Simple Summary
Endometrial cancer (EC) is the second most common gynecologic malignancy, with its incidence rising due to demographic changes. Current diagnostic methods are invasive and insufficiently specific, highlighting the need for accurate, non-invasive biomarkers. In this study, we applied mass spectrometry-based multi-steroid profiling and machine learning to analyze systemic steroid levels—focusing on androgens, 11-oxyandrogens, glucocorticoids and mineralocorticoids—as potential diagnostic and prognostic biomarkers. Our cohort included 62 patients with EC and 70 controls with benign uterine conditions. We identified distinct steroid level alterations between cases and controls. While steroids alone had limited diagnostic and prognostic value, a multivariate model combining classic androgens, CA-125, HE4, BMI and parity achieved an AUC of 0.87, 79.1% sensitivity and 74.7% specificity in distinguishing EC from benign conditions. This model outperformed those based on CA-125, HE4 or their combination with BMI. These findings underscore the potential of integrating steroid profiling with established biomarkers to enhance EC detection.
Abstract
Objective: To evaluate the diagnostic and prognostic potential of preoperative serum steroid levels in endometrial cancer (EC) alone and in combination with clinical parameters and biomarkers CA-125 and HE4. Methods: This single-center observational study included 62 patients with EC and 70 controls with benign uterine conditions who underwent surgery between June 2012 and February 2020. Preoperative serum levels of classic androgens, 11-oxyandrogens, glucocorticoids and mineralocorticoids were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Machine learning was used to assess their diagnostic and prognostic value alone and combined with clinical parameters and tumor biomarkers. Results: Patients with EC had significantly higher serum levels of classic androgens (androstenedione, testosterone), 11-oxyandrogens (11β-hydroxy-androstenedione, 11β-hydroxy-testosterone) and glucocorticoids (17α-hydroxy-progesterone, 11-deoxycortisol) compared to controls. While individual steroids had limited diagnostic value, a multivariate model including classic androgens, CA-125, HE4, BMI and parity achieved an AUC 0.87, 79.1% sensitivity and 74.7% specificity in distinguishing EC from benign uterine condition. This model outperformed our previously published model based on CA-125, HE4 and BMI (AUC: 0.81, p < 0.0001). Prognostically, HE4 was the strongest marker for lymphovascular space invasion (LVSI) (AUC: 0.79) and deep myometrial invasion (MI) (AUC: 0.71). Among steroids, androstenedione was the most predictive of LVSI (AUC: 0.67), while 11β-hydroxy-testosterone was the strongest predictor of deep MI (AUC: 0.64). Conclusions: Patients with EC exhibit distinct steroid hormone profiles. While steroids alone offer modest diagnostic and prognostic value, integrating them into multivariate models improves diagnostic accuracy.
1. Introduction
Endometrial cancer (EC) is the sixth most common cancer affecting women, with approximately 420,000 new cases diagnosed worldwide in 2022 [1]. Its incidence is rising globally, driven by factors such as population growth, aging, increasing obesity rates and a higher human development index [2]. Traditionally, EC was classified into two groups: type I (endometrioid) and type II (non-endometrioid, including serous, clear-cell and un/dedifferentiated EC) [3]. However, this classification does not align well with molecular characteristics, limiting its use in precision medicine. The Cancer Genome Atlas (TCGA) introduced over a decade ago a molecular classification that stratifies EC into four groups: POLE-altered (POLE-alt), mismatch repair deficient (dMMR), non-specific molecular profile (NSMP) and TP53-altered (TP53-alt) tumors [4]. The first three groups mostly include low-grade, clinically favorable endometrioid tumors, while TP53-alt tumors are typically high-grade endometrioid or serous with a higher risk of recurrence [4,5,6]. Both classification systems are currently used in clinical practice in Europe [5,7].
EC often presents early, typically with postmenopausal bleeding, but this red flag symptom is nonspecific, with only 5–10% of symptomatic women being ultimately diagnosed with the disease [8]. A definitive diagnosis requires histological examination of an endometrial tissue sample, usually obtained when transvaginal ultrasound shows an endometrial thickness greater than 5 mm. While this threshold is highly sensitive (over 90%), it has low specificity (around 50%) in postmenopausal women, leading to a high rate of false positives [9]. Therefore, there is a clear need for less invasive and more accurate diagnostic tools. The Risk of Endometrial Malignancy B (REM-B) algorithm, which combines age, presence of abnormal uterine bleeding, BMI, HE4 levels and ultrasound findings, shows excellent sensitivity and specificity (>90%) but requires further validation [10]. Promising diagnostic models using omics approaches (e.g., proteomics, metabolomics) and machine learning have also emerged [11,12,13,14]. However, no blood-based diagnostic tests for EC are currently included in European clinical guidelines [5].
Once EC is confirmed, preoperative biopsy findings, such as tumor type and grade guide surgical decisions [5]. Preoperative imaging, such as magnetic resonance imaging (MRI) aids in assessing myometrial and cervical involvement and detecting enlarged lymph nodes and disease spread [15]. However, risk stratification remains imprecise, as biopsy results often do not align with post-hysterectomy findings [16,17,18], highlighting the need for more accurate prognostic tools. A recent study found that molecular classification of preoperative biopsy samples, combined with imaging tests, predicts advanced disease more reliably than histotype or grade [19]. This molecular information can be used preoperatively to help tailor surgical treatment. Additionally, biomarkers such as cancer antigen 125 (CA-125) and human epididymis protein 4 (HE4) have been shown to improve preoperative risk stratification [20,21,22]. HE4 is also useful for predicting deep myometrial invasion (MI) [21,23] and lympho-vascular space invasion (LVSI) [23], though further validation in prospective studies is needed.
The present study had two main objectives: (1) to evaluate preoperative serum levels of steroids, including classic androgens, 11-oxyandrogens, glucocorticoids and mineralocorticoids, in patients with EC and control patients, and (2) to examine their diagnostic and prognostic potential as individual biomarkers and in combination with other steroids, CA-125, HE4 and patient clinical characteristics. For this purpose, we measured preoperative serum levels of steroid hormones in 62 patients with EC and 70 women with benign uterine conditions using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Machine learning was applied to evaluate the diagnostic and prognostic utility of these hormones.
2. Materials and Methods
2.1. Study Population
This single-center case-control observational study included 132 women who underwent surgical treatment at the Department of Obstetrics and Gynecology, University Medical Centre Ljubljana, Slovenia, between June 2012 and February 2020. Based on clinical and histopathological findings, participants were stratified into two groups: patients with endometrial adenocarcinoma (n = 62) and a control group of women with benign uterine conditions, such as uterine prolapse or myomas (n = 70).
Inclusion criteria for the case group were age ≥40 years, a diagnosis of endometrial adenocarcinoma (any histotype, grade or International Federation of Gynecology and Obstetrics (FIGO) stage) and available serum sample for steroid quantification. For the control group, inclusion criteria were age ≥40 years, a diagnosis of benign uterine conditions and available serum sample for steroid quantification. Exclusion criteria for both groups included a diagnosis of polycystic ovary syndrome (PCOS), while the control group also excluded participants with endometrial hyperplasia. The study was approved by the Medical Ethics Committee of the Republic of Slovenia (Approval ID: 0120-487/2020/3) and all participants provided written informed consent prior to enrollment.
Patients were recruited by senior gynecologists with assistance from study nurses. Morning blood samples were collected 1 to 7 days before surgery, along with detailed information on lifestyle, medication use and gynecological and clinical status. Sample collection and processing followed a strict standard operating procedure specifically designed for metabolomic studies [24]. Blood samples (6 mL) were obtained via venipuncture using clot-activator tubes (BD Vacutainer, Franklin Lakes, NJ, USA, #368815). The samples were centrifuged at 2000 g for 15 min. Serum was carefully aspirated, divided into 200 µL aliquots and stored at −80 °C in 1.8 mL cryotubes (Nalgen Nunc International, Roskilde, Denmark, #375418) until analysis.
2.2. Multi-Steroid Profiling of Serum Samples by LC-MS/MS
Multi-steroid profiling was performed using an LC-MS/MS method developed and validated by Schiffer and collaborators at the Steroid Metabolome Analysis Core (SMAC) at the University of Birmingham [25].
