Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search and Data Collection
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Studies and Patient Characteristics
3.2. Technical and Post-Operative Outcomes
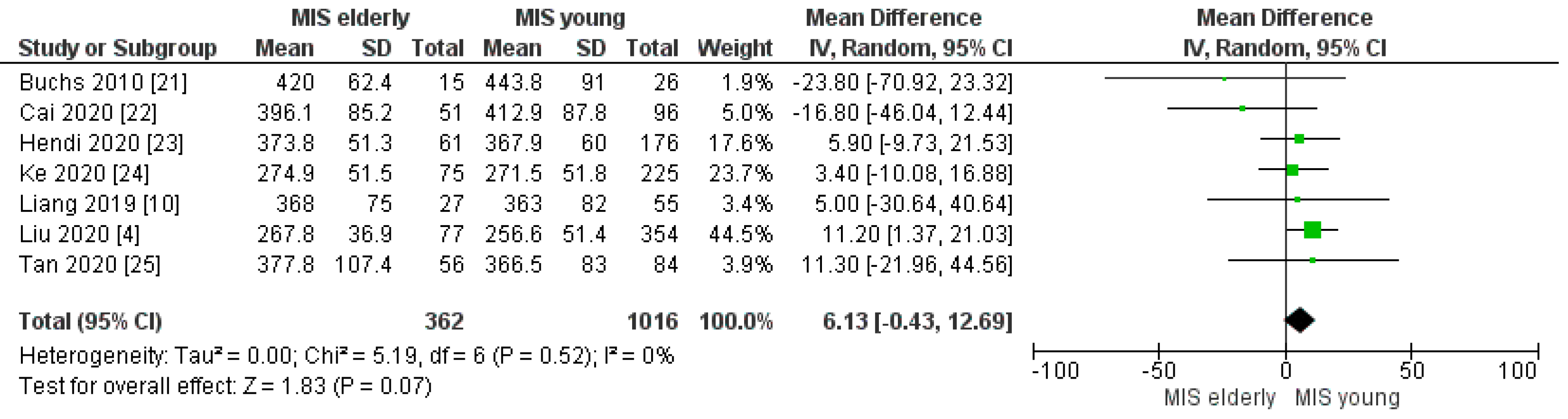
3.2.1. Operating Time
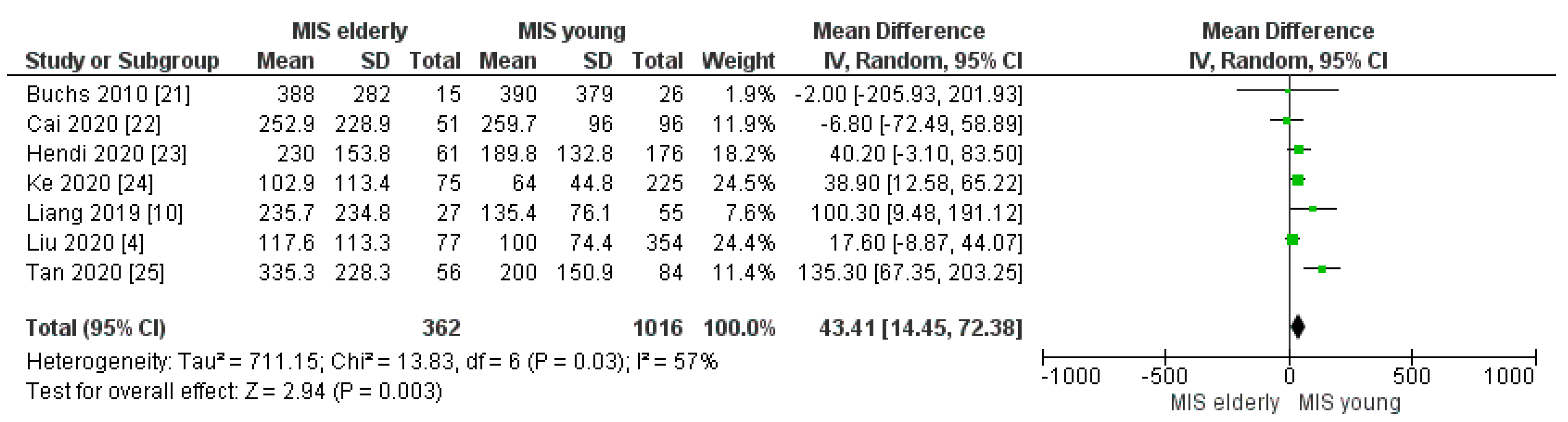
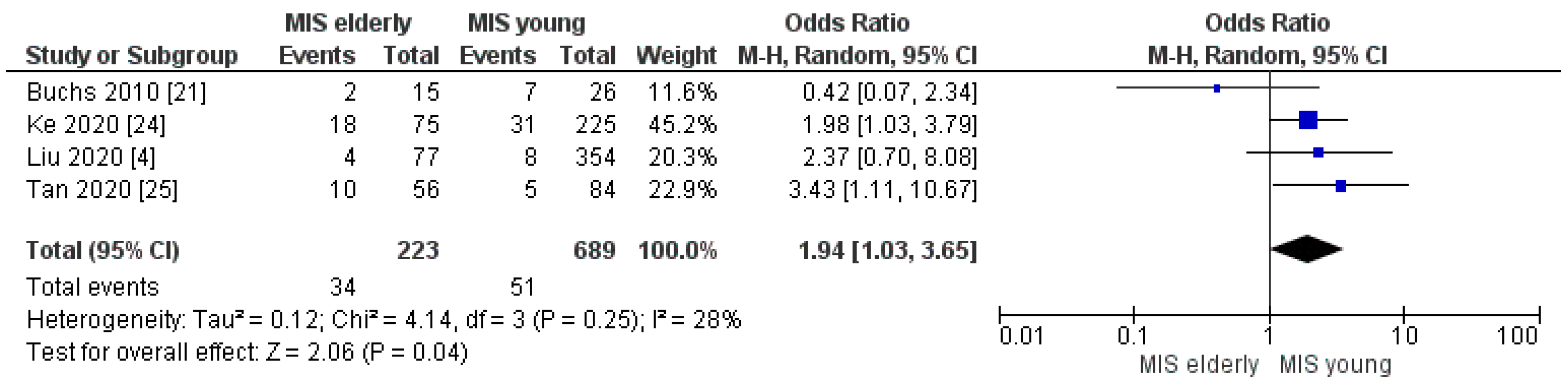
3.2.2. Intraoperative Blood Loss and Intraoperative Red Blood Cell (RBC) Transfusion Rate
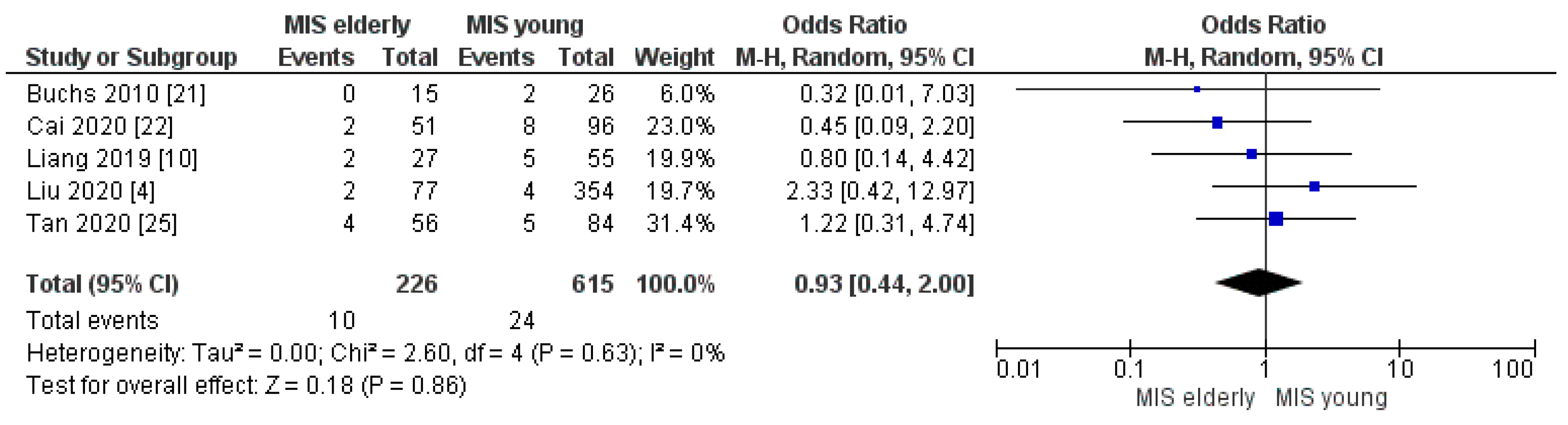
3.2.3. Conversion to Open Surgery and Reoperation Rate
3.2.4. Peri-Operative Mortality Rate
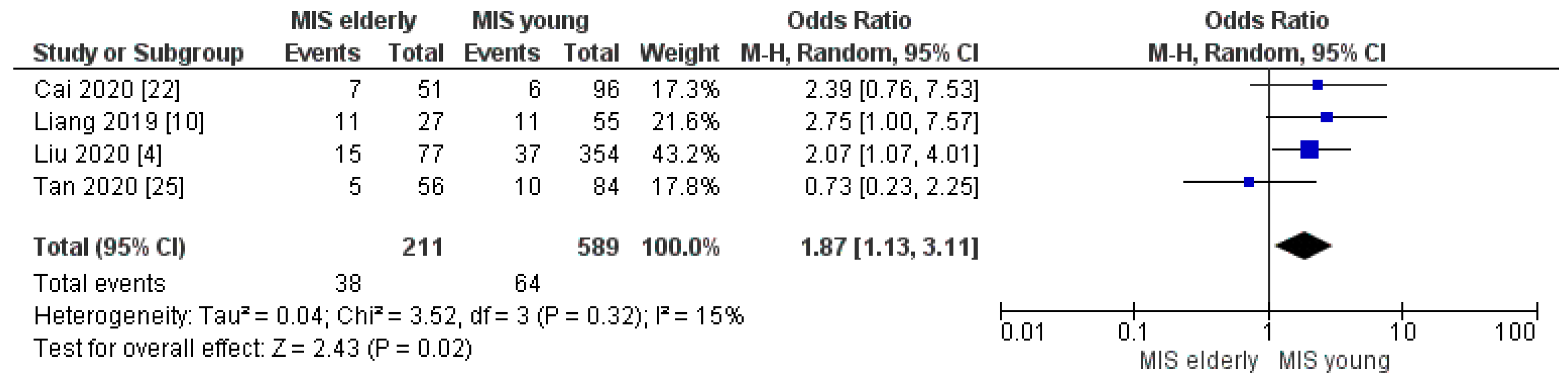
3.2.5. Complication Rate
3.2.6. Post-Operative Pancreatic Fistula Grade > A and Biliary Leakage Rate
3.2.7. Post-Op Bleeding, Delayed Gastric Empty and Abdominal Collection Rates
3.2.8. Lung Morbidity Rate
3.2.9. R0 Margin Rate and Mean Number of Harvested Lymphnodes
3.2.10. Readmission Rate
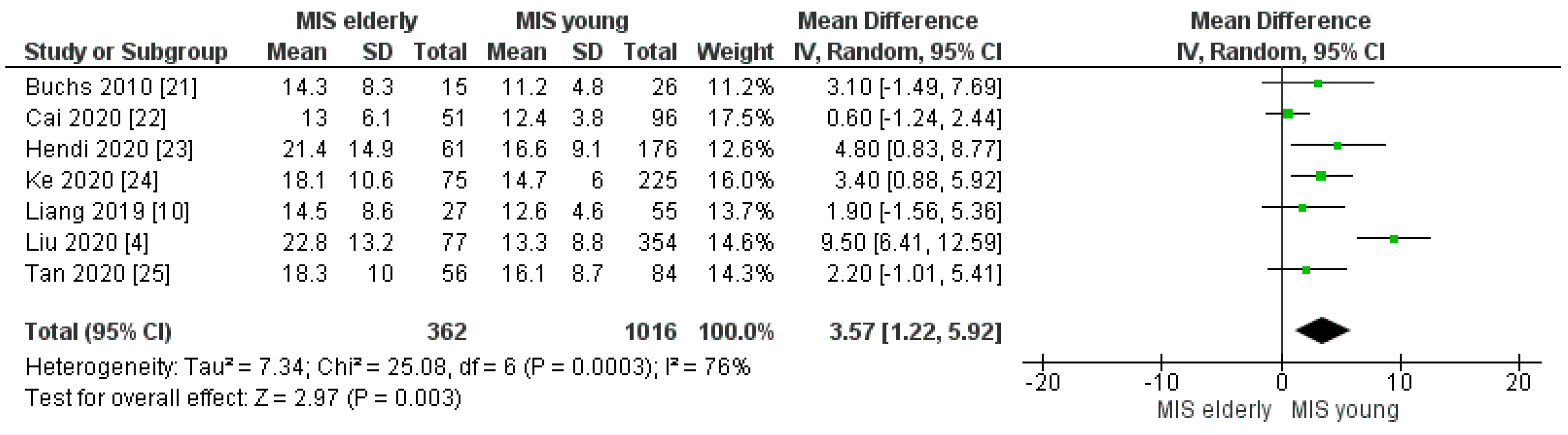
3.2.11. Length of Hospital Stay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Ballarin, R.; Spaggiari, M.; Di Benedetto, F.; Montalti, R.; Masetti, M.; De Ruvo, N.; Romano, A.; Guerrini, G.P.; De Blasiis, M.G.; Gerunda, G.E. Do Not Deny Pancreatic Resection to Elderly Patients. J. Gastrointest. Surg. 2009, 13, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Damodaran Prabha, R.; Chua, T.C.; Arena, J.; Kotecha, K.; Mittal, A.; Gill, A.J.; Samra, J.S. Safety and Efficacy of Pancreaticoduodenectomy in Octogenarians. Front. Surg. 2021, 8, 617286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, Z.; Zhang, X.; Zhao, G.; Tan, X.; Gao, Y.; Lau, W.Y.; Liu, R. Robotic Pancreaticoduodenectomy in Elderly and Younger Patients: A Retrospective Cohort Study. Int. J. Surg. 2020, 81, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gagner, M.; Pomp, A. Laparoscopic Pylorus-Preserving Pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in General Surgery: Personal Experience in a Large Community Hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef]
