Simple Summary
The COVID-19 pandemic severely disrupted healthcare systems, particularly affecting cancer care due to delayed diagnoses and treatments. To assess the impact of such crises, this study developed a set of hospital-based quality indicators (QIs) for four cancer types: breast cancer, hepatocellular carcinoma, gynecological cancers (excluding ovarian cancer), and peritoneal carcinomatosis. A multidisciplinary team followed a structured process, including a literature review, expert panel validation using the RAND/UCLA method, and final selection by a steering committee. Among 150 initially identified indicators, 49 were validated, with most focusing on care processes such as diagnosis, treatment, and therapeutic delays. Two indicators were common to all four cancers: multidisciplinary team discussions and psychological support consultations. This study highlights the feasibility of developing QIs tailored to health crises. The next steps will involve real-time implementation, international validation, and integration into healthcare policies to enhance crisis preparedness and ensure continuous quality improvement in cancer care.
Abstract
Background/Objectives: The COVID-19 pandemic led to significant disruptions in healthcare systems, particularly impacting cancer care through delays in diagnoses and treatments. Quality indicators (QIs) are essential tools for monitoring healthcare performance, yet existing QIs may not be suited for crises. This study aimed to develop a set of hospital-based QIs tailored to assess the impact of care reorganization during health crises across four cancer types: breast cancer, hepatocellular carcinoma, gynecological cancers (excluding ovarian cancer), and peritoneal carcinomatosis. Methods: A multidisciplinary steering committee (SC) conducted a five-stage process, including a literature review, indicator selection, content validation via the RAND/UCLA method, final validation by the SC, and a pilot feasibility study. QIs were assessed based on clinical relevance, reproducibility, sensitivity to change, and feasibility. Expert panels evaluated and validated the indicators in two rounds of voting. Results: Among 150 initially identified QIs, 49 were validated: 12 for breast cancer, 11 for hepatocellular carcinoma, 8 for gynecological cancers, and 18 for peritoneal carcinomatosis. Most (92%) were process indicators, covering diagnosis, treatment, and care delays. Two common indicators were identified across all four cancers: multidisciplinary team meeting discussions and psychological support consultations. Conclusions: This study demonstrates the feasibility of developing crisis-responsive QIs to monitor cancer care during health system disruptions. Future work will focus on their real-time implementation, validation in international settings, and integration into healthcare policies to enhance crisis preparedness.
1. Introduction
The COVID-19 pandemic, which began in 2020, had a significant impact on healthcare systems worldwide, leading to widespread lockdowns, travel restrictions, and reorganizations among healthcare providers to limit virus transmission and address increased pressure on health services.
In France, a series of successive and graduated national directives mandated extensive rescheduling of medical procedures across all healthcare establishments. On 16 March 2020, the government ordered the “postponement of any non-urgent surgical or medical activity, while taking into account potential risks to patient outcomes” [1]. Furthermore, during the initial lockdown, organized screening programs for breast, colorectal, and cervical cancer were suspended [2,3].
The effects on cancer care were particularly severe due to the potential negative consequences of delayed diagnoses, the complexities of cancer treatment pathways, the frequent need for high-risk surgeries and intensive care, and the heightened vulnerability of cancer patients to COVID-19 [4,5]. In early 2020, the first lockdown in France led to a dramatic decline in cancer screenings, diagnoses, and treatments, with the notable exception of chemotherapy [6,7,8,9]. In contrast, subsequent public health measures, including a second lockdown in late 2020, did not significantly impact cancer care delivery [10].
During times of healthcare system strain, as evidenced by the COVID-19 pandemic, monitoring care quality becomes crucial for informing and supporting local and national organizational initiatives. Real-time quality of care indicators were used to adapt health policies and provide operational guidance for healthcare systems [11,12]. Quality indicators assess specific healthcare processes or outcomes, and their key attributes include reliability (absence of measurement bias), validity (accurately measuring what they are intended to assess), relevance, actionability (usefulness for policymaking, monitoring, or strategy development), and feasibility [13]. A widely recognized conceptual framework for health system performance measurement, developed by the OECD, helps member countries prioritize areas for improving care quality [13].
Indicators that monitor cancer patient outcomes, such as five-year survival rates, are routinely used in many countries. While improving survival remains the ultimate goal, data on intermediate outcomes, processes, and healthcare structures are essential for guiding health system policies. A wide range of cancer quality indicators exists, covering aspects such as diagnosis, treatment, prevention, follow up, palliative care, rehabilitation, and even research [14,15]. However, in many countries, these indicators are not routinely available and require significant effort to collect, limiting their usefulness in informing real-time health system policies during crises, like the COVID-19 pandemic [16]. In this context, the development of indicators specifically adapted to disruptions in care delivery during global health crises may represent a relevant approach. The indicators developed should be designed not only as quality indicators but also as true Key Performance Indicators (KPIs), capable of guiding strategic decision-making during times of crisis. Indeed, when specifically designed to be sensitive to unstable contexts, quality indicators can serve a dual purpose: monitoring clinical practices while acting as strategic management tools for decision-makers [17,18]. These indicators, therefore, aim not only to assess the quality of oncology care but also to measure the responsiveness, resilience, and adaptability of healthcare structures. This hybrid positioning reflects an integrated approach that combines clinical relevance with organizational utility.
The primary challenge in creating cancer-specific indicators lies in the heterogeneity of cancer as a disease, with each tumor type following a distinct care pathway. This article aims to describe the process of identifying and developing a set of hospital quality of care indicators for four cancer types to monitor the impact of care reorganization during health crises.
2. Materials and Methods
This study was conducted in four regional hospitals: Grenoble University Hospital, Léon Bérard Cancer Center, Médipôle Lyon-Villeurbanne, and Hospices Civils de Lyon, ensuring that the impact of the epidemic on the Rhône and Isère regions was adequately represented.
In 2020, a multidisciplinary steering committee (SC) was established to oversee the project. The committee included two clinicians, a project manager, two public health specialists, and a biostatistician. The SC decided to develop quality indicators (QIs) for four patient cohorts corresponding to distinct cancer sites, each expected to exhibit significant heterogeneity in the impact of the COVID-19 crisis on patient workflows: breast cancer, hepatocellular carcinoma, gynecological cancers (excluding ovarian cancer), and peritoneal carcinosis. Ovarian cancer was excluded from the gynecological cancer cohort due to its natural history, which is characterized by frequent dissemination into the peritoneal cavity. In this study, a dedicated cohort for peritoneal carcinomatosis, regardless of the primary tumor site, was established. Therefore, including ovarian cancer in the gynecological cancer group was considered redundant. Its analysis was integrated into the peritoneal cancer cohort to ensure nosological and analytical consistency.
