Simple Summary
Everolimus is an oral drug used in patients with advanced hormone receptor positive, HER2 negative breast cancer. In this study based on a national French real-world cohort of more than 22,000 patients, we sought to evaluate the impact of everolimus on overall survival. Using statistical methods fit for real-world data, our findings suggest that the use of everolimus may favorably impact overall survival, and that it is very likely underused in this common clinical setting.
Abstract
Everolimus is the first oral targeted therapy widely used in advanced HR+/HER2− breast cancer. We sought to evaluate the impact of everolimus-based therapy on overall survival in the ESME-MBC database, a national metastatic breast cancer cohort that collects retrospective data using clinical trial-like methodology including quality assessments. We compared 1693 patients having received everolimus to 5928 patients not exposed to everolimus in the same period. Overall survival was evaluated according to treatment line, and a propensity score with the inverse probability of treatment weighting method was built to adjust for differences between groups. Crude and landmark overall survival analyses were all compatible with a benefit from everolimus-based therapy. Adjusted hazard ratios for overall survival were 0.34 (95% CI: 0.16–0.72, p = 0.0054), 0.34 (95% CI: 0.22–0.52, p < 0.0001), and 0.23 (95% CI: 0.14–0.36, p < 0.0001) for patients treated with everolimus in line 1, 2, and 3 and beyond, respectively. No clinically relevant benefit on progression-free survival was observed. Causes for everolimus discontinuation were progressive disease (56.2%), adverse events (27.7%), and other miscellaneous reasons. Despite the limitations inherent to such retrospective studies, these results suggest that adding everolimus-based therapy to the therapeutic sequences in patients with advanced HR+/HER2− breast cancer may favorably affect overall survival.
1. Introduction
Breast cancer is the second most common cancer worldwide and the most frequent cancer in women [1]. About 70% of breast cancers are hormone receptor positive (HR+) and HER2 negative (HER2−). In patients with advanced HR+/HER2− breast cancer, past and current guidelines strongly recommend endocrine-based treatments unless there is a “visceral crisis”. In addition, European (ABC5) and American (NCCN) recommendations advise exhausting endocrine therapy lines before chemotherapy, again except in cases of rapid progression or endocrine resistance as defined by disease progression in the first 6 months of endocrine therapy for advanced disease [2,3]. However, all patients eventually suffer from progressive disease, and in order to circumvent endocrine resistance, many targeted therapies have been developed. Everolimus, an mTOR inhibitor, was the first targeted therapy to obtain its Marketing Authorization in France (July 2012), for the treatment of patients with advanced HR+ breast cancers resistant to nonsteroidal aromatase inhibitors based on the pivotal Bolero-2 trial [4], and the drug was reimbursed in October 2014. The clinical results were confirmed by many real-world cohort studies across many countries [5,6,7,8,9], all showing a median progression-free survival of 8–9 months in patients with endocrine-resistant metastatic breast cancer (mBC). Most interestingly, the benefit in progression-free survival with everolimus-based therapy appears highly conserved across treatment lines, underlining the consistent efficacy of everolimus in patients with endocrine resistant mBC, and thus suggesting a potential favorable effect on overall survival, however not demonstrated in the Bolero-2 trial.
In 2014, the UNICANCER group (including the 18 French Comprehensive Cancer Centers, which care for over one third of all patients with breast cancer nationwide) launched the Epidemiological Strategy and Medical Economics (ESME) academic initiative in order to investigate real-world data in oncology [10]. Real-world data give the opportunity to retrospectively assess the activity of specific drugs outside clinical trials [11]. Based on this large real-life cohort, the first global results of endocrine therapy sequences have been reported, demonstrating the absence of improvement in overall survival in advanced HR+/HER2− breast cancer [12] while underlining the underuse of endocrine-based therapies in this common clinical setting [13]. These first analyses of the ESME cohort, however, did not specifically describe the evolution of the patients who received everolimus, nor specifically explored the impact on overall survival of specific therapies. We focused the present research on patients treated with an everolimus-based combination. The incidence and context of use of everolimus were presented in an early report [14], suggesting that everolimus was used at some point in less than 20% of patients with HR+/HER2− mBC, mostly with fewer visceral metastasis, mainly in advanced treatment lines, and almost exclusively after official approval by French regulatory authorities. We report here updated data with long-term overall survival analyses, focusing on patients treated from 2012 onwards. The objectives of this study were to describe the impact of everolimus on overall survival and progression-free survival according to treatment line, and to evaluate its positioning in the therapeutic strategy in a real-life setting.