- Ballarin, R.; Magistri, P.; Tarantino, G.; Assirati, G.; Pecchi, A.; Guerrini, G.P.; Di Benedetto, F. Laparoscopic Pancreaticoduodenectomy for Tumors of the Head of the Pancreas; 10 Cases for a Single Center Experience. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 858–859. [Google Scholar] [PubMed]
- Nigri, G.; Petrucciani, N.; La Torre, M.; Magistri, P.; Valabrega, S.; Aurello, P.; Ramacciato, G. Duodenopancreatectomy: Open or Minimally Invasive Approach? Surgeon 2014, 12, 227–234. [Google Scholar] [CrossRef]
- Gall, T.M.; Pencavel, T.D.; Cunningham, D.; Nicol, D.; Jiao, L.R. Transition from Open and Laparoscopic to Robotic Pancreaticoduodenectomy in a UK Tertiary Referral Hepatobiliary and Pancreatic Centre—Early Experience of Robotic Pancreaticoduodenectomy. HPB 2020, 22, 1637–1644. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, L.; Jiang, C.; Hu, P.; Wang, H.; Cai, Z.; Wang, W. Laparoscopic Pancreaticoduodenectomy in Elderly Patients. Surg. Endosc. 2020, 34, 2028–2034. [Google Scholar] [CrossRef]
- Zureikat, A.H.; Postlewait, L.M.; Liu, Y.; Gillespie, T.W.; Weber, S.M.; Abbott, D.E.; Ahmad, S.A.; Maithel, S.K.; Hogg, M.E.; Zenati, M.; et al. A Multi-Institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann. Surg. 2016, 264, 640–649. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Zhao, Z.-M.; Tan, X.-L.; Zhao, G.-D.; Zhang, X.; Xu, Y. The Surgical Outcomes of Robot-Assisted Laparoscopic Pancreaticoduodenectomy versus Laparoscopic Pancreaticoduodenectomy for Periampullary Neoplasms: A Comparative Study of a Single Center. Surg. Endosc. 