Simple Summary
The sentinel lymph node is a surgical technique developed in oncological surgery to identify and analyze fewer lymph nodes than a conventional lymph node dissection in order to limit the morbidity and mortality of such an extensive procedure without compromising the patients’ outcomes. This concept seems to also be useful in radiation oncology that treats lymph node areas. This may help radiation oncologists to treat their patients more precisely by targeting more accurately pathological sites and sparing healthy tissues. The aim of this review is to highlight the feasibility and level of proof regarding the use of this technique for treatment planning in radiation oncology.
Abstract
The sentinel lymph node technique is minimally invasive and used routinely by surgeons, reducing the need for morbid extensive lymph node dissections, which is a significant advantage for cancer staging and treatment decisions. The sentinel lymph node could also help radiation oncologists to identify tumor drainage for each of their patients, leading to a more personalized radiotherapy, instead of a probabilistic irradiation based on delineation atlases. The aim is both to avoid recurrence in unexpected areas and to limit the volume of irradiated healthy tissues. The aim of our study is to evaluate the impact of sentinel lymph node mapping for radiation oncologists. This concept, relying on sentinel lymph node mapping for treatment planning, is known as lymph-flow-guided radiotherapy. We present an up-to-date narrative literature review showing the potential applications of the sentinel lymph node technique for radiotherapy, as well as the limits that need to be addressed before its routine usage.
1. Introduction
The lymphatic vasculature is a system draining the organs through vessels and lymph nodes that act as filters. Its complexity stems from the numerous anatomical variants described in studies during cadaver dissections or surgical explorations, but knowledge of these variations is becoming increasingly available [1]. The metastatic spreading of cancer was described in some models to start with the invasion of the first draining loco-regional nodes, which then became known as sentinel lymph nodes (SLNs) [2]. The idea of using these SLNs thus emerged, based on a sentinel lymph node mapping (SLNM) using colorimetric, fluorescent or radioisotope tracers to identify the area of drainage of an anatomical territory, followed by sentinel lymph node biopsies (SLNBs) to assess whether a node is pathological (pSLN) or not (nSLN) [3]. The concept of SLNs was a major breakthrough for surgeons, allowing for a decrease in the number of morbid extensive lymph node dissections (ELND). SLNB is a minimally invasive technique, used routinely in penile cancer, breast cancer and melanoma thanks to its reliable performance and proven safety, and plays a key role in cancer staging and treatment decisions [4,5].
In order to better personalize radiotherapy treatments, we hypothesized that SLNM and SLNB could be used to provide more personalized radiotherapy by identifying the drainage of each patient’s tumor, making it possible to not miss unexpected areas or to preserve healthy tissues. This concept is called “lymph-flow-guided radiotherapy” (LFGRT), and we aim to present a current review of the rather sparse literature exploring this hypothesis. We will discuss localizations of cancers for which the SLN has been evaluated in surgery and in which radiotherapy could play a role, both in well-established areas such as head and neck cancers, or areas currently under investigation such as renal cancers.
2. Breast Cancer
2.1. Axillary Lymph Node Dissection (ALND) Can Be Avoided Thanks to SLNB
In patients with nSLN, ALND can be avoided, as no difference was observed in overall and disease-free survival, or in axillary failure, which was low and reported in 0.7–0.8% of patients [6,7].
In patients with pSLN presenting a tumor smaller than 5 cm and no palpable adenopathy (cT1-T2 cN0), ALND could be avoided in patients with micrometastasis (<2 mm), since the IBCSG 23-01 trial showed no inferiority in disease-free survival after 10 years [8], as well as in patients with up to two macrometastasis but no capsular effraction, since the ACOSOG Z0011 (Alliance) trial showed no inferiority in overall survival after 10 years [9]. ALND was also proposed to be replaced by axillary radiotherapy, since the AMAROS and OTOASOR trials showed no difference in overall and disease-free survival between ALND and axillary radiotherapy [10,11]. A retrospective study compared 260 patients who received axillary radiotherapy versus those who did not and found no significant difference: 5-year overall survival was 93.4% versus 96.8% (p = 0.19), respectively, and 5-year disease-free survival was 92.3% versus 100% (p = 1.06), respectively [12].
A systematic review highlighted that ALND induced significantly more lymphedemas and shoulder dysfunctions in comparison with observation or axillary radiotherapy [13]. For most patients with nSLN or with pSLN (up to two metastasis) and cT1-T2 cN0 tumors, ALND should be avoided to decrease morbidity. Axillary radiotherapy is worth discussing in case of risk factors.