2.2.1. Sample Preparation
Steroids were extracted from serum samples by liquid–liquid extraction, as described previously [25,26]. Briefly, 180 µL of sample, calibrator or quality control (QC) was mixed with 10 µL of the internal standard mixture, followed by protein precipitation with 50 µL of acetonitrile (#75-05-8, Biosolve, Dieuze, France). Steroid extraction was performed with 1 mL tert-butyl methyl ether (MTBE) (#1634-04-4, Acros Organics, Fisher Scientific UK Ltd., Loughborough, UK), the mixtures were vortex-mixed at 1000 rpm for 10 min and the phases were allowed to separate for a further 20 min. Subsequently, the organic phase was transferred to a 96-well plate (Porvair Sciences Ltd., Wrexham, UK) with 700 µL glass inserts (Randox, Crumlin, UK) and dried under a nitrogen stream at 45 °C. The dried extract was reconstituted in 125 µL of 50% methanol (#136841, Biosolve, Dieuze, France) and water (#232141, Biosolve, Dieuze, France) prior to LC-MS/MS analysis.
2.2.2. LC-MS/MS Analysis
LC-MS/MS analysis was performed on a Waters Acquity UHPLC (Waters Ltd., Wilmslow, UK) coupled to an XEVO TQ XS mass spectrometer (Waters Ltd., Wilmslow, UK). The detailed method description can be found in the original article [25]. Briefly, chromatographic separation was performed using a Phenomenex Luna Omega column, 1.6 µm, polar C18, 100 Å, 2.1 × 50 mm (Phenomenex, Macclesfield, UK) at 60 °C. Ten microliters of the reconstituted sample was injected. A linear gradient from 45% to 75% of mobile phase B (UHPLC grade methanol) was applied for five minutes at a flow rate of 0.6 mL/min, followed by a washing step with 95% B for 5.2 min and equilibration at starting conditions until injection of the next sample. Ammonium fluoride (6 mM) was introduced into the flow path via post-column infusion at a rate of 5 µL/min to enhance ionization. The autosampler was maintained at 10 °C. The eluate was then injected into the mass spectrometer operated in positive electrospray ionization (ESI) mode. The ion source temperature was 150 °C, the desolvation temperature and gas flow were 600 °C and 1200 L/h, respectively, and the cone gas was 150 L/h.
16 steroids were quantified, including progesterone (Chemical Abstracts Service (CAS) number: CAS: 57-83-0; #P8783, Sigma Aldrich GmbH, Roedermark, Germany), dehydroepiandrosterone (DHEA; CAS: 53-43-0; #D4000, Sigma Aldrich GmbH, Roedermark, Germany), androstenedione (A4, CAS: 63-05-8; #46033, Sigma Aldrich GmbH, Roedermark, Germany), testosterone, (T; CAS: 58-22-0; #T6147, Sigma Aldrich GmbH, Roedermark, Germany), 5α-dihydrotestosterone (DHT; CAS: 521-18-6; #A8380, Sigma Aldrich GmbH, Roedermark, Germany), 11β-hydroxy-androstenedione (11OHA4, CAS: 382-44-5; #A3009, Sigma Aldrich GmbH, Roedermark, Germany), 11-keto-androstenedione (11KA4, CAS: 382-45-6; #284998, Sigma Aldrich GmbH, Roedermark, Germany), 11β-hydroxy-testosterone (11OHT; CAS: 1816-85-9; #A5760, Sigma Aldrich GmbH, Roedermark, Germany), 11-keto-testosterone (11KT, CAS: 564-35-2; #A6720, Steraloids, Newport, R.I., USA), 17α-hydroxy-progesterone (CAS: 68-96-2; #H5752, Sigma Aldrich GmbH, Roedermark, Germany), 11-deoxycortisol (CAS: 152-58-9; #R0500, Sigma Aldrich GmbH, Roedermark, Germany), cortisol (CAS: 50-23-7; #H4001, Sigma Aldrich GmbH, Roedermark, Germany), cortisone (CAS: 53-06-5; #C2755, Sigma Aldrich GmbH, Roedermark, Germany), 11-deoxycorticosterone (CAS: 64-85-7; #D6875, Sigma Aldrich GmbH, Roedermark, Germany), corticosterone (CAS: 50-22-6; #C2505, Sigma Aldrich GmbH, Roedermark, Germany) and aldosterone (CAS: 52-39-1; #Q2000-000, Steraloids, Newport, R.I., USA).
Data processing and quantification were performed using TargetLynx software version 4.2 (Waters Ltd., Wilmslow, UK). For T, 3% of the samples were below the lower limit of quantification (LLOQ), for 11-deoxycortisol, 3.8%, for 11OHT, 4.6%, and for 17α-hydroxy-progesterone, 12.12%. In these cases, values below the LLOQ were assigned as LLOQ/2. The analytes 11-deoxycorticosterone, DHT, aldosterone and progesterone were below the LLOQ in 95%, 83%, 79% and 74% of samples, respectively, and were not analyzed further.
2.3. Measurement of Serum CA-125 and HE4 Levels
Serum levels of CA-125 and HE4 for samples collected from June 2012 to December 2014 were previously determined by our group and are reported by Knific and collaborators [23]. Specimens collected from December 2014 to February 2020 were analyzed separately for the present study. In both cases, analysis of CA-125 and HE4 levels was performed at the Clinical Institute for Clinical Biochemistry, University Medical Center, Ljubljana, using clinically validated electroluminescent immunoassays (ECLIAs) specific for CA-125 (REF: 11776223190, Roche Diagnostics GmbH, Mannheim, Germany) and HE4 (REF: 05950929190, Roche Diagnostics GmbH, Mannheim, Germany), on a Cobas e411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
2.4. Statistical Analysis
The data were numerically anonymized and were collected in Microsoft Office Excel 2010 spreadsheets. Statistical analysis was performed in R studio version 4.3.0 or higher. Probability of <0.05 was defined as statistically significant and all tests were two sided. Continuous variables were expressed as median values with interquartile range (IQR) and were compared using the Mann–Whitney U test. Categorical variables were expressed as frequencies with percentages and compared using the Chi-square or the Fisher exact test. Power analysis was performed to assess the adequacy of the sample size for group comparisons using GPower version 3.1. The power for detecting small, medium and large effect sizes (Cohen’s d = 0.2, 0.5, 0.8) between cases and controls was calculated as 20%, 79.4% and 99.4%, respectively, indicating sufficient power to detect medium to large effects.
Machine learning was performed in R Studio (version 4.3.0 or higher) using caret library [27]. We tested 21 variables, including 12 steroid hormones (DHEA, A4, T, 11OHA4, 11OHT, 11KA4, 11KT, 17α-hydroxy-progesterone, 11-deoxycortisol, cortisol, cortisone and corticosterone); 3 steroid combinations, including classic androgen pool (sum of DHEA, A4 and T), 11-oxyandrogen pool (sum of 11OHA4, 11OHT, 11KA4 and 11KT) and glucocorticoid pool (sum of 17α-hydroxy-progesterone, 11-deoxycortisol, cortisol and cortisone); 2 proteins (HE4 and CA-125); and 4 clinical characteristics (age, menopause status, BMI and parity).
All continuous variables were first ln-transformed for normality, followed by standardization (mean subtraction and division by standard deviation) to ensure comparability across different variables. Logistic regression models, both univariate and multivariate, were trained using a 5 × 5-fold cross-validation scheme, with 1000 iterations to ensure stability and robustness. For the multivariate logistic regression models, first we employed feature selection using the function stepAIC() from MASS library in both directions to identify the most informative variables associated with the classification outcome.
The minimum sample size for the logistic regression models was calculated based on Peduzzi et al. [28] using the formula: N = 10 × k/p, where N is the minimum required number of samples, k is the number of covariates and p is the smallest proportion of negative or positive cases in the cohort. Using this approach, we calculated the minimum sample size needed for the diagnostic univariate logistic regression models to be 22, for the most complex multivariate diagnostic model (five-variable logistic regression model) to be 106 samples. Our cohort of 132 patients exceeded this minimum, ensuring adequate power for the diagnostic models.