2017, 31, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-Analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal Literature Search for Systematic Reviews in Surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Buchs, N.C.; Addeo, P.; Bianco, F.M.; Gangemi, A.; Ayloo, S.M.; Giulianotti, P.C. Outcomes of Robot-Assisted Pancreaticoduodenectomy in Patients Older than 70 Years: A Comparative Study. World J. Surg. 2010, 34, 2109–2114. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; Cai, Y.; Wang, X.; Ouyang, G.; Li, Y.; Meng, L.; Peng, B. The Effect of Age on Short- and Long-Term Outcomes in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Laparoscopic Pancreaticoduodenectomy. Pancreas 2020, 49, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Hendi, M.; Mou, Y.; Lu, C.; Pan, Y.; Zhang, B.; Chen, K.; Xu, X.; Zhang, R.; Zhou, Y.; Jin, W. Laparoscopic Pancreaticodoudenectomy. Medicine 2020, 99, e22175. [Google Scholar] [CrossRef]
- Ke, J.; Liu, Y.; Liu, F.; Ji, B. Application of Laparoscopic Pancreatoduodenectomy in Elderly Patients. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tang, T.; Zhang, Y.; Zu, G.; An, Y.; Chen, W.; Wu, D.; Sun, D.; Chen, X. Laparoscopic vs. Open Pancreaticoduodenectomy: A Comparative Study in Elderly People. Updates Surg. 2020, 72, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yu, J.; Zhang, Y.; Liang, Z.; Han, X. Perioperative and Oncological Outcomes Following Minimally Invasive versus Open Pancreaticoduodenectomy for Pancreatic Duct Adenocarcinoma. Surg. Endosc. 2021, 35, 2273–2285. [Google Scholar] [CrossRef]
- Tan, E.; Song, J.; Lam, S.; D’Souza, M.; Crawford, M.; Sandroussi, C. Postoperative Outcomes in Elderly Patients Undergoing Pancreatic Resection for Pancreatic Adenocarcinoma: A Systematic Review and Meta-Analysis. Int. J. Surg. 2019, 72, 59–68. [Google Scholar] [CrossRef]
- Fung, G.; Sha, M.; Kunduzi, B.; Froghi, F.; Rehman, S.; Froghi, S. Learning Curves in Minimally Invasive Pancreatic Surgery: A Systematic Review. Langenbecks Arch. Surg. 2022, 407, 2217–2232. [Google Scholar] [CrossRef]