2.2. SLNM/SLNB Indicates Nodal Irradiation
Regional nodal irradiation, in addition to breast/chest wall irradiation, is currently indicated in case of clinical or pathological node involvement but deserves to be challenged. In fact, two phase III trials demonstrated that nodal irradiation (including axillary, infra/supraclavicular, internal mammary nodes) reduced breast cancer recurrence and specific mortality but did not significantly improve overall survival [14,15]. Moreover, a recent trial randomized 735 patients who received nodal irradiation, both including and excluding the internal mammary nodes, and found no benefit to irradiating this area, with the exception of a subgroup of patients with medial/central tumors [16], explained by their pattern of lymphatic drainage. Some authors suggested performing SLNM with the acquisition of SPECT/CT images to identify the drainage of each tumor, knowing that up to 50% of patients can present drainage in both axillary and internal mammary nodes, depending on the tumor’s location [17]. Figure 1 shows two examples of drainage in internal mammary nodes. However, in practice, only axillary nodes are noticed because they matter in surgery. Given recent findings, knowing the specific drainage of the cancer would help radiation oncologists delineate lymphatic areas, notably the internal mammary nodes, for relevant prophylactic irradiation [18].
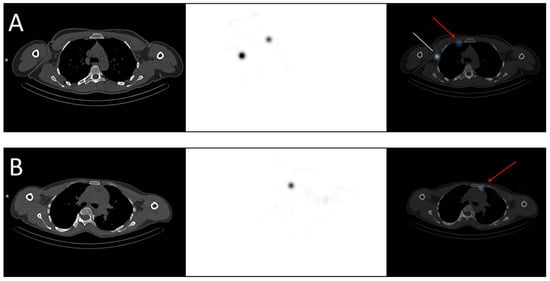
Figure 1.
Examples of breast cancer drainage in the internal mammary nodes visualized in SPECT-CT. (A) A 36-year-old woman was diagnosed with two malignant nodules in the right breast, localized in the inner quadrants. The sentinel lymph node mapping revealed drainage in both the axillary (green line) and the internal mammary nodes (red arrow). (B) A 40-year-old woman was diagnosed with a malignant nodule in the left breast, localized in the upper-inner quadrant. The sentinel lymph node mapping revealed drainage in the internal mammary nodes (red arrow).
While SLNM identifies lymphatic drainage of the breast tumor after peritumoral tracer injection, axillary reverse mapping (ARM) identifies drainage of the upper limb after arm injection. ARM was initially developed for surgeons to preserve the main nodes draining the arm and not the tumor during ALND 6, but ARM seemed to be applicable for axillary irradiation too [19]. A dosimetric evaluation pointed out that all the nodes identified by ARM received the prescribed dose during standard axillary radiotherapy, explaining the rate of arm lymphedema [20]. A pilot study showed the feasibility of combining SLNM and ARM to preserve the main nodes, draining the arm while conserving the good coverage of the SLN sites in 5/6 of the patients. In the remaining patient, it was not possible to preserve these nodes because the SLNM and ARM overlapped [21]. The next step is to conduct trials to evaluate the oncological outcomes and their impact on lymphedema when reducing axillary irradiation volumes.
2.3. The Role of SLNB Needs to Be Redefined in a Neoadjuvant Setting
In cN0 patients, SLNB after neoadjuvant chemotherapy demonstrated a comparable performance to SLNB in upfront surgery and reduced the need to perform an ALND [22].
In patients with nodes confirmed by histology, the SN FNAC trial validated SLNB after neoadjuvant chemotherapy [23]. In case of residual nodal disease (ypN+), the guidelines recommend treating the axillary nodes [24]. For these patients, the ongoing ALLIANCE A011202 trial aims to determine the optimal treatment by comparing ALND and axillary radiotherapy with axillary radiotherapy alone (ClinicalTrials.gov number: NCT01901094). In case of complete nodal response (ypNO), the need for adjuvant nodal treatment is more debatable; hence, the ongoing NSAPB B-51 trial compares nodal irradiation with observation (ClinicalTrials.gov number: NCT01872975).
In conclusion, there is a clear decrease in ALND in breast cancer thanks to SLNB, in cases of nSLN but also in selected cases of pSLN. The results of SLNB indicate nodal irradiation, but SLNM may also provide some information on specific tumor drainage (especially internal mammary drainage) to help define which volumes should be targeted in radiotherapy. Moreover, ARM identifies the lymphatic nodes that drain the arm instead of the tumor and is worth exploring to reduce radiation-induced lymphedema. LFGRT is thus appealing as an effective method of irradiation with lower toxicity.