2. Materials and Methods
2.1. Study Design and Data Source
We conducted a noninterventional, retrospective study to describe the outcome of patients with HR+/HER2− MBC treated with everolimus, selected in the ESME-MBC database. The ESME-MBC database is a national metastatic breast cancer cohort that collects retrospective data using clinical trial-like methodology, including quality assessments. The ESME-MBC database was built from existing information systems, treatment databases, and patients’ electronic medical records, with homogenous onsite-collected information and high-level quality control. The whole methodology was previously extensively detailed in [10].
The present analyses were approved by the Institutional Review Boards of participating institutions. Per French regulations, no formal dedicated informed consent was required, but all patients had approved the use of their electronically recorded data. The ESME analyses were approved by an independent Ethics Committee (Comité De Protection Des Personnes Sud-Est II-2015-79). In compliance with French regulations, the ESME-MBC database was authorized by the French data protection authority and managed by R&D UNICANCER in accordance with the current best practice guidelines [10,12].
2.2. Study Population
All consecutive women and men over 18 years diagnosed with HR+ HER2− metastatic breast cancer between January 2012 and December 2017 in the 18 French comprehensive cancer centers were selected (n = 7825). Among them, 1897 received at least one dose of everolimus (everolimus) at some point in their therapeutic sequence (study population), 1693 patients were evaluable for successive lines of treatment, and 5928 patients never received everolimus during the course of metastatic disease (comparative population).
2.3. Evaluation Criteria
The primary endpoint was overall survival (OS) in patients who received everolimus. Secondary endpoints were the impact of everolimus on overall survival and progression-free survival (PFS) in relation to treatment line (line 1 (L1), line 2 (L2), or line 3 and more (L3+)), the description of patient characteristics at metastatic diagnosis and at the initiation of each treatment line, the position of everolimus in the therapeutic strategy (L1, L2, L3+) and in relation to CDK4/6 inhibitors after 2016 (date of marketing authorization in France), and the description and quantification of the causes of treatment discontinuation.
2.4. Statistical Considerations
Descriptive statistics were used to summarize patient characteristics at diagnosis of metastatic disease, and at time of the start of metastatic treatment line (L1, L2, and L3+). Comparisons between everolimus or noneverolimus groups were performed using a chi-square or Fisher’s exact test for categorical data and t-test or nonparametric Wilcoxon test for continuous data; a p value < 0.05 was considered statistically significant.
Overall survival was defined as the time between the diagnosis of metastatic disease and the date of death (from any cause) or censored to the date of latest news. Progression-free survival was defined as the time from the starting date of treatment until the disease progression or death or the date of latest news. Progression was defined as any of the following events: local/locoregional relapse, progression of known metastases, new metastatic sites, death. A line was defined as a treatment change at least one month after initiation and/or after disease progression. Due to the definition of treatment lines by the ESME team, some patients were not classifiable in lines. Therefore, a treatment initiation more than 12 months after progression was not considered, which explains the final number of patients of 1693.
