Cell-Free DNA as a Biomarker at Diagnosis and Follow-Up in 256 B and T-Cell Lymphomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Design
2.2. Sample Collection, cfDNA Isolation, and Quantification
2.3. Next-Generation Sequencing (NGS)
2.4. Statistical Analysis
3. Results
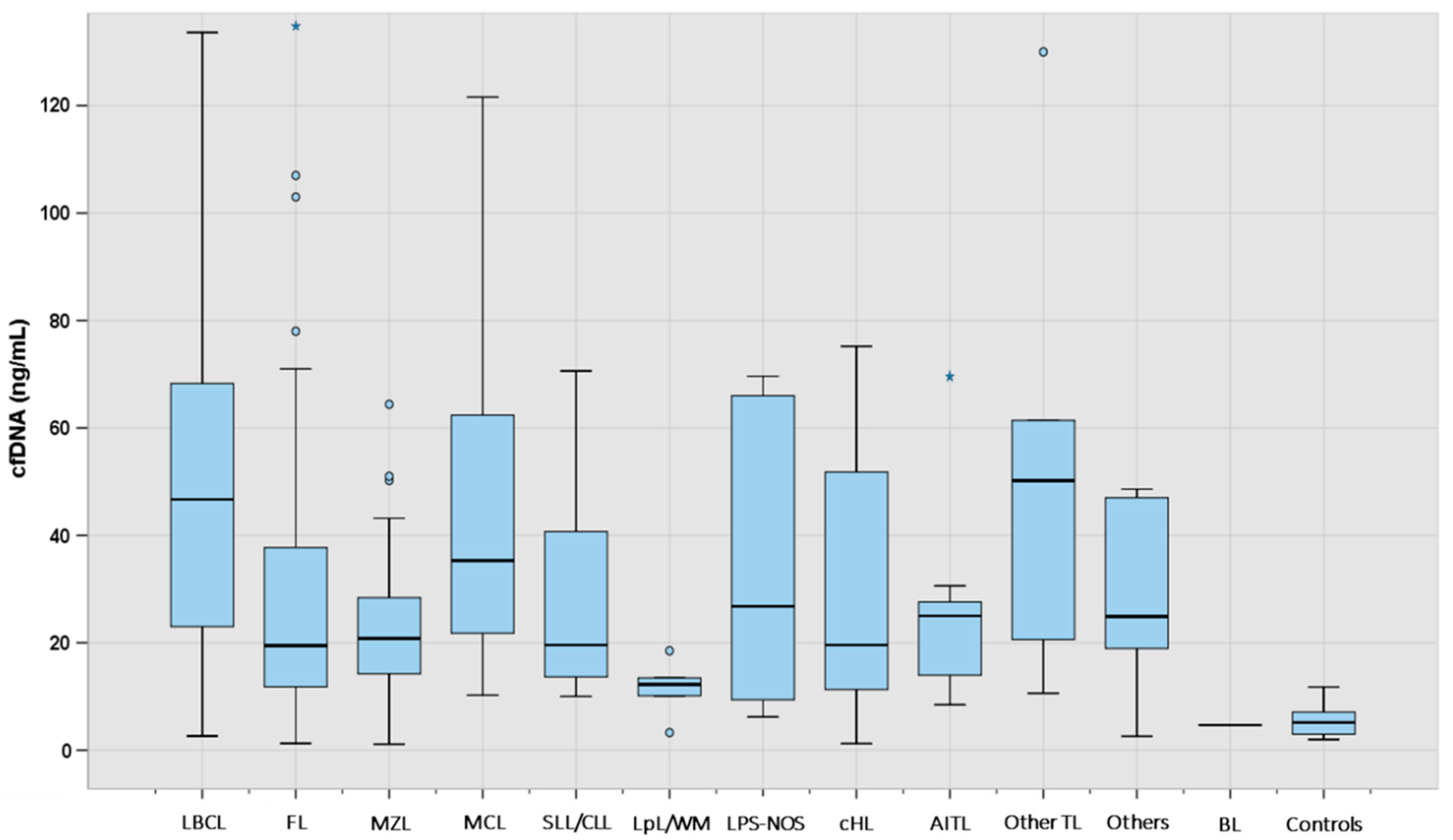
3.1. Patient Characteristics and Levels of cfDNA According to Lymphoma Subtype
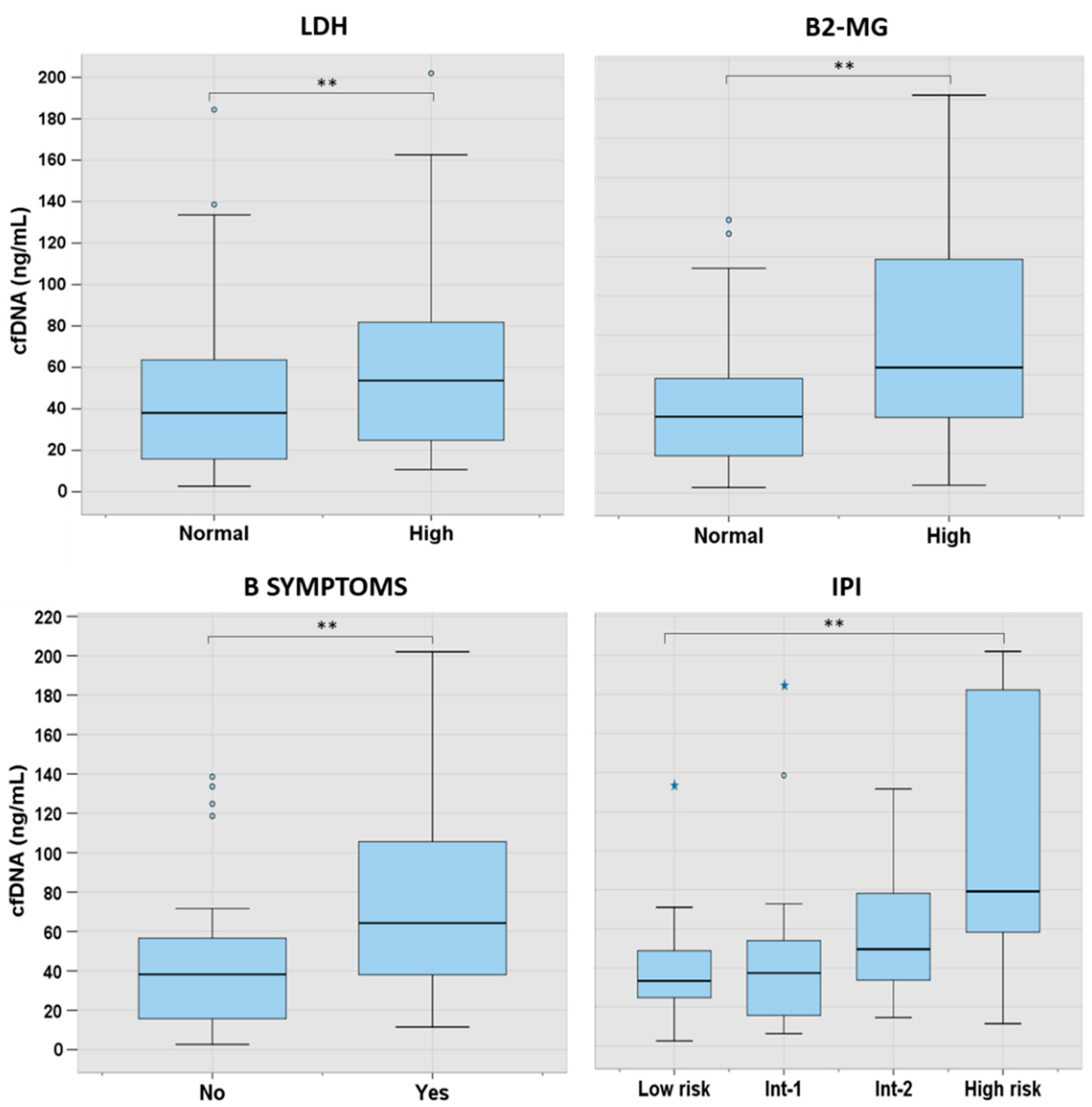
3.2. cfDNA Levels According to Characteristics at Presentation in the Main Lymphoma Subtypes
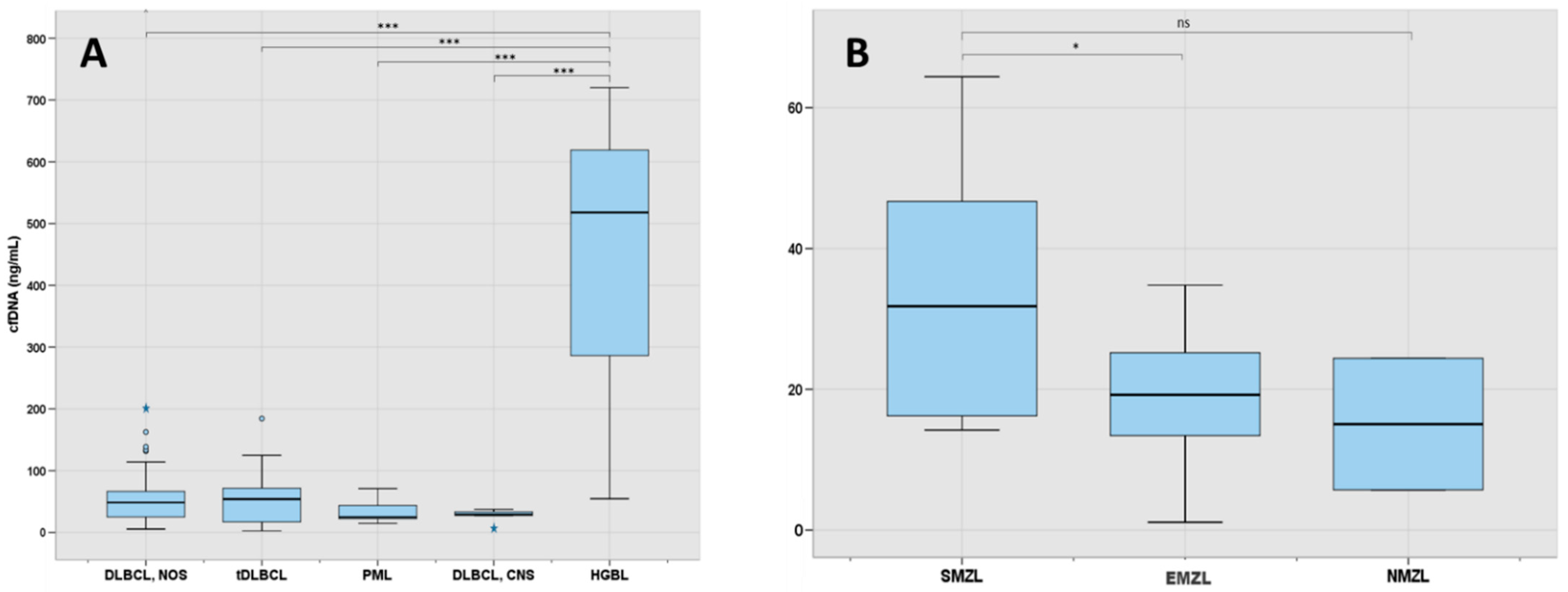
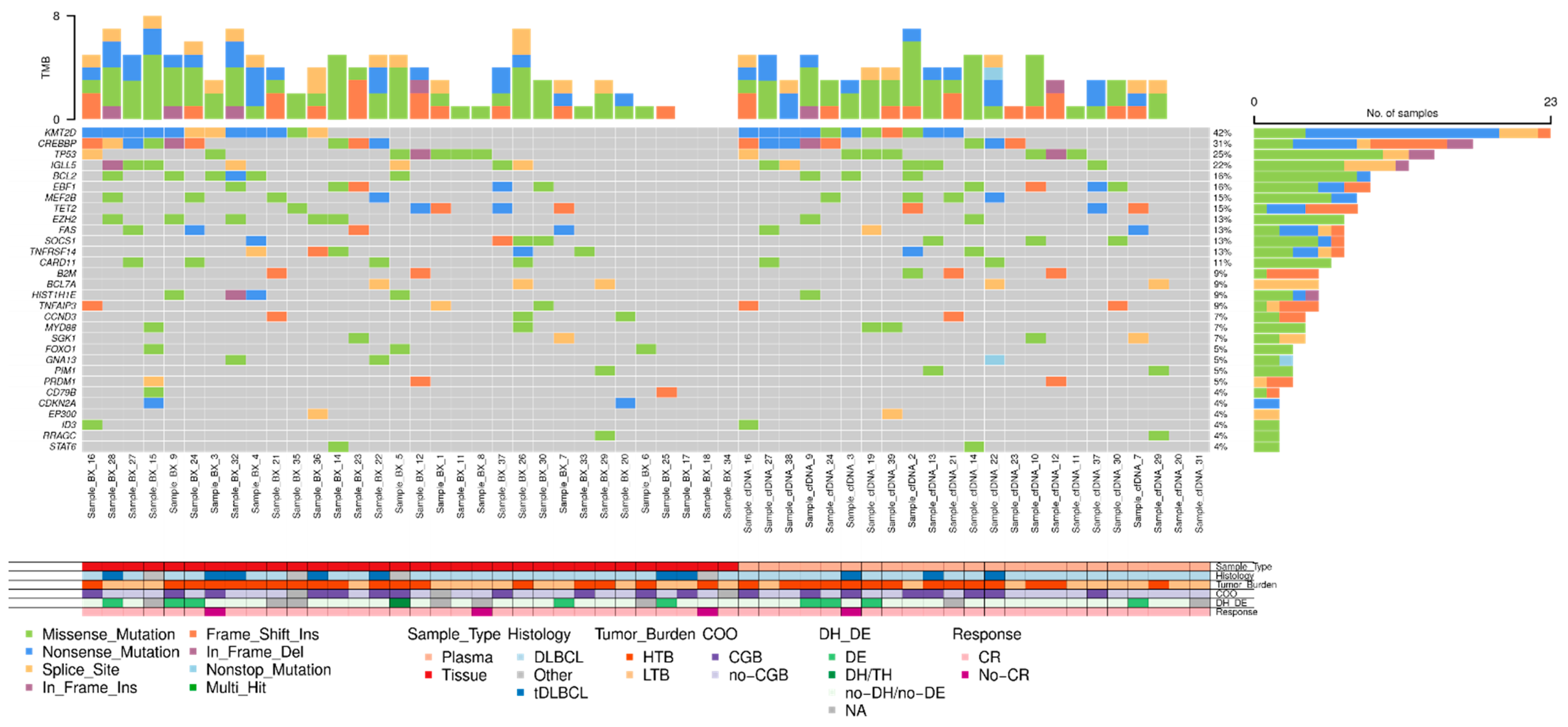
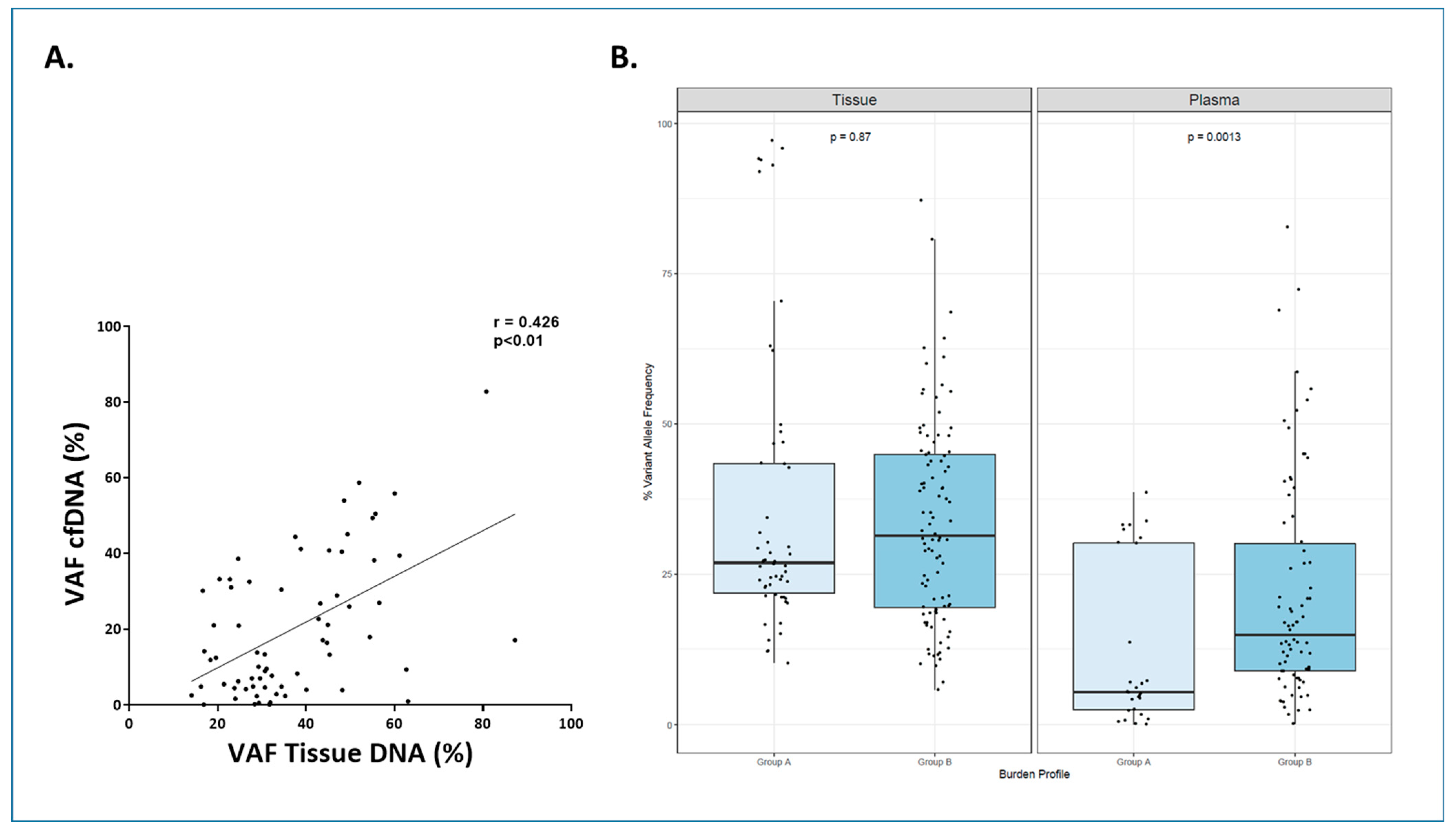
3.3. Molecular Characterization at Diagnosis Using cfDNA and Tissue-DNA in LBCL (n = 49)
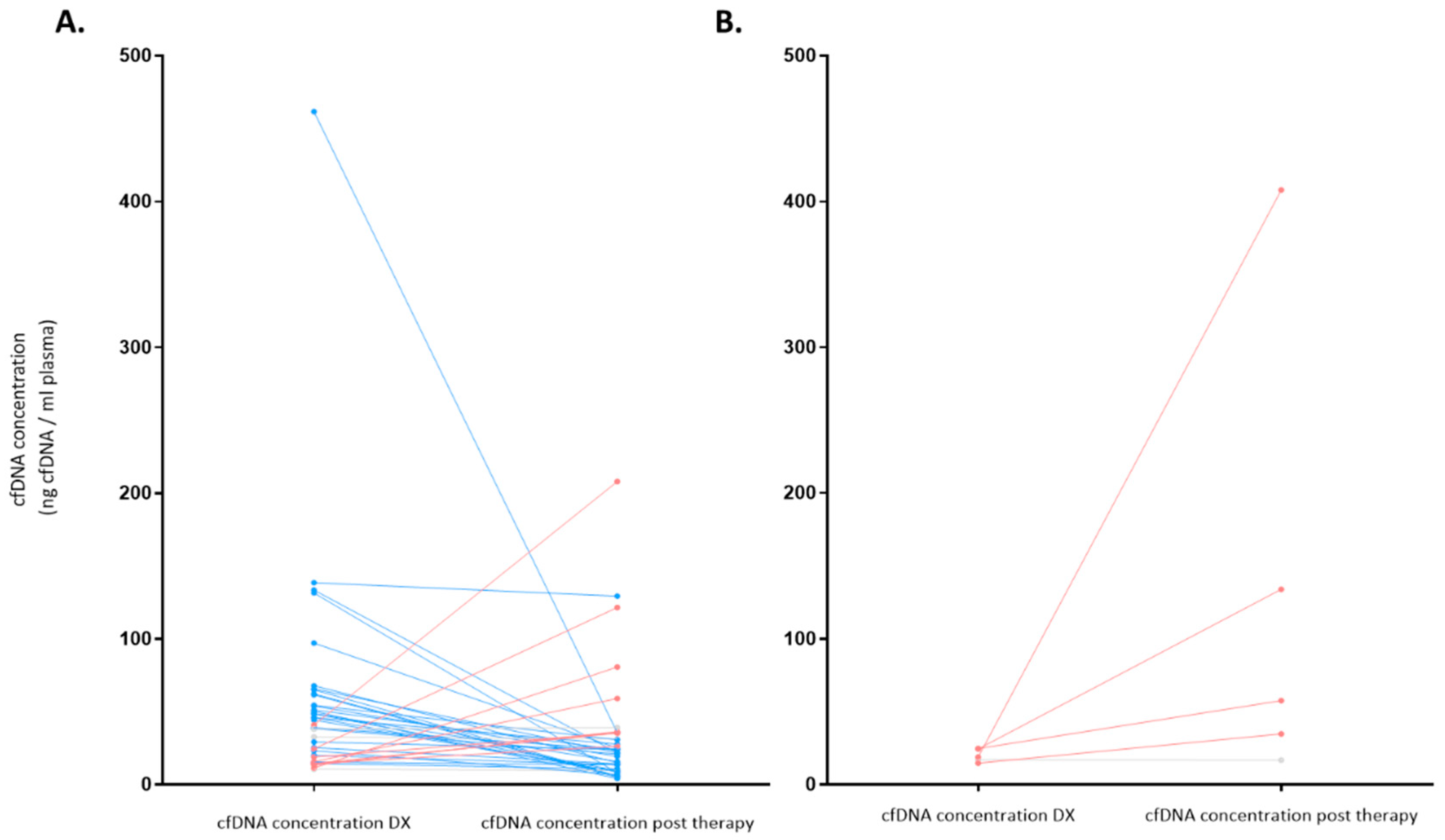
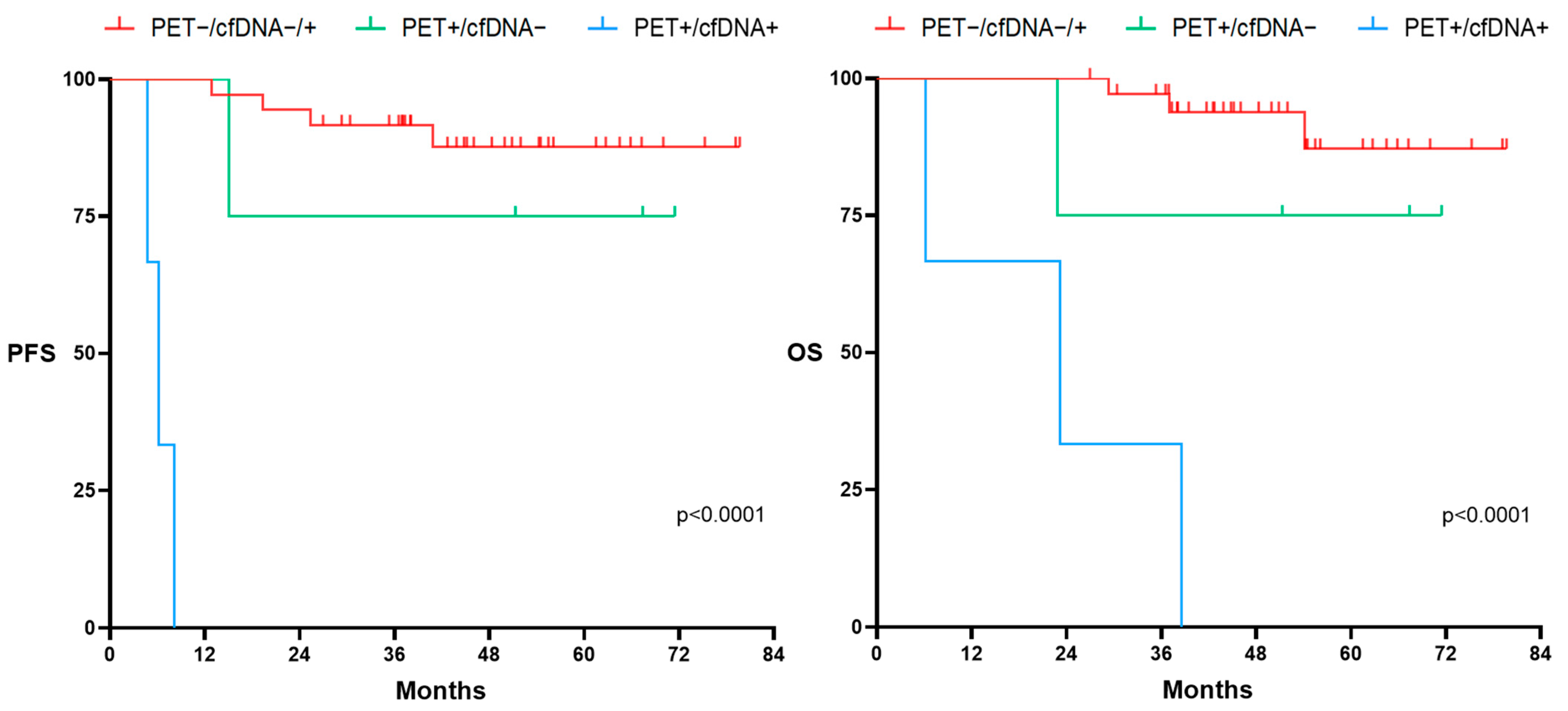
3.4. cfDNA Concentration as a Prognosis Biomarker before and after Therapy for LBCL (n = 49)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gormally, E.; Caboux, E.; Vineis, P.; Hainaut, P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat. Res. Rev. Mutat. Res. 2007, 635, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Jahr, K.R.S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar] [PubMed]
- Trino, S.; Lamorte, D.; Caivano, A.; De Luca, L.; Sgambato, A.; Laurenzana, I. Clinical relevance of extracellular vesicles in hematological neoplasms: From liquid biopsy to cell biopsy. Leukemia 2021, 35, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.Y.; Chik, K.W.; Chiu, R.W.; Ho, C.Y.; Lam, C.W. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin. Chem. 2002, 48, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.M.W.; Kircher, M.; Hill, A.J.; Daza, R.M. Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-of-Origin. Cell 2016, 164, 57–68. [Google Scholar] [CrossRef]
- Garcia-Gisbert, N.; Fernández-Ibarrondo, L.; Fernández-Rodríguez, C.; Gibert, J.; Andrade-Campos, M.; Arenillas, L.; Camacho, L.; Angona, A.; Longarón, R.; Salar, A.; et al. Circulating cell-free DNA improves the molecular characterisation of Ph-negative myeloproliferative neoplasms. Br. J. Haematol. 2021, 192, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 1. [Google Scholar] [CrossRef]
