Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
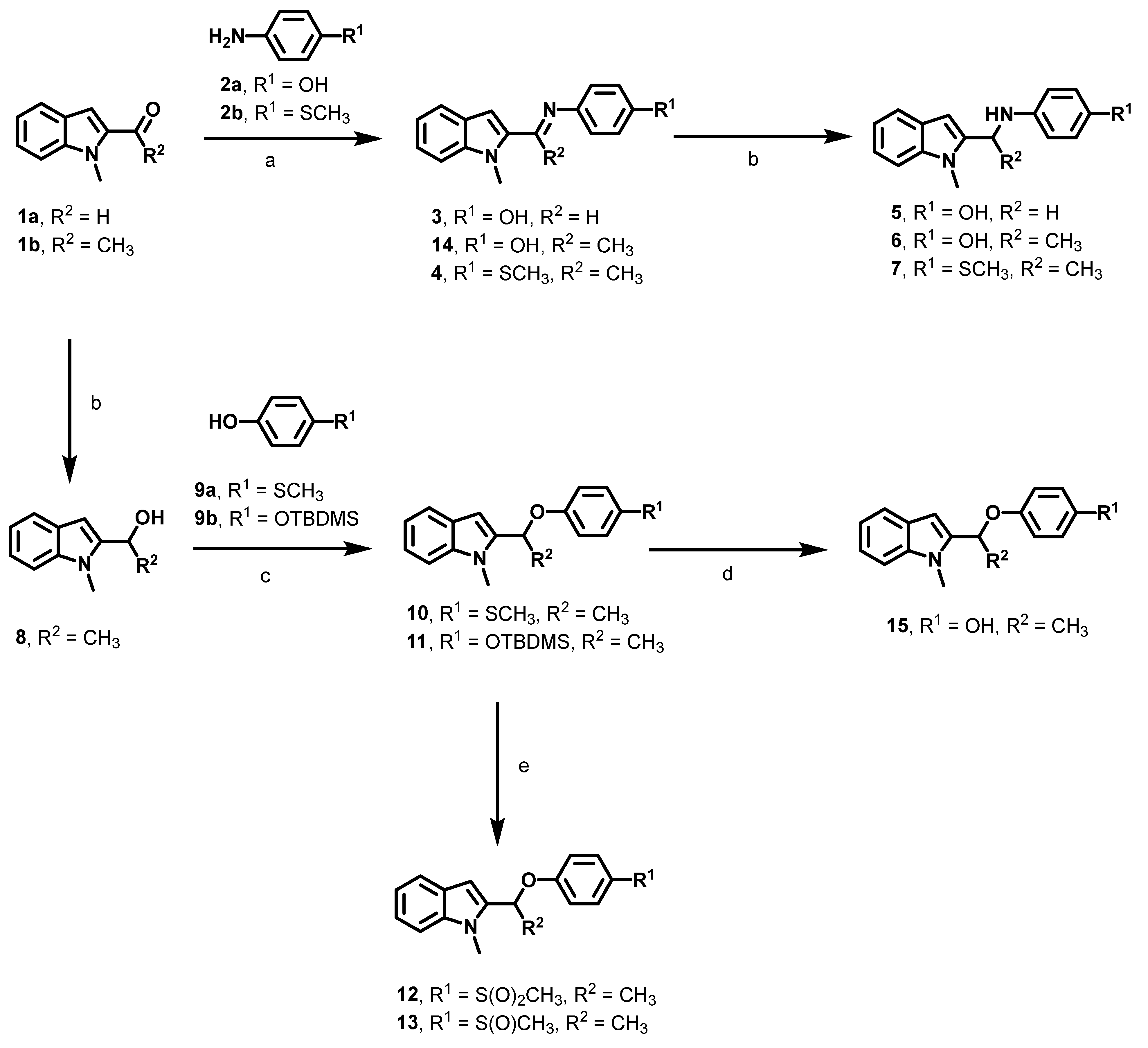
2.2. Synthesis
2.3. Cell Culture and Reagents
2.4. Treatments
2.5. Cell Migration Assays
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
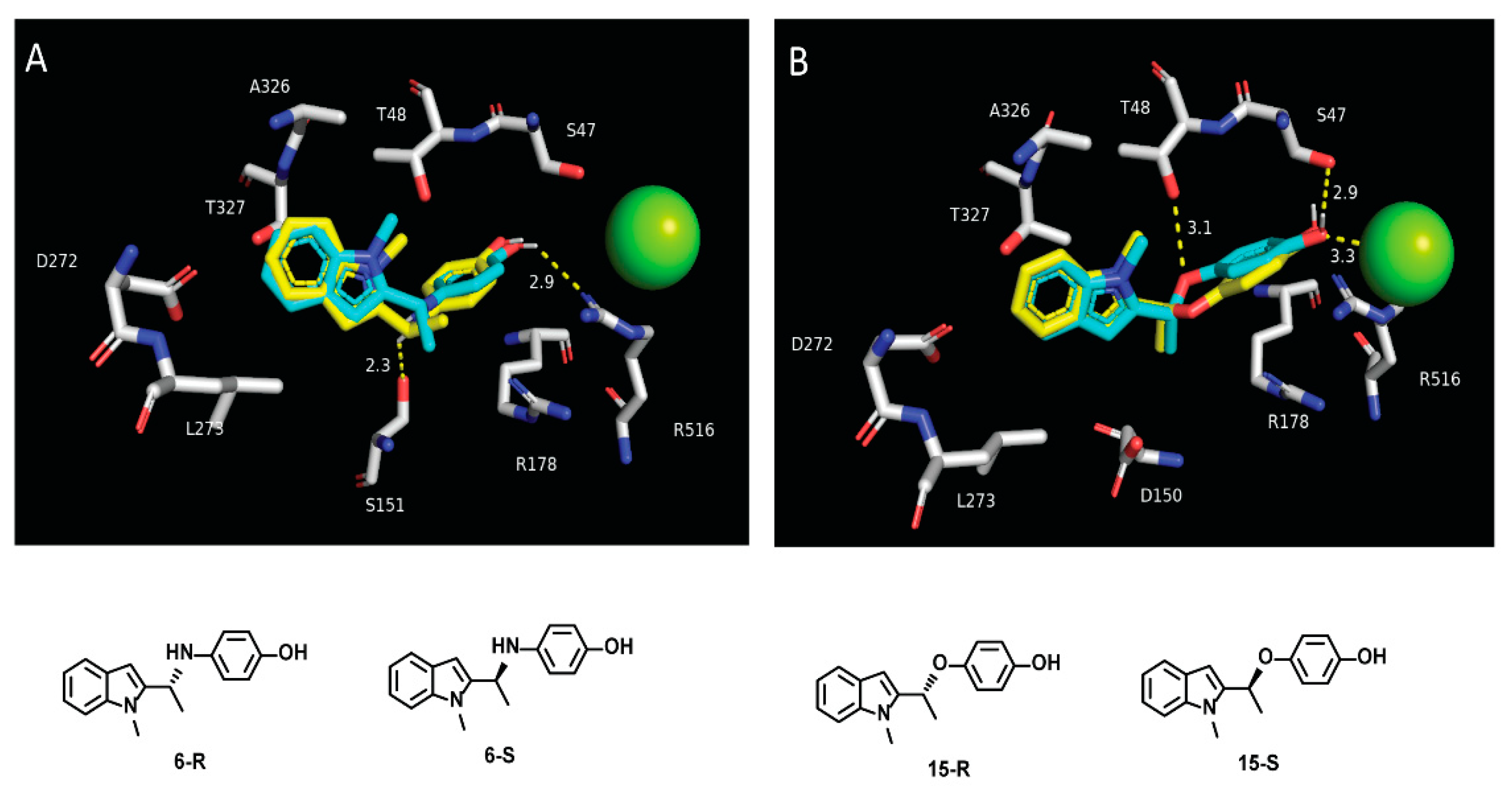
3.1. Optimization of the First Generation Gαi2 Inhibitors
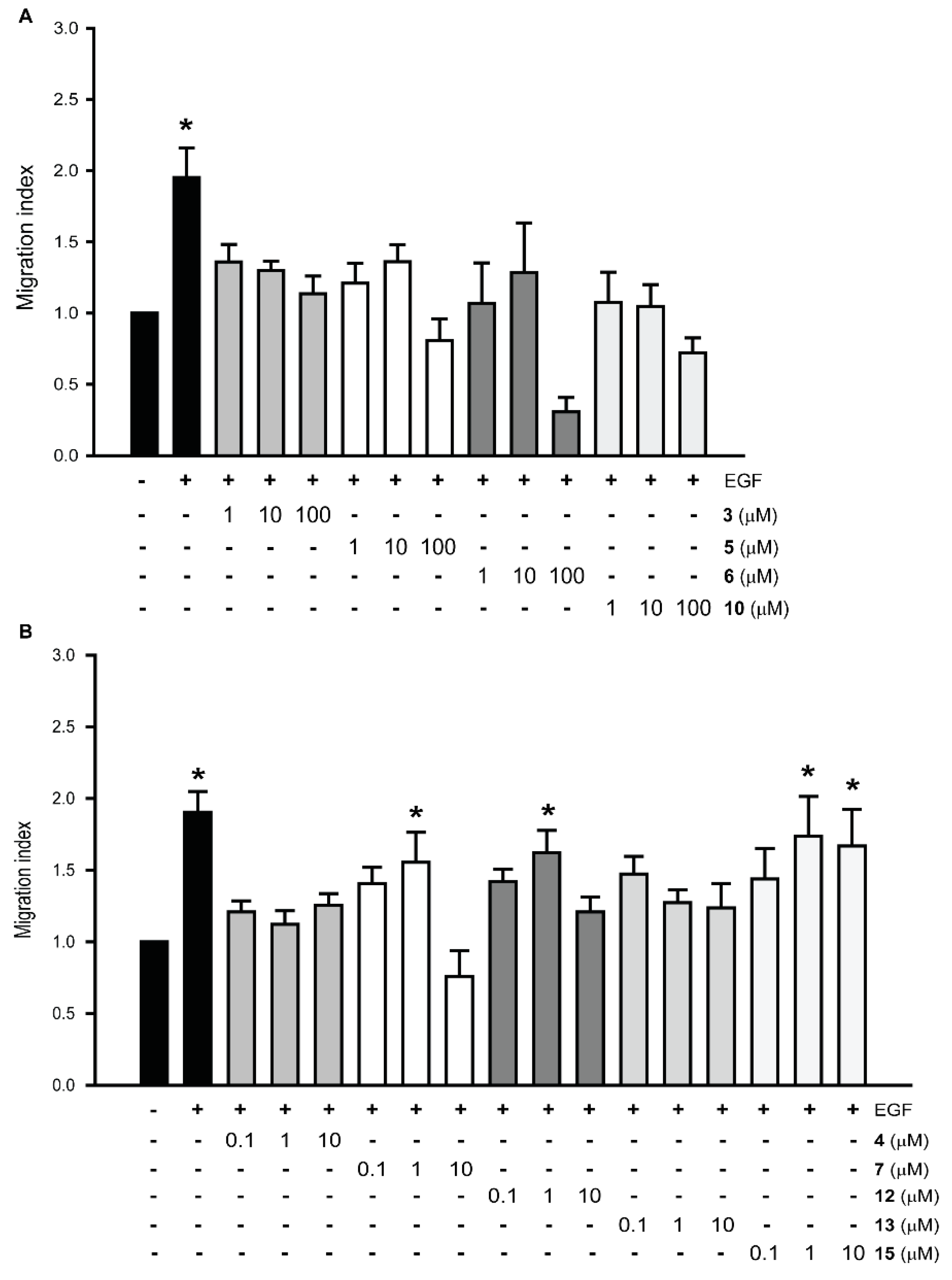
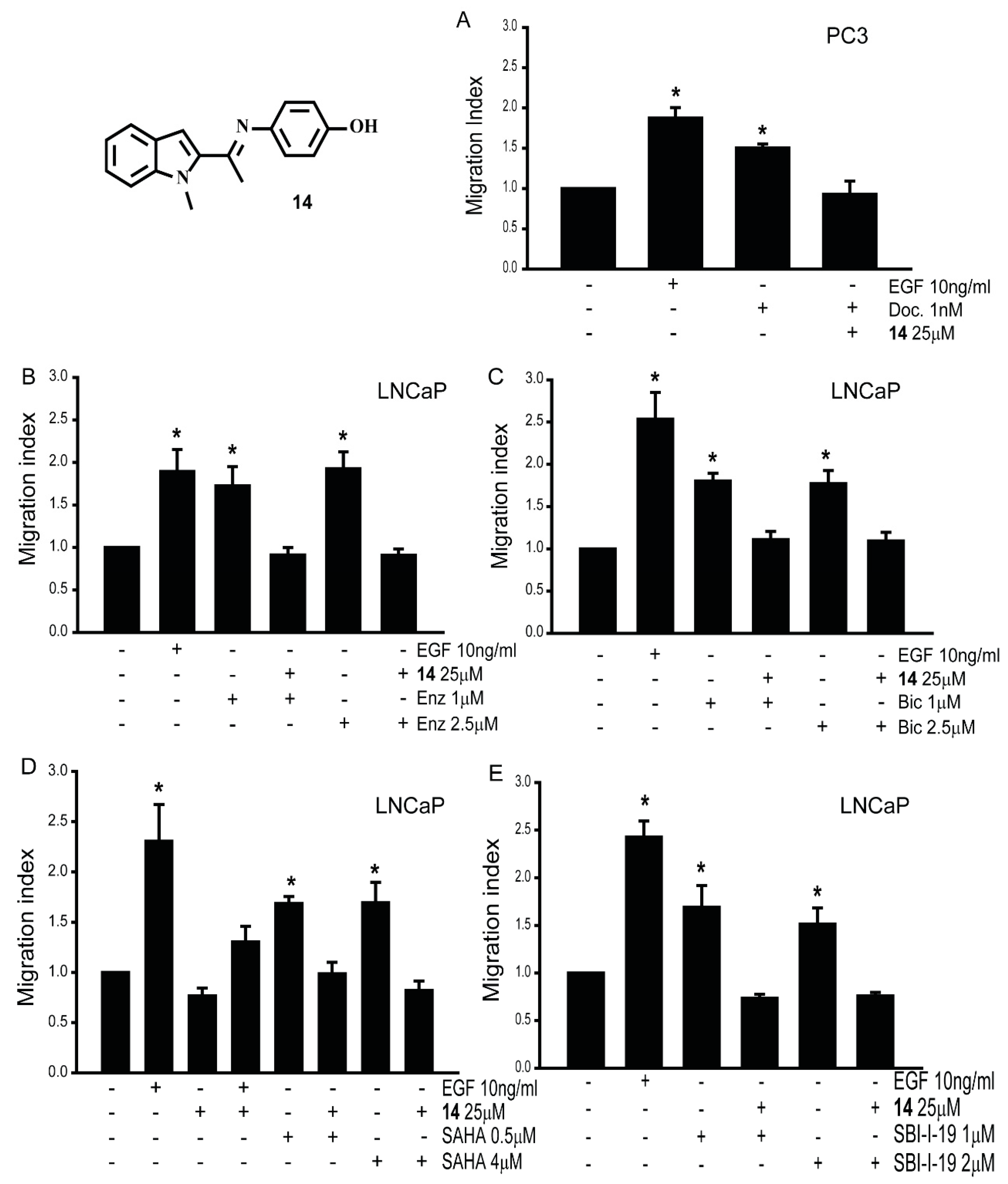
3.2. Effect of New Compounds on the Migration of Prostate Cancer Cell Lines
3.3. Evaluation of In Vitro Stability of Representative Compounds
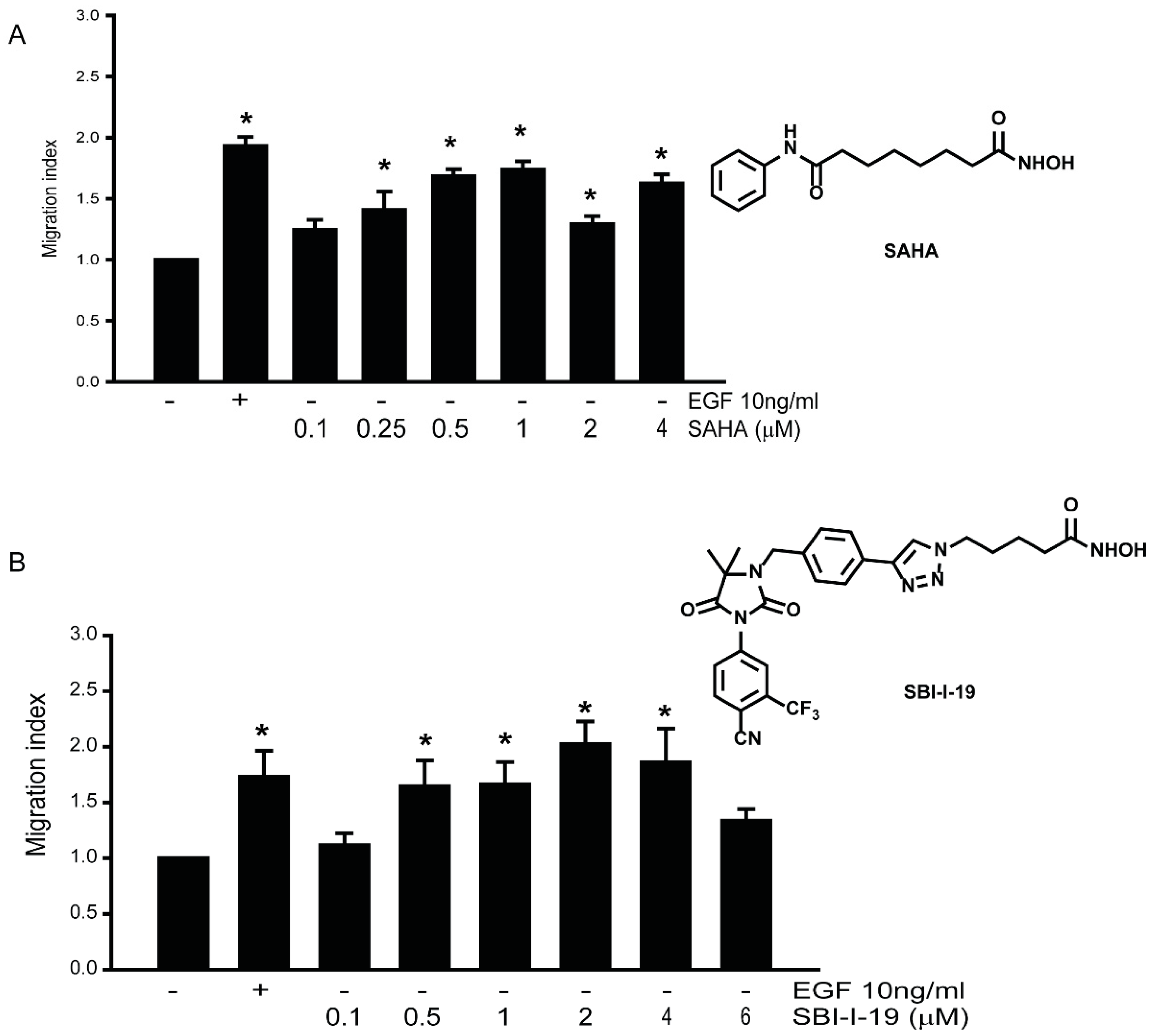
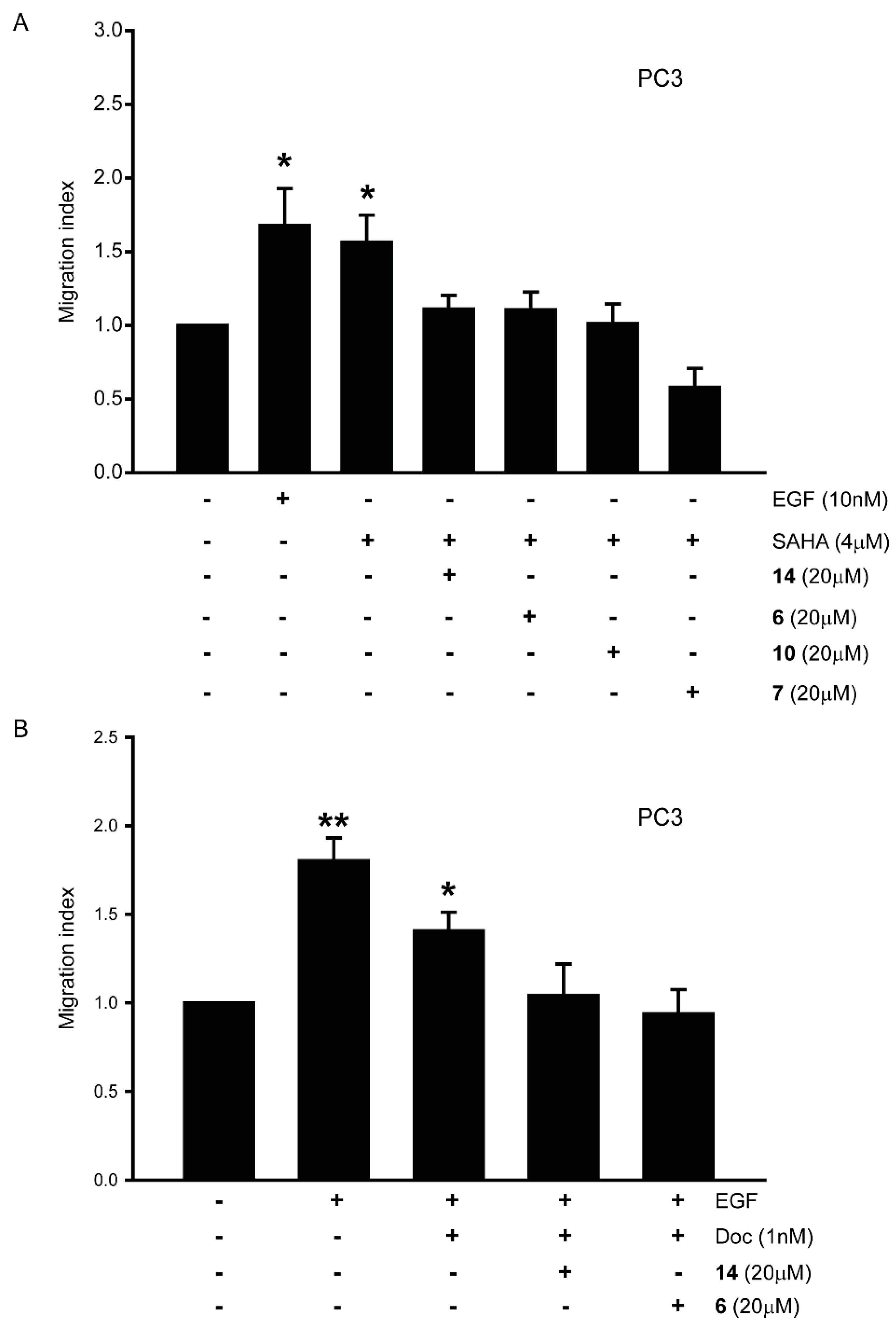
3.4. HDACi Induce Cell Migration in LNCaP Prostate Cancer Cell Lines
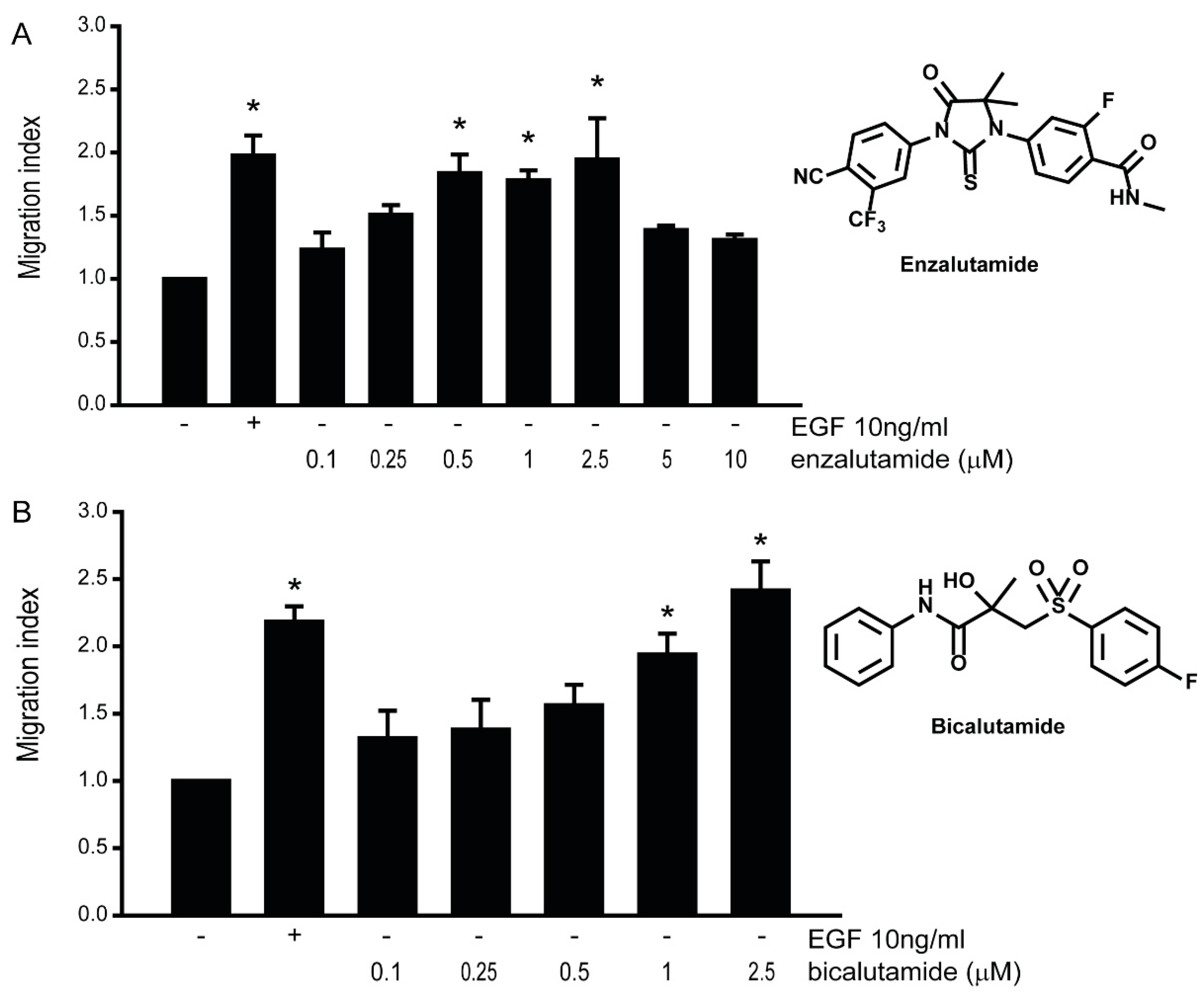
3.5. Anti-Androgens Induce Cell Migration in AR-Positive LNCaP Prostate Cancer Cells
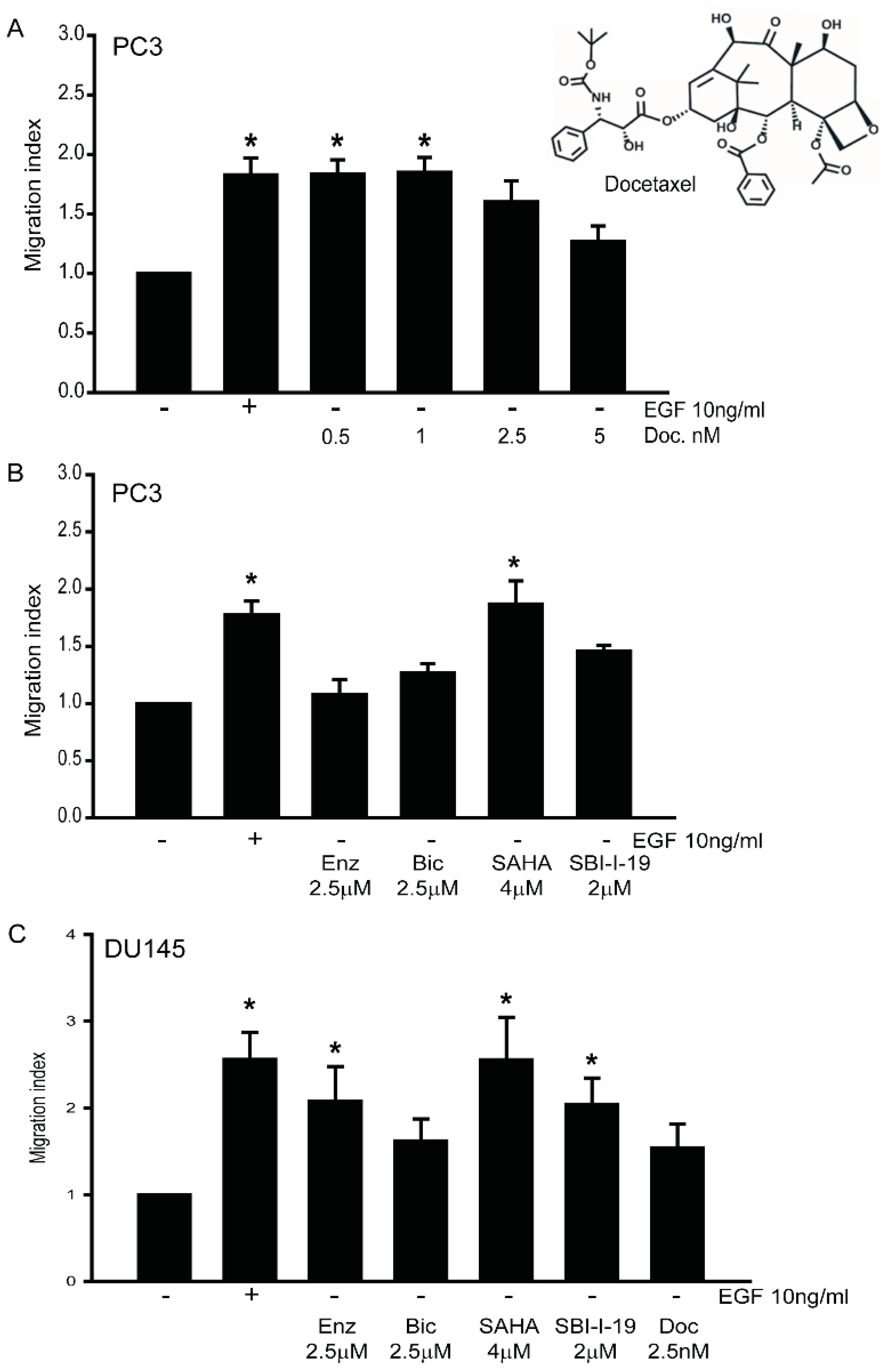
3.6. Effects of Chemotherapy on Cancer Cell Migration in AR-Negative DU145 and PC3 Cells
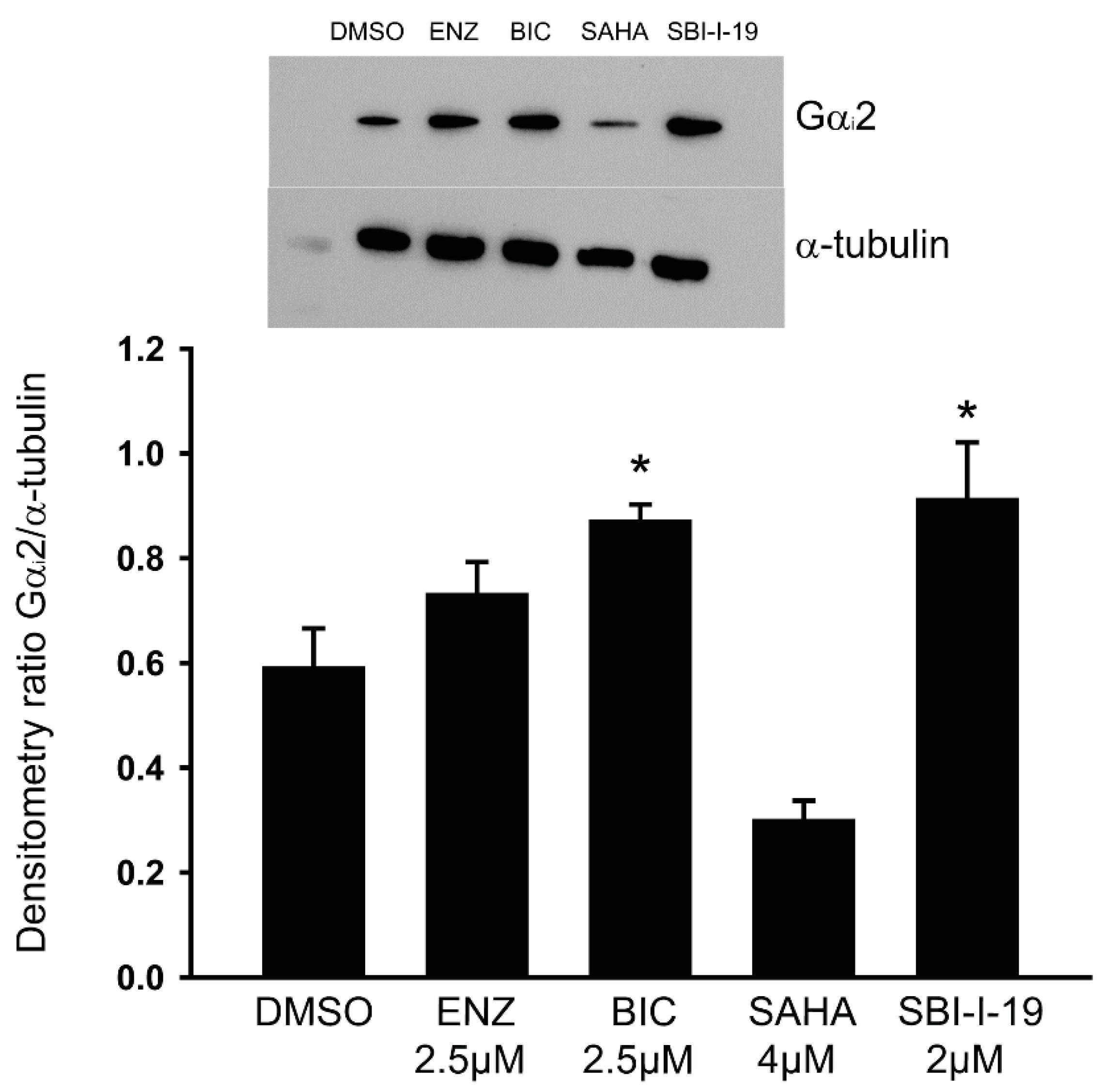
3.7. Anti-Androgens Upregulate the Expression of Gαi2 Protein in LNCaP Cells
3.8. Gαi2 Inhibitors Block the Effects of the Chemotherapeutic Drugs on Prostate Cancer Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanharanta, S.; Massague, J. Origins of metastatic traits. Cancer Cell 2013, 24, 410–421. [Google Scholar] [CrossRef]