3. Gynecologic Cancers
3.1. SLNB Is a Well-Documented Technique in Vulvar Cancers
Locally advanced vulvar carcinomas are usually treated conservatively thanks to chemoradiation, whereas early-stage treatment consists of radical resection with nodal assessment and can be followed by adjuvant radiotherapy. Lymph node staging is a major prognostic factor in vulvar cancers [25]. For FIGO IB to II and lateral lesions (≥2 cm from vulvar midline) with clinically/radiologically node negative tumors, SLNB is recommended, since nSLN is associated with low morbidity, groin recurrence and disease-specific mortality, while being more cost-effective than extensive lymphadenectomy [26].
In case of pSLN, the management of ipsilateral groin with lymphadenectomy and radiotherapy should be discussed [27]. The GROINSS-V-II trial studied 322 patients with pSLN to evaluate whether groin dissection could be replaced by inguinofemoral radiotherapy. Due to high groin recurrence, the protocol had to be amended to allow for patients with SLN > 2 mm (macrometastasis) to undergo lymphadenectomy as the standard of care, but patients with SLN ≤ 2 mm (micrometastasis) could continue to receive radiotherapy. The 2-year groin recurrence rate was low for patients with micrometastasis (1.6%), but high for patients with macrometastasis when treated by radiotherapy (22%) compared to those treated by lymphadenectomy (6.9%). Ipsilateral inguinofemoral irradiation appears to be a low-morbidity option for patients with micrometastasis but should not be the first intention in case of macrometastasis [28].
How to manage contralateral groin remains unclear. Two retrospective monocentric studies suggested not treating contralateral groin, since they found very low rates of contralateral involvement: 0% (0/28) patients and 5.3% (1/19) patients, respectively [29,30]. However, a recent study reported a higher rate of contralateral involvement, at 22.2% (4/18) of patients, after an initial diagnosis of unilateral metastasis, supporting current guidelines in favor of contralateral prophylactic treatment by either lymphadenectomy or radiotherapy [31].
For larger tumors (greater than 4 cm), the negative predictive value deteriorates, so there is no strong evidence to recommend using the SLN technique [30].
3.2. SLNB Is Not the Standard Reference for Node Staging in Cervical Cancers at Present
Lymph node status leads the indication for radiotherapy in cervical cancers. The treatment is exclusively chemoradiation if metastatic lymph nodes are detected before radical surgery, or adjuvant chemoradiation if detected after resection. SLNB is currently employed in addition to pelvic node dissection but not alone, despite some interesting performances [32]. Indeed, questions have been raised about the ability to detect micrometastasis, reliability in intraoperative detection and the limited evidence obtained from prospective studies [33]. The SENTIX trial evaluated intraoperative SLN frozen section and SLNB without pelvic node dissection in 395 patients: SLN pathological examinations achieved high detection for node staging, but the intraoperative SLN frozen section failed to detect about 50% of pathological nodes [34]. Ongoing SENTICOL III and PHENIX trials are enrolling patients with early-stage cervical cancer. The SENTICOL III trial follows the SENTICOL II trial, which showed the decreased morbidity of SLNB alone [35] and randomizes patients between SLNB alone (experimental arm) and SLNB plus pelvic node dissection (reference arm). In the PHENIX trial, all patients undergo SLNB and are allocated into either the PHENIX-I (if nSLN) or PHENIX-II (if pSLN) cohorts. Patients in each cohort are randomized after the SLNB between observation (experimental arm) and pelvic node dissection (reference arm). The primary outcome of these two trials is disease-free survival to demonstrate non-inferiority, and results are expected in 2026 [36,37].
For more advanced cervical cancers, higher than FIGO 2018 stage Ib3, the involvement of para-aortic nodes needs to be assessed to guide irradiation volumes. This assessment is based on FDG PET-CT and para-aortic lymphadenectomy. The role played by SLNB is little documented and thus cannot be recommended [38,39].