- Renz, B.W.; Khalil, P.N.; Mikhailov, M.; Graf, S.; Schiergens, T.S.; Niess, H.; Boeck, S.; Heinemann, V.; Hartwig, W.; Werner, J.; et al. Pancreaticoduodenectomy for Adenocarcinoma of the Pancreatic Head Is Justified in Elderly Patients: A Retrospective Cohort Study. Int. J. Surg. 2016, 28, 118–125. [Google Scholar] [CrossRef]
- Shamali, A.; De’Ath, H.D.; Jaber, B.; Abuawad, M.; Barbaro, S.; Hamaday, Z.; Abu Hilal, M. Elderly Patients Have Similar Short Term Outcomes and Five-Year Survival Compared to Younger Patients after Pancreaticoduodenectomy. Int. J. Surg. 2017, 45, 138–143. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, N.; Tian, E.; Li, M.; Zhang, H.; Zhao, G.; Tan, X.; Wang, W.; Han, B.; Yuan, J.; et al. Short-Term Outcomes of Robotic versus Open Pancreaticoduodenectomy in Elderly Patients: A Multicenter Retrospective Cohort Study. Int. J. Surg. 2022, 104, 106819. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Levi Sandri, G.B.; Amore Bonapasta, S.; Farsi, M.; Coratti, A. The Role of Robotics in Widening the Range of Application of Minimally Invasive Surgery for Pancreaticoduodenectomy. Pancreatology 2016, 16, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, V.; Livingstone, A. Is Age Just a Number: Pancreaticoduodenectomy in Elderly Patients? Hepatobiliary Pancreat. Dis. Int. 2016, 15, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Valle, V.; Fernandes, E.; Mangano, A.; Aguiluz, G.; Bustos, R.; Bianco, F.; Giulianotti, P.C. Robotic Whipple for Pancreatic Ductal and Ampullary Adenocarcinoma: 10 Years Experience of a US Single-Center. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, e2135. [Google Scholar] [CrossRef] [PubMed]