- Diehl, F.C.M.; Schmidt, K. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef]
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef]
- Roschewski, M.; Dunleavy, K.; Pittaluga, S.; Moorhead, M.; Pepin, F.; Kong, K.; Shovlin, M.; Jaffe, E.S.; Staudt, L.M.; Lai, C.; et al. Comparative Study of Circulating Tumor DNA and Computerized Tomography Monitoring in Untreated Diffuse Large B-Cell Lymphoma. Lancet Oncol. 2015, 16, 541–549. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Scherer, F.; Jin, M.C.; Soo, J.; Craig, A.F.M.; Esfahani, M.S.; Chabon, J.J.; Stehr, H.; Liu, C.L.; Tibshirani, R.; et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J. Clin. Oncol. 2018, 36, 2845–2853. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, Y.J.; Lee, D.; Cho, D.; Ko, Y.H.; Cho, J.; Park, W.Y.; Park, D.; Kim, S.J.; Kim, W.S. Analysis of circulating tumor DNA by targeted ultra-deep sequencing across various non-Hodgkin lymphoma subtypes. Leuk. Lymphoma 2019, 60, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.K.; Hartung, K.; Botzen, A.; Brobeil, A.; Rummel, M.; Kurch, L.; Georgi, T.; Jox, T.; Bielack, S.; Burdach, S.; et al. Genotyping circulating tumor DNA of pediatric Hodgkin lymphoma. Leukemia 2020, 34, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Hohaus, S.; Giachelia, M.; Massini, G.; Mansueto, G.; Vannata, B.; Bozzoli, V.; Criscuolo, M.; D’Alò, F.; Martini, M.; Larocca, L.M.; et al. Cell-free circulating DNA in Hodgkin’s and non-Hodgkin’s lymphomas. Ann. Oncol. 2009, 20, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Cervena, K.; Vodicka, P.; Vymetalkova, V. Diagnostic and prognostic impact of cell-free DNA in human cancers: Systematic review. Mutat. Res. Rev. Mutat. Res. 2019, 781, 100–129. [Google Scholar] [CrossRef]
- Swerdlow, T.J.S.H.; Campo, E.; Harris, N.L.; Pileri, S.A.; Jaffe, E.S.; Stein, H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Tatarczuch, M.; Waltham, M.; Shortt, J.; Polekhina, G.; Hawkes, E.A.; Ho, S.-J.; Trotman, J.; Brasacchio, D.; Co, M.; Li, J.; et al. Molecular associations of response to the new generation BTK inhibitor zanubrutinib in marginal zone lymphoma. Blood Adv. 2023, 7, 3531–3539. [Google Scholar] [CrossRef]
