Simple Summary
Of the worldwide population, 0.5 to 1% of people are carrying a heterozygous mutation of Ataxia–Telangiectasia Mutated (ATM) gene. While the clinical radiosensitivity of carriers of germline biallelic inactivation of the ATM gene is well described, controversies are observed for monoallelic carriers of ATM mutation. The aim of this study is to evaluate acute and late toxicities after adjuvant breast radiation therapy in ATM pathogenic variant carriers. This observational retrospective study showed an absence of significative acute and late toxicities after breast radiation therapy among patients carrying a heterozygous rare variant of the ATM gene. Single nucleotide polymorphism rs1801516 (G/A), described as associated with late subcutaneous fibrosis, was not associated with this late adverse event in our study.
Abstract
The Ataxia–Telangiectasia Mutated (ATM) gene is implicated in DNA double-strand break repair. Controversies in clinical radiosensitivity remain known for monoallelic carriers of the ATM pathogenic variant (PV). An evaluation of the single-nucleotide polymorphism (SNP) rs1801516 (G-A) showed different results regarding late subcutaneous fibrosis after breast radiation therapy (RT). The main objective of this study was to evaluate acute and late toxicities in carriers of a rare ATM PV or predicted PV and in carriers of minor allele A of rs1801516 facing breast RT. Fifty women with localized breast cancer treated with adjuvant RT between 2000 and 2014 at Institut Curie were selected. Acute and late toxicities in carriers of a rare PV or predicted PV (n= 9), in noncarriers (n = 41) and in carriers of SNP rs1801516 (G-A) (n = 8), were examined. The median age at diagnosis was 53 years old and 82% of patients had an invasive ductal carcinoma and 84% were at clinical stage I–IIB. With a median follow-up of 13 years, no significant difference between carriers and noncarriers was found for acute toxicities (p > 0.05). The same results were observed for late toxicities without an effect from the rs1801516 genotype on toxicities. No significant difference in acute or late toxicities was observed between rare ATM variant carriers and noncarriers after breast RT for localized breast cancer.
1. Introduction
Breast cancer remains a global health challenge, accounting for over two million new cancer cases annually. Breast cancer mortality rates ranked first among all cancers in 2020, with seven hundred thousand deaths [1]. Non-metastatic breast cancer represents 70 to 80% of all breast cancers diagnosed every year, and its management is based on a multimodal approach [2]. Radiation therapy (RT) is a key part of this multimodal treatment, especially in adjuvant loco-regional breast cancer by reducing the risk of local recurrence by 50% [3]. With advances in genomic exploration over the past two decades, some germline variants have been described as associated with an increased risk of breast cancer, such as pathogenic variants (PVs) in the BRCA1, BRCA2, PALB2, TP53 and ATM genes [4]. Identifying such germline PVs has major implications in daily oncologist practice, thanks to the development of specific targeted treatments such as PARP inhibitors.
The ATM (Ataxia–Telangiectasia Mutated) gene is located on 11q22.3. It encodes a serine/threonine kinase which acts a key regulator of signaling following DNA double-strand breaks [5]. Individuals with the biallelic ATM PV (either homozygous PV carriers or compound heterozygous PV carriers) present with Ataxia–Telangiectasia (A-T) syndrome. A-T patients have a hypersensitivity to ionizing radiation and agents that cause DNA double-strand breaks [6,7,8]. It is estimated that 0.5 to 1% of the general population carry an ATM variant classified as pathogenic for A-T disease, and studies conducted in hereditary breast and ovarian cancer families or early-onset breast cancer cases showed that such variants confer a 2 to 4-fold increase in breast cancer risk as compared to noncarriers [9,10,11]. Therefore, it is estimated that over 4% of all breast cancer cases may carry a so-called PV, which represents over 80,000 new cases per year worldwide.
Over the last decade, a number of studies have been conducted in cancer patients to assess the role of ATM variants as a risk factor for normal tissue complications after RT. Before clinical data, in vivo studies have shown a lower survival rate after 2 Gray (Gy) irradiation of lymphoblastic cells of monoallelic carriers of ATM than non-mutated donor cells [12,13]. Those data have been correlated with in vivo studies showing an increase in chromatid abnormalities after a 1 Gy irradiation of ATM monoallelic knock-out mice [14,15]. In addition to rare PVs, the common single-nucleotide polymorphism (SNP) rs1801516 (c.55557G>A; p.Asp1853Asn) has been investigated over the years for its potential association with late radiation-induced complications, which has led to controversial results. Three systematic reviews and meta-analyses have addressed its impact on normal tissue injuries after RT, of which two showed a significantly increased risk of acute toxicity and radiation-induced fibrosis, respectively, among carriers of the minor allele A, while another study found no association [16,17,18]. Regarding the role of rare ATM PVs in such toxicities, inconsistent results have been reported as well [19,20,21,22].