- Berx, G.; Raspe, E.; Christofori, G.; Thiery, J.P.; Sleeman, J.P. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin. Exp. Metastasis 2007, 24, 587–597. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. Metastatic colonization: Settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin. Cancer Biol. 2011, 21, 99–106. [Google Scholar] [CrossRef]
- Roussos, E.T.; Condeelis, J.S.; Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer 2011, 11, 573–587. [Google Scholar] [CrossRef]
- Schaks, M.; Giannone, G.; Rottner, K. Actin dynamics in cell migration. Essays Biochem. 2019, 63, 483–495. [Google Scholar]
- Zhong, M.; Clarke, S.; Vo, B.T.; Khan, S.A. The essential role of Gialpha2 in prostate cancer cell migration. Mol. Cancer Res. 2012, 10, 1380–1388. [Google Scholar] [CrossRef]
- Caggia, S.; Chunduri, H.; Millena, A.C.; Perkins, J.N.; Venugopal, S.V.; Vo, B.T.; Li, C.; Tu, Y.; Khan, S.A. Novel role of Gialpha2 in cell migration: Downstream of PI3-kinase-AKT and Rac1 in prostate cancer cells. J. Cell. Physiol. 2018, 234, 802–815. [Google Scholar] [CrossRef]
- Caggia, S.; Tapadar, S.; Wu, B.; Venugopal, S.V.; Garrett, A.S.; Kumar, A.; Stiffend, J.S.; Davis, J.S.; Oyelere, A.K.; Khan, S.A. Small Molecule Inhibitors Targeting Gαi2 Protein Attenuate Migration of Cancer Cells. Cancers 2020, 12, 1631. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Manna, F.; Karkampouna, S.; Zoni, E.; De Menna, M.; Hensel, J.; Thalmann, G.N.; Kruithof-de Julio, M. Metastases in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2019, 9, a033688. [Google Scholar] [CrossRef]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. S1), S3–S8. [Google Scholar]
- Huang, J.; Lin, B.; Li, B. Anti-Androgen Receptor Therapies in Prostate Cancer: A Brief Update and Perspective. Front. Oncol. 2022, 12, 865350. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Mout, L.; Moll, J.M.; Chen, M.; de Morree, E.S.; de Ridder, C.M.A.; Gibson, A.; Stuurman, D.; Aghai, A.; Erkens-Schulze, S.; Mathijssen, R.H.J.; et al. Androgen receptor signalling impairs docetaxel efficacy in castration-resistant prostate cancer. Br. J. Cancer 2020, 123, 1715–1719. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, Y.S.; Kummar, S.; Giaccone, G.; Trepel, J.B. Histone deacetylase inhibitors in cancer therapy. Curr. Opin. Oncol. 2008, 20, 639–649. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Gryder, B.E.; Austin, L.A.; Tene Defo, B.A.; Hayden, S.C.; Pi, M.; Quarles, L.D.; Oyelere, A.K.; El-Sayed, M.A. Antiandrogen gold nanoparticles dual-target and overcome treatment resistance in hormone-insensitive prostate cancer cells. Bioconjug. Chem. 2012, 23, 1507–1512. [Google Scholar] [CrossRef]