- Volckmar, A.-L.A.R.; Holger Sültmann, P.S.; Thoas Fioretos, V.E.; Albrecht Stenzinger, S.D. A Field Guide for Cancer Diagnostics using cell-free DNA: From Principles to Practice and Clinical Applications. Genes Chromosomes Cancer 2018, 57, 123–139. [Google Scholar] [CrossRef]
- Bellosillo, B.; Montagut, C. High-accuracy liquid biopsies. Nat. Med. 2019, 25, 1820–1821. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Green, M.R.; Bratman, S.V.; Scherer, F.; Liu, C.L.; Kunder, C.A.; Takahashi, K.; Glover, C.; Keane, C.; Kihira, S.; et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 2015, 125, 3679–3687. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Q.; Yang, J.; Zhang, M.; Liu, X.; Zhang, H.; Huang, Y.; Li, J.; Bao, J.; Wang, J.; et al. Application of circulating tumour DNA in terms of prognosis prediction in Chinese follicular lymphoma patients. Front. Genet. 2023, 14, 1066808. [Google Scholar] [CrossRef] [PubMed]
- Hatipoğlu, T.; Esmeray Sönmez, E.; Hu, X.; Yuan, H.; Danyeli, A.E.; Şeyhanlı, A.; Önal-Süzek, T.; Zhang, W.; Akman, B.; Olgun, A.; et al. Plasma Concentrations and Cancer-Associated Mutations in Cell-Free Circulating DNA of Treatment-Naive Follicular Lymphoma for Improved Non-Invasive Diagnosis and Prognosis. Front. Oncol. 2022, 12, 870487. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Jardin, F. pharmaceuticals Cell-Free DNA for the Management of Classical Hodgkin Lymphoma. Pharmaceuticals 2021, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Viennot, M.; Lequesne, J.; Viailly, P.J.; Bohers, E.; Bessi, L.; Marcq, B.; Etancelin, P.; Dubois, S.; Picquenot, J.M.; et al. Targeted genotyping of circulating tumor DNA for classical Hodgkin lymphoma monitoring: A prospective study. Haematologica 2021, 106, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Darrah, J.M.; Herrera, A.F. Updates on Circulating Tumor DNA Assessment in Lymphoma. Curr. Hematol. Malig. Rep. 2018, 13, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.; Manoochehrabadi, S.; Pashaiefar, H.; Zaimy, M.A.; Ahmadvand, M. Clinical significance of cell-free DNA as a prognostic biomarker in patients with diffuse large B-cell lymphoma. Blood Res. 2019, 54, 114–119. [Google Scholar] [CrossRef]