- Nassour, I.; Tohme, S.; Hoehn, R.; Adam, M.A.; Zureikat, A.H.; Alessandro, P. Safety and Oncologic Efficacy of Robotic Compared to Open Pancreaticoduodenectomy after Neoadjuvant Chemotherapy for Pancreatic Cancer. Surg. Endosc. 2021, 35, 2248–2254. [Google Scholar] [CrossRef]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; Reid-Lombardo, K.M.; Truty, M.J.; Nagorney, D.M.; Kendrick, M.L. Total Laparoscopic Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma: Oncologic Advantages over Open Approaches? Ann. Surg. 2014, 260, 633–640. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| n. | Author | Region | Year | Study Period | Study Design and Cut-Off Age | Sample Size | Age (Years) | Groups | MINORS (Quality) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MISe | MISy | MISe | MISy | ||||||||
| 1 | Buchs [21] | USA | 2010 | 2007–2010 | OCS (P)—70 | 15 | 26 | 76.8 | 56.3 | RPDe vs. RPDy | 22 |
| 2 | Liang [10] | China | 2019 | 2015–2018 | OCS (R)—70 | 27 | 55 | 74.0 | 59.0 | LPDe vs. LPDy | 22 |
| 3 | Cai [22] | China | 2020 | 2012–2019 | OCS (R)—70 | 51 | 96 | 75.2 | 56.1 | LPDe vs. LPDy | 21 |
| 4 | Hendi [23] | China | 2020 | 2012–2017 | OCS (P)—75 | 61 | 176 | 75.7 | 55.7 | LPDe vs. LPDy | 23 |
| 5 | Ke [24] | China | 2020 | 2015–2019 | OCS (R)—65 | 75 | 225 | >65 | <65 | LPDe vs. LPDy | 22 |
| 6 | Liu [4] | China | 2020 | 2018–2019 | OCS (R)—75 | 77 | 354 | 77.0 | 57.9 | RPDe vs. RPDy | 23 |
| 7 | Tan [25] | China | 2020 | 2015–2017 | OCS (R)—70 | 56 | 84 | 75.2 | 60.7 | LPDe vs. LPDy | 23 |
| MISe | MISy | Studies (n) | |
|---|---|---|---|
| Total patients included | 362 | 1016 | 1378 (7) |
| Age (years) | 75.7 | 57.6 | 6 |
| Male/Female (%) | 214/148 (59.1) | 590/426 (58.1) | 7 |
| BMI | 23.0 | 23.6 | 7 |
| Total Bilirubin (mmol/L) | 134.0 | 146.7 | 2 |
| CA 19.9 U/ml | 187.5 | 133.6 | 2 |
| Pre-op Biliary drainage (%) | 16 (18.0) | 42 (17.0) | 2 |
| ASA I/II (%) | 213 (61.0) | 865 (87.0) | 6 |
| ASA III/IV (%) | 134 (39.0) | 125 (13.0) | 6 |