3.3. SLNB Could Guide the Indication of Adjuvant Radiotherapy in Endometrial Cancers
For endometrial cancers, decisions regarding adjuvant treatments such as radiotherapy, brachytherapy or chemotherapy is influenced by nodal assessment, but also depend on other histopathological factors and, more recently, on molecular and genomic profiles [40,41]. For nodal assessment, a Cochrane meta-analysis validates SLNB as an accurate technique with high sensitivity [42], and some researchers have developed an algorithm using data from 247 patients, including SLNB, that can identify the involved lymph nodes with a sensitivity of 98% and negative predictive value of 99.5% to replace extensive lymphadenectomy [43]. A recent meta-analysis pooled the results from 14 studies evaluating SLNB and analyzing over 2000 patients with low- and intermediate-risk endometrial cancers and found about 10% of pathological involvement with a high detection rate and negative predictive value [44]. In high-risk cancers, the use of SLNB is more discussed [45]. Nevertheless, a multi-institutional retrospective study showed similar disease-free survival and overall survival for patients undergoing SLNB with and without back-up lymphadenectomy [46], and a review identified only retrospective studies, but suggested the non-inferiority of SLNB compared to lymphadenectomy [47]. Thanks to these good performances, SLNB could guide the indication of adjuvant radiotherapy. For example, a study showed that the decision of adjuvant radiotherapy changed for some patients based on the SLNB results [48].
In conclusion, lymph node staging is a major prognosis factor for vulvar cancers. For FIGO IB to II, <4 cm, and cN0 tumors, SLNB is recommended. In case of pSLN, ipsilateral groin should be treated by either radiotherapy (if micrometastasic disease) or lymphadenectomy (if macrometastatic disease), whereas the treatment of contralateral groin is more open to discussion. In cervical cancers, SLNB alone cannot be recommended in routine treatment at present, and complements the pelvic node dissection that remains the standard until the results of ongoing trials are reported. In endometrial cancers, SLNB could help in the radiotherapy decision-making process.
4. Urologic Cancers
4.1. Penile Cancers Represent a Leading Indication of SLNB
In penile cancers, SLNB is a highly recommended procedure for the management of clinically node-negative patients based on the European Association of Urology guidelines [49]. Systematic reviews have confirmed the relevance of SLNB in this cancer, which has a very stereotyped echelon-based pattern of lymphatic drainage [50,51]. SLNs are detected during surgery with a high sensitivity and specificity of about 77% and 100%, respectively [52], especially when using blue dye and radiotracer in combination [53]. Performances appear even better when acquiring 3D-imaging in SPECT/CT before surgical detection, to increase the detection rate [54] and decrease the rate of false-positive nodes [55].
However, no studies investigated the use of SLNM and SLNB in radiation oncology, mainly because the benefits of nodal irradiation have not been demonstrated. In the absence of nodal involvement, prophylactic inguinal irradiation at 50 Gy showed no decrease in recurrences compared to surveillance [56]. If lymph nodes are involved, inguinal dissection is performed, and adjuvant radiotherapy might be offered in case of bad prognosis factors. The use of adjuvant radiotherapy is under debate since a systematic review showed no benefits, and thus a standard recommendation cannot be made [57].
4.2. SLNB Is Non-Mature in Bladder, Testicular, and Renal Cancers
SLNB has been described in bladder cancers for more than 20 years, but still presents non-negligible rates of false-negative lymph nodes, thus requiring further investigation, notably regarding radiotracers and detection techniques [58,59].
In testicular cancers, SLNB appears safe in prospective studies, but its value for guiding adjuvant treatment remains to be demonstrated [60,61].
In renal cancers, SLNM is not an easy technique to reproduce because it can be non-contributory in 30% of cases due to a lack of drainage of the radiotracer through lymphatic vessels [62]. Aside from these technical difficulties, its ability to detect and then treat lymph nodes is debated since it does not seem to change overall survival according to a recent meta-analysis [63].
4.3. SLNB Could Help Redefine Irradiation Volumes in Prostate Cancers
In prostate cancers, extended pelvic lymph node dissection remains the gold standard for lymph node staging, but SLNB demonstrated comparable results with high sensitivity and specificity and a low rate of false-negative nodes [64]. Nodal staging was improved, with up to 94% accuracy achieved with the combination of SLNB and a PSMA PET-CT [65]. Although not used routinely [66], the SLNB appears to be an accurate technique with low morbidity that could help in treatment decisions in intermediate- and high-risk prostate cancers [67]; for instance, to extend androgen deprivation therapy in cases where pathological nodes were detected or to better define volumes in radiotherapy [68].