The primary objective of this study was to evaluate whether women with a monoallelic rare ATM PV or predicted PV and who underwent RT for non-metastatic breast cancer are at higher risk of acute and late toxicities than noncarriers. We also examined the effect of the rs1801516 genotype on radio-induced toxicity.
2. Materials and Methods
2.1. Study Population
The study population consisted of female breast cancer patients enrolled in two French national studies: the familial case–control study GENESIS [23] and the ongoing familial prospective cohort, CoF-AT2 [24]. GENESIS (GENE SISters) was designed to investigate familial predisposition to breast cancer. Index cases were enrolled through the national network of family cancer clinics (Genetics and Cancer Group of UNICANCER). Eligible index cases were cases diagnosed with infiltrating mammary or ductal adenocarcinoma, tested negative for BRCA1 and BRCA2 PVs, and had a sister with breast cancer. Female cancer-free friends or colleagues of the index cases were also enrolled and served as controls. Inclusions started in February 2007 and ended in December 2013. Clinical, epidemiological, familial data and biological samples are centralized at Institut Curie. Blood samples were collected at inclusion.
The prospective cohort CoF-AT2 was initiated in 2003 to include Ataxia–Telangiectasia (A-T) patients’ relatives. The study protocol was amended in 2017 in order to enroll new participants from cancer-prone families segregating an ATM pathogenic or predicted pathogenic variant (see definitions of PV and predicted PV in the next paragraph) through the national network of family cancer clinics. Epidemiological, familial and clinical data, together with biological samples of participants, were collected. A genetic test targeting the ATM variant identified in the index case was performed in the Department of Genetics of Institut Curie for all relatives enrolled in CoF-AT2. All blood samples were collected before RT treatment.
For the present retrospective study, we selected women from GENESIS and CoF-AT2 affected with breast cancer and those included were at least treated with breast RT for local and/or loco-regional breast cancer at Institut Curie Paris between 2000 and 2014. Exclusion criteria were women aged under 18 years, patients with metastatic breast cancer and patients with prior RT treatment involving patients’ breasts in treatment fields. Extraction of data from medical records was performed by two of the coauthors (AB and RB) and both were blinded of the ATM status of the patients.
2.2. ATM Variants Identification and Classification
In GENESIS, the entire coding sequence of ATM was sequenced in blood DNA of participants in the context of a large case–control study investigating the contribution of rare variants in DNA repair genes in breast cancer susceptibility. Detailed information on sequencing procedures and variant filtering and annotation is described in Girard et al.’s research [25]. For the present study, as in the original study, only loss-of-function variants (i.e., indels frameshift, stop gain, stop loss, start loss and canonical splice variants predicted to result in a truncated protein) and missense variants with a minor allele frequency below 0.05% in GENESIS controls were retained. We further filtered missense variants to keep only those predicted as deleterious using the in silico prediction tool CADD [26,27] and Align-GVGD classifier [28,29]. Align-GVGD categorizes missense substitutions into seven grades ordered from evolutionarily most likely (C0) to least likely (C65). Missense variants with a PHRED CADD score equal or above 10 and/or classified as C45, C55 or C65 by Align-GVGD were retained. In CoF-AT2, relatives of index cases were genotyped for the ATM variant identified in the index case. The same rules as in GENESIS were used to select eligible ATM variants.
In our analyses, all loss-of-function variants and missense variants classified as pathogenic for A-T disease were considered as “pathogenic variants (PVs)”, and other retained missense variants were considered as “predicted pathogenic variants (PPVs)”.
In total, forty-seven breast cancer cases from GENESIS and three breast cancer cases from CoF-AT treated by RT at the Institut Curie were included in this study; nine of them were heterozygous for an ATM PV or PPV (seven in GENESIS and two in CoF-AT2). Among the nine carriers of a PV or PPV, five had the genotype rs1801516-GG, one had the genotype rs1801516-GA, and the rs1801516 genotype was not available for three of them. Among the 41 noncarriers of a PV or PPV, 25 had the genotype rs1801516-GG, 7 had the genotype rs1801516-GA and the rs1801516 genotype was not available for nine of them.
2.3. Radiation Therapy Treatment Characteristics and Follow-Up
All women underwent a dosimetric computed tomography (CT) scanner without injection in the radiation therapy department of Institut Curie Paris in order to prepare the RT plan of treatment. Delineation of clinical tumor volume (CTV) and organ at risk (OAR) was performed following international recommendations. Dose prescriptions were under clinician appreciation and ranged between 45 Gy and 50 Gy for whole breast irradiation and up to 14 to 16 Gy when a sequential boost was performed on the tumoral surgery bed. Each patient had weekly clinics during the RT. Then, all patients were followed up with every three months with clinical examination and annual ultrasound and mammograms. Acute toxicities were defined as appearance of dermatitis, dysphagia or lymphoedema in the three months after RT. Late toxicities were defined as subcutaneous fibrosis, telangiectasia, lymphoedema or heart disease occurring more than three months after the end of RT treatment. Toxicities were graduated using the CTCAE v.5 scale [30].