Both OS and PFS were estimated using the Kaplan–Meier method. The reverse Kaplan–Meier method was used to estimate the median follow-up durations. Hazard ratios are presented with a 95% CI. The landmark approach was used to limit the immortality bias for the analyses of overall survival. We built a propensity score to adjust for differences between groups for specific analyses of OS and PFS according to treatment line, as baseline characteristics of both populations at the initiation of each line could differ according to the chosen treatment. This method reduces biases in the estimation of treatment effects associated with nonrandom observational data (prespecified prognostic factors) and is useful for observational studies in which baseline characteristics differ and when the number of characteristics or potential confounders is relatively large [15,16]. We used the inverse probability of treatment weighting (IPTW) method with stabilized weights on OS [17]. The selected variables in relation to survival outcomes and allocation of everolimus treatment were gender time interval between primary diagnosis and metastatic relapse (de novo metastatic versus <2 years versus >2 years), recurrence (no recurrence versus local recurrence versus loco-regional recurrence), the modality of diagnosis of metastatic disease (systematic examination symptoms), SBR grade (I versus II versus III versus undetermined/not available), age at the initiation of the studied line (<52 years versus ≥52 years), number of metastatic sites at the initiation of the studied line (<3 versus ≥3), and type of metastatic sites at the initiation of the studied line (brain visceral versus non brain visceral versus nonvisceral). Specifically, the logistic model with all covariables gives a propensity score π. For a patient with treatment (everolimus) the weight is , and for a patient without treatment the weight is . In order to preserve the sample size of the original data, we stabilized weights by using a logistic model without covariable [17]. We ultimately obtained ᴘ, which is the probability of treatment by not taking account of a given covariable. Finally, for a patient with treatment (everolimus) the stabilized weight is , while for a patient without treatment (everolimus) the stabilized weight is . To evaluate the model, we used the Harrell’s C index and a graphic representation of overlapping scores by plotting the kernel density estimate (KDE) of the residuals corresponding to the regression of each component of x on β⊺ x grouping by the response y. Models with good fit result in plots in which the KDE curves for different values of y are similar in shape and location (see Figure S1 in the Supplementary Material section). As the log-rank test is inadequate when propensity score weight is taken into account, a robust variance estimator in the Cox model was used.
3. Results
3.1. Patient Characteristics
Of the 23,698 patients in the ESME-MBC database, 7825 with positive hormone receptor and negative HER2 were diagnosed after 2012, including 1897 who received at least one dose of everolimus. Of these, 1693 patients had identifiable successive lines of treatment (Figure 1, and Table 1, Table 2, Table 3 and Table 4).
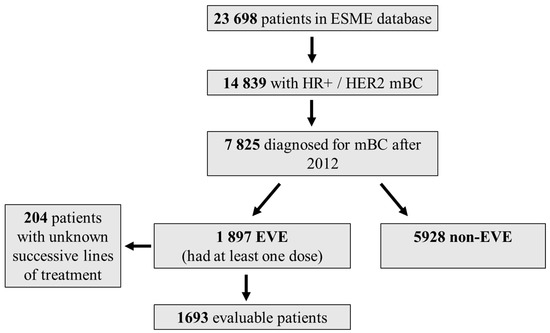

Table 1.
Patient characteristics at onset of metastatic disease, according to everolimus exposure.

Table 2.
Patient characteristics at the initiation of line 1.

Table 3.
Patient characteristics at the initiation of line 2.

Table 4.
Patient characteristics at the initiation of line 3 and beyond.
Median age at metastatic diagnosis was 63 years (22–03). Patients having received everolimus were slightly younger than noneverolimus patients (25.9% and 23.7% under 52 years, respectively, p = 0.057). Everolimus-treated patients had more frequent nonvisceral metastases (52.3% vs. 47.9%, p < 0.0001) and bone-only metastases (38.5% vs. 31.3%, p < 0.0001) at metastatic diagnosis, and less frequent clinical symptoms (42.4% vs. 47.5%, p = 0.0001) than noneverolimus patients. The everolimus–exemestane regimen was the most frequently used endocrine therapy combination (94.3%). Among the 7825 patients, 1897 (24.2%) received at least one dose of everolimus. In the first line setting population, 4.2% received everolimus, 17.9% in the L2 setting population, and 21.4% in the L3+ setting population. Patients who received everolimus as a first treatment line were slightly older (83.6% over 52 years in the everolimus group vs. 76.6% in the noneverolimus group, p = 0.01), as well as when everolimus was prescribed in L2 (81.2% over 52 years vs. 76.5%, p = 0.005). The age differences between groups was not statistically significant for the L3+ population. Among patients who received everolimus, when prescribed in an L1 setting, 51.6% had nonvisceral metastasis, 35.1% in an L2 setting, and 26.6% in an L3+ setting. Bone-only metastasis at the initiation of everolimus was observed in 39.2%, 24.5%, and 16.9% of patients in L1, L2, and L3+, respectively. Overall, everolimus patients had less frequent additional nonvisceral metastases compared to noneverolimus patients (52.3% nonvisceral metastasis vs. 47.9%, respectively, p < 0.0001). In the L3+ population, everolimus patients had less frequent additional nonvisceral metastases compared to noneverolimus patients (26.6% nonvisceral metastasis vs. 17.1%, respectively, p < 0.0001) and more frequent bone-only metastasis (16.9% vs. 9.3 respectively, p < 0.0001)
3.2. Overall Survival
Median follow-up was 47.9 months (0–98.7) and 61.4 months (2.1–98.7) for the overall and everolimus-treated populations, respectively. Median OS in the overall population was 46.8 months (95% CI, 45.5–47.9). Crude and landmark (6 and 12 months) OS analyses all suggested a benefit from everolimus-based therapy (all p values < 0.0001). For the everolimus population, the crude HR for overall survival was 0.68 (95% CI: 0.63–0.72) when compared to patients who had not been exposed to everolimus (Figure 2).