Prophylactic pelvic elective node irradiation (ENI), in addition to prostate gland irradiation, has indications for unfavorable intermediate- and high-risk cN0 prostate cancers [69], even if the benefits of pelvic irradiation remain controversial [70,71]. Some research organizations, such as the RTOG or the UK CRUK PIVOTAL, have suggested delineation atlases to contour lymph node areas that classically include the distal common iliac, external and internal iliac, and obturator lymph nodes [72]. The French group GETUG identified some rarely irradiated regions, which are nevertheless at risk of invasion [73], such as the proximal common iliac, para-rectal, peri-vesical, peri-vesicular, pre-sacral, pudendal, inguinal and retroperitoneal drainage regions, as described in SPECT-CT [74], and more recently in PET-CT [75,76,77]. The undercoverage of these regions could explain some patterns of disease recurrence in proximal common iliac [78] or retroperitoneal and inguinal regions [79], whereas in-field recurrence is observed less often [80]. The difficulty of obtaining an overall recommendation could come from the fact that lymphatic drainage varies considerably depending on the intraprostatic localization of the tumor (base or apex, ventral or dorsal, central or lateral) [81].
A phase I proof-of-concept study evaluated SLNM’s ability to include relevant lymph nodes in the target volume in six patients for a more personalized irradiation [82]. A phase II study showed good biochemical control (73.8%) at 5 years for 61 patients when the mapped drainage areas were irradiated in addition to the usually recommended areas [83]. An in silico study simulated radiotherapy plans according to RTOG delineation guidelines in 57 patients who had an SLNM procedure from a previous trial: 305 pelvic nodes were identified (mean of 5.4 “hot” nodes per patient); 67/305 (22%) would not have been taken into consideration by standard delineation. Despite the margins around the delineated areas, 42 of these 67 nodes (63%) would not have received at least 95% of the prescribed doses and would have been mistreated [84]. Figure 2 illustrates this point thanks to examples of atypical drainage in some prostate cancers. There is a need to change the paradigm of “one-size-fits-all”. SLNM is a path to explore, but some questions remain: would it be better to only treat draining nodes that are identified to reduce the irradiated volumes and what benefits would there be in terms of decreasing morbidity? Alternatively, would it be best to treat the nodes identified in addition to the usually described nodal areas and what would the oncological outcomes be? Clinical trials at a larger scale to answer these questions are lacking.
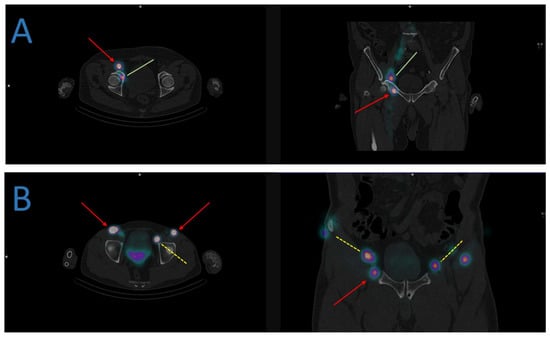
Figure 2.
Examples of atypical lymphatic drainage in some prostate cancers. (A) The sentinel lymph node mapping identifies a drainage in the right obturator (green lines) and inguinal nodes (red arrows). (B) The sentinel lymph node mapping identifies a bilateral drainage in the external iliac (yellow dashed lines) and inguinal nodes (red arrows).
In conclusion, SLNB is a cornerstone for the management of penile cancers from a surgeon’s point of view, but its use in radiation oncology is almost inexistent, as the use of radiotherapy to treat lymph nodes has no validated indications at present. For bladder, testicular and renal cancers, the literature is much sparser, and the performances of SLN techniques in these indications remain very uncertain and experimental. SLNM and SLNB are still under investigation for prostate cancers, but the results are more promising and could help to better define irradiation volumes for more personalized radiotherapy.
5. Anal Cancer
5.1. SLNB Shows Better Performances Than FDG PET-CT for Detecting Metastases in Inguinal Nodes
The standard treatment of anal cancers is based on radiotherapy for T1 N0 tumors or concurrent radiochemotherapy (most often with 5FU and mitomycine C) for the others. Irradiation concerns the gross tumor, pelvic nodes, and inguinal nodes. Cancer staging currently relies on FDG PET-CT due to its high sensitivity. For instance, a study showed the perfect sensitivity of PET-CT, which did not miss any metastatic inguinal nodes, but reported a significant number of false-positive images, leading to a poor positive predictive value of only 43% [85]. Another study evaluated the SLNB of inguinal nodes and found this technique to be superior to FDG PET-CT, with fewer false-positive and false-negative patients [86]. In addition to better accuracy, a study revealed that pSLN was associated with oncological outcomes and a much better prognosis factor than positive inguinal uptake in FDG PET-CT. In fact, inguinal pSLN was significantly associated with a decrease in disease-free (21 vs. 56 months; p = 0.046) and overall (28 vs. 59 months; p = 0.028) survival [87]. Inguinal SLNB should be used more [88], as several literature reviews have reported good reproducibility and performance and acceptable rates of complications, but its deployment is limited by a lack of trials with a large population, notably because anal cancer is a rather rare cancer [89,90].