2.4. Statistical Analysis
Follow-up was calculated from the date of the end of RT to the date of last news. The median follow-up was estimated by the inverted Kaplan–Meier method. Baseline characteristics were summarized as numbers and percentages for qualitative data and as means and standard deviations or medians with the minimum and maximum (or inter-quartile range) for continuous variables. The Chi 2 test or Fisher test was used for the analysis of the contingency tables. The risk of late toxicity, such as fibrosis, was assessed within a competing risks framework, recognizing death as a significant competing event. Cumulative incidence functions were used for this analysis, and a Fine–Gray model was implemented to appropriately handle the complexity introduced by competing risks. All p-values were two-sided, and a 5% level of significance was used. Analyses were carried out using software R 4.2.2. (http://cran.r-project.org; accessed on 1 October 2022).
3. Results
3.1. Population Characteristics
The median follow-up was 13 years (range 1.6 to 21.9). In this study, 50 patients (9 ATM rare variant carriers and 41 noncarriers) have been analyzed and their main characteristics are summarized in Table 1. The rare ATM variant carriers and noncarrier patients had the same baseline characteristics. The median age was 53 years old (range: 35–77) and the median Body Mass Index (BMI) was 24.6 kg/m2 (range: 18.2–34.9). More than two-thirds of the patients had no antecedent of current and/or former smoking history (70%). Only one patient had a skin disorder which was diagnosed as psoriasis and was not clinically present in the RT area. Repartition of right or left breast cancer was equivalent. The most represented tumor localization in the breast was in the External Upper Quadrant (27/50, 54%), followed by the Union of Upper Quadrant (8/50, 16%) and the Internal Upper Quadrant (6/50, 12%). The main clinical stage (as described according to the American Joint Committee on Cancer (AJCC) 2017 v8 guidelines) was stage II (22/50, 44%) [31]. In total, 82% of the tumors were invasive ductal, overexpressing the Estrogen Receptor (ER) in 80% of cases, and without presence of embolus for two-thirds of them.

Table 1.
Baseline characteristics of the 50 breast cancer patients included in the analyses.
3.2. Characteristics of Genetic Variants
The rare ATM variants identified in the investigated series are described in Table 2. Among the nine rare variant carriers, three had a pathogenic variant, five a variant of unknown signification (VUS) and one had a benign variant according to the ClinVar classification.

Table 2.
Description of ATM variants identified in patients. All patients are heterozygous variant carriers.
Additionally, eight patients were heterozygous for the minor allele A of rs1801516 (c.55557G>A; p.Asp1853Asn). Information regarding this common variant was missing for four women. Of note, one patient with the genotype rs1801516-GA also carried the rare variant c.4853G>A.
3.3. Treatment Details
The treatment characteristics are given in Table 3. The two groups had no significative difference between the received treatments. Eighty percent (40/50) of patients underwent a breast conserving surgery and sixty-eight percent (34/50) had an axillary lymph node dissection (ALND). Data on axillary surgery were not available for four patients. None of the patients had an immediate breast reconstruction in case of mastectomy. Neo-adjuvant treatment was performed for nine patients (18%) and consisted of Epirubicine/Cyclophosphamide (EC)-Taxotere, 5FU-Navelbine or FEC100 protocols. Residual Cancer Burden score (RCB score) was evaluated as RCB0 for two patients (22%), RCB I and II for one patient (11%), respectively, and RCB III for five patients (56%).

Table 3.
Treatment details.
Adjuvant treatment was delivered to 22 patients (44%); it was composed of adjuvant chemotherapy (36%) or targeted therapy (8%) with Trastuzumab. Adjuvant hormonotherapy was delivered to 32 patients (64%). One patient had an ovariectomy for the purposes of anti-hormonal treatment.
Regarding RT characteristics, 3D-CRT (Conformal RT) was used for 70% of patients and Isocentric lateral decubitus (ILD) for 30% of treated women. The mean dose of RT was 56 Gy (range: 45 to 71 Gy) and the mean RT duration was 42.7 days (range: 28 to 81). Three different RT sources were used: 60Cobalt (18/50, 36%), X-ray (13/50, 26%) or a mixed treatment of 60Cobalt and electron (2/50, 4%) or X-ray and electron (17/50, 34%). Only two patients had concomitant chemotherapy during RT. The treated volumes of RT were as follows: whole breast irradiation in 44% (22/50), or whole breast associated with RT of lymph nodes (area I–I–III–IV ± IMNI) (28/50, 56%), with or without a boost of the tumor bed (22/50, 44%).