- Arzuaga-Mendez, J.; Prieto-Fernández, E.; Lopez-Lopez, E.; Martin-Guerrero, I.; García-Ruiz, J.C.; García-Orad, A. Cell-free DNA as a biomarker in diffuse large B-cell lymphoma: A systematic review. Crit. Rev. Oncol. Hematol. 2019, 139, 7–15. [Google Scholar] [CrossRef]
- Melani, C.; Wilson, W.H.; Roschewski, M. Monitoring Clinical Outcomes in Aggressive B-Cell Lymphoma: From Imaging Studies to Circulating Tumor DNA; Elsevier Ltd.: Cham, Swizterland, 2018; Volume 31. [Google Scholar] [CrossRef]
- Melani, M.R.C. Molecular Monitoring of Cell-Free Circulating Tumor DNA in Non-Hodgkin Lymphoma. Oncology 2016, 30, 731–744. [Google Scholar]
- Scherer, F.; Kurtz, D.M.; Newman, A.M.; Stehr, H.; Craig, F.M.; Esfahani, M.S.; Lovejoy, A.F.; Chabon, J.J.; Klass, D.M.; Liu, C.L.; et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci. Transl. Med. 2016, 8, 364ra155. [Google Scholar] [CrossRef]
- Cirillo, M.; Borchmann, S. An update on disease biomarkers for Hodgkin lymphoma. Expert Rev. Hematol. 2020, 13, 481–488. [Google Scholar] [CrossRef]
- Spina, V.; Bruscaggin, A.; Cuccaro, A.; Martini, M.; Di Trani, M.; Forestieri, G.; Manzoni, M.; Condoluci, A.; Arribas, A.; Terzi-Di-Bergamo, L.; et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood 2018, 131, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Delfau-Larue, M.H.; Van Der Gucht, A.; Dupuis, J.; Jais, J.P.; Nel, I.; Beldi-Ferchiou, A.; Hamdane, S.; Benmaad, I.; Laboure, G.; Verret, B.; et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: Distinct prognostic value in follicular lymphoma. Blood Adv. 2018, 2, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, C.; Huet, S.; Carlton, V.E.H.; Fabiani, B.; Delmer, A.; Jardin, F.; Delfau-Larue, M.H.; Hacini, M.; Ribrag, V.; Guidez, S.; et al. The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma. Oncotarget 2017, 8, 8765–8774. [Google Scholar] [CrossRef]
- Distler, A.; Lakhotia, R.; Phelan, J.D.; Pittaluga, S.; Melani, C.; Muppidi, J.R.; Simard, J.; Pradhan, A.; Hillsman, A.; Yang, Y.; et al. A Prospective Study of Clonal Evolution in Follicular Lymphoma: Circulating Tumor DNA Correlates with Overall Tumor Burden and Fluctuates over Time without Therapy. Blood 2021, 138, 1328. [Google Scholar] [CrossRef]