5.2. SLNB Could Spare Groin Irradiation and Its Toxicities
The selection of patients for groin irradiation currently depends on tumor size: T1 tumors are not a systematic indication, while groin irradiation is generally indicated for T2 or higher tumors. These rules present two problems: first, some T1 tumors may have occult inguinal metastasis whereas some T2 tumors may not, and second, groin irradiation can be poorly tolerated. The idea of adjusting radiation fields based on SLNB is not new in anal cancers [91]. A pilot study tested the feasibility of performing inguinal SLNB on patients with T1 or T2 anal tumors and irradiating the groin only in cases of pSLN [92]. The results of SLNB changed management in half (10/20) of their patients: 4 patients with a T1 tumor and pSLN received groin irradiation that was not initially indicated, and 6 patients with a T2 tumor and nSLN avoided groin irradiation that was initially indicated. Nevertheless, treatment de-escalation requires caution because a prospective study agreed with the feasibility of SLNB but also reported the cases of 2 out of 14 patients with nSLN who were spared groin irradiation, and who then developed inguinal metastasis at one year and two years, respectively [93]. Another study combined the use of FDG PET-CT and SLNB for inguinal staging, and patients presenting no sign of inguinal involvement in both exams avoided groin irradiation, and then presented significantly less inguinal dermatitis, especially severe dermatitis (grades 1–2: 12% vs. 50% and grades 3–4: 0% vs. 17%; p < 0.05) [94]. A retrospective study confirmed the difference between patients with nSLN and pSLN in terms of prognosis for disease-free and overall survival, and showed that it seemed safe not to target inguinal nodes in cases of nSLN, as none of their patients presented inguinal recurrence after a mean follow-up of 43 months [95].
In conclusion, disease staging in anal cancers is currently based on FDG PET-CT, which has shown good performances for pelvic nodes or visceral metastasis. However, FDG PET-CT has its limits for inguinal status, with a high rate of false positives. Inguinal SLNB can be seen as a more reliable alternative to inguinal staging, as well as to “LFGRT” where radiation fields are tailored to each patient. As anal cancers are uncommon, data on oncologic outcomes are lacking and comparative trials are needed.
6. Head and Neck Cancers
6.1. SLNB Is a Promising Procedure for Head and Neck Cancers
SLNB appears to be a well-accepted technique at present, providing a significantly lower surgical and postoperative morbidity when compared to elective neck dissection [96]. Several studies showed the performance of SLNB, leading to a meta-analysis of 26 studies (and 766 patients) by Thompson et al., who found a pooled sensitivity and a negative predictive value of 95% and 96%, respectively, for all head and neck cancers, and of 94% and 96%, respectively, for the subgroup of oral cavity cancer [97].
A recent randomized phase III trial further demonstrated an oncologic equivalence between SLNB and neck dissection in T1-T2 N0 oral and oropharyngeal cancers, with no significant difference at 2 and 5 years in neck node recurrence, disease-specific mortality, or overall survival [98]. A retrospective cohort study of 816 patients with a lateralized or paramedian early-stage oral cancer even suggested better outcomes with SLNB compared to ipsilateral elective neck dissection. The results showed more contralateral regional recurrences in patients undergoing ipsilateral neck dissection versus SLNB (3.8% vs. 1.3%; p = 0.018), and statistical analyses found a significantly higher risk of these patients presenting with contralateral recurrence (Hazard Ratio = 2.585; p = 0.030). The patients with contralateral node recurrence seemed to have a worse prognosis than those whose occult contralateral metastasis were detected earlier thanks to SLNB, with a disease-specific survival rate at five years of 42% versus 88% (p = 0.066) [99].
6.2. Nodal Irradiation Is a Key Treatment in Head and Neck Cancers
In contrast to surgeons, radiation therapists treat head and neck cancers that can be more advanced in terms of size (T1 to T4 tumors) and nodal involvement. Irradiation is delivered to both sides of the neck in most cases. This is a long-standing practice [100,101], mainly because empirical convention is used to avoid contralateral recurrence, rather than evidence-based medicine [102,103]. However, it is now well-documented that bilateral irradiation engenders more frequent and more severe acute and late toxicities, degrading the patients’ quality of life due to complications such as fibrosis, dysphonia, xerostomia, sticky saliva and dysphagia [104,105,106,107].