3.4. Acute Toxicities
The acute toxicities of rare monoallelic ATM PV or predicted PV carriers are presented in Table 4. Over the nine carriers, the main acute toxicity was dermatitis for eight of them (89%). No significative difference between ATM PV or predicted PV carriers and noncarriers was observed when looking at acute dermatitis (p = 0.98), dysphagia (p = 1) or lymphoedema (p = 1) (Figure 1). The group of rare monoallelic ATM PV carriers was composed of three patients, of which two experienced grade 1 dermatitis and one woman experienced grade 2. No other acute toxicity was found.

Table 4.
Occurrence of acute and late toxicities after radiation therapy among rare ATM variant carriers and noncarriers.
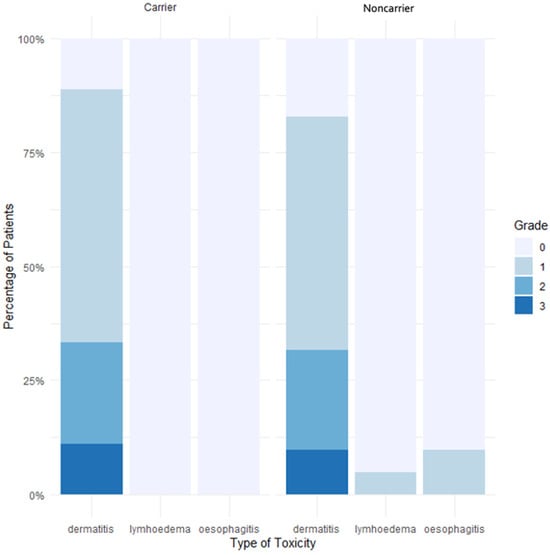
Figure 1.
Percentage of acute toxicities in carriers and noncarriers of a rare ATM PV or predicted PV. Abbreviations: Carrier: patients with a rare ATM pathogenic variant (PV) or predicted PV, Noncarrier: patients with no rare ATM PV or predicted PV.
We next compared the manifestation of acute toxicities between the group of eight patients with the genotype rs1801516-GA and the group of 25 patients with the genotype rs1801516-GG and not carrying a rare PV or predicted PV. After a median follow-up of 13 years, no significative difference regarding acute dermatitis (p = 0.23), dysphagia (p = 1) and lymphoedema (p = 0.88) was demonstrated (Figure S1). The unique patient carrying both genotype rs1801516-GA and the predicted PV c.4853G>A presented grade 1 acute dermatitis and no other acute toxicity.
When focusing on the 16 patients carrying either a rare monoallelic PV or predicted PV and/or the minor allele A of SNP rs1801516, the main acute toxicity was dermatitis, impacting 75% of women, representing twelve women above this subgroup. Regarding acute toxicities, no significant difference was observed between the carriers of a rare monoallelic PV or predicted PV ATM carriers and carriers of the minor allele A of SNP rs1801516 as compared to noncarriers (dermatitis: p = 0.65/ dysphagia: p = 1/ lymphoedema: p = 1) (Figure S2).
3.5. Late Toxicities
Late toxicities for the nine rare ATM PV or predicted PV carriers are detailed in Table 4. The main late toxicity was subcutaneous fibrosis for two out of nine patients (25%). With a median follow-up of 13 years, no significant difference was found for subcutaneous fibrosis (p = 0.16), telangiectasia (p = 0.33), lymphoedema (p = 0.72) and heart disease (p = 1) (Figure 2). No grade 3 and higher late toxicities were reported in both groups. No late plexopathy was found. The group of rare monoallelic ATM PV carriers was composed of three patients who did not experience any late toxicity.
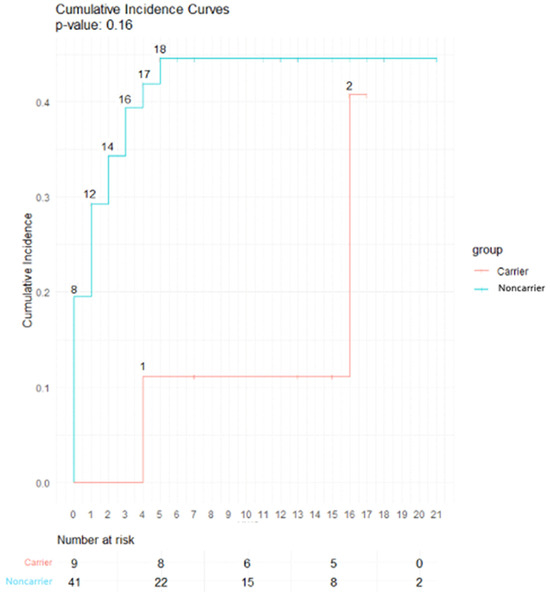
Figure 2.