In addition to cross-validation, internal validation was performed via bootstrapping to assess the robustness of the models. We generated 1000 bootstrap samples with replacement from the original dataset and refitted the logistic regression models on each resample to estimate the variability in model performance.
Subset analysis was performed to distinguish between patients without and with LVSI, without and with deep MI, and between low- and high-grade tumors, using the same variables as for the diagnostic models. The prognostic models for LVSI were evaluated using 5 × 5 cross-validation on a dataset of 12 positive and 50 negative cases. The prognostic models for deep MI were tested using 5 × 5 cross-validation on 16 positive and 44 negative cases (data were unavailable for 2 patients). The prognostic models for tumor grade prediction were evaluated on 46 low-grade cases and 16 high-grade cases using 5 × 5 cross-validation. Due to small sample sizes and class imbalance, only univariate logistic regression was applied. For the univariate prognostic models, the calculated minimum sample sizes were 52 patients for LVSI status and 37 for deep MI status. In both cases, the number of samples in the sub-cohort exceeded the calculated minimum, ensuring sufficient power for these models as well.
We assessed model performance using AUC (area under the receiver operating (ROC) curve), sensitivity, specificity, precision, F1 score, accuracy and Akaike information criteria (AIC). ROC curves were used for visualization of classification performance.
3. Results
3.1. Description of the Cohort
In this study, we included 62 patients with endometrial cancer (EC) and 70 women with benign uterine conditions (Figure 1). The median age at diagnosis was 64.5 years (interquartile range [IQR]: 59.3–71.0) in the case group and 64.0 years (IQR: 55.0–71.0) in the control group. The median body mass index (BMI) was 30.4 kg/m2 (IQR: 27.2–35.2) for the EC group and 27.4 kg/m2 (range: 23.8–30.5) for the control group (p = 0.005). No significant differences were found between the groups in terms of age, menopausal status, presence of type 2 diabetes, arterial hypertension, use of hormonal therapy, medication intake or smoking status (Table 1). In terms of tumor biomarkers, the median CA-125 level was 13.0 kU/L (IQR: 9.1–19.0 kU/L) in the control group, compared to 21.7 kU/L (IQR: 13.4–34.5 kU/L) in the case group (p < 0.0001). The median HE4 level in the control group was 55.6 pmol/L (IQR: 45.6–69.7 pmol/L), while in the case group it was 86.0 pmol/L (IQR: 64.7–130.4 pmol/L) (p < 0.0001). None of the patients received neoadjuvant chemotherapy.
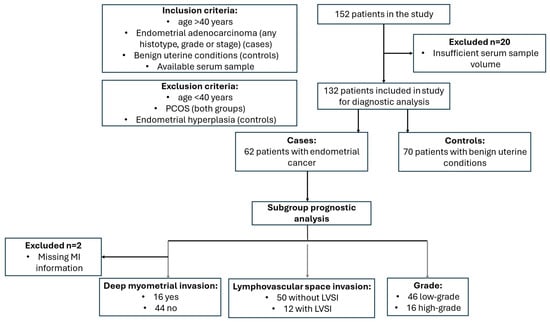
Figure 1.
Study flow diagram. LVSI, lymphovascular space invasion; MI, myometrial invasion.

Table 1.
Clinical characteristics of the study participants.
Among the patients with EC, 55 had endometrioid EC, 6 had serous EC and 1 had clear-cell EC. The average number of lymph nodes removed during lymphadenectomy was 19.36 (range: 1–41), with an average of 4.4 tumor-positive lymph nodes (range: 1–13, n = 5). Fifty-four patients had no tumor-positive lymph nodes, and for three patients, this information was missing. LVSI was identified in 12 patients (19%). MI was classified as follows: deep invasion (more than 50% of the myometrium) in 16 patients (26%), less than 50% invasion in 30 patients (48%) and no invasion in 14 patients (23%). Information on MI was missing for 2 patients. Based on the FIGO classification, the distribution of EC stages was as follows: Stage IA (n = 40, 64.5%), Stage IB (n = 11, 17.7%), Stage IIA (n = 1, 1.6%), Stage IIB (n = 1, 1.6%), Stage IIIA (n = 1, 1.6%), Stage IIIB (n = 1, 1.6%), Stage IIIC (n = 2, 3.2%) and Stage IVB (n = 3, 4.8%). Data on FIGO stage were missing for two patients. Detailed histopathological characteristics are provided in Supplementary Table S1.
3.2. Preoperative Steroid Hormone Levels Differ Between Patients with EC and Women with Benign Uterine Conditions
We used a validated LC-MS/MS method to determine preoperative serum levels of steroid hormones, including classic and 11-oxyandrogens, glucocorticoids, mineralocorticoids and progesterone (see Supplementary Figure S1 for steroid synthesis pathways).
Univariate statistical analysis identified significant differences in six steroid hormones between patients with EC and controls (Table 2). More specifically, levels of 11β-hydroxylated androgens (11OHA4, 11OHT) and their classical androgen counterparts (A4, T, respectively) were significantly higher in cases compared to controls (p = 0.01–0.03). Likewise, glucocorticoid precursors 17α-hydroxy-progesterone and 11-deoxycortisol were also elevated in cases compared to controls (p = 0.04 and 0.01, respectively), while cortisol levels showed a borderline increase (p = 0.055).

Table 2.
Preoperative serum concentrations of the measured steroid hormones in patients with EC and control women with benign uterine pathologies.
3.3. Preoperative 11-Oxyandrogen Levels Differ Between Tumor Grades
We next investigated the association between steroid hormone levels and key clinicopathological features of EC, including histological subtype, tumor grade, metastasis, LVSI and deep MI.
Regarding histological subtypes, we observed significant differences in 11KA4 (p = 0.014) and CA-125 (p = 0.006) levels between endometrioid and serous EC. Additionally, 11OHA4 levels were higher in serous cases, though this difference was borderline significant (p = 0.059) (Supplementary Table S2).
When analyzing tumor grade, we found that high-grade tumors (grade 3 endometrioid and non-endometrioid EC) exhibited significantly higher levels of 11OHA4 (p = 0.012), 11KA4 (p = 0.008), CA-125 (p < 0.001) and HE4 (p = 0.005) compared to low-grade tumors (grade 1–2 endometrioid EC). Within the endometrioid subgroup, steroid hormone levels did not differ significantly between low- and high-grade tumors, although CA-125 (p = 0.003) and HE4 (p = 0.013) remained elevated in high-grade cases (Supplementary Table S2).
In terms of metastasis, LVSI and deep MI, we found no significant differences in steroid hormone levels between affected and unaffected patients (Table 3). However, CA-125 and HE4 were significantly elevated in metastatic cases (p = 0.002 and p = 0.001, respectively) and in patients with LVSI (p = 0.018 and p = 0.001, respectively). Additionally, HE4 levels were significantly higher in cases with deep MI (p = 0.004).

Table 3.
Clinicopathological characteristics related to median steroid hormone levels (interquartile range) in patients with EC. Statistically significant p-values (<0.05) in bold.
3.4. Development of Machine Learning Diagnostic Models Based on Preoperative Serum Steroid Levels
We applied machine learning to evaluate the diagnostic and prognostic potential of steroid hormones, steroid pools (classic androgens, 11-oxyandrogens, glucocorticoids) and their combinations with CA-125, HE4 and clinical parameters (age, BMI, menopause status, parity). The models’ performance was evaluated with cross-validation.
In univariate analysis, HE4 was the strongest EC predictor (AUC: 0.778, 95% CI: 0.773–0.782), while among steroids, 11OHT performed best (AUC: 0.629) (Figure 2a, Supplementary Table S3). The performance of other steroids is given in Supplementary Table S3. For multivariate analysis, we first used a stepwise approach to identify the top predictive variables. The best two-variable model (HE4 + classic androgen pool) performed similarly to the Knific et al. model based on CA-125, HE4 and BMI (AUC: 0.811 vs. 0.813, p = 0.881). Adding BMI, parity and CA-125 to the best two-variable model sequentially improved performance, with the final five-variable model (HE4, classic androgen pool, BMI, parity and CA-125) achieving the highest AUC (0.866, 95% CI: 0.863–0.869), sensitivity (79.1%) and specificity (74.7%), significantly outperforming CA-125 alone (p < 0.0001), HE4 alone (p = 0.004) and the Knific et al. model (p < 0.0001) (Figure 2b). The Knific et al. model’s performance in our cohort (AUC: 0.813) differed slightly from the original study (AUC: 0.804), likely due to differences in 20 samples between this cohort and the original one.