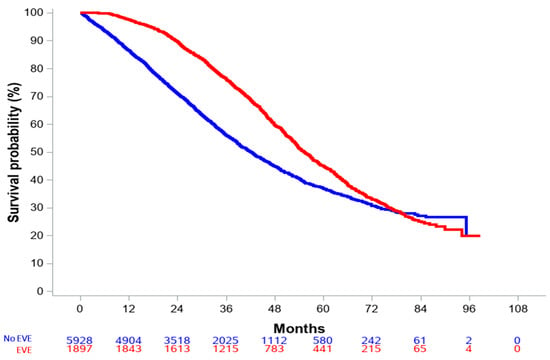
Figure 2.
Overall survival in the entire population, according to everolimus exposure.
The 6-month and 12-landmark OS analyses are shown in Figure 3. For patients with at least a 6-month or 12-month follow-up, 6-month and 12-month HR were 0.74 (95% CI: 0.69–0.80, Figure 3A) and 0.81 (95% CI: 0.75–0.88, Figure 3B), respectively.
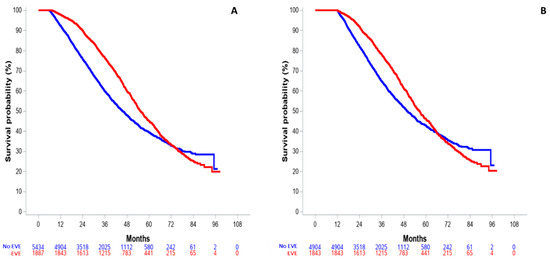
Figure 3.
Landmark analysis of overall survival in the entire population, according to minimal follow-up. (A) 6-month landmark OS. (B) 12-month landmark OS.
To account for imbalance between the everolimus- and non-everolimus-treated groups, we then focused our investigation on adjusted survival analyses, including lines of treatment as a key parameter. Overall, comparing everolimus-treated and non-everolimus-treated patients suggested a striking benefit on overall survival of everolimus-based therapy. Survival curves are presented in Figure 4.
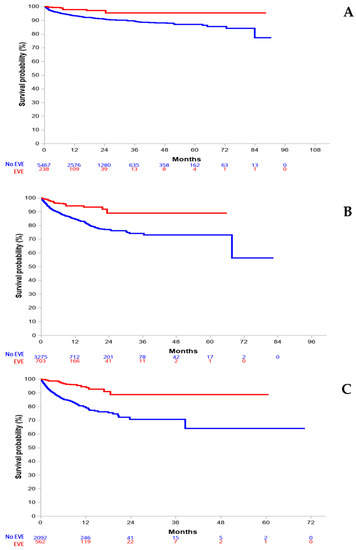
Figure 4.
IPTW-adjusted overall survival, according to line of treatment with everolimus. (A) first line; (B) second line; (C) third line and beyond.
Survival analyses based on IPTW demonstrated a consistent benefit on OS with everolimus treatment when administered in lines 1, 2, and 3+ with respective HR values of 0.34 (95% CI: 0.16–0.72, p = 0.0054), 0.34 (95% CI: 0.22–0.52, p < 0.0001), and 0.23 (95% CI: 0.14–0.36, p < 0.0001). Of note, Harrell’s C index always overlapped for the three analyses, allowing these adjusted analyses (Figure S1, Supplementary Material).