Incidence curve of late subcutaneous fibrosis in carriers and noncarriers of a rare ATM PV or predicted PV. Abbreviations: Carrier: patients with a rare ATM Pathogenic variant (PV) or predicted PV, Noncarrier: patients with no rare ATM PV or predicted PV.
With a median follow-up of 13 years, 50% (4/8) of patients with the genotype rs1801516-GA experienced late subcutaneous fibrosis, but this proportion did not differ in the group of patients with the genotype rs1801516-GG (p = 0.87) (Figure S3). No significant difference was observed for other late RT-related complications such as late lymphoedema (p = 0.65), late telangiectasia (p = 1) and late heart disease (p = 1). Late subcutaneous fibrosis did not differ between carriers of a rare ATM PV or predicted PV and/or minor allele A of rs1801516 and noncarriers (p = 0.67) (Figure S4).
4. Discussion
In 2017, the National Comprehensive Cancer Network (NCCN) made recommendations regarding adjuvant RT of breast cancer for women carrying a monoallelic ATM pathogenic variant. The NCCN recommends not to avoid adjuvant RT, which is consistent with our present results showing an absence of supplementary acute or late toxicities in this specific population [32].
Our retrospective study is one of the most recent studies with long term follow-up evaluating the incidence of acute and late radiation toxicities in women with breast cancer and heterozygous for a rare PV or predicted PV or heterozygous for allele A of the common SNP rs1801516. Our results are consistent with those of Bremer et al., showing no difference in acute and late cutaneous toxicity after treatment for early breast cancer with 3D-CRT between a group of ten patients carrying a known ATM PV leading to a frameshift or missense [20].
In the present work, we focused our analyses not only on carriers of a rare variant reported as pathogenic for A-T or classified as pathogenic for HBOC in ClinVar but we also included a few patients carrying a predicted PV according to in silico tools. This is because such variants have also been associated with breast cancer in large case–control studies, and the risk estimates are close to the risk estimates associated with the so-called PV [25,29]. National and international initiatives are ongoing to clarify the role of such variants and of other VUSs identified through multi-gene panel testing [33,34,35,36].
Moreover, we did not observe an association between ATM variant status of the patient and clinical stage and histology criteria associated with aggressive tumors (for example, embolus, high mitotic index, triple-negative histological subtype), which is interesting in the discussion of the adjuvant RT treatment schedule of carriers of ATM PV or PPV who do not seem to have more aggressive histological characteristics than noncarrier patients. An exploration of a possible difference in histological subtypes of breast cancer in ATM variant carriers compared to other genes implicated in DNA double-strand break repair, such as BRCA1 and BRCA2, was conducted by Abdulrahman et al. in 2018 and showed that tumors of ATM VUS carriers seem smaller, with lower pathologic T stages at diagnosis and greater surrogate molecular subtypes [37]. In a previous study, we performed a systematic pathology review of breast tumors from 21 ATM PV carriers from A-T families and 18 PV or predicted PV carriers from Hereditary Breast and Ovary Cancer families (including patients enrolled in CoF_AT2 and GENESIS), and we found that ATM-associated breast tumors belong mostly to the luminal B subtype in a retrospective tumor [38].
One relevant piece of information from our study is the exploration of the well-studied SNP rs1801516 (c.5557G>A, p.Asp1853Asn), leading to controversy especially regarding late subcutaneous fibrosis after RT. Andreassen et al. investigated seven patients with the heterozygous genotype of rs1801516 and showed a significant association between this SNP and grade 3 subcutaneous fibrosis after breast RT at 50 Gy dose (incidence in individuals with the GA genotype: 37% vs. incidence in individuals with the GG genotype: 16%, p = 0.03) [19]. Their results are in accordance with the results of the study by Ho et al. including 131 patients, with 15 of them carrying the A allele. In the latter study, 53% of the carriers and 27% of the noncarriers had grade 2 to 4 subcutaneous side effects (OR: 3.1, 95% CI 1.1–9.4) [22]. Our results do not confirm these findings, but this may be due to a lack of power given our limited sample.
This study has several limitations: the retrospective analysis which is associated with a risk of bias and especially a survivorship bias since women are the prevalent cases and the small sample of patients carrying a well-known ATM PV, thus rendering power of statistical analysis quite unsatisfactory for this particular population. Other limitations are the technique of RT (majority of 3D-CRT) and the type of energy used (60Cobalt), which is not the standard treatment nowadays for RT for breast cancer with the outcome of IMRT (Intensity Modulated Radiotherapy). Nonetheless, our findings could be interesting in daily practice, given the few adverse events observed in this population, especially in long-term follow-up of patients treated with 3D-CRT and 60Cobalt, which are more associated with adverse events.