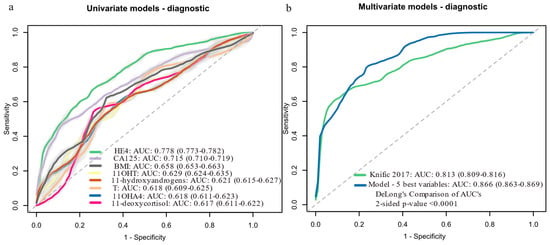
Figure 2.
Logistic regression models for predicting EC probability (cases, n = 62; controls, n = 70): (a) Univariate models with AUC above 0.6. (b) Multivariate models: Knific 2017 [23] = 0.884 (ln[CA-125]) + 2.402 (ln[HE4]) + 3.142 (ln[BMI]) − 23.284; model with 5 best variables = 2.868 (ln[HE4]) + 1.116 (ln[Androgen pool]) + 2.929 (ln[BMI]) − 0.835 (Parity) + 1.292 (ln[CA-125]) − 27.214. Data in brackets represent 95% confidence intervals (CI). Light grey color around the ROC curves shows standard deviation. 11OHA4, 11β-hydroxy-androstenedione; 11OHT, 11β-hydroxy-testosterone; AUC, area under the receiver operating curve; BMI, body mass index; CA-125, cancer antigen 125; HE4, human epididymis protein 4; ln, natural logarithm; ROC, receiver operator curve; T, testosterone.
To further validate model robustness, we performed internal validation using bootstrapping (1000 resamples) (Supplementary Table S4). The five-variable model performance remained robust (mean AUC: 0.872; 95% CI: 0.809–0.924; mean sensitivity: 79.4%; mean specificity: 75.5%). The Knific et al. model yielded a bootstrap AUC of 0.822 (95% CI: 0.747–0.891). These results support the stability of our findings across different validation approaches.
3.5. Development of Machine Learning Prognostic Models Based on Preoperative Serum Steroid Levels
We also evaluated the prognostic potential of steroid hormones for predicting LVSI, deep MI and tumor grade. The models’ performance was evaluated with cross-validation. Steroid hormones alone had modest predictive value for LVSI (Figure 3a, Supplementary Table S5), deep MI (Figure 3b, Supplementary Table S6) and tumor grade (Supplementary Table S7) (AUC < 0.70). HE4 was the strongest predictor of LVSI (AUC: 0.791) and deep MI (AUC: 0.709), while A4 (AUC: 0.667) and 11OHT (AUC: 0.641) were the top-performing steroids, respectively. Differences in HE4′s performance for LVSI (AUC: 0.810) and deep MI (AUC: 0.776) compared to the Knific et al. study [23] were most likely due to differences in 20 samples between this cohort and the original one. For tumor grade, CA-125 was the best predictor (AUC: 0.810), with 11KA4 as the top-performing steroid (AUC: 0.690).
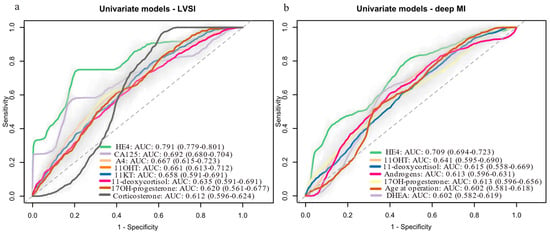
Figure 3.
Univariate logistic regression models for predicting: (a) LVSI status (LVSI-positive: n = 12; LVSI-negative: n = 50), (b) deep MI (deep MI present: n = 16; absent: n = 44). Data are shown for variables with AUCs above 0.6. Data in brackets represent 95% confidence intervals (CI). Light grey color around the ROC curves shows standard deviation. 11OHT, 11β-hydroxy-testosterone; 11KT, 11-ketotestosterone; AUC, area under the receiver operating curve; CA-125, cancer antigen 125; DHEA, dehydroepiandrosterone; HE4, human epididymis protein 4; LVSI, lympho-vascular space invasion; MI, myometrial invasion; ROC, receiver operator curve; T, testosterone.
4. Discussion
In this study, we profiled preoperative serum steroid levels in 62 patients with EC and 70 women with benign uterine pathologies using LC-MS/MS. To our knowledge, this is the first study to evaluate the diagnostic and prognostic potential of systemic steroids in EC using machine learning. This is also the first case-control study evaluating systemic 11-oxyandrogen levels in EC.
We found that patients with EC exhibit distinct alterations in systemic steroid hormone profiles, including elevated levels of classic androgens, 11-oxyandrogens and glucocorticoids. In particular, the marked increase in 11β-hydroxylated androgens could be the result of enhanced CYP11B1 activity, an adrenal-specific enzyme that catalyzes the 11β-hydroxylation of A4 and T and is also responsible for cortisol synthesis. Indeed, we observed concurrent elevations of 11OHA4, 11OHT, cortisol and corticosterone (a CYP11B1/2 product) in cases supporting the hypothesis of a systemic adrenal response to tumor-related stress or immune activation.
An additional explanation may lie in the role of gut microbiota, which is increasingly recognized for its involvement in steroid metabolism. Microbial enzymes can generate androgens such as DHEA and T from precursors like pregnenolone and 17α-hydroxy-pregnenolone [29] and may also contribute to the production of 11-oxyandrogens from cortisol or other C21 steroids [30,31,32]. The distinct alterations in the gut microbiota in patients with EC [33], might contribute to altered systemic steroid levels as well, although this need to be investigated further.
A third explanation of altered 11-oxyandrogen levels could be the tumor itself. Although Dahmani et al. reported that systemic levels of 11-oxyandrogens decline after tumor removal, suggesting tumor-driven regulation [34], our recent findings show that CYP11B1 is not expressed in EC tumors or in vitro cell lines [35]. We also showed that local intra-tumoral production of 11-oxyanrogens cannot happen from classic androgen precursors [35]. Consequently, the postoperative drop in systemic 11OHA4 and 11OHT levels cannot be solely attributed to tumor excision and likely reflects larger systemic changes.
Finally, the adipose tissue can be an important regulator of steroid hormone levels. Given that higher BMI is characteristic of patients with EC [36], it is plausible that expanded adipose depots in these individuals further drive alterations in both classic and 11-oxyandrogen levels. Indeed, the adipose tissue has been shown to express steroid metabolizing enzymes, like AKR1C3 [37,38,39].
Moving on to steroid diagnostic potential, we found that individual serum steroids had limited diagnostic accuracy, but adding them to CA-125, HE4 and BMI significantly improved both sensitivity and specificity. This biologically meaningful enhancement suggests that including steroid hormones provides a more complete biochemical signature than CA-125 and HE4, as steroids offer a direct readout of the endocrine dysregulation and intracrine activity that underpin EC pathogenesis. These diagnostic improvements align with extensive epidemiological data showing androgens, like DHEA-sulfate [40,41], A4 [40,41], T [41] and some androgen glucuronides [40] to be elevated in patients with EC, although not all studies agree [40,42,43]. Moreover, endometrial tumors, especially endometrioid ones, are known to express steroid-metabolizing enzymes and receptors [44,45,46,47,48,49,50], and to form locally androgens, 11-oxyandrogens and estrogens [35,51], suggesting that this intra-tumoral activity may also contribute to the improved diagnostic performance of the models.
Regarding prognostic potential, we found that individual serum steroids had limited prognostic accuracy for LVSI and deep MI. Unfortunately, due to the limited sample size of this sub-cohort, we were unable to investigate them in a multi-variate manner. The best prognostic predictors of LVSI and MI in our cohort were HE4 and CA-125, consistent with prior studies [21,22,52,53,54,55,56] and systematic reviews and meta analyses [57,58], which report strong performance of these markers for LVSI and MI prediction.