For each cohort, a clinical referent was appointed based on their expertise. The SC, in collaboration with the clinical referents, was responsible for assembling both the bibliography panel and the expert panel.
This study followed five key stages for each cohort as follows:
1. Literature Review: A bibliography panel, composed of two to three clinical experts supported by public health specialists and methodologists from the SC, conducted a literature review using PubMed and Google Scholar to identify relevant studies and reviews on QIs. Additional searches were conducted on official state websites and documents to gather indicators developed by national organizations recognized for promoting patient care and safety. For each identified QI, the panel recorded its title, calculation method, inclusion and exclusion criteria, and bibliographic references.
2. Selection of Indicators: Based on the indicators identified in the previous stage, the SC selected those deemed appropriate for this study. Indicators were excluded if they did not assess hospital-based quality of care, were too similar to others, or posed significant measurement challenges (e.g., involving multiple components or lacking clarity).
3. Content Validation: Content validation was conducted using the RAND/UCLA method [19,20], a modified Delphi technique involving a multidisciplinary panel of experts and anonymous scoring cycles. This method helps identify areas of agreement and disagreement among medical experts [20]. The validation process involved consensus opinions from an expert panel of 5 to 11 clinical specialists, along with a methodological expert. This study was conducted regionally, with participation from professionals across the four hospitals mentioned.
Two rounds of consensus were implemented. The first round was conducted via an electronic questionnaire sent by email. The second round took place through videoconferencing or in-person meetings. The questionnaire, created using the Mesydel platform (2021), included five closed questions per indicator, with responses measured on a 10-point Likert scale. The selection criteria assessed were (a) clinical relevance, (b) inter-institutional variability, (c) reproducibility, (d) sensitivity to change, (e) measurability within a short timeframe, and (f) suitability for assessing the impact of the COVID-19 crisis. Each indicator aimed to measure the effect of the COVID-19 crisis on care quality within the cohort.
Experts rated their agreement with the six selection criteria on a 9-point Likert scale (1 = strongly disagree, 9 = strongly agree). A criterion was validated if it achieved a median score of ≥7 and demonstrated consensus among voting members (“consensus to retain”). An indicator was considered validated when all selection criteria met the required threshold.
QIs that failed to achieve validation in the first round were reviewed in the second round. Experts were provided with a summary of their initial responses, along with anonymous group responses, to facilitate informed re-evaluation. After discussing criteria that had not reached consensus, experts were invited to re-vote on the same selection criteria.
4. Final Selection: QIs that achieved validation for clinical relevance and sensitivity to change but did not meet other criteria were submitted to the SC for a final decision. The final set of selected QIs was shared with both the bibliography panel and the expert panel for feedback.
5. Pilot Study: A pilot study for reliability analysis is currently ongoing and falls beyond the scope of this manuscript.
3. Results
3.1. Quality Indicator Selection Process
The selection of quality indicators (QIs) took place between November 2020 and June 2021. A total of 11 clinicians participated in the bibliographic panels, with representation as follows: breast cancer (n = 3), hepatocellular carcinoma (n = 3), gynecological cancer (n = 3), and peritoneal carcinomatosis (n = 2). Each panel was supported by at least one methodologist (Table 1).

Table 1.
Expert panel compositions.
The bibliographic panels initially identified 150 indicators: 80 for breast cancer, 24 for hepatocellular carcinoma, 20 for gynecological cancer, and 26 for peritoneal carcinomatosis. Following discussions, the steering committee selected 74 potential indicators and submitted them to expert groups for content validation. Although the bibliographic panels worked independently, several QIs were common across all four cancer types, particularly those related to care relevance, delays between diagnosis and therapeutic procedures, treatment modalities, and management processes (Figure 1).
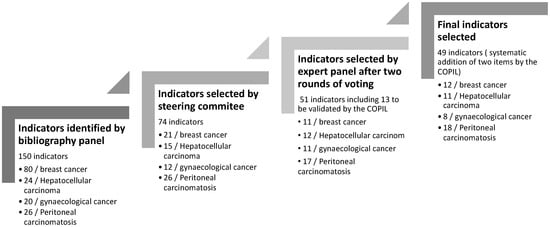
Figure 1.
Indicator selection process.
A total of 23 clinical experts participated in the expert panels, distributed as follows: breast cancer (n = 6), hepatocellular carcinoma (n = 7), gynecological cancer (n = 6), and peritoneal carcinomatosis (n = 5). The expert panels validated 38 indicators after two rounds of voting. Additionally, 13 more QIs were considered valid based on clinical relevance and sensitivity to change, even though they did not meet all selection criteria. Ultimately, the steering committee selected 49 indicators (Figure 1).
3.2. Nature of Validated Indicators
The validated indicators covered all phases of the patient care pathway and were categorized according to the three domains of the Lancet Global Health High-Quality Health Systems framework: foundation, care process, and quality impact [21]. The foundation domain includes the facilities, personnel, and tools necessary for delivering care. The care process domain encompasses indicators related to competent, timely, and effective care, as well as patient experience. The quality impact domain reflects positive health outcomes, such as reductions in morbidity and mortality.
A significant majority of the selected QIs (92%) focused on monitoring hospital quality of care through the care process, while only 8% pertained to quality impact. Notably, no foundation indicators were validated in this study.
Despite variability in indicators by disease type, there were notable similarities in the proportions of different indicator types selected. Specifically, indicators related to the care process were particularly well represented, with proportions ranging from 82% to 100% (Figure 2).
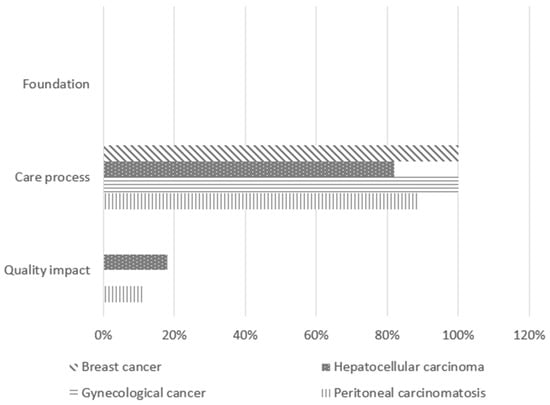
Figure 2.
Nature of the validated quality indicators.
The twelve QIs selected for the breast cancer pathway (see Appendix A, Table A1) were all process indicators: three focused on the diagnostic process, three on treatment modalities, five on delays before or between treatments, and one on staging.