- Lakhotia, R.; Melani, C.; Dunleavy, K.; Pittaluga, S.; Saba, N.; Lindenberg, L.; Mena, E.; Bergvall, E.; Lucas, A.N.; Jacob, A.; et al. Circulating tumor DNA predicts therapeutic outcome in mantle cell lymphoma. Blood Adv. 2022, 6, 2667–2680. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chan, Y.C.; Tam, C.S.; Hunter, T.; Vassiliadis, D.; Teh, C.E.; Thijssen, R.; Yeh, P.; Wong, S.Q.; Ftouni, S.; et al. Dynamic molecular monitoring reveals that SWI–SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat. Med. 2019, 25, 119–129. [Google Scholar] [CrossRef]
- Chong Wei, D.Z.; Wei, W.; Yan, Z.; Danqing, Z.; Wei, Z. Mutation profiling, tumour burden assessment, outcome prediction and disease monitoring by circulating tumour DNA in peripheral T-cell lymphoma. Br. J. Hematol. 2023, 1, 86–95. [Google Scholar]
- Sakata-Yanagimoto, M.; Nakamoto-Matsubara, R.; Komori, D.; Nguyen, T.B.; Hattori, K.; Nanmoku, T.; Kato, T.; Kurita, N.; Yokoyama, Y.; Obara, N.; et al. Detection of the circulating tumor DNAs in angioimmunoblastic T-cell lymphoma. Ann. Hematol. 2017, 96, 1471–1475. [Google Scholar] [CrossRef]
- Camus, V.; Viennot, M.; Lévêque, E.; Viailly, P.-J.; Tonnelet, D.; Veresezan, E.-L.; Drieux, F.; Etancelin, P.; Dubois, S.; Stamatoullas, A.; et al. Circulating tumor DNA in primary mediastinal large B-cell lymphoma versus classical Hodgkin lymphoma: A retrospective study. Leuk. Lymphoma 2022, 63, 834–844. [Google Scholar] [CrossRef]
- Rivas-Delgado, A.; Nadeu, F.; Andrade-Campos, M.; López, C.; Enjuanes, A.; Mozas, P.; Frigola, G.; Colomo, L.; Sanchez-Gonzalez, B.; Villamor, N.; et al. Cell-Free DNA for Genomic Analysis in Primary Mediastinal Large B-Cell Lymphoma. Diagnostics 2022, 12, 1575. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Delgado, A.; Nadeu, F.; Enjuanes, A.; Casanueva-Eliceiry, S.; Mozas, P.; Magnano, L.; de Anta, N.C.; Rovira, J.; Dlouhy, I.; Martín, S.; et al. Mutational landscape and tumor burden assessed by cell-free DNA in diffuse large B-cell lymphoma in a population-based study. Clin. Cancer Res. 2021, 27, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Diop, F.; Spaccarotella, E.; Monti, S.; Zanni, M.; Rasi, S.; Deambrogi, C.; Spina, V.; Bruscaggin, A.; Favini, C.; et al. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood 2017, 129, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Lauer, E.M.; Mutter, J.; Scherer, F. Circulating tumor DNA in B-cell lymphoma: Technical advances, clinical applications, and perspectives for translational research. Leukemia 2022, 36, 2151–2164. [Google Scholar] [CrossRef]
- Strijker, M.; Soer, E.C.; de Pastena, M.; Creemers, A.; Balduzzi, A.; Beagan, J.J.; Busch, O.R.; van Delden, O.M.; Halfwerk, H.; van Hooft, J.E.; et al. Circulating tumor DNA quantity is related to tumor volume and both predict survival in metastatic pancreatic ductal adenocarcinoma. Int. J. Cancer 2020, 146, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, J.; Wang, G.; Zhao, J.; Xu, J.; Han, J.; Zhao, Z.; Zhao, J.; Zhu, B.; Zhuo, M.; et al. Allele Frequency–Adjusted Blood-Based Tumor Mutational Burden as a Predictor of Overall Survival for Patients With NSCLC Treated With PD-(L)1 Inhibitors. J. Thorac. Oncol. 2020, 15, 556–567. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Soo, J.; Co Ting Keh, L.; Alig, S.; Chabon, J.J.; Sworder, B.J.; Schultz, A.; Jin, M.C.; Scherer, F.; Garofalo, A.; et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat. Biotechnol. 2021, 39, 1537–1547. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; Liu, H.; Zhao, J.; Zhou, H.; Su, X.; Liu, X.; Li, L.; Qiu, L.; Qian, Z.; et al. Tracking the evolution of untreated high-intermediate/high-risk diffuse large B-cell lymphoma by circulating tumour DNA. Br. J. Haematol. 2022, 196, 617–628. [Google Scholar] [CrossRef]
- Frank, M.J.; Hossain, N.M.; Bukhari, A.; Dean, E.; Spiegel, J.Y.; Claire, G.K.; Kirsch, I.; Jacob, A.P.; Mullins, C.D.; Wee Lee, L.; et al. Monitoring of Circulating Tumor DNA Improves Early Relapse Detection After Axicabtagene Ciloleucel Infusion in Large B-Cell Lymphoma: Results of a Prospective Multi-Institutional Trial. J. Clin. Oncol. 2021, 39, 3034–3043. [Google Scholar] [CrossRef]