3.3. Progression-Free Survival
Evaluation of PFS according to everolimus-based therapy and line of treatment was an important secondary objective of the study. IPTW analyses suggested a longer progression-free survival with everolimus when administered in the L3+ setting (HR = 0.82 (95% CI: 0.75–0.90), p < 0.0001) (Figure S2, Supplementary Material). However, adjusted PFS for patients receiving everolimus either in line 1 or line 2 were not statistically significant (HR 0.99 (95% CI, 0.84–1.17), p = 0.92, and 1.02 (95% CI, 0.94–1.10), p = 0.69 respectively).
3.4. Treatment Landscape
We examined the landscape of treatment with everolimus in this unselected and large population of patients with advanced ER+/HER2− breast cancer. Causes of everolimus discontinuation were recorded in the 1897 patients having received at least one dose of everolimus. Expectedly, disease progression (54%) and adverse events (26.6%) were the two main causes of discontinuation of everolimus. Other reasons were physician’s choice (7%), patient’s choice (2.3%), and miscellaneous (6.1%). Of note, the median duration of everolimus treatment was remarkably stable across lines of treatment: 5.2 months (interquartile range, IQR, 2.4–10.8), 4.8 months (IQR 2.7–8.9), and 4.8 months (IQR 2.8–8.8) for L1, L2, and L3+, respectively.
We finally focused on the variation of everolimus prescription over time. The prescription rate of everolimus increased from 1.2% in 2012 (when access to the drug was made possible) to 19.5% in 2017 (Figure S3, see Supplementary Material). We observed a limited but steady increase in the proportion of patients receiving everolimus from 2012 to 2017, when CDK4/6 inhibitors became available and were entered into guidelines [3,18]. An exploratory analysis showed that 998 everolimus-treated patients (52.6%) also received a CDK4/6-inhibitor-based treatment, mostly after everolimus therapy (n = 826, 87%). Very interestingly, the median duration of CDK4/6 inhibitor therapy for those patients was 4.6 months (IQR 2.9–8.7).
4. Discussion
In this study, we harnessed the real-life data from the national ESME program in order to describe the survival outcomes of patients with HR+/HER2− mBC and treated with an everolimus-based combination. We compared the outcomes of these patients to a contemporary population of patients with advanced HR+/HER2− breast cancer, also included in the ESME database but not exposed to everolimus. A striking benefit in overall survival was observed for patients exposed to everolimus, particularly when treated in the second line or third line and beyond settings. In order to limit biases due to the numerical imbalance in some important prognostic parameters such as the metastatic profile at initiation of a line of treatment (Table 2, Table 3 and Table 4), we developed adjusted survival analyses based on a propensity score and adjustment based on inverse probability of treatment weighting. These techniques are widely used and recognized as powerful tools for the analysis of real-world cohorts [19,20], and the present report is the first to use such methods to evaluate the clinical utility of everolimus in a real-world setting, together with a very-long-term follow-up. We included many potential confounding factors such as gender, time interval between primary diagnosis and metastatic relapse, recurrence, metastatic disease diagnosis context, SBR grade, and age, number, and type of metastatic sites at the initiation of the line of interest. Very interestingly, IPTW-adjusted analyses for OS confirmed a benefit from the everolimus-based therapy when administered in lines 2 and 3. In the first line setting, the HR was 0.34 (95% CI, 0.16–0.72) with a p value of 0.054, a trend for benefit of borderline significance.
Furthermore, IPTW analyses suggested a significantly longer progression-free survival with everolimus when administered in an L3+ setting. More globally, it is striking to observe that the initial [13] and presently updated ESME real-word data are in line with the survival outcomes that were reported in the prospective trials, whether everolimus was combined with exemestane [4], tamoxifen [21], or fulvestrant [22]. The BOLERO-2 final PFS analysis showed a median PFS of 7.8 months at median follow up of 18 months in a nonsteroidal aromatase-inhibitor-resistant population with the everolimus–exemestane combination. As for the GINECO [21] and TREND [22] studies, the median PFS were 8.6 months and 7.4 months, respectively, in postmenopausal women with hormone receptor-positive, HER2-negative, aromatase-inhibitor-resistant mBC. Our data also confirm the clinically meaningful benefit from the everolimus-based therapy found in the BALLET study [6], among patients with advanced HR+/HER2− mBC.