The hepatocellular carcinoma pathway included eleven indicators (see Appendix A, Table A2), comprising two quality impact indicators and nine care process indicators: three focused on the diagnostic process, five on treatments, and one on delays before or between treatments.
The gynecological cancer pathway (excluding ovarian cancer) featured eight process indicators (see Appendix A, Table A3): three related to the diagnostic process, three to treatments, and two to delays before or between treatments.
The peritoneal carcinomatosis pathway included eighteen indicators (see Appendix A, Table A4), of which two were quality impact indicators and sixteen were process indicators: one focused on the diagnostic process, nine on treatments, and six on delays before or between treatments.
Notably, two indicators were common across all four pathways: the number of new cases presented at cancer multidisciplinary team meetings and the number of consultations with psychologists or psychiatrists.
These indicators collectively encompass all stages of the treatment process: diagnosis (10/49; 20.4%), treatment (36/49; 73.5%), staging (1/49; 2%), counseling (1/49; 2%), follow up (1/49; 2%), and therapeutic delays (2/49; 4.1%).
4. Discussion
The present study demonstrates the feasibility of developing a multidisciplinary set of QIs tailored to multiple cancer cohorts in the context of a global disruption of healthcare systems. We identified a total of forty-nine QIs across four cancer care pathways: twelve for breast cancer, eleven for hepatocellular carcinoma, eight for gynecological cancers, and eighteen for peritoneal carcinomatosis.
This approach to improving the quality and safety of care was guided by a steering committee and a dedicated team of experts. The establishment of a multidisciplinary team of clinicians allowed for the integration of diverse perspectives and strengthened collaboration among the participating hospitals. The selected indicators underwent professional consensus and align with the existing literature and best practice recommendations. The RAND/UCLA method provided a robust methodological framework for thoroughly evaluating each indicator. Through a comprehensive, structured, and evidence-based approach, we identified specific indicators relevant to the four cancers studied, covering the entire patient care pathway and facilitating coordination among various stakeholders.
Currently, these QIs are being assessed for their feasibility of implementation and their ability to reliably measure relevant outcomes.
Our study revealed a high proportion of process indicators (92%) compared to historical QI sets in oncology [15,21,22]. This finding can be attributed to our focus on indicators specifically designed to assess the effects of global healthcare disruptions, such as those experienced during the COVID-19 pandemic. Foundational indicators may have been considered less likely to be affected by such disruptions, as they primarily evaluate organizational dimensions, which fell outside the scope of this study. Quality impact indicators were selected less frequently, likely because many can only be assessed after a significant post-treatment interval, making them less effective for real-time monitoring of care quality.
Process indicators offer several advantages in this context. They can be readily extracted from patient records or other data sources (such as cohorts and registries), and some could potentially be derived from medico-administrative data [23]. These indicators are particularly useful for evaluating changes in healthcare practices [24] and identifying deficiencies in patient care over time. Importantly, only quality indicators supported by scientific evidence on process and outcome evaluation were selected. Additionally, process indicators are typically easier for healthcare providers to interpret, offering actionable insights that facilitate the replication of corrective interventions and the generation of generalizable knowledge for implementing complex healthcare improvements [25]. Beyond these general advantages, the indicators developed in this study were designed with the specific challenges of healthcare crises in mind. They focus on critical steps in the care pathway that are highly vulnerable to disruption, such as diagnostic and treatment delays. Their selection was based on feasibility for rapid data collection and real-time use, making them particularly relevant for monitoring care quality under emergency conditions [17,18]. The inclusion of a crisis-specific criterion in the RAND/UCLA validation process further ensures their contextual appropriateness, while their methodological simplicity supports future replicability and use in preparedness strategies.
The integration of both process and outcome indicators enables a comprehensive assessment of care relevance and coordination, thereby illustrating the tangible impact of the COVID-19 crisis and potential future crises. It is crucial to recognize that all indicator classifications are interconnected; thus, indicators should not be analyzed in isolation but rather within a holistic framework that considers the entire patient care pathway.
At this stage, the selected QIs have yet to be collected for the COVID-19 pandemic year and preceding years. Consequently, their reliability and feasibility for data collection remain to be evaluated. A previous study proposed breast cancer-specific QIs that were automated using the French real-life medico-administrative cancer database to develop a standardized set for breast cancer care [23]. In that study, 10 indicators were selected compared to 12 in our study, despite both being derived from similar international research [21]. Notably, only four QIs were common to both studies, highlighting the need to tailor indicator sets to their intended use and feasibility of data collection, as a standardized set may not be universally applicable across different healthcare settings.
A significant limitation of our study was the absence of patient partners in the expert panels due to the health context at the time of this study’s initiation. Including patients in this type of research is strongly recommended [25,26], as their perspectives could have fostered a more patient-centered approach. Their participation would have allowed for a better consideration of key aspects of patients’ lived experiences, such as their journey through the care pathway and the impact on their quality of life. Their absence may, therefore, have influenced the weighting or selection of certain indicators closely linked to patients’ subjective perceptions, particularly those related to treatment delays or access to supportive care services [27]. As such, the selected QIs will require validation by patient panels in future studies.
Additionally, it is important to note that all experts involved were affiliated with French institutions, and the applicability of the selected QIs may vary in other national contexts.
While this study was conducted within the French healthcare system, adapting and validating the selected indicators in non-French-speaking or resource-limited contexts represents an important area for future work. Most of the process indicators focus on core steps of the oncology care pathway, such as diagnosis, treatment, and follow up, which are broadly applicable across healthcare systems. However, contextual adaptation will be necessary, including cultural and organizational validation, assessment of data collection feasibility, and alignment with local clinical relevance. A two-phase approach could be envisioned: initial adaptation through consultation with local experts and patient representatives followed by pilot testing to evaluate feasibility, reproducibility, and sensitivity to change in diverse settings.
The pandemic has underscored the critical importance of preparedness in the healthcare sector to ensure optimal patient care and equitable access to medical services. Developing indicators specifically designed to monitor care quality during periods of healthcare disruption is a key component of crisis preparedness. These indicators must be validated, implemented, and embraced by the medical community to be effectively utilized in future crises.
The next phase of this project will focus on assessing the reliability and reproducibility of the validated indicators, as well as evaluating the feasibility of real-time data collection. Conducting this study across four regional hospitals will provide a representative overview of the pandemic’s impact. Ultimately, the goal is to expand this research into an international study, introducing standardized indicators while considering the specific characteristics and cultural contexts of each country.
5. Conclusions
This study demonstrates the feasibility of developing crisis-responsive QIs to monitor cancer care during health system disruptions. Future work will focus on their real-time implementation, validation in international settings, and integration into healthcare policies to enhance crisis preparedness.