Several lines of evidence provide a rationale for why steroid hormones should be taken into consideration into prognostic models. For example, Tangen et al. reported that higher preoperative plasma levels of DHEA, DHEA-sulfate, progesterone (and its 21-hydroxylated metabolite) and estrone sulfate were linked to longer overall survival in patients with EC [59]. Conversely, lower concentrations of 17-OH-progesterone, 11-deoxycortisol and A4 correlated with more aggressive tumor features and poorer disease-specific survival [60]. Moreover, 11-oxyandrogen metabolites appear to carry independent prognostic information: elevated preoperative 11-ketoandrosterone predicted a higher risk of recurrence, while increased postoperative 11-hydroxyandrosterone was associated with both greater recurrence rates and reduced survival [34].
Beyond their diagnostic and prognostic potential, changes in the systemic steroid profile of patients with EC also have significant implications for tumor biology. Forsse and colleagues found that tumors from patients with low plasma levels of 17α-hydroxyprogesterone and 11-deoxycortisol had increased expression of genes related to cell proliferation, while tumors from patients with high plasma levels of these hormones showed upregulated estrogen signaling and increased expression of genes associated with inflammation [60]. Additionally, estradiol levels were positively correlated with the expression of genes involved in estrogen receptor signaling within tumors [59,60]. Our recent work also highlighted differences in the intra-tumoral metabolism of 11-oxyandrogen precursors (11OHA4 and 11KA4) between low- and high-grade EC tumors. Low-grade tumors were more efficient in producing 11KT, a metabolite capable of activating the androgen receptor, which has been associated with better survival outcomes [35].
Currently, there are a considerable number of studies focusing on biomarkers for EC detection and prognosis, although most of these studies are in discovery phase, and only a few have been validated in independent, multi-centric cohorts. These studies explore a wide range of biomolecules across various biological fluids—including plasma, serum, cervicovaginal fluid and uterine aspirates—and increasingly apply machine learning approaches (reviewed in [61,62]). Among the biomolecules studied are proteins (hormones, cancer-associated antigens, plasma glycoproteins, plasma lipoproteins, enzymes, growth factors) [11,63,64,65,66,67,68,69], lipids and metabolites (glycerophospholipids, sphingolipids, fatty acids, amino acids) ([12,14,70,71,72,73,74,75,76,77,78,79,80,81]), micro RNAs [82,83], circulating tumor DNA [84,85,86]. Additionally, routine blood count parameters—reflecting systemic immune or inflammatory responses—have shown promising diagnostic potential [87,88,89].
Among these studies, two approaches currently show the greatest potential for clinical implementation: the REM-B algorithm [10] and the metabolomic panel developed by Troisi et al. [81]. The REM-B algorithm integrates patient age, presence of abnormal uterine bleeding, BMI, HE4 levels and ultrasound findings and achieves a sensitivity and specificity of over 90% It has already been validated in an independent cohort, but multicenter validation is still needed to confirm its clinical utility. Similarly, the ensemble models based on metabolomic panel by Troisi et al. show a remarkable diagnostic performance with a sensitivity of 100% and specificity of 96% in detecting EC.
In our study we identified androgens as novel biomarker candidates for EC diagnosis. When incorporated into more comprehensive diagnostic models, these biomarkers can help improve diagnostic accuracy. Nonetheless, our study has several limitations. First, although the selection criteria for this study were rigorous, some potential biases may have influenced the results. More specifically, all study participants were from a single clinical center and primarily of Slovenian ethnicity, which may limit the generalizability of the findings to other populations with greater ethnic and demographic diversity. Additionally, the under-representation of cases with high-grade EC could introduce bias, as this may not fully reflect the disease spectrum seen in broader clinical settings. Second, measurement bias should be taken into consideration: although all samples were collected in the morning to minimize circadian effects, residual time-of-day variation may persist. To address this, we adhered to standardized protocols for sample collection, randomized sample processing and blinded analysts to clinical data ensuring that the analysis remained objective and free from potential interpretive bias. Furthermore, steroid measurements were based on a single preoperative time point, and finally, LC-MS/MS technical constraints limited the quantification of low-abundance hormones like DHT and progesterone.
Despite these limitations, our study also has notable strengths, such as including patients with benign uterine conditions as a control group, rather than healthy women, which are unlikely to require differential diagnosis. Moreover, we employed a multiplexed mass spectrometry method for steroid quantification, which is currently the gold standard for steroid analysis. Finally, the integration of steroids and proteins into diagnostic models represents a novel approach that could be easily translated into clinical practice once validated in larger, multi-center cohorts.
5. Conclusions
Our study shows that patients with EC have distinct steroid hormone profiles compared to women with benign uterine conditions. While individual steroid hormones had limited diagnostic value, combining them in multivariate models significantly improved diagnostic performance. Our multivariate model, incorporating classic androgens, CA-125, HE4, BMI and parity, achieved AUC value of 0.87, 79.1% sensitivity and 74.7% specificity in distinguishing EC from benign uterine conditions, significantly outperforming CA-125 and HE4 alone, or their combination with BMI. This highlights the potential of steroid profiling as a valuable addition to EC diagnostics.
Integrating steroid profiling into routine diagnostics could enhance early detection and improve patient outcomes; however, further validation in larger independent cohorts is needed to confirm the clinical utility of the proposed diagnostic model.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17101679/s1, Figure S1: Measured steroid hormones (blue boxes) and their positions and relations in steroid biosynthesis; Table S1: Histopathological characteristics of patients with EC included in this study; Table S2: Clinical-pathological characteristics related to median steroid hormone levels (interquartile range) in patients with EC; Table S3: Logistic regression models for EC diagnosis; Table S4: Evaluation of best performing multivariate diagnostic models with bootstraping; Table S5: Logistic regression models for predicting LVSI; Table S6: Logistic regression models for predicting deep MI; Table S7: Logistic regression models for preoperative risk assessment.
Author Contributions
M.G.: Conceptualization, Data curation; Formal analysis, Investigation; Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. A.E.T.: Methodology, Writing—review & editing. Š.S.: Writing—review and editing. T.L.R.: Conceptualization, Funding acquisition, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovenian Research Agency (Grant numbers J3-2535 and J3-3069) to T.L.R., research program funding P1-0390 and P3-0449, and practical skills grant from the Society of Endocrinology to M.G.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Republic of Slovenia (ID: 0120-487/2020/3 on 17 November 2020).
Informed Consent Statement
All participants involved in the study were provided with an informed consent form, which they read and signed prior to participation.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.