5. Conclusions
This study showed no association between rare ATM PVs or predicted PVs and manifestation of acute or late toxicities after breast RT for localized breast cancer in heterozygous variant carriers. In this small series of patients, the minor allele A of rs1801516 was not associated with late subcutaneous fibrosis. Further larger and prospective investigations are needed to confirm our findings in order to better personalize RT for patients carrying a monoallelic alteration of ATM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16071417/s1, Figure S1: Acute toxicities in carriers and noncarriers of allele A of SNP rs1801516; Figure S2: Acute toxicities between carriers and noncarriers of a rare monoallelic ATM PV or predicted PV and/or of allele A of SNP rs1801516; Figure S3: Incidence curve of late subcutaneous fibrosis according to genotype for SNP rs1801516; Figure S4: Incidence curve of late subcutaneous fibrosis in carriers and noncarriers of a rare monoallelic ATM PV or predicted PV and/or of allele A of SNP rs1801516.
Author Contributions
A.B. and R.B. were responsible for the analyses and conducted the statistical analyses. A.B., R.B., N.A., A.F., M.W., M.L.M., F.L., D.S.-L. and Y.K. wrote the manuscript. D.S.-L. and N.A. led the GENESIS and CoF-AT2 studies and contributed to the protocol, design and search for funding. E.C. contributed to the protocol and design of the GENESIS study. N.A. and S.E.-M. were responsible for the coordination of GENESIS and CoF-AT2. S.E.-M., E.C. and D.L.G. were responsible for the inclusion of participants, data collection and data entry. A.B. and R.B. completed information on the radiotherapeutics treatments from the Institut Curie medical records. F.L. was responsible for the biological resource center and for the strategy to identify and classify the ATM gene variants, was involved in the study design and contributed to the writing of the manuscript. N.A., D.S.-L. and F.L. provided critical readings of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for GENESIS was provided by the Ligue Nationale contre le Cancer (3 grants: PRE05/DSL and PRE07/DSL to D. Stoppa-Lyonnet; PRE11/NA to N. Andrieu), the French National Institute of Cancer (INCa, Grant b2008-029/LL-LC) and the comprehensive cancer center SiRIC (Site de Recherche Intégrée sur le Cancer: Grant INCa-DGOS-4654) to N. Andrieu. Sequencing data: France Génomique to F. Lesueur and CNRGH. Financial support for CoF-AT was provided by Inserm and Ministère de la Recherche (01P0751–01P0752–01P0753–01P0754–01P0755), Electricité de France (conseil scientifique de Radioprotection d’EDF, grant EP 2002–03, EP 2004-03 RB 2016–22), Fondation de France (grants 2001009761 and 2005011201), La Ligue (grants PRE04/NA, PRE07/NA and PRE2015 LNCC/NA), La Ligue Comité du Maine et Loire, MGEN Union, ITMO Santé Publique d’AVIESAN (grant AAP12-COH-110), Institut Curie (CEST NC2013-015) and Institut National du Cancer (grant INCa-9578). This work has been also supported by the Fondation ARC pour la recherche sur le cancer (www.fondation-arc.org; accessed on 1 January 2003) (Grant ARCPGA2022120005732_6344).
Institutional Review Board Statement
The GENESIS study protocol was approved by the appropriate ethics committee (Comité de Protection des Personnes Ile-de-France III, 3 October 2006, agreement n°2373). The CoF-AT study protocol was approved by the appropriate ethics committee (Comité de Protection des Personnes Ile-de-France III).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments
We wish to thank former and current members of the genetic epidemiology platform (the PIGE, Plateforme d’Investigation en Génétique et Epidémiologie: M. Marcou, L. Toulemonde, J. Beauvallet, N. Mebirouk) and past members of the GENESIS biological resource center (C. Verny-Pierre, L. Barjhoux, V. Sornin, N. Mebirouk). We also thank members of the Genetic department of Institut Curie, Paris, who followed up with the patients or retrieved their clinical data (M. Gauthier-Villars, B. Buecher, A. de Pauw, M. Belotti, C. Colas, E. Mouret-Fourme and H. Delhomelle).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; André, F.; Baselga, J.; et al. Tailoring Therapies—Improving the Management of Early Breast Cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10 801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [CrossRef] [PubMed]