In order to avoid either over- or under-treating, the applications for SLNM and SLNB were explored in the field of radiotherapy with two aims: to determine tumor lymphatic drainage in each individual patient and to detect occult metastasis. Some prospective phase I-II monocentric studies tested LFGRT, which consisted of targeting only the lymph node levels with radiotracer fixation. De Veij Mestdagh et al. first evaluated SLNM in patients with lateralized cT1-3 N0-2b head and neck cancers thanks to radiolabelled 99mTc-colloid, and found that contralateral drainage affected 11/54 (20%) of the patients involving node levels II (88%), III (25%), and IV (13%), and was significantly associated with T3 tumors compared to T1 and T2 tumors (45% vs. 14%; p = 0.035) [108]. They then showed some dosimetric benefits to only treating the mapped drainage areas with median dose reductions in the contralateral parotid and submandibular glands, larynx, and thyroid gland [109], which translated into clinical benefits with significant reductions in dysphagia, gastric tube placement, and late xerostomia [110]. In the studies cited above, the patients did not undergo a pathological examination of their hot nodes, so they designed the ongoing SUSPECT-2 trial for patients with cT1-4 N0-2b lateralized tumors, where a pathological examination is performed in case of contralateral SLN, and bilateral neck irradiation will be indicated only if malignant involvement is histologically proven [111]. A Russian study of 26 patients with a cT1-2 N0 tongue cancer found 10/26 (38.5%) patients with bilateral drainage. The experimental LFGRT plans made significant reductions possible in both the irradiated volume and the mean doses received by the spinal cord, as well as the contralateral parotid gland, when compared to virtual plans for bilateral irradiation according to standard guidelines [112]. A Belgian study of 44 patients with cN0 head and neck cancers found 48% of patients with unilateral drainage and 16% with unexpected drainage that would not have been covered by usual delineations. The experimental plans also showed some dosimetric benefits and had positive clinical outcomes for dysphagia, xerostomia, hypothyroidism and patients’ quality of life [113].
In conclusion, SLN techniques are used increasingly in head and neck cancers and will become a major indication. This could be an opportunity for the use of “LFGRT” to decide between a unilateral or bilateral nodal irradiation, and to determine which drainage areas should be included or spared.
7. Discussion and Future Directions
The role of SLN in treatment planning presents a certain level of interest and has some feasibility, but we may wonder how to use it concretely in daily practice. An important step would be to conduct clinical trials comparing standard delineated volumes and tailored volumes. The objectives would be to decrease radiation toxicities and improve oncological outcomes by treating unexpected drainage areas, while noting that reducing the volumes too much could be a pitfall. A difficulty would be calculating each indication to determine which would be most effective and detect a difference in efficacy and/or toxicity. Table 1 indicates the main ongoing trials that will provide some answers.

Table 1.
Ongoing trials evaluating the role of the sentinel lymph node in treatment planning in radiotherapy. We searched for currently active trials registered on ClinicalTrials.gov with the following tags: “sentinel” and “radiotherapy”. Abbreviations: head and neck squamous cell cancer (HNSCC).
This review aimed to provide an overview of the use of SLN techniques to individualize radiotherapy treatment by reviewing the current knowledge, but is limited in its power to reach definitive conclusions, given the sparse literature. The impact of SLNM is difficult to accurately evaluate and will differ according to the localizations. It should be most significant in head and neck cancers, since many studies showed both dosimetric and clinical benefits with decreased toxicity and could be routinely used. It should also be meaningful to avoid loco-regional recurrence in prostate cancer, with several studies showing better target coverage. In other localizations, we acknowledge that its impact is more theoretical, because the majority of studies found in the literature only show dosimetric improvements, while studies verifying whether such results will translate into clinical outcomes are lacking.
Another point that should be stressed is the need for a strong collaboration between the different actors and departments (notably surgery, nuclear medicine and radiotherapy) to obtain a smooth workflow. Additionally, developing studies on lymph-flow-guided radiotherapy is not a simple task, since surgical studies are needed in order to first demonstrate that the indications of SLN are safe and effective. This could be a great opportunity to reinforce collaboration between the oncological specialties and to conduct more joint studies.
