.8) | 73.9 (44.2; 126.0) | +3.8 | 0.629 |
| SMA (cm2) | 122.8 (106.9; 137.0) | 122 (108.7; 128.2) | −4.3 | 0.001 |
| SMI (cm2/m2) | 45.1 (40.9; 49.7) | 44.0 (38.1; 47.6) | −4.3 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Imbimbo, G.; Pisegna, S.; Scagnoli, S.; Alabiso, C.; Ardovino, M.; Gallicchio, C.; Rizzo, V.; Botticelli, A. Impact of Body Composition Changes on Treatment-Related Toxicities and Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Patients Receiving Trastuzumab Deruxtecan. Cancers 2025, 17, 3063. https://doi.org/10.3390/cancers17183063
Molfino A, Imbimbo G, Pisegna S, Scagnoli S, Alabiso C, Ardovino M, Gallicchio C, Rizzo V, Botticelli A. Impact of Body Composition Changes on Treatment-Related Toxicities and Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Patients Receiving Trastuzumab Deruxtecan. Cancers. 2025; 17(18):3063. https://doi.org/10.3390/cancers17183063
Chicago/Turabian StyleMolfino, Alessio, Giovanni Imbimbo, Simona Pisegna, Simone Scagnoli, Claudia Alabiso, Massimiliano Ardovino, Carmen Gallicchio, Veronica Rizzo, and Andrea Botticelli. 2025. "Impact of Body Composition Changes on Treatment-Related Toxicities and Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Patients Receiving Trastuzumab Deruxtecan" Cancers 17, no. 18: 3063. https://doi.org/10.3390/cancers17183063
APA StyleMolfino, A., Imbimbo, G., Pisegna, S., Scagnoli, S., Alabiso, C., Ardovino, M., Gallicchio, C., Rizzo, V., & Botticelli, A. (2025). Impact of Body Composition Changes on Treatment-Related Toxicities and Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Patients Receiving Trastuzumab Deruxtecan. Cancers, 17(18), 3063. https://doi.org/10.3390/cancers17183063