- Gryder, B.E.; Akbashev, M.J.; Rood, M.K.; Raftery, E.D.; Meyers, W.M.; Dillard, P.; Khan, S.; Oyelere, A.K. Selectively targeting prostate cancer with antiandrogen equipped histone deacetylase inhibitors. ACS Chem. Biol. 2013, 8, 2550–2560. [Google Scholar] [CrossRef][Green Version]
- Chandrasekaran, B.; Tapadar, S.; Wu, B.; Saran, U.; Tyagi, A. Antiandrogen-Equipped Histone Deacetylase Inhibitors Selectively Inhibit Androgen Receptor (AR) and AR-Splice Variant (AR-SV) in Castration-Resistant Prostate Cancer (CRPC). Cancers 2023, 15, 1769. [Google Scholar] [CrossRef]
- Deng, G.; Wang, R.; Sun, Y.; Huang, C.P.; Yeh, S.; You, B.; Feng, C.; Li, G.; Ma, S.; Chang, C. Targeting androgen receptor (AR) with antiandrogen Enzalutamide increases prostate cancer cell invasion yet decreases bladder cancer cell invasion via differentially altering the AR/circRNA-ARC1/miR-125b-2-3p or miR-4736/PPARgamma/MMP-9 signals. Cell Death Differ. 2021, 28, 2145–2159. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.G.; Houthuijzen, J.M.; Cirkel, G.A.; Roodhart, J.M.; Shaked, Y.; Voest, E.E. Treatment-induced host-mediated mechanisms reducing the efficacy of antitumor therapies. Oncogene 2014, 33, 1341–1347. [Google Scholar] [CrossRef]