- Bastos-Oreiro, M.; Sanz-Villanueva, L.; Muñiz, P.; Bailén, R.; Chicano, M.; Oarbeskoa, G.; Gómez, I.; Gutiérrez, A.; de la Iglesia, I.; Carbonell, D.; et al. Cell-Free DNA Dynamic Concentration and Other Variables Are Predictors of Early Progression after Chimeric Antigen Receptor T Cell Therapy in Patients with Diffuse Large B Cell Lymphoma. Transplant Cell Ther. 2023, 29, 472.e1–472.e4. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Tang, S.S.; Wolfe, J.; Kaley, T.J.; Daras, M.; Pentsova, E.I.; Piotrowski, A.F.; Stone, J.; Lin, A.; Nolan, C.P.; et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019, 133, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.M. The many facets of liquid biopsies in lymphoma. Blood 2022, 139, 1780–1781. [Google Scholar] [CrossRef] [PubMed]
- Meriranta, L.; Alkodsi, A.; Pasanen, A.; Lepistö, M.; Mapar, P.; Blaker, Y.N.; Jørgensen, J.; Karjalainen-Lindsberg, M.-L.; Fiskvik, I.; Mikalsen, L.T.G.; et al. Molecular features encoded in the ctDNA reveal heterogeneity and predict outcome in high-risk aggressive B-cell lymphoma. Blood 2022, 139, 1863–1877. [Google Scholar] [CrossRef]

| LBCL (N = 88) | FL (N = 47) | MZL (N = 30) | MCL (N = 14) | SLL/CLL (N = 7) | LpL/WM (N = 5) | BL (N = 1) | LPS-NOS (N = 6) | cHL (N = 30) | AITL (N = 9) | Other TL (N = 6) | Others (N = 6) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years (range) | 67 (19–91) | 62 (36–86) | 71 (43–93) | 71 (49–84) | 77 (46–82) | 70 (41–89) | 34 | 81 (47–87) | 40 (18–86) | 62 (28–91) | 59 (45–79) | 48 (35–76) |
| Gender | ||||||||||||
| Male | 58% | 45% | 50% | 86% | 71% | 100% | – | 17% | 53% | 67% | 67% | 100% |
| Female | 42% | 55% | 50% | 14% | 29% | – | 100% | 83% | 47% | 33% | 33% | – |
| AA stage | ||||||||||||
| I–II | 43% | 37% | 57% | – | – | – | 100% | – | 53% | – | 23% | 17% |
| III–V | 57% | 63% | 43% | 100% | 100% | 100% | – | 100% | 47% | 100% | 77% | 83% |
| B symptoms | 41% | 22% | 10% | 38% | 25% | 0 | 0 | 0% | 47% | 33% | 17% | 83% |
| LDH elevated | 52% | 11% | 10% | 36% | 0 | 0 | 0 | 17% | 7% | 33% | 40% | 83% |
| BM involvement | 15% | 34% | 30% | 86% | 100% | 100% | 0 | 31% | 20% | 22% | 17% | 17% |
| Type of Lymphoma | N | Median (ng/mL) | IQR (ng/mL) |
|---|---|---|---|
| LBCL | 88 | 46.0 | 23.2–68.2 |