Author Contributions
Conceptualization, J.P. and J.H.; methodology, P.M., J.P. and J.H.; steering committee and cancer cohort experts, V.K., G.L., C.C. and J.P.; indicators for final selection, P.M., J.P. and J.H.; project coordination, A.S.B.; writing—original draft preparation, N.P., A.S.B. and J.P.; writing—review and editing, all authors; funding acquisition, J.P. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by four different sponsors: Direction de la Recherche HCL, Fondation HCL (Grant “Dons Urgence COVID”, COLLAT-COVID), Fondation ARC (Grant N° COVID202001340), and Canceropôle Auvergne Rhône Alpe (CLARA) (ONCOSTARTER IMCOCA 04/21).
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article (See Appendix A).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| QIs | Quality indicators |
Appendix A
Indicators Selected for the Four Cohort Cancers

Table A1.
Specific quality of care indicators for breast cancer.
Table A1.
Specific quality of care indicators for breast cancer.
| Indicator Title | Type of Indicator | Numerator | Denominator | Inclusion Criteria | Exclusion Criteria | Data Sources | Bibliographic References |
|---|---|---|---|---|---|---|---|
| Stage at diagnosis | Care process Staging | Number of women at each stage by UICC 8th edition | Number of women diagnosed with invasive non-metastatic breast cancer | Age > 18 Invasive non-metastatic carcinoma of the breast | Cancer at another location during the year Neoadjuvant chemotherapy or hormone therapy | Electronic Health Record (EHR) | [28,29] |
| Proportion of women with MRI in invasive lobular carcinoma | Care process Diagnosis | Number of patients with non-metastatic invasive lobular carcinoma who had an MRI with treatment | Number of patients treated for non-metastatic invasive lobular carcinoma | Age > 18 Diagnosis of invasive lobular carcinoma | Metastatic cancer | EHR SNDS (National Health Data System) | [30] |
| Number of women diagnosed with invasive non-metastatic breast cancer/week | Care process Diagnosis | Number of women diagnosed with invasive non-metastatic breast cancer/week | Number of women diagnosed with breast cancer/week | Age > 18 Invasive non-metastatic breast carcinoma | EHR | Added by the bibliographic panel. | |
| Proportion of women who had their first treatment (surgery, chemotherapy, or hormone therapy) within 6 weeks or less of the date of the mammogram preceding treatment | Care process Therapeutic delay | Number of women who had their first treatment within 6 weeks or less of the date of the mammogram | Number of women having had a mammogram followed by treatment | Age > 18 Ductal carcinoma in situ or invasive non-metastatic invasive carcinoma of the breast Have had a mammogram Have had a biopsy Have received at least one treatment | History of contralateral breast cancer Other cancers diagnosed in the same year Chaining problem between different AMDB stays | EHR SNDS | [21,31] |
| Proportion of women with ductal carcinoma in situ or invasive non-metastatic breast cancer whose time between surgery and first additional treatment (chemotherapy or radiotherapy) is within the expected timeframe | Care process Therapeutic delay | Time between surgery and radiotherapy < 12 weeks of radiotherapy Time between surgery and chemotherapy < 6 weeks if chemotherapy | Invasive breast carcinoma (CIM-10): C50; C500; C506; C508; C509 + Chirurgie (CCAM): QEFA001; QEFA003; QEFA004; QEFA005; QEFA007; QEFA008; QEFA010; QEFA012; QEFA013; QEFA017; QEFA020. QEFA015 | Age > 18 Invasive non-metastatic breast carcinoma operated on and having had additional treatment | History of contralateral breast cancer Cancer at another location during the year | SNDS | [30,32,33,34,35] |
| Proportion of neoadjuvant chemotherapy | Care process Treatment | Number of women receiving intravenous chemotherapy before surgery for invasive breast cancer | Number of women diagnosed with invasive non-metastatic breast cancer | Age > 18 Invasive non-metastatic breast carcinoma | History of breast cancer Cancer at another location during the year | SNDS | [32] |
| Time between biopsy and first surgery (excluding neoadjuvant chemotherapy) | Care process Therapeutic delay | Number of women who had surgery within 4 weeks or less of the date of the pathological report | Number of women who had a mammogram followed by a diagnosis of invasive non-metastatic breast cancer treated by primary surgery (no neoadjuvant chemotherapy or hormone therapy) during the given period | Age > 18 Invasive non-metastatic breast carcinoma | Neoadjuvant chemotherapy or hormone therapy Cancer at another location during the year | SNDS | [21,30] |
| Proportion of women for whom the time between the end of adjuvant chemotherapy and the start of radiotherapy was less than 6 weeks | Care process Therapeutic delay | Number of women with invasive non-metastatic breast carcinoma who had radiotherapy within 6 weeks of completing adjuvant chemotherapy | Number of women with invasive non-metastatic breast carcinoma who have had chemotherapy followed by radiotherapy | Age > 18 Invasive non-metastatic breast carcinoma Have had adjuvant chemotherapy Have had radiotherapy after adjuvant chemotherapy | History of contralateral breast cancer Cancer at another location during the year Surgical revision (mastectomy or axillary dissection) between chemotherapy and radiotherapy | SNDS | [30] |
| Proportion of women with a delay between the initial biopsy and the first surgery of less than 3 months | Care process Therapeutic delay | Number of women a delay between the initial biopsy with a diagnosis of cancer and surgery below 3 months | Number of women with invasive non-metastatic breast carcinoma treated with upfront surgery | Age > 18 Invasive non-metastatic breast carcinoma with upfront surgery | Cancer at another location during the year | EHR SNDS | [21,31] |
| Proportion of women who received radiotherapy after breast-conserving surgery for ductal carcinoma in situ or invasive non-metastatic breast cancer | Care process Treatment | Number of women with ductal carcinoma in situ or invasive non-metastatic breast cancer who had radiotherapy after conservative surgery | Number of women with ductal carcinoma in situ or invasive non-metastatic breast cancer who have undergone conservative surgery | Age > 18 Ductal carcinoma in situ or invasive non-metastatic breast cancer | History of contralateral breast cancer Cancer at another location during the year | SNDS | [30] |
| Number of consultations with a psychologist or psychiatrist | Care process Treatment | Number of consultations with a psychologist or psychiatrist (SF-12 mental, HAD, EORTC) | Number of women with ductal carcinoma in situ or invasive non-metastatic breast cancer | Age > 18, justifying psychological, psychiatric, or psychiatric care | _ | EHR | Added by the bibliographic panel |
| Number of new files presented to specialist MTDMs | Care process Diagnosis | Number of new files presented to specialist MTDMs | Number of women diagnosed with breast cancer | All new patients presented to specialist MTDMs for breast cancer | _ | EHR | Added by the bibliographic panel |

Table A2.