We also looked at to how everolimus was used in the successive therapeutic sequences, including during the early phase of the CDK4/6 inhibitors era. It is somewhat startling to observe that, at the time when everolimus was the first and only approved targeted therapy in advanced HR+/HER2− breast cancer, it was prescribed at some point in the course of the disease in only 24.2% of all cases, and mainly in the L3+ setting. In line with the consistent PFS and OS results, the median duration of treatment with everolimus was very stable at about 5 months for each treatment line, including very-long-term responders in very advanced patients. Remarkably, all these results complement an earlier ESME report [12] that demonstrated that overall survival did not improve in the decade preceding the introduction of CDK4/6 inhibitors. Taken together, these data strongly suggest that everolimus is a valuable and underused drug in patients with advanced HR+/HER2− breast cancer. Lastly, it seems interesting to note that in patients who received both CDK4/6 inhibitors and an everolimus combination (mostly CDK4/6 inhibitors after everolimus at the time of the study), the median duration of therapy with CDK4/6 inhibitors was much shorter (4.8 months) than observed in the literature [23,24,25,26]. This might again strengthen the potential interest in positioning everolimus later in the disease course, as also suggested by recent reports [7,9,27]. This is in accordance with recommendations suggesting a prescription of everolimus from the second line and later during the course of the disease [18].
We are fully aware of the limitations of the present report. Our study is limited by its retrospective nature, and the lack of individual information on important clinical features. For instance, patient weight variations, performance status, or LDH levels at the time of metastatic disease diagnosis or at each treatment line appeared to be scarcely collected in electronic medical records and are consequently not exploitable. Furthermore, the potential toxicity of everolimus needs to be taken into account, as in this series the treatment was discontinued in 26.6% of cases owing to adverse events, although it might be mitigated by individual dose escalation [28]. Consequently, patient selection by oncologists, according to individual risk–benefit balance, may favor the fittest, which could have an influence on overall survival. Likewise, despite adjusted IPTW analyses, our findings seem to suggest that the patients who received everolimus had less severe diseases, which might also explain part of the benefit observed on overall survival. Nevertheless, the adjusted PFS analyses showed this same benefit, suggesting an obvious contribution of everolimus to survival outcomes. Finally, the clinical utility of everolimus in patients pretreated with CDK4/6 inhibitors remains to be thoroughly evaluated [29].
5. Conclusions
Taken together and despite the limitations of such retrospective real-world data, it is our belief that the present report brings important information on the role of everolimus in the management of patients with advanced HR+/HER2− breast cancer. The favorable outcomes we report here in a large real-life population suggest a strong benefit from everolimus-based therapy. These data support current guidelines and should prompt oncologists to consider including everolimus in the therapeutic strategy for patients with advanced HR+, HER2− breast cancer, particularly from the third line onwards, while the benefit–risk balance must be assessed on a case-by-case basis.
Supplementary Materials
The following are available online at www.mdpi.com/10.3390/cancers15041191/s1, Figure S1. Harrell’s C index kernel density plotting. A. Patients in L1. B. Patients in line 2. C. Patients in line 3 and beyond. OS: overall survival. Nonevero_ps: propensity score distribution of patients who did not receive everolimus (blue curve). Evero_ps: propensity score distribution of patients exposed to everolimus (red curve). Figure S2. Progression-free survival as per inverse probability of treatment weighting analysis. A. Patients in L1. B. Patients in line 2. C. Patients in line 3 and beyond. PFS: progression-free survival. No: patients who did not receive everolimus (blue curves). Yes: patients exposed to everolimus (red curves). Figure S3. Proportion of patients receiving everolimus in the advanced setting between 2012 and 2019.