- Hamdan, D.; Nguyen, T.T.; Leboeuf, C.; Meles, S.; Janin, A.; Bousquet, G. Genomics Applied to the Treatment of Breast Cancer. Oncotarget 2019, 10, 4786–4801. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Paull, T.T. ATM Activation by DNA Double-Strand Breaks through the Mre11-Rad50-Nbs1 Complex. Science 2005, 308, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y. ATM and Related Protein Kinases: Safeguarding Genome Integrity. Nat. Rev. Cancer 2003, 3, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia Telangiectasia: A Review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.; Hecht, B.K. Cancer in Ataxia-Telangiectasia Patients. Cancer Genet. Cytogenet. 1990, 46, 9–19. [Google Scholar] [CrossRef]
- Angèle, S.; Romestaing, P.; Moullan, N.; Vuillaume, M.; Chapot, B.; Friesen, M.; Jongmans, W.; Cox, D.G.; Pisani, P.; Gérard, J.-P.; et al. ATM Haplotypes and Cellular Response to DNA Damage: Association with Breast Cancer Risk and Clinical Radiosensitivity. Cancer Res. 2003, 63, 8717–8725. [Google Scholar]
- Thorstenson, Y.R.; Roxas, A.; Kroiss, R.; Jenkins, M.A.; Yu, K.M.; Bachrich, T.; Muhr, D.; Wayne, T.L.; Chu, G.; Davis, R.W.; et al. Contributions of ATM Mutations to Familial Breast and Ovarian Cancer. Cancer Res. 2003, 63, 3325–3333. [Google Scholar]
- Renwick, A.; Thompson, D.; Seal, S.; Kelly, P.; Chagtai, T.; Ahmed, M.; North, B.; Jayatilake, H.; Barfoot, R.; Spanova, K.; et al. ATM Mutations That Cause Ataxia-Telangiectasia Are Breast Cancer Susceptibility Alleles. Nat. Genet. 2006, 38, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.; Birrell, G.; Lavin, M. Testing for Mutations of the Ataxia Telangiectasia Gene in Radiosensitive Breast Cancer Patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1998, 47, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.; Arutyunyan, R.; Stumm, M.; Dörk, T.; Bendix, R.; Bremer, M.; Varon, R.; Sauer, R.; Gebhart, E. Radiosensitivity of Ataxia Telangiectasia and Nijmegen Breakage Syndrome Homozygotes and Heterozygotes as Determined by Three-Color FISH Chromosome Painting. Radiat. Res. 2002, 157, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.M.; Kittrell, F.S.; Yu, Y.; McCarthy, M.; Zabriskie, R.C.; Ullrich, R.L. Radiation Induces Genomic Instability and Mammary Ductal Dysplasia in Atm Heterozygous Mice. Oncogene 2001, 20, 4409–4411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Q.; Howard, M.E.; Tu, X.; Zhu, Q.; Denbeigh, J.M.; Remmes, N.B.; Herman, M.G.; Beltran, C.J.; Yuan, J.; Greipp, P.T.; et al. Inhibition of ATM Induces Hypersensitivity to Proton Irradiation by Upregulating Toxic End Joining. Cancer Res. 2021, 81, 3333–3346. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.G.R.; Bellon, J.R.; Shannon, K.M.; Gadd, M.A.; Dunn, S.; Rosenstein, B.S.; Ho, A.Y. ATM Variants in Breast Cancer: Implications for Breast Radiation Therapy Treatment Recommendations. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cui, J.; Tang, F.; Cong, X.; Han, F. Ataxia Telangiectasia-Mutated Gene Polymorphisms and Acute Normal Tissue Injuries in Cancer Patients after Radiation Therapy: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1090–1098. [Google Scholar] [CrossRef]
- Terrazzino, S.; Cargnin, S.; Deantonio, L.; Pisani, C.; Masini, L.; Canonico, P.L.; Genazzani, A.A.; Krengli, M. Impact of ATM Rs1801516 on Late Skin Reactions of Radiotherapy for Breast Cancer: Evidences from a Cohort Study and a Trial Sequential Meta-Analysis. PLoS ONE 2019, 14, e0225685. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Overgaard, J.; Alsner, J.; Overgaard, M.; Herskind, C.; Cesaretti, J.A.; Atencio, D.P.; Green, S.; Formenti, S.C.; Stock, R.G.; et al. ATM Sequence Variants and Risk of Radiation-Induced Subcutaneous Fibrosis after Postmastectomy Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 776–783. [Google Scholar] [CrossRef]
- Bremer, M.; Klöpper, K.; Yamini, P.; Bendix-Waltes, R.; Dörk, T.; Karstens, J.H. Clinical Radiosensitivity in Breast Cancer Patients Carrying Pathogenic ATM Gene Mutations: No Observation of Increased Radiation-Induced Acute or Late Effects. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2003, 69, 155–160. [Google Scholar] [CrossRef]