Specific quality of care indicators for hepatocellular carcinoma (HCC).
Table A2.
Specific quality of care indicators for hepatocellular carcinoma (HCC).
| Indicator Title | Type of Indicator | Numerator | Denominator | Inclusion Criteria | Exclusion Criteria | Data Sources | Bibliographic References |
|---|---|---|---|---|---|---|---|
| Proportion of patients diagnosed with hepatocellular carcinoma (HCC) who received curative treatment | Care process Treatment | Number of patients with HCC receiving curative treatment (resection/local ablation/liver transplantation, LT) | Total number of patients with HCC | All new HCC + resection/local ablation/LT within 1 year | _ | SNDS (MID) Health data warehouse (HDW) | [36,37,38,39] |
| Proportion of patients on the HCC transplant waiting list eligible for waiting treatment and treated during the waiting phase | Care process Treatment | Number of patients on the transplant waiting list for active HCC with treatment on hold | Number of patients on the transplant waiting list with active HCC and preserved liver function | Patient on the transplant waiting list with active HCC | Registration with a “non-treatable HCC” component | ABM, excluding an untreatable HCC component | [36,37,38,39] |
| Proportion of patients receiving post-treatment monitoring after resection or TPC | Care process Treatment | Number of patients with cross-sectional abdominal imaging/3 months within 2 years of resection/local ablation | Number of patients treated by resection/local ablation/ transplant | Patient treated by resection/local ablation/ transplant | _ | EHR | [36,40] |
| Perioperative mortality (90 days) after liver resection in cirrhotic patients | Quality impact | Number of deaths within 90 days of HCC resection | Number of resections for HCC | All new HCC + surgical resection | _ | SNDS | [39] |
| Perioperative mortality (90 days) after liver transplantation for HCC | Quality impact | Number of deaths within 90 days of liver transplantation for HCC | Number of transplants for HCC | Liver transplant for HCC | _ | SNDS | [39] |
| Number of new HCC files presented to specialist liver MDTMs | Care process Diagnosis | Number of new HCC files presented to specialist liver MDTMs | Number of patients diagnosed with HCC | All new HCC presented to the liver specialist MDTMs | _ | EHR | [41] |
| Time between first diagnostic imaging and MDTM presentation | Care process Diagnosis | Time between the first imaging describing a liver nodule and the date of the first MDTM presentation | Number of patients with a first imaging exam describing a liver nodule who were subsequently presented at an MDTM during the given period | All new HCC presented to the liver specialist MDTMs | _ | EHR | [42] |
| Time between first MDTMs and first treatment | Care process Therapeutic delay | Time between the first presentation at MDTMs and the first treatment | Number of patients who were presented at an MDTM and subsequently received a first treatment during the given period | All new HCC presented to the liver specialist MDTMs | _ | EHR | [42] |
| Percentage of patients with histological evidence of HCC | Care process Diagnosis | Percentage of patients with histological confirmation of HCC | Total number of patients diagnosed with HCC | All new HCC presented to the liver specialist MDTMs | _ | DPI | [42] |
| Proportion of patients on the list for HCC transplants | Care process Treatment | Number of patients transplanted for HCC | Number of patients on the list for HCC | Patient on the list for HCC | MELD > 20 | ABM | [36,37,38,39] |
| Number of consultations with a psychologist or psychiatrist | Care process Treatment | Number of consultations with a psychologist or psychiatrist (SF-12 mental, HAD, EORTC) | Number of patients treated for HCC | Age > 18, justifying psychological, psychiatric, or psychiatric care | _ | EHR | Added by the bibliographic panel |

Table A3.
Specific quality of care indicators for gynecological cancer (excluding ovarian cancer).
Table A3.
Specific quality of care indicators for gynecological cancer (excluding ovarian cancer).
| Indicator Title | Type of Indicator | Numerator | Demoninator | Inclusion Criteria | Exclusion Criteria | Data Sources | Bibliographic References |
|---|---|---|---|---|---|---|---|
| Time between the date of surgery and the date of the first radiotherapy session | Care process Therapeutic delay | Time between surgery and adjuvant radiotherapy | Number of patients with gynecological cancer who underwent surgery and received adjuvant radiotherapy during the given period | Woman with gynecological cancer (excluding ovarian cancer) | _ | SNDS | [43] |
| Annual percentage of women treated with radiotherapy or radiochemotherapy as first-line treatment for cervical cancer | Care process Treatment | Annual number of women with cervical cancer receiving radiotherapy or radiochemotherapy | Annual number of women diagnosed with cervical cancer | Woman with with cervical cancer in the first-line treatment | _ | SNDS | [44] |
| Time between first consultation and date of biopsy | Care process Diagnosis | Time between first consultation and date of biopsy | Number of women diagnosed with gynecological cancer during the given period | Age > 18, requiring diagnostic biopsy | Biopsy available at the first consultation | EHR | Added by the expert panel. |
| Time between diagnosis and surgery for cervical cancer | Care process Therapeutic delay | Time between diagnosis and surgery | Number of women diagnosed with cervical cancer and treated with upfront surgery during the given period | Hysterectomy or trachelectomy for stage Ia-IIa cervical cancer | Pre-cancerous cells Surgery other than hysterectomy or trachelectomy | EHR SNDS | [45,46] |
| Percentage of patients for whom surgery is indicated who have received neoadjuvant treatment | Care process Treatment | Number of patients receiving neoadjuvant treatment (radiotherapy, chemotherapy, hormone therapy) | Number of patients with a surgical indication during the given period | Woman with gynecological cancer (excluding ovarian cancer) | Patient with no indication for surgery | EHR | [47] |
| Time between the first symptom reported by the patient and the first consultation | Care process Diagnosis | Time between first symptom and first consultation in gynecology | Number of women diagnosed with gynecologic cancer and with at least one reported symptom at the time of diagnosis during the given period | Woman with gynecological cancer (excluding ovarian cancer) | _ | EHR | Added by the expert panel. |
| Number of new files presented to specialist MDTMs | Care process Diagnosis | Number of new files presented to specialist MDTMs | Number of newly diagnosed cancer patients | All new women presented to specialist MDTMs | _ | EHR | [48] |
| Number of consultations with a psychologist or psychiatrist | Care process Treatment | Number of consultations with a psychologist or psychiatrist (SF-12 mental, HAD, EORTC) | Number of patients treated for cancer | Age > 18, justifying psychological, psychiatric, or psychiatric care | _ | EHR | [48] |

Table A4.