Acknowledgments
We thank the study participants for donating blood for this study. We also thank Vesna Sekelj-Rangus, Milena Osredkar, Irena Tonin and Leon Meglič from the Department of Gynecology and Obstetrics, University Medical Center, Ljubljana, Joško Osredkar and Vera Troha Poljančič from the Clinical Institute of Chemistry and Clinical Biochemistry, University Medical Center, Ljubljana and Robert Marijan from the Institute of Biochemistry and Molecular Genetics, Faculty of Medicine, University of Ljubljana.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 11KA4 | 11-keto-androstenedione |
| 11OHA4 | 11β-hydroxy-androstenedione |
| 11OHT | 11β-hydroxytestosterone |
| A4 | Androstenedione |
| AUC | Area under the receiver operating curve |
| BMI | Body mass index |
| CA-125 | Cancer antigen 125 |
| DHEA | Dehydroepiandrosterone |
| DHT | 5α-dihydrotestosterone |
| dMMR | missmatch repair deficient |
| DSS | Disease-specific survival |
| EC | Endometrial cancer |
| ESI | Electrospray ionization |
| FIGO | International Federation of Gynecology and Obstetrics |
| HE4 | Human epidydymis protein 4 |
| IQR | Interquartile range |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LLOQ | Lower limit of quantification |
| LVSI | Lymphovascular space invasion |
| MI | Myometrial invasion |
| MRI | Magnetic resonance imaging |
| MTBE | tert-butyl methyl ether |
| NSMP | Non-specific molecular profile |
| PCOS | Polycystic ovary syndrome |
| QC | Quality control |
| REM | Risk of Endometrial Malignancy |
| SMAC | Steorid Metabolome Analysiss Core |
| T | Testosterone |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12. [Google Scholar] [CrossRef]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef]
- Long, B.; Clarke, M.A.; Morillo, A.D.M.; Wentzensen, N.; Bakkum-Gamez, J.N. Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: Systematic review and meta-analysis. Gynecol. Oncol. 2020, 157, 624–633. [Google Scholar] [CrossRef]
- Plotti, F.; Capriglione, S.; Scaletta, G.; Luvero, D.; Lopez, S.; Nastro, F.F.; Terranova, C.; De Cicco Nardone, C.; Montera, R.; Angioli, R. Implementing the Risk of Endometrial Malignancy Algorithm (REM) adding obesity as a predictive factor: Results of REM-B in a single-center survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 51–56. [Google Scholar] [CrossRef]
- Njoku, K.; Pierce, A.; Chiasserini, D.; Geary, B.; Campbell, A.E.; Kelsall, J.; Reed, R.; Geifman, N.; Whetton, A.D.; Crosbie, E.J. Detection of endometrial cancer in cervico-vaginal fluid and blood plasma: Leveraging proteomics and machine learning for biomarker discovery. EBioMedicine 2024, 102, 105064. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, J.; Zhang, J.; Cao, J.; Hu, X.; Huang, Y.; Wang, R.; Wu, J.; Di, W.; Qian, K.; et al. Identification and validation of serum metabolite biomarkers for endometrial cancer diagnosis. EMBO Mol. Med. 2024, 16, 988–1003. [Google Scholar] [CrossRef]
- Troisi, J.; Raffone, A.; Travaglino, A.; Belli, G.; Belli, C.; Anand, S.; Giugliano, L.; Cavallo, P.; Scala, G.; Symes, S.; et al. Development and Validation of a Serum Metabolomic Signature for Endometrial Cancer Screening in Postmenopausal Women. JAMA Netw. Open 2020, 3, e2018327. [Google Scholar] [CrossRef] [PubMed]
- Knific, T.; Vouk, K.; Smrkolj, Š.; Prehn, C.; Adamski, J.; Rižner, T.L. Models including plasma levels of sphingomyelins and phosphatidylcholines as diagnostic and prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018, 178, 312–321. [Google Scholar] [CrossRef]
- Lee, M.S.; Moon, M.H.; Kim, S.Y.; Jang, S.; Oh, S.; Cho, J.Y. Preoperative risk stratification in women with endometrial cancer: A comparison of contrast-enhanced MR imaging and diffusion-weighted MR imaging. Eur. J. Radiol. 2022, 150, 110276. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Singh, D.K.; Meyer, L.; Smith, D.H.; Wertheim, I.; Resnik, E.; Bodurka, D.C. Predictors of final histology in patients with endometrial cancer. Gynecol. Oncol. 2004, 95, 463–468. [Google Scholar] [CrossRef]
- Visser, N.C.M.; Reijnen, C.; Massuger, L.F.A.G.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J.M.A. Accuracy of Endometrial Sampling in Endometrial Carcinoma: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef]
- Body, N.; Lavoué, V.; De Kerdaniel, O.; Foucher, F.; Henno, S.; Cauchois, A.; Laviolle, B.; Leblanc, M.; Levêque, J. Are preoperative histology and MRI useful for classification of endometrial cancer risk? BMC Cancer 2016, 16, 498. [Google Scholar] [CrossRef]
- Cabrera, S.; Bebia, V.; López-Gil, C.; Luzarraga-Aznar, A.; Denizli, M.; Salazar-Huayna, L.; Abdessayed, N.; Castellví, J.; Colas, E.; Gil-Moreno, A. Molecular classification improves preoperative risk assessment of endometrial cancer. Gynecol. Oncol. 2024, 189, 56–63. [Google Scholar] [CrossRef]
- Reijnen, C.; Visser, N.C.; Kasius, J.C.; Boll, D.; Geomini, P.M.; Ngo, H.; Van Hamont, D.; Pijlman, B.M.; Vos, M.C.; Bulten, J.; et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J. Gynecol. Oncol. 2019, 30, e70. [Google Scholar] [CrossRef]
- Brennan, D.J.; Hackethal, A.; Metcalf, A.M.; Coward, J.; Ferguson, K.; Oehler, M.K.; Quinn, M.A.; Janda, M.; Leung, Y.; Freemantle, M.; et al. Serum HE4 as a prognostic marker in endometrial cancer—A population based study. Gynecol. Oncol. 2014, 132, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.; Jamieson, A.; Chiu, D.; Leung, S.; Lum, A.; Kommoss, S.; Huntsman, D.G.; Talhouk, A.; Gilks, C.B.; McAlpine, J.N. Serum CA125 levels in the context of ProMisE molecular classification provides pre-operative prognostic information that can direct endometrial cancer management. Gynecol. Oncol. 2025, 193, 1–11. [Google Scholar] [CrossRef]
- Knific, T.; Osredkar, J.; Smrkolj, Š.; Tonin, I.; Vouk, K.; Blejec, A.; Frković Grazio, S.; Rižner, T.L. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol. Oncol. 2017, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rizner, T.L.; Adamski, J. Paramount importance of sample quality in pre-clinical and clinical research—Need for standard operating procedures (SOPs). J. Steroid Biochem. Mol. Biol. 2019, 186, 1–3. [Google Scholar] [CrossRef]
- Schiffer, L.; Shaheen, F.; Gilligan, L.C.; Storbeck, K.-H.; Hawley, J.M.; Keevil, B.G.; Arlt, W.; Taylor, A.E. Multi-steroid profiling by UHPLC-MS/MS with post-column infusion of ammonium fluoride. J. Chromatogr. B 2022, 1209, 123413. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Kempegowda, P.; Sitch, A.J.; Adaway, J.E.; Shaheen, F.; Ebbehoj, A.; Singh, S.; McTaggart, M.P.; O’Reilly, M.W.; Prete, A.; et al. Classic and 11-oxygenated androgens in serum and saliva across adulthood: A cross-sectional study analyzing the impact of age, body mass index, and diurnal and menstrual cycle variation. Eur. J. Endocrinol. 2023, 188, lvac017. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Pernigoni, N.; Zagato, E.; Calcinotto, A.; Troiani, M.; Mestre, R.P.; Calì, B.; Attanasio, G.; Troisi, J.; Minini, M.; Mosole, S.; et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 2021, 374, 216–224. [Google Scholar] [CrossRef]
- Devendran, S.; Méndez-García, C.; Ridlon, J.M. Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J. Lipid Res. 2017, 58, 916–925. [Google Scholar] [CrossRef]