| Overall Comorbidity (%) | 112 (59.0) | 206 (33.0) | 3 |
| Hypertension (%) | 95 (42.0) | 186 (24.0) | 4 |
| CAD (%) | 28 (10.0) | 56 (6.0) | 4 |
| Diabetes (%) | 30 (18.0) | 94 (16.0) | 3 |
| Neurological disease (%) | 4 (4.0) | 2 (1.0) | 2 |
| Lung comorbidity (%) | 10 (6.0) | 25 (4.0) | 3 |
| Benign disease (%) | 56 (15.0) | 225 (22.0) | 7 |
| Malignant disease (%) | 306 (85.0) | 791 (78.0) | 7 |
| Maximum tumor diameter (cm) | 2.66 | 2.66 | 5 |
| Whipple procedure (%) | 308 (99.0) | 908 (99.0) | 6 |
| Pylorus-preserving procedure (%) | 3 (1.00) | 12 (1.00) | 6 |
| TNM 1a (%) | 7 (11.0) | 9 (8.00) | 2 |
| TNM 1b (%) | 33 (53.0) | 61 (53.0) | 2 |
| TNM 2a (%) | 10 (16.0) | 23 (20.0) | 2 |
| TNM 2b (%) | 8 (13.0) | 19 (16.0) | 2 |
| TNM 3 (%) | 4 (6.0) | 4 (3.0) | 2 |
| TNM 4 (%) | 0 | 0 | 2 |
| Surgical Outcome | Type of Surgery | Observations (n) | Mean or % | Studies Included (n) | p-Value |
|---|---|---|---|---|---|
| Operating time (min) | MISe | 362 | 354.06 | 7 | 0.07 |
| MISy | 1016 | 354.49 | |||
| Blood loss (mL) | MISe | 362 | 237.49 | 7 | 0.003 |
| MISy | 1016 | 191.27 | |||
| Intra-op Transfusion rate | MISe | 34/223 | 15.0% | 4 | 0.04 |
| MISy | 51/689 | 7.0% | |||
| Conversion to Open rate | MISe | 10/226 | 4.0% | 5 | 0.86 |
| MISy | 24/615 | 4.0% | |||
| Reoperation rate | MISe | 15/362 | 4.0% | 7 | 0.75 |
| MISy | 40/1016 | 4.0% | |||
| Peri-op Mortality rate | MISe | 13/362 | 4.0% | 7 | 0.02 |
| MISy | 14/1016 | 1.0% | |||
| Overall Complication rate | MISe | 96/204 | 47.0% | 4 | 0.08 |
| MISy | 229/652 | 35.0% | |||
| Clavien–Dindo I/II rate | MISe | 112/211 | 53.0% | 4 | 0.86 |
| MISy | 238/589 | 40.0% | |||
| Clavien–Dindo ≥ III rate | MISe | 38/211 | 18.0% | 4 | 0.02 |
| MISy | 64/589 | 11.0% | |||
| POPF grade > A rate | MISe | 49/362 | 14.0% | 7 | 0.24 |
| MISy | 110/1016 | 11.0% | |||