Specific quality of care indicators for peritoneal carcinomatosis.
Table A4.
Specific quality of care indicators for peritoneal carcinomatosis.
| Indicator Title | Type of Indicator | Numerator | Denominator | Inclusion Criteria | Exclusion Criteria | Data Sources | Bibliographic References |
|---|---|---|---|---|---|---|---|
| Time between consultation for curative indication and CRS +/− HIPEC | Care process Therapeutic delay | Time between consultation for curative indication and CRS ± HIPEC | Number of patients who had a consultation for curative intent and underwent CRS ± HIPEC during the given period | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Time between MDTM decision and CRS +/− HIPEC | Care process Therapeutic delay | Time between MDTM decision and CRS ± HIPEC | Number of patients for whom CRS ± HIPEC was decided at an MDTM and performed during the given period | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Time without treatment (chemotherapy or CRS +/− HIPEC) | Care process Therapeutic delay | Time without treatment (chemotherapy or CRS +/− HIPEC) | Number of patients who had a documented treatment interruption or treatment delay during the given period | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Proportion of interventions (CRS +/− HIPEC) postponed | Care process Treatment | Number of patients operated on | Total number of patients planned for CRS +/− HIPEC | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin with CRS +/− HIPEC postponed | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Proportion of conversions to non-resectability | Care process Treatment | Number of exploratory laparotomies without CRS +/− HIPEC | Total number of patients planned for CRS +/− HIPEC | Age > 18 Peritoneal carcinosis of digestive or gynecological origin, which has become unresectable due to waiting times | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | EHR | [49,50] |
| Proportion of patients progressing after deferral or cancellation (morphological assessment/markers) | Quality impact | Number of patients with clinical, biological, or morphological progression | Total number of patients planned for CRS +/− HIPEC | Age > 18 Peritoneal carcinosis of digestive or gynecological origin with clinical, biological, or morphological progression due to waiting time | Age < 18 | EHR | [51,52] |
| Total duration of chemotherapy (weeks) or number of cycles of chemotherapy | Care process Treatment | Number of chemotherapy cycles administered per patient | Number of patients who received chemotherapy during the given period | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | SNDS | [53] |
| Rate of additional cycles of chemotherapy compared with the initial number | Care process Treatment | Number of additional chemotherapies | Number of chemotherapy treatments initially planned | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Proportion of patients cancelled on the same day | Care process Treatment | Number of patients cancelled on the same day | Number of chemotherapy treatments initially planned | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR | [54,55] |
| Proportion of patients with shortened prehabilitation (<3 weeks) | Care process Treatment | Number of patients planned for CRS +/− HIPEC with prehabilitation < 3 weeks | Number of chemotherapy treatments initially planned | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | EHR | [50] |
| Morbidity–mortality rate within 30 days of surgery after CRS +/− HIPEC | Quality impact | Number of patients who experienced severe post-operative complications or died within 30 days after CRS ± HIPEC | Number of patients who underwent CRS ± HIPEC during the given period | Age > 18 Resectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | SNDS | [56] |
| Time between consultation for indication of PIPAC and first PIPAC | Care process Therapeutic delay | Time between consultation for indication of PIPAC and first PIPAC | Number of patients for whom PIPAC was indicated and performed during the given period | Age > 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Time between MDTM decision (indication given) and first PIPAC | Care process Therapeutic delay | Time between MDTM decision (indication given) and first PIPAC | Number of patients for whom a PIPAC was indicated in an MDTM and performed during the given period | Age > 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | EHR SNDS | Added by the bibliographic panel. |
| Time without active treatment (chemotherapy or surgery) during the therapeutic pathway | Care process Therapeutic delay | Time without active treatment (chemotherapy or surgery) during the therapeutic pathway | Number of patients treated during the given period | Age > 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | EHR | [53] |
| Proportion of interventions (PIPACs) reported | Care process Treatment | Actual number of patients operated on | Total number of patients included in the PIPAC pathway | Age > 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | EHR | Added by the bibliographic panel. |
| Proportion of patients treated with PIPAC alone | Care process Treatment | Number of patients treated by PIPAC alone | Total number of patients included in the PIPAC pathway | Age > 18 Unresectable peritoneal carcinosis of digestive or gynecological origin | Age < 18 Resectable peritoneal carcinosis of digestive or gynecological origin | SNDS | Added by the bibliographic panel. |
| Number of new files presented to specialist MDTMs | Care process Diagnosis | Number of new files presented to specialist MDTMs | Number of new patients diagnosed | All new patients presented to specialist MDTMs | _ | EHR | [48] |
| Number of consultations with a psychologist or psychiatrist | Care process Treatment | Number of consultations with a psychologist or psychiatrist (SF-12 mental, HAD, EORTC) | Number of patients treated for cancer | Age > 18, justifying psychological, psychiatric, or psychiatric care | _ | EHR | [48,50] |
References
- Milon, A.; Deroche, C.; Jomier, B.; Vermeillet, S. Santé Publique: Pour un Nouveau Eépart–Leçons de L’épidémie de COVID-19; Sénat: Paris, France, 2020; Rapport d’information No. 199 (2020–2021); Available online: https://www.senat.fr/rap/r20-199-1/r20-199-110.html#toc645 (accessed on 25 December 2023).
- French National Cancer Institute. Préconisations Pour L’adaptation de L’offre de Soins des Établissements Accueillant les Patients Atteints de Cancer Dans le Contexte de L’épidémie de COVID-19; Réseau de Prévention des Infections Associées aux Soins (REPIAS): Saint-Maurice, France, 2020; Available online: https://www.preventioninfection.fr/document/preconisations-pour-ladaptation-de-loffre-de-soins-des-etablissements-accueillant-les-patients-atteints-de-cancer-dans-le-contexte-de-lepidemie-de-covid-19/ (accessed on 25 December 2023).