- Iannuzzi, C.M.; Atencio, D.P.; Green, S.; Stock, R.G.; Rosenstein, B.S. ATM Mutations in Female Breast Cancer Patients Predict for an Increase in Radiation-Induced Late Effects. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Fan, G.; Atencio, D.P.; Green, S.; Formenti, S.C.; Haffty, B.G.; Iyengar, P.; Bernstein, J.L.; Stock, R.G.; Cesaretti, J.A.; et al. Possession of ATM Sequence Variants as Predictor for Late Normal Tissue Responses in Breast Cancer Patients Treated with Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sinilnikova, O.M.; Dondon, M.-G.; Eon-Marchais, S.; Damiola, F.; Barjhoux, L.; Marcou, M.; Verny-Pierre, C.; Sornin, V.; Toulemonde, L.; Beauvallet, J.; et al. GENESIS: A French National Resource to Study the Missing Heritability of Breast Cancer. BMC Cancer 2016, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Janin, N.; Andrieu, N.; Ossian, K.; Laugé, A.; Croquette, M.F.; Griscelli, C.; Debré, M.; Bressac-de-Paillerets, B.; Aurias, A.; Stoppa-Lyonnet, D. Breast Cancer Risk in Ataxia Telangiectasia (AT) Heterozygotes: Haplotype Study in French AT Families. Br. J. Cancer 1999, 80, 1042–1045. [Google Scholar] [CrossRef][Green Version]
- Girard, E.; Eon-Marchais, S.; Olaso, R.; Renault, A.-L.; Damiola, F.; Dondon, M.-G.; Barjhoux, L.; Goidin, D.; Meyer, V.; Le Gal, D.; et al. Familial Breast Cancer and DNA Repair Genes: Insights into Known and Novel Susceptibility Genes from the GENESIS Study, and Implications for Multigene Panel Testing. Int. J. Cancer 2019, 144, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A General Framework for Estimating the Relative Pathogenicity of Human Genetic Variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the Deleteriousness of Variants throughout the Human Genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Tavtigian, S.V.; Byrnes, G.B.; Goldgar, D.E.; Thomas, A. Classification of Rare Missense Substitutions, Using Risk Surfaces, with Genetic- and Molecular-Epidemiology Applications. Hum. Mutat. 2008, 29, 1342–1354. [Google Scholar] [CrossRef]
- Tavtigian, S.V.; Oefner, P.J.; Babikyan, D.; Hartmann, A.; Healey, S.; Le Calvez-Kelm, F.; Lesueur, F.; Byrnes, G.B.; Chuang, S.-C.; Forey, N.; et al. Rare, Evolutionarily Unlikely Missense Substitutions in ATM Confer Increased Risk of Breast Cancer. Am. J. Hum. Genet. 2009, 85, 427–446. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA. Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, F.; Easton, D.F.; Renault, A.-L.; Tavtigian, S.V.; Bernstein, J.L.; Kote-Jarai, Z.; Eeles, R.A.; Plaseska-Karanfia, D.; Feliubadaló, L.; Spanish ATM Working Group; et al. First International Workshop of the ATM and Cancer Risk Group (4–5 December 2019). Fam. Cancer 2022, 21, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, F.; Eon-Marchais, S.; Bonnet-Boissinot, S.; Beauvallet, J.; Dondon, M.-G.; Golmard, L.; Rouleau, E.; Garrec, C.; Martinez, M.; Toulas, C.; et al. TUMOSPEC: A Nation-Wide Study of Hereditary Breast and Ovarian Cancer Families with a Predicted Pathogenic Variant Identified through Multigene Panel Testing. Cancers 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Eccles, D.M.; Romero, I.L.; Al-Mulla, F.; Balmaña, J.; Biancolella, M.; Bslok, R.; Caligo, M.A.; Calvello, M.; Capone, G.L.; et al. Genetic Testing and Clinical Management Practices for Variants in Non-BRCA1/2 Breast (and Breast/Ovarian) Cancer Susceptibility Genes: An International Survey by the Evidence-Based Network for the Interpretation of Germline Mutant Alleles (ENIGMA) Clinical Working Group. JCO Precis. Oncol. 2018, 2, PO.18.00091. [Google Scholar] [CrossRef]
- Porras, L.-M.; Padilla, N.; Moles-Fernández, A.; Feliubadaló, L.; Santamariña-Pena, M.; Sánchez, A.T.; López-Novo, A.; Blanco, A.; de la Hoya, M.; Molina, I.J.; et al. A New Set of in Silico Tools to Support the Interpretation of ATM Missense Variants Using Graphical Analysis. J. Mol. Diagn. JMD 2024, 26, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.A.; Heintzelman, R.C.; Corbman, M.; Garcia, F.U. Invasive Breast Carcinomas with ATM Gene Variants of Uncertain Significance Share Distinct Histopathologic Features. Breast J. 2018, 24, 291–297. [Google Scholar] [CrossRef]
- Renault, A.-L.; Mebirouk, N.; Fuhrmann, L.; Bataillon, G.; Cavaciuti, E.; Le Gal, D.; Girard, E.; Popova, T.; La Rosa, P.; Beauvallet, J.; et al. Morphology and Genomic Hallmarks of Breast Tumours Developed by ATM Deleterious Variant Carriers. Breast Cancer Res. BCR 2018, 20, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