- Daenen, L.G.; Roodhart, J.M.; van Amersfoort, M.; Dehnad, M.; Roessingh, W.; Ulfman, L.H.; Derksen, P.W.; Voest, E.E. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011, 71, 6976–6985. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-Induced Metastasis: Molecular Mechanisms, Clinical Manifestations, Therapeutic Interventions. Cancer Res. 2019, 79, 4567–4576. [Google Scholar] [CrossRef]
- Liang, Y.; McDonnell, S.; Clynes, M. Examining the relationship between cancer invasion/metastasis and drug resistance. Curr. Cancer Drug Targets 2002, 2, 257–277. [Google Scholar] [CrossRef]
- Norouzi, S.; Gorgi Valokala, M.; Mosaffa, F.; Zirak, M.R.; Zamani, P.; Behravan, J. Crosstalk in cancer resistance and metastasis. Crit. Rev. Oncol. Hematol. 2018, 132, 145–153. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Mohamed, S.G.; Al-Hamdani, A.A.S.; Mahendradhany, A.P.; Ko, Y.G. Acyclic and cyclic imines and their metal complexes: Recent progress in biomaterials and corrosion applications. RSC Adv. 2018, 8, 23294–23318. [Google Scholar] [CrossRef]
- Kumar, J.; Rai, A.; Raj, V. A comprehensive review on pharmacological activity of schiff base containing derivatives. Org. Med. Chem. 2017, 1, 555564. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Manhas, M.S.; Hoffman, W.H.; Lal, B.; Bose, A.K. Steroids. Part X. A convenient synthesis of alkyl aryl ethers. J. Chem. Soc. Perkin Trans. 1 1975, 5, 461–463. [Google Scholar] [CrossRef]