- You, B.; Ravaud, A.; Canivet, A.; Ganem, G.; Giraud, P.; Guimbaud, R.; Kaluzinski, L.; Krakowski, I.; Mayeur, D.; Grellety, T.; et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020, 21, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.; van Wifferen, F.; Sun, Z.; de Jonge, L.; Lew, J.-B.; Greuter, M.J.; Puttelaar, R.v.D.; Feletto, E.; Lansdorp-Vogelaar, I.; Coupé, V.M.; et al. Potential global loss of life expected due to COVID-19 disruptions to organised colorectal cancer screening. eClinicalMedicine 2023, 62, 102081. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: An international, prospective, cohort study. Lancet Oncol. 2021, 22, 1507–1517. [Google Scholar] [CrossRef]
- De Vincentiis, L.; Carr, R.A.; Mariani, M.P.; Ferrara, G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: An audit study from cellular pathology. J. Clin. Pathol. 2021, 74, 187–189. [Google Scholar] [CrossRef]
- Le Bihan-Benjamin, C.; Rocchi, M.; Putton, M.; Méric, J.-B.; Bousquet, P.J. Estimation of Oncologic Surgery Case Volume Before and After the COVID-19 Pandemic in France. JAMA Netw. Open 2023, 6, e2253204. [Google Scholar] [CrossRef]
- Walker, M.J.; Wang, J.; Mazuryk, J.; Skinner, S.-M.; Meggetto, O.; Ashu, E.; Habbous, S.; Rad, N.N.; Espino-Hernández, G.; Wood, R.; et al. Delivery of Cancer Care in Ontario, Canada, During the First Year of the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e228855. [Google Scholar] [CrossRef]
- Belmont, A.-S.; Sajous, C.; Bruyas, A.; Calattini, S.; Cartalat, S.; Chauvenet, M.; Colombel, M.; Dalle, S.; Dagonneau, T.; Darrason, M.; et al. Impact of the First Wave of the COVID-19 Pandemic on the Lyon University Hospital Cancer Institute (IC-HCL). Cancers 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.L.B.; Simonnet, J.-A.; Rocchi, M.; Khati, I.; Ménard, E.; Houas-Bernat, E.; Méric, J.-B.; Bousquet, P.-J. Monitoring the impact of COVID-19 in France on cancer care: A differentiated impact. Sci. Rep. 2022, 12, 4207. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Koul, S.; Olivecrona, G.K.; Götberg, M.; Tydén, P.; Rydberg, E.; Scherstén, F.; Alfredsson, J.; Vasko, P.; Omerovic, E.; et al. Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart 2020, 106, 1812–1818. [Google Scholar] [CrossRef]
- Mafham, M.M.; Spata, E.; Goldacre, R.; Gair, D.; Curnow, P.; Bray, M.; Hollings, S.; Roebuck, C.; Gale, C.P.; A Mamas, M.; et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020, 396, 381–389. [Google Scholar] [CrossRef]
- Carinci, F.; Van Gool, K.; Mainz, J.; Veillard, J.; Pichora, E.C.; Januel, J.M.; Arispe, I.; Kim, S.M.; Klazinga, N.; Haelterman, M.; et al. Towards actionable international comparisons of health system performance: Expert revision of the OECD framework and quality indicators. Int. J. Qual. Health Care 2015, 27, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; McCartney, A.; Ponti, A.; Marotti, L.; Vrieling, C.; Eniu, A.; Sousa, B.; Ripamonti, C.; Travado, L.; Spitz, S.; et al. European Society of Breast Cancer Specialists/Advanced Breast Cancer Global Alliance quality indicators for metastatic breast cancer care. Eur. J. Cancer 2023, 187, 105–113. [Google Scholar] [CrossRef] [PubMed]
- La Torre, G.; Mannocci, A.; Cocchiara, R.A.; D’Egidio, V.; Sestili, C.; Lia, L.; Cianfanelli, S.; Backhaus, I.; Dorelli, B.; Ricciardi, M. Systematic Review of the Quality Indicators (Qis) to Evaluate the CCCN Approach in the Management of Oncologic Patients. 2019. Available online: https://www.ipaac.eu/res/file/outputs/wp10/quality-indicators-systematic-review-evaluation-comprehensive-cancer-care-network.pdf (accessed on 16 April 2025).
- Linck, P.-A.; Garnier, C.; Depetiteville, M.-P.; MacGrogan, G.; Mathoulin-Pélissier, S.; Quénel-Tueux, N.; Charitansky, H.; Boisserie-Lacroix, M.; Chamming’s, F. Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre. Eur. Radiol. 2021, 32, 1644–1651. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-Quality Health Systems in the Sustainable Development Goals Era: Time for a Revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Fernandes, Ó.B.; de Lange, M.; Lingsma, H.; Klazinga, N.; Kringos, D. Changes in the quality of cancer care as assessed through performance indicators during the first wave of the COVID-19 pandemic in 2020: A scoping review. BMC Health Serv. Res. 2022, 22, 786. [Google Scholar] [CrossRef] [PubMed]
- Dudley, L.; Mamdoo, P.; Naidoo, S.; Muzigaba, M. Towards a harmonised framework for developing quality of care indicators for global health: A scoping review of existing conceptual and methodological practices. BMJ Health Care Inform. 2022, 29, e100469. [Google Scholar] [CrossRef]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R.; Lazaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual; RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kühn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef]
- Maes-Carballo, M.; Gómez-Fandiño, Y.; Hermida, A.R.; Estrada-López, C.R.; Martín-Díaz, M.; Khan, K.S.; Bueno-Cavanillas, A. Quality indicators for breast cancer care: A systematic review. Breast 2021, 59, 221–231. [Google Scholar] [CrossRef]
- Houzard, S.; Courtois, E.; Benjamin, C.L.B.; Erbault, M.; Arnould, L.; Barranger, E.; Coussy, F.; Couturaud, B.; Cutuli, B.; de Cremoux, P.; et al. Monitoring Breast Cancer Care Quality at National and Local Level Using the French National Cancer Cohort. Clin. Breast Cancer 2022, 22, e832–e841. [Google Scholar] [CrossRef]
- French Health Ministry. National Health Strategy 2018–2022. Published 17 December 2017. Available online: https://sante.gouv.fr/systeme-de-sante/strategie-nationale-de-sante/article/la-strategie-nationale-de-sante-2018-2022 (accessed on 25 December 2023).
- Moore, G.F.; Audrey, S.; Barker, M.; Bond, L.; Bonell, C.; Hardeman, W.; Moore, L.; O’Cathain, A.; Tinati, T.; Wight, D.; et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015, 350, h1258. [Google Scholar] [CrossRef]
- Westby, M.D.; Marshall, D.; Jones, C. Development of quality indicators for hip and knee arthroplasty rehabilitation. Osteoarthr. Cartil. 2018, 26, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Bombard, Y.; Baker, G.R.; Orlando, E.; Fancott, C.; Bhatia, P.; Casalino, S.; Onate, K.; Denis, J.-L.; Pomey, M.-P. Engaging Patients to Improve Quality of Care: A Systematic Review. Implement. Sci. 2018, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Institut National du Cancer. Cancer du Sein: Indicateurs de Qualité et de Sécurité des Soins. Collection les Données [Internet]. 2019. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Cancer-du-sein-indicateurs-de-qualite-et-de-securite-des-soins (accessed on 25 March 2025).