Author Contributions
Conceptualization, H.F.-M., P.C., T.B. and S.C.; methodology, A.L.-C. and M.C.; validation, S.C. and P.C.; formal analysis, H.F.-M., A.L.-C., S.C. and P.C.; investigation, P.C.; resources, B.P., C.L., V.D., J.-S.F., S.G., M.-A.M.-R., A.M., J.-C.E., T.P., M.U., I.D., P.A., L.U., M.D., J.-M.F., F.C., A.G., M.C. and P.C.; data curation, H.F.-M., A.L.-C. and S.C.; writing—original draft preparation, H.F.-M., T.B., S.C. and P.C.; writing—review and editing, H.F.-M., T.B., S.C. and P.C.; supervision, M.C. and S.C.; project administration, M.C.; funding acquisition, M.C. All authors contributed to interpretation of the data, completion, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The ESME-MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai, and Daiichi Sankyo). Data collection, analysis, and publication are managed entirely by UNICANCER independently of the industrial consortium.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME local coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Group and Strategic Committee for their ongoing support. The 18 Participating French Comprehensive Cancer Centers (FCCC) are as follows: I. Curie, Paris/Saint-Cloud; G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes; C.F. Baclesse, Caen, ICM Montpellier; C.L. Bérard, Lyon; C.G.-F. Leclerc, Dijon; C.H. Becquerel, Rouen; I.C. Regaud, Toulouse; C.A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C.E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C.J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C.P. Strauss, Strasbourg; I.J. Godinot, Reims; C.O. Lambret, Lille.
Conflicts of Interest
JSF declares consulting fees from Pfizer, Lilly, Novartis, AstraZeneca, Clovis Oncology, GSK, Gilead, Daiichi Sankyo, Seagen, Exact Science; honoraria for lectures from Lilly, Novartis, Gilead, Daiichi Sankyo, Seagen; travel support and meeting attendance from Pfizer, Milly, Novartis, AstraZeneca, Clovis Oncology, GSK, Gilead, Daiichi Sankyo, Seagen. AG declares Institutional research funding from MSD, BMS, Novartis, Boehringer Ingelheim, Roche Genentech, AstraZeneca, Sanofi, and Daiichi Sankyo. PC declares consulting fees and honoraria from Pfizer, Roche, Lilly; Institutional research funding from Novartis, Pfizer; travel support and meeting attendance from Roche, Pfizer, and Daiichi Sankyo. All other authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Noguchi, S.; Pritchard, K.I.; Burris, H.A., III; Baselga, J.; Gnant, M.; Hortobagyi, G.N.; Campone, M.; Pistilli, B.; Piccart, M.; et al. Everolimus Plus Exemestane in Postmenopausal Patients with HR+ Breast Cancer: BOLERO-2 Final Progression-Free Survival Analysis. Adv. Ther. 2013, 30, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Chocteau-Bouju, D.; Chakiba, C.; Mignot, L.; Madranges, N.; Pierga, J.-Y.; Beuzeboc, P.; Quenel-Tueux, N.; Diéras, V.; Bonnefoi, H.; Debled, M.; et al. Efficacy and tolerance of everolimus in 123 consecutive advanced ER positive, HER2 negative breast cancer patients. A two center retrospective study. Breast 2015, 24, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Mariani, G.; Ciruelos, E.M.; Martin, M.; Tjan-Heijnen, V.C.G.; Neven, P.; Gavila, J.G.; Michelotti, A.; Montemurro, F.; Generali, D.; et al. Safety of everolimus plus exemestane in patients with hormone-receptor–positive, HER2–negative locally advanced or metastatic breast cancer progressing on prior non-steroidal aromatase inhibitors: Primary results of a phase IIIb, open-label, single-arm, expanded-access multicenter trial (BALLET). Ann. Oncol. 2016, 27, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Renna, C.E.; Moore, H.C.F.; Abraham, J.; Kruse, M.L.; Montero, A.J.; LeGrand, S.B.; Wang, L.; Budd, G.T. Real-World Outcomes of Everolimus and Exemestane for the Treatment of Metastatic Hormone Receptor-Positive Breast Cancer in Patients Previously Treated With CDK4/6 Inhibitors. Clin. Breast Cancer 2022, 22, 143–148. [Google Scholar] [CrossRef]