- Kupwade, R.V.; Khot, S.S.; Lad, U.P.; Desai, U.V.; Wadgaonkar, P.P. Catalyst-free oxidation of sulfides to sulfoxides and diethylamine catalyzed oxidation of sulfides to sulfones using Oxone as an oxidant. Res. Chem. Intermed. 2017, 43, 6875–6888. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Liu, M.; Liby, T.; Bayani, N.; Bucher, E.; Chiotti, K.; Derrick, D.; Chauchereau, A.; Heiser, L.; Alumkal, J.; et al. Enzalutamide response in a panel of prostate cancer cell lines reveals a role for glucocorticoid receptor in enzalutamide resistant disease. Sci. Rep. 2020, 10, 21750. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Miller, D.S.; Blessing, J.A.; Krasner, C.N.; Mannel, R.S.; Hanjani, P.; Pearl, M.L.; Waggoner, S.E.; Boardman, C.H. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: A study of the Gynecologic Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Holen, K.D.; Saltz, L.B. New therapies, new directions: Advances in the systemic treatment of metastatic colorectal cancer. Lancet Oncol. 2001, 2, 290–297. [Google Scholar] [CrossRef]
- Fontebasso, Y.; Dubinett, S.M. Drug Development for Metastasis Prevention. Crit. Rev. Oncog. 2015, 20, 449–473. [Google Scholar] [CrossRef]
- Ji, M.; Lee, E.J.; Kim, K.B.; Kim, Y.; Sung, R.; Lee, S.J.; Kim, D.S.; Park, S.M. HDAC inhibitors induce epithelial-mesenchymal transition in colon carcinoma cells. Oncol. Rep. 2015, 33, 2299–2308. [Google Scholar] [CrossRef]