- Perry, N.; Broeders, M.; de Wolf, C. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition summary document. Ann. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Ferrua, M. Development and feasibility of a set of quality indicators relative to the timeliness and organisation of care for new breast cancer patients undergoing surgery. BMC Health Serv. Res. 2012, 12, 167. [Google Scholar] [CrossRef]
- Andreano, A.; Anghinoni, E.; Autelitano, M. Indicators based on registers and administrative data for breast cancer: Routine evaluation of oncologic care pathway can be implemented. J. Eval. Clin. Pract. 2016, 22, 62–70. [Google Scholar] [CrossRef]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Blanc, J.; Barbare, J.C.; Baumann, A.S. «Carcinome hépatocellulaire». [Internet]. Thésaurus National de Cancérologie Digestive. 2019. Available online: http://www.tncd.org (accessed on 25 March 2025).
- Inca HAS. Guide–Affection de Longue Durée. ALD 30-Tumeur Maligne, Affection Maligne du Tissu Lymphatique ou Hématopoïétique. Cancer Primitif du Foie. 2010. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2010-02/ald_30_lap_ksein_vd.pdf (accessed on 25 March 2025).
- Dhanasekaran, R.; Talwalkar, J.A. Quality of Cancer Care in Patients with Cirrhosis and Hepatocellular Carcinoma. Curr. Gastroenterol. Rep. 2015, 17, 34. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Berenguer, M.; Burra, P.; Ghobrial, M. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Amaddeo, G.; Brustia, R.; Allaire, M. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021, 3, 100199. [Google Scholar] [CrossRef]
- Plan Cancer 2014–2019 [Internet]. p. 27. Available online: https://www.iccp-portal.org/sites/default/files/plans/Summary-plan-cancer-2014-2019-Anglais.pdf (accessed on 25 March 2025).
- Wang, Y.; Zhang, S.; Wei, L. Recommendations on management of gynecological malignancies during the COVID-19 pandemic: Perspectives from Chinese gynecological oncologists. J. Gynecol. Oncol. 2020, 31, e68. [Google Scholar] [CrossRef]
- Akladios, C.; Azais, H.; Ballester, M.; Bendifallah, S.; Bolze, P.A.; Bourdel, N.; Bricou, A.; Canlorbe, G.; Carcopino, X.; Chauvet, P.; et al. Recommendations for the surgical management of gynecological cancers during the COVID-19 pandemic-FRANCOGYN group for the CNGOF. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101729. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Novatt, H.; Matsuzaki, S.; Hom, M.S.; Castaneda, A.V.; Licon, E.; Nusbaum, D.J.; Roman, L.D. Wait-time for hysterectomy and survival of women with early-stage cervical cancer: A clinical implication during the coronavirus pandemic. Gynecol. Oncol. 2020, 158, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, G.; Golfier, F.; Peron, J.; Moret, S.; Chene, G.; Nohuz, E.; Lebon, M.; Dubernard, G.; Cortet, M. Impact de la pandémie de COVID-19 sur les modifications thérapeutiques des patientes atteintes de cancers gynécologiques [Impact of the COVID-19 Outbreak on the management of patients with gynecological cancers]. Gynecol. Obstet. Fertil. Senol. 2020, 48, 777–783. [Google Scholar]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef]
- Fadavi, P.; Houshyari, M.; Yousefi Kashi, A.S.; Jarrahi, A.M.; Roshanmehr, F.; Broomand, M.A.; Sandoughdaran, S.; Taghizadeh-Hesary, F. Review on the Oncology Practice in the Midst of COVID-19 Crisis: The Challenges and Solutions. Asian Pac. J. Cancer Prev. 2021, 22, 19–24. [Google Scholar] [CrossRef]
- Birgisson, H.; Enblad, M.; Artursson, S.; Ghanipour, L.; Cashin, P.; Graf, W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2020, 46, 2283–2291. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Concin, N.; Planchamp, F.; Morice, P.; Vergote, I.; du Bois, A.; Querleu, D. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. Int. J. Gynecol. Cancer 2020, 30, 436–440. [Google Scholar] [CrossRef]
- Glehen, O.; Kepenekian, V.; Bouché, O.; Gladieff, L.; Honore, C.; RENAPE-BIG-RENAPE. Treatment of primary and metastatic peritoneal tumors in the COVID-19 pandemic. Proposals for prioritization from the RENAPE and BIG-RENAPE groups. J. Visc. Surg. 2020, 157, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.D.; Tran Cao, H.S.; Roland, C.L.; Teshome, M.; Bednarski, B.K.; Ikoma, N.; Graham, P.H.; Keung, E.Z.; Scally, C.P.; Katz, M.H.G.; et al. Surgical decision-making and prioritization for cancer patients at the onset of the COVID-19 pandemic: A multidisciplinary approach. Surg. Oncol. 2020, 34, 182–185. [Google Scholar] [CrossRef]
- Cavaliere, D.; Parini, D.; Marano, L.; Cipriani, F.; Di Marzo, F.; Macrì, A.; D’Ugo, D.; Roviello, F.; Gronchi, A.; SICO (Italian Society of Surgical Oncology). Surgical management of oncologic patient during and after the COVID-19 outbreak: Practical recommendations from the Italian society of Surgical Oncology. Updates Surg. 2021, 73, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.M.; Dolivet, G.; Evrard, S.; Ferron, G.; Lécuru, F.; Leufflen, L.; Rivoire, M.; Sgarbura, O. Recommandations de la Société française de chirurgie oncologique (SFCO) pour l’organisation de la chirurgie oncologique durant l’épidémie de COVID-19 [French Society for Surgical Oncology (SFCO) guidelines for the management of surgical oncology in the pandemic context of COVID 19]. Bull. Cancer 2020, 107, 524–527. [Google Scholar] [PubMed]
- Shariff, F.; Bischof, D.; Govindarajan, A.; Prince, R.; Burkes, R.; Haase, E.; Mack, L.; Temple, W.; Hebbard, P.; Boulanger-Gobeil, C.; et al. Evidence-Based Strategies for the Treatment of Peritoneal Malignancies during Health Care Resource Restriction: The COVID-19 Pandemic. Curr. Oncol. 2020, 28, 40–51. [Google Scholar] [CrossRef]
- Alyami, M.; Kim, B.J.; Villeneuve, L.; Vaudoyer, D.; Képénékian, V.; Bakrin, N.; Gilly, F.N.; Cotte, E.; Glehen, O.; Passot, G. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int. J. Hyperth. 2018, 34, 532–537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).