| DLBCL, NOS | 60 | 48.5 | 24.8–67.0 |
| Transformed LBCL | 13 | 54.0 | 15.9–95.1 |
| PML | 7 | 24.8 | 21.2–48.6 |
| DLBCL, CNS | 5 | 29.4 | 16.9–35.4 |
| HGBL | 3 | 518.0 | 286.3–619.0 |
| FL | 47 | 19.5 | 11.7–39.2 |
| MZL | 30 | 20.8 | 14.2–29.3 |
| NMZL | 2 | 15.1 | 5.7–24.4 |
| EMZL | 17 | 19.2 | 13.2–26.7 |
| SMZL | 11 | 31.8 | 15.8–50.2 |
| MCL | 14 | 35.3 | 19.5–77.2 |
| SLL/CLL | 7 | 19.6 | 11.5–53.6 |
| LpL/WM | 5 | 12.2 | 6.7–16.0 |
| BL | 1 | 4.68 | NA |
| LPS-NOS | 6 | 26.8 | 8.6–66.9 |
| cHL | 30 | 19.6 | 11.0–53.4 |
| AITL | 9 | 25.0 | 14.0–27.6 |
| Other TL | 6 | 50.2 | 26.3–6.3 |
| Others | 6 | 24.9 | 20.2–41.8 |
| All cases | 249 | 27.8 | 14.5–56.0 |
| Controls | 33 | 5.2 | 2.8–7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Feijóo, R.; Andrade-Campos, M.; Gibert, J.; Sánchez-González, B.; Fernández-Ibarrondo, L.; Fernández-Rodríguez, C.; Garcia-Gisbert, N.; Camacho, L.; Lafuente, M.; Vázquez, I.; et al. Cell-Free DNA as a Biomarker at Diagnosis and Follow-Up in 256 B and T-Cell Lymphomas. Cancers 2024, 16, 321. https://doi.org/10.3390/cancers16020321
Diez-Feijóo R, Andrade-Campos M, Gibert J, Sánchez-González B, Fernández-Ibarrondo L, Fernández-Rodríguez C, Garcia-Gisbert N, Camacho L, Lafuente M, Vázquez I, et al. Cell-Free DNA as a Biomarker at Diagnosis and Follow-Up in 256 B and T-Cell Lymphomas. Cancers. 2024; 16(2):321. https://doi.org/10.3390/cancers16020321
Chicago/Turabian StyleDiez-Feijóo, Ramón, Marcio Andrade-Campos, Joan Gibert, Blanca Sánchez-González, Lierni Fernández-Ibarrondo, Concepción Fernández-Rodríguez, Nieves Garcia-Gisbert, Laura Camacho, Marta Lafuente, Ivonne Vázquez, and et al. 2024. "Cell-Free DNA as a Biomarker at Diagnosis and Follow-Up in 256 B and T-Cell Lymphomas" Cancers 16, no. 2: 321. https://doi.org/10.3390/cancers16020321
APA StyleDiez-Feijóo, R., Andrade-Campos, M., Gibert, J., Sánchez-González, B., Fernández-Ibarrondo, L., Fernández-Rodríguez, C., Garcia-Gisbert, N., Camacho, L., Lafuente, M., Vázquez, I., Colomo, L., Salar, A., & Bellosillo, B. (2024). Cell-Free DNA as a Biomarker at Diagnosis and Follow-Up in 256 B and T-Cell Lymphomas. Cancers, 16(2), 321. https://doi.org/10.3390/cancers16020321