- Diaz-Nunez, M.; Diez-Torre, A.; De Wever, O.; Andrade, R.; Arluzea, J.; Silio, M.; Arechaga, J. Histone deacetylase inhibitors induce invasion of human melanoma cells in vitro via differential regulation of N-cadherin expression and RhoA activity. BMC Cancer 2016, 16, 667. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Wang, X.; Xu, C.; Zhang, N.; Di, W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020, 472, 59–69. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Hemann, M.T. Chemotherapeutic resistance: Surviving stressful situations. Cancer Res. 2011, 71, 5062–5066. [Google Scholar] [CrossRef]
- Shiao, S.L.; Ganesan, A.P.; Rugo, H.S.; Coussens, L.M. Immune microenvironments in solid tumors: New targets for therapy. Genes Dev. 2011, 25, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.D.; Stover, D.G.; Hai, T. Chemotherapy-Exacerbated Breast Cancer Metastasis: A Paradox Explainable by Dysregulated Adaptive-Response. Int. J. Mol. Sci. 2018, 19, 3333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coleman, M.; Brekken, R.A. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Filippi, I.; Morena, E.; Aldinucci, C.; Carraro, F.; Sozzani, S.; Naldini, A. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1alpha and PI3K/Akt pathway. J. Cell. Physiol. 2014, 229, 2067–2076. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