- Winter, J.; Morris, G.N.; O’Rourke-Locascio, S.; Bokkenheuser, V.D.; Mosbach, E.H.; Cohen, B.I.; Hylemon, P.B. Mode of action of steroid desmolase and reductases synthesized by Clostridium “scindens” (formerly Clostridium strain 19). J. Lipid Res. 1984, 25, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Gong, R.; Xi, Y. Gut microbiome dysbiosis in patients with endometrial cancer vs. healthy controls based on 16S rRNA gene sequencing. Current microbiology 2023, 80, 239. [Google Scholar] [CrossRef]
- Dahmani, C.; Caron, P.; Simonyan, D.; Turcotte, V.; Grégoire, J.; Plante, M.; Guillemette, C. Circulating adrenal 11-oxygenated androgens are associated with clinical outcome in endometrial cancer. Front. Endocrinol. 2023, 14, 1156680. [Google Scholar] [CrossRef] [PubMed]
- Gjorgoska, M.; Šturm, L.; Lanišnik Rižner, T. Pre-receptor regulation of 11-oxyandrogens differs between normal and cancerous endometrium and across endometrial cancer grades and molecular subtypes. Front. Endocrinol. 2024, 15, 1404804. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Schiffer, L.; Oestlund, I.; Snoep, J.L.; Gilligan, L.C.; Taylor, A.E.; Sinclair, A.J.; Singhal, R.; Freeman, A.; Ajjan, R.; Tiganescu, A.; et al. Inhibition of the glucocorticoid-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 drives concurrent 11-oxygenated androgen excess. FASEB J. 2024, 38, e23574. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Kempegowda, P.; Walsh, M.; Taylor, A.E.; Manolopoulos, K.N.; Allwood, J.W.; Semple, R.K.; Hebenstreit, D.; Dunn, W.B.; Tomlinson, J.W.; et al. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3327–3339. [Google Scholar] [CrossRef]
- Turcu, A.F.; Rege, J.; Auchus, R.J.; Rainey, W.E. 11-Oxygenated androgens in health and disease. Nat. Rev. Endocrinol. 2020, 16, 284–296. [Google Scholar] [CrossRef]
- Lukanova, A.; Lundin, E.; Micheli, A.; Arslan, A.; Ferrari, P.; Rinaldi, S.; Krogh, V.; Lenner, P.; Shore, R.E.; Biessy, C.; et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int. J. Cancer 2004, 108, 425–432. [Google Scholar] [CrossRef]
- Michels, K.A.; Brinton, L.A.; Wentzensen, N.; Pan, K.; Chen, C.; Anderson, G.L.; Pfeiffer, R.M.; Xu, X.; Rohan, T.E.; Trabert, B. Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women’s Health Initiative Observational Study. JNCI Cancer Spectr. 2019, 3, pkz029. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Hertzmark, K.; Koenig, K.L.; Lundin, E.; Rinaldi, S.; Johnson, T.; Krogh, V.; Hallmans, G.; Idahl, A.; Lukanova, A.; et al. Premenopausal Circulating Androgens and Risk of Endometrial Cancer: Results of a Prospective Study. Horm. Cancer 2016, 7, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pavlič, R.; Vidic, S.; Anko, M.; Knific, T.; Büdefeld, T.; Marton, K.; Sinreih, M.; Poschner, S.; Jäger, W.; Frković-Grazio, S.; et al. Altered Profile of E1-S Transporters in Endometrial Cancer: Lower Protein Levels of ABCG2 and OSTβ and Up-Regulation of SLCO1B3 Expression. Int. J. Mol. Sci. 2021, 22, 3819. [Google Scholar] [CrossRef] [PubMed]
- Sinreih, M.; Hevir, N.; Rižner, T.L. Altered expression of genes involved in progesterone biosynthesis, metabolism and action in endometrial cancer. Chem. Biol. Interact. 2013, 202, 210–217. [Google Scholar] [CrossRef]
- Hojnik, M.; Kenda Šuster, N.; Smrkolj, Š.; Frković Grazio, S.; Verdenik, I.; Rižner, T.L. AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas. J. Clin. Med. 2020, 9, 4105. [Google Scholar] [CrossRef]
- Ito, K.; Suzuki, T.; Akahira, J.-i.; Moriya, T.; Kaneko, C.; Utsunomiya, H.; Yaegashi, N.; Okamura, K.; Sasano, H. Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int. J. Cancer 2002, 99, 652–657. [Google Scholar] [CrossRef]
- Šmuc, T.; Rižner, T.L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol. Cell. Endocrinol. 2009, 301, 74–82. [Google Scholar] [CrossRef]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef]
- Tangen, I.L.; Onyango, T.B.; Kopperud, R.; Berg, A.; Halle, M.K.; Øyan, A.M.; Werner, H.M.J.; Trovik, J.; Kalland, K.H.; Salvesen, H.B.; et al. Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget 2016, 7, 49289–49298. [Google Scholar] [CrossRef]
- Pavlič, R.; Gjorgoska, M.; Hafner, E.; Sinreih, M.; Gajser, K.; Poschner, S.; Jäger, W.; Rižner, T.L. In the Model Cell Lines of Moderately and Poorly Differentiated Endometrial Carcinoma, Estrogens Can Be Formed via the Sulfatase Pathway. Front. Mol. Biosci. 2021, 8, 743403. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.E.; Njoku, K.; Jones, E.R.; Crosbie, E.J. Serum CA125 and HE4 as Biomarkers for the Detection of Endometrial Cancer and Associated High-Risk Features. Diagnostics 2022, 12, 2834. [Google Scholar] [CrossRef]
- Antonsen, S.L.; Høgdall, E.; Christensen, I.J.; Lydolph, M.; Tabor, A.; Loft Jakobsen, A.; Fagö-Olsen, C.L.; Andersen, E.S.; Jochumsen, K.; Høgdall, C. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: A prospective multicenter study (ENDOMET). Acta Obstet. Gynecol. Scand. 2013, 92, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Quan, Q.; Meng, Y.; Wu, J.; Yang, S.; Hu, J.; Mu, X. The value of serum HE4 and CA125 levels for monitoring the recurrence and risk stratification of endometrial endometrioid carcinoma. Heliyon 2023, 9, e18016. [Google Scholar] [CrossRef]
- O’Toole, S.A.; Huang, Y.; Norris, L.; Power Foley, M.; Shireen, R.; McDonald, S.; Kamran, W.; Ibrahim, N.; Ward, M.; Thompson, C.; et al. HE4 and CA125 as preoperative risk stratifiers for lymph node metastasis in endometrioid carcinoma of the endometrium: A retrospective study in a cohort with histological proof of lymph node status. Gynecol. Oncol. 2021, 160, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Barr, C.E.; Crosbie, E.J. HE4 as a Biomarker for Endometrial Cancer. Cancers 2021, 13, 4764. [Google Scholar] [CrossRef]
- Degez, M.; Caillon, H.; Chauviré-Drouard, A.; Leroy, M.; Lair, D.; Winer, N.; Thubert, T.; Dochez, V. Endometrial cancer: A systematic review of HE4, REM and REM-B. Clin. Chim. Acta 2021, 515, 27–36. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Ma, C.X.; Kang, Y.H. Role of Human Epididymis Protein 4 (HE4) in Determining Survival of Patients With Endometrial Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2020, 19, 1533033820971660. [Google Scholar] [CrossRef]
- Tangen, I.L.; Fasmer, K.E.; Konings, G.F.; Jochems, A.; Delvoux, B.; Xanthoulea, S.; Stokowy, T.; Strand, E.; Berg, H.F.; Auriola, S.; et al. Blood steroids are associated with prognosis and fat distribution in endometrial cancer. Gynecol. Oncol. 2019, 152, 46–52. [Google Scholar] [CrossRef]
- Forsse, D.; Forsse, D.; Tangen, I.L.; Tangen, I.L.; Fasmer, K.E.; Fasmer, K.E.; Halle, M.K.; Halle, M.K.; Viste, K.; Almås, B.; et al. Blood steroid levels predict survival in endometrial cancer and reflect tumor estrogen signaling. Gynecol. Oncol. 2019, 156, 400–406. [Google Scholar] [CrossRef]
- Romano, A.; Rižner, T.L.; Werner, H.M.J.; Semczuk, A.; Lowy, C.; Schröder, C.; Griesbeck, A.; Adamski, J.; Fishman, D.; Tokarz, J. Endometrial cancer diagnostic and prognostic algorithms based on proteomics, metabolomics, and clinical data: A systematic review. Front. Oncol. 2023, 13, 1120178. [Google Scholar] [CrossRef] [PubMed]
- Tea Lanišnik Rižner, A.R. The discovery of biomarkers for endometrial cancer: Update over the last years. Expert Rev. Mol. Diagn. IERO 2025, IERO 2505546. [Google Scholar] [CrossRef]