| Abdominal Collection rate | MISe | 21/155 | 14.0% | 3 | 0.26 |
| MISy | 50/505 | 10.0% | |||
| Biliary Leakage rate | MISe | 13/279 | 5.0% | 5 | 0.22 |
| MISy | 30/877 | 3.0% | |||
| Post-op Bleeding rate | MISe | 24/287 | 8.0% | 6 | 0.22 |
| MISy | 53/791 | 7.0% | |||
| DGE rate | MISe | 33/301 | 11.0% | 6 | 0.20 |
| MISy | 76/840 | 9.0% | |||
| Lung Morbidity rate | MISe | 20/167 | 12.0% | 3 | 0.02 |
| MISy | 42/605 | 7.0% | |||
| R0-margin rate | MISe | 187/195 | 96.0% | 4 | 0.34 |
| MISy | 401/411 | 98.0% | |||
| N. harvested lymphnodes | MISe | 270 | 14.4 | 5 | 0.98 |
| MISy | 636 | 14.3 | |||
| Readmission rate | MISe | 12/235 | 5.0% | 4 | 0.67 |
| MISy | 44/718 | 6.0% | |||
| Hospital stay (days) | MISe | 362 | 17.5 | 7 | 0.003 |
| MISy | 1016 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballarin, R.; Esposito, G.; Guerrini, G.P.; Magistri, P.; Catellani, B.; Guidetti, C.; Di Sandro, S.; Di Benedetto, F. Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis. Cancers 2024, 16, 323. https://doi.org/10.3390/cancers16020323
Ballarin R, Esposito G, Guerrini GP, Magistri P, Catellani B, Guidetti C, Di Sandro S, Di Benedetto F. Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis. Cancers. 2024; 16(2):323. https://doi.org/10.3390/cancers16020323
Chicago/Turabian StyleBallarin, Roberto, Giuseppe Esposito, Gian Piero Guerrini, Paolo Magistri, Barbara Catellani, Cristiano Guidetti, Stefano Di Sandro, and Fabrizio Di Benedetto. 2024. "Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis" Cancers 16, no. 2: 323. https://doi.org/10.3390/cancers16020323
APA StyleBallarin, R., Esposito, G., Guerrini, G. P., Magistri, P., Catellani, B., Guidetti, C., Di Sandro, S., & Di Benedetto, F. (2024). Minimally Invasive Pancreaticoduodenectomy in Elderly versus Younger Patients: A Meta-Analysis. Cancers, 16(2), 323. https://doi.org/10.3390/cancers16020323