| Compound | Plasma (t1/2 = Min) | Microsome (t1/2 = Min) | ||
|---|---|---|---|---|
| Human | Mouse | Human | Mouse | |
| 6 | >120 | 27 | 118 | 47 |
| 7 | >120 | >120 | 45 | <10 |
| 10 | >120 | >120 | 35 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caggia, S.; Johnston, A.; Walunj, D.T.; Moore, A.R.; Peer, B.H.; Everett, R.W.; Oyelere, A.K.; Khan, S.A. Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration. Cancers 2024, 16, 296. https://doi.org/10.3390/cancers16020296
Caggia S, Johnston A, Walunj DT, Moore AR, Peer BH, Everett RW, Oyelere AK, Khan SA. Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration. Cancers. 2024; 16(2):296. https://doi.org/10.3390/cancers16020296
Chicago/Turabian StyleCaggia, Silvia, Alexis Johnston, Dipak T. Walunj, Aanya R. Moore, Benjamin H. Peer, Ravyn W. Everett, Adegboyega K. Oyelere, and Shafiq A. Khan. 2024. "Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration" Cancers 16, no. 2: 296. https://doi.org/10.3390/cancers16020296
APA StyleCaggia, S., Johnston, A., Walunj, D. T., Moore, A. R., Peer, B. H., Everett, R. W., Oyelere, A. K., & Khan, S. A. (2024). Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration. Cancers, 16(2), 296. https://doi.org/10.3390/cancers16020296