- Ura, B.; Capaci, V.; Aloisio, M.; Di Lorenzo, G.; Romano, F.; Ricci, G.; Monasta, L. A Targeted Proteomics Approach for Screening Serum Biomarkers Observed in the Early Stage of Type I Endometrial Cancer. Biomedicines 2022, 10, 1857. [Google Scholar] [CrossRef]
- Ura, B.; Biffi, S.; Monasta, L.; Arrigoni, G.; Battisti, I.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Celsi, F.; Addobbati, R.; et al. Two Dimensional-Difference in Gel Electrophoresis (2D-DIGE) Proteomic Approach for the Identification of Biomarkers in Endometrial Cancer Serum. Cancers 2021, 13, 3639. [Google Scholar] [CrossRef] [PubMed]
- Enroth, S.; Berggrund, M.; Lycke, M.; Lundberg, M.; Assarsson, E.; Olovsson, M.; Stålberg, K.; Sundfeldt, K.; Gyllensten, U. A two-step strategy for identification of plasma protein biomarkers for endometrial and ovarian cancer. Clin. Proteom. 2018, 15, 38. [Google Scholar] [CrossRef]
- Tarney, C.M.; Wang, G.; Bateman, N.W.; Conrads, K.A.; Zhou, M.; Hood, B.L.; Loffredo, J.; Tian, C.; Darcy, K.M.; Hamilton, C.A.; et al. Biomarker panel for early detection of endometrial cancer in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial. Am. J. Obstet. Gynecol. 2019, 221, 472.e1–472.e10. [Google Scholar] [CrossRef]
- Celsi, F.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Aloisio, M.; Di Lorenzo, G.; Romano, F.; Ricci, G.; Ura, B. Gel-Based Proteomic Identification of Suprabasin as a Potential New Candidate Biomarker in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 2076. [Google Scholar] [CrossRef]
- Ceylan, Y.; Akpınar, G.; Doger, E.; Kasap, M.; Guzel, N.; Karaosmanoglu, K.; Kopuk, S.Y.; Yucesoy, I. Proteomic analysis in endometrial cancer and endometrial hyperplasia tissues by 2D-DIGE technique. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101652. [Google Scholar] [CrossRef]
- Akkour, K.; Alanazi, I.O.; Alfadda, A.A.; Alhalal, H.; Masood, A.; Musambil, M.; Rahman, A.M.A.; Alwehaibi, M.A.; Arafah, M.; Bassi, A.; et al. Tissue-Based Proteomic Profiling in Patients with Hyperplasia and Endometrial Cancer. Cells 2022, 11, 2119. [Google Scholar] [CrossRef]
- Hishinuma, E.; Shimada, M.; Matsukawa, N.; Shima, Y.; Li, B.; Motoike, I.N.; Shibuya, Y.; Hagihara, T.; Shigeta, S.; Tokunaga, H.; et al. Identification of predictive biomarkers for endometrial cancer diagnosis and treatment response monitoring using plasma metabolome profiling. Cancer Metab. 2023, 11, 16. [Google Scholar] [CrossRef]
- Ihata, Y.; Miyagi, E.; Numazaki, R.; Muramatsu, T.; Imaizumi, A.; Yamamoto, H.; Yamakado, M.; Okamoto, N.; Hirahara, F. Amino acid profile index for early detection of endometrial cancer: Verification as a novel diagnostic marker. Int. J. Clin. Oncol. 2014, 19, 364–372. [Google Scholar] [CrossRef]
- Njoku, K.; Campbell, A.E.; Geary, B.; MacKintosh, M.L.; Derbyshire, A.E.; Kitson, S.J.; Sivalingam, V.N.; Pierce, A.; Whetton, A.D.; Crosbie, E.J. Metabolomic Biomarkers for the Detection of Obesity-Driven Endometrial Cancer. Cancers 2021, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Audet-Delage, Y.; Villeneuve, L.; Grégoire, J.; Plante, M.; Guillemette, C. Identification of Metabolomic Biomarkers for Endometrial Cancer and Its Recurrence after Surgery in Postmenopausal Women. Front. Endocrinol. 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Sarno, L.; Landolfi, A.; Scala, G.; Martinelli, P.; Venturella, R.; Di Cello, A.; Zullo, F.; Guida, M. Metabolomic Signature of Endometrial Cancer. J. Proteome Res. 2018, 17, 804–812. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Lugade, A.; Field, J.; Al-Wahab, Z.; Han, B.; Mandal, R.; Bjorndahl, T.C.; Turkoglu, O.; Graham, S.F.; Wishart, D.; et al. Metabolomic prediction of endometrial cancer. Metabolomics 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, X.; Sun, Y.; Wang, L.; Shu, H.; Qian, C. A metabolomic signature of FIGO stage I and II endometrial cancer. Neoplasma 2021, 68, 1283–1291. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, W.; Wei, J.; Yao, Y.; Sun, G.; Wang, L.; Zhang, W.; Chen, S.; Zhou, W.; Zhao, H.; et al. A serum lipidomics study for the identification of specific biomarkers for endometrial polyps to distinguish them from endometrial cancer or hyperplasia. Int. J. Cancer 2022, 150, 1549–1559. [Google Scholar] [CrossRef]
- Schuhn, A.; Tobar, T.W.; Gahlawat, A.W.; Hauke, J.; Baumann, L.; Okun, J.G.; Nees, J. Potential of blood-based biomarker approaches in endometrium and breast cancer: A case-control comparison study. Arch. Gynecol. Obstet. 2022, 306, 1623–1632. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Chen, K.; Chiu, C.-Y.; Lu, K.-Y.; Lu, H.-Y.; Chiang, M.-H.; Tsai, C.-K.; Lo, C.-J.; Cheng, M.-L.; Chang, T.-C.; et al. Metabolomic biomarkers in cervicovaginal fluid for detecting endometrial cancer through nuclear magnetic resonance spectroscopy. Metabolomics 2019, 15, 146. [Google Scholar] [CrossRef]
- Yi, R.; Xie, L.; Wang, X.; Shen, C.; Chen, X.; Qiao, L. Multi-Omic Profiling of Multi-Biosamples Reveals the Role of Amino Acid and Nucleotide Metabolism in Endometrial Cancer. Front. Oncol. 2022, 12, 861142. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Mollo, A.; Lombardi, M.; Scala, G.; Richards, S.M.; Symes, S.J.K.; Travaglino, A.; Neola, D.; de Laurentiis, U.; Insabato, L.; et al. The Metabolomic Approach for the Screening of Endometrial Cancer: Validation from a Large Cohort of Women Scheduled for Gynecological Surgery. Biomolecules 2022, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cao, M.; Liu, C.; Zhang, C.; Li, C.; Cheng, W.; Zhang, S.; Zhang, H.; Zhu, W. Three plasma-based microRNAs as potent diagnostic biomarkers for endometrial cancer. Cancer Biomark. 2021, 31, 127–138. [Google Scholar] [CrossRef]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int. J. Cancer 2013, 132, 1633–1645. [Google Scholar] [CrossRef]
- Liu, J.; Hu, D.; Lin, Y.; Chen, X.; Yang, R.; Li, L.; Zhan, Y.; Bao, H.; Zang, L.; Zhu, M.; et al. Early detection of uterine corpus endometrial carcinoma utilizing plasma cfDNA fragmentomics. BMC Med. 2024, 22, 310. [Google Scholar] [CrossRef]
- Zou, J.; Wang, E. eTumorType, An Algorithm of Discriminating Cancer Types for Circulating Tumor Cells or Cell-Free DNAs in Blood. Genom. Proteom. Bioinform. 2017, 15, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.W.; Selenica, P.; Patel, J.; Wu, M.; Nincevic, J.; Lakhman, Y.; Zhou, Q.; Shah, R.H.; Berger, M.F.; Da Cruz Paula, A.; et al. High-Sensitivity Mutation Analysis of Cell-Free DNA for Disease Monitoring in Endometrial Cancer. Clin. Cancer Res. 2023, 29, 410–421. [Google Scholar] [CrossRef]
- Yayla Abide, C.; Bostanci Ergen, E.; Cogendez, E.; Kilicci, C.; Uzun, F.; Ozkaya, E.; Karateke, A. Evaluation of complete blood count parameters to predict endometrial cancer. J. Clin. Lab. Anal. 2018, 32, e22438. [Google Scholar] [CrossRef]
- Marin, A.-G.; Filipescu, A.G.; Petca, R.C.; Vlădăreanu, R.; Petca, A. Clinical Correlations between Serological Markers and Endometrial Cancer. Cancers 2024, 16, 1935. [Google Scholar] [CrossRef]
- Ronsini, C.; Iavarone, I.; Vastarella, M.G.; Della Corte, L.; Andreoli, G.; Bifulco, G.; Cobellis, L.; De Franciscis, P. SIR-EN-New Biomarker for Identifying Patients at Risk of Endometrial Carcinoma in Abnormal Uterine Bleeding at Menopause. Cancers 2024, 16, 3567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).