- Bilici, A.; Uysal, M.; Menekse, S.; Akin, S.; Yildiz, F.; Turan, M.; Goksu, S.S.; Beypinar, I.; Sakalar, T.; Değirmenci, M.; et al. Real-Life Analysis of Efficacy and Safety of Everolimus Plus Exemestane in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Metastatic Breast Cancer Patients: A Turkish Oncology Group (TOG) Study. Cancer Investig. 2021, 40, 199–209. [Google Scholar] [CrossRef]
- Rozenblit, M.; Mun, S.; Soulos, P.; Adelson, K.; Pusztai, L.; Mougalian, S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021, 23, 14. [Google Scholar] [CrossRef]
- Pérol, D.; Robain, M.; Arveux, P.; Mathoulin-Pélissier, S.; Chamorey, E.; Asselain, B.; Berchery, D.; Gourgou, S.; Breton, M.; Delaine-Clisant, S.; et al. The ongoing French metastatic breast cancer (MBC) cohort: The example-based methodology of the Epidemiological Strategy and Medical Economics (ESME). BMJ Open 2019, 9, e023568. [Google Scholar] [CrossRef]
- Cottu, P.; Ramsey, S.D.; Solà-Morales, O.; Spears, P.A.; Taylor, L. The emerging role of real-world data in advanced breast cancer therapy: Recommendations for collaborative decision-making. Breast 2021, 61, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Grinda, T.; Antoine, A.; Jacot, W.; Blaye, C.; Cottu, P.-H.; Diéras, V.; Dalenc, F.; Gonçalves, A.; Debled, M.; Patsouris, A.; et al. Evolution of overall survival and receipt of new therapies by subtype among 20,446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open 2021, 6, 100114. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Lardy-Cleaud, A.; Frank, S.; Debled, M.; Cottu, P.H.; Pistilli, B.; Vanlemmens, L.; Leheurteur, M.; Lévy, C.; Laborde, L.; et al. Assessment of the efficacy of successive endocrine therapies in hormone receptor–positive and HER2-negative metastatic breast cancer: A real-life multicentre national study. Eur. J. Cancer 2019, 118, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cottu, P.H.; Lardy-Cleaud, A.; Frank, S.; Le Saux, O.; Chabaud, S.; Parent, D.; Pistilli, B.; Debled, M.; Mailliez, A.; Veyret, C.; et al. Use of everolimus in advanced hormone receptor–positive metastatic breast cancer in a multicenter national observational study. J. Clin. Oncol. 2017, 35, e12548. [Google Scholar] [CrossRef]
- D’Agostino, R.B.J. Propensity Score Methods for Bias Reduction in the Comparison of a Treatment to a Non-Randomized Control Group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Franchetti, Y. Use of Propensity Scoring and Its Application to Real-World Data: Advantages, Disadvantages, and Methodological Objectives Explained to Researchers Without Using Mathematical Equations. J. Clin. Pharmacol. 2022, 62, 304–319. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2−metastatic breast cancer. npj Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized Phase II Trial of Everolimus in Combination with Tamoxifen in Patients with Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer with Prior Exposure to Aromatase Inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, S.; Romond, E.; Black, E.P.; Van Meter, E.; Shelton, B.; Kadamyan-Melkumian, V.; Stevens, M.; Elledge, R. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)-positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res. Treat. 2014, 143, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Rugo, H.S.; Im, S.-A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2− ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Jeong, H.; Jeong, J.H.; Kim, J.E.; Ahn, J.-H.; Jung, K.H.; Kim, S.-B. Comparison of the Effectiveness and Clinical Outcome of Everolimus Followed by CDK4/6 Inhibitors with the Opposite Treatment Sequence in Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Cancer Res. Treat. 2022, 54, 469–477. [Google Scholar] [CrossRef]
- Schmidt, M.; Lübbe, K.; Decker, T.; Thill, M.; Bauer, L.; Müller, V.; Link, T.; Furlanetto, J.; Reinisch, M.; Mundhenke, C.; et al. A multicentre, randomised, double-blind, phase II study to evaluate the tolerability of an induction dose escalation of everolimus in patients with metastatic breast cancer (DESIREE). ESMO Open 2022, 7, 100601. [Google Scholar] [CrossRef]
- Cook, M.M.; Al Rabadi, L.; Kaempf, A.J.; Saraceni, M.M.; Savin, M.A.; Mitri, Z.I. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy. Oncologist 2021, 26, 101–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).