8. Conclusions
After providing a major breakthrough for surgeons, SLN techniques have shown promising results as far as radiotherapy treatment planning is concerned. SLNM and SLNB appear to be safe procedures that help redefine the irradiation volumes that should be used for each individual, avoiding the use of a probabilistic method, thus avoiding under- or over-treatment. Some indications have a head start, such as head and neck cancers, where several trials can be found in the literature, whereas other indications are still theoretical, as only retrospective or in silico studies have been published. In any case, “LFGRT” cannot be used in routine treatments for individualization of the treatments, and should currently be seen as an exploratory technique, given the lack of published phase III randomized trials or meta-analyses with large numbers of patients. It nonetheless remains an inspiring concept, which should be further developed soon.
Author Contributions
L.A.-T.: Writing—Original Draft. C.R.: Methodology, Writing—Original Draft, Supervision. M.A.: Writing—Review and Editing. A.C.: Writing—Review and Editing. M.D.: Writing—Review and Editing. A.M.: Writing—Review and Editing. L.V.: Writing—Review and Editing. S.S.: Conceptualization, Writing—Original Draft, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Patients were informed for using their imaging data. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
No datasets were created or analyzed to write the present article.
Acknowledgments
The authors would like to thanks their coworkers in the two departments of radiotherapy and nuclear medicine for fruitful advices regarding this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leong, S.P.; Pissas, A.; Scarato, M.; Gallon, F.; Pissas, M.H.; Amore, M.; Wu, M.; Faries, M.B.; Lund, A.W. The lymphatic system and sentinel lymph nodes: Conduit for cancer metastasis. Clin. Exp. Metastasis 2022, 39, 139–157. [Google Scholar] [CrossRef]
- Jakobsen, J.K. Sentinel node biopsy in uro-oncology: A history of the development of a promising concept. Urol. Oncol. 2015, 33, 486–493. [Google Scholar] [CrossRef]
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.; Setit, A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; History, evolution and current applications. Breast Dis. 2021, 40, 219–225. [Google Scholar] [CrossRef]
- Nieweg, O.E.; Uren, R.F.; Thompson, J.F. The history of sentinel lymph node biopsy. Cancer J. 2015, 21, 3–6. [Google Scholar] [CrossRef]
- Moncayo, V.M.; Alazraki, A.L.; Alazraki, N.P.; Aarsvold, J.N. Sentinel Lymph Node Biopsy Procedures. Semin. Nucl. Med. 2017, 47, 595–617. [Google Scholar] [CrossRef]
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef]
- Noguchi, M.; Inokuchi, M.; Noguchi, M.; Morioka, E.; Ohno, Y.; Kurita, T. Axillary surgery for breast cancer: Past, present, and future. Breast Cancer 2021, 28, 9–15. [Google Scholar] [CrossRef]
- Galimberti, V.; Cole, B.F.; Viale, G.; Veronesi, P.; Vicini, E.; Intra, M.; Mazzarol, G.; Massarut, S.; Zgajnar, J.; Taffurelli, M.; et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1385–1393. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Sávolt, Á.; Péley, G.; Polgár, C.; Udvarhelyi, N.; Rubovszky, G.; Kovács, E.; Győrffy, B.; Kásler, M.; Mátrai, Z. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment of the Axilla–Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur. J. Surg. Oncol. 2017, 43, 672–679. [Google Scholar] [CrossRef]
- Ortega Expósito, C.; Falo, C.; Pernas, S.; Pérez Carton, S.; Gil Gil, M.; Ortega, R.; Pérez Montero, H.; Stradella, A.; Martinez, E.; Laplana, M.; et al. The effect of omitting axillary dissection and the impact of radiotherapy on patients with breast cancer sentinel node macrometastases: A cohort study following the ACOSOG Z0011 and AMAROS trials. Breast Cancer Res. Treat. 2021, 189, 111–120. [Google Scholar] [CrossRef]
- Castelo, M.; Hu, S.Y.; Dossa, F.; Acuna, S.A.; Scheer, A.S. Comparing Observation, Axillary Radiotherapy, and Completion Axillary Lymph Node Dissection for Management of Axilla in Breast Cancer in Patients with Positive Sentinel Nodes: A Systematic Review. Ann. Surg. Oncol. 2020, 27, 2664–2676. [Google Scholar] [CrossRef]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef]
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef]
- Kim, Y.B.; Byun, H.K.; Kim, D.Y.; Ahn, S.J.; Lee, H.S.; Park, W.; Kim, S.S.; Kim, J.H.; Lee, K.C.; Lee, I.J.; et al. Effect of Elective Internal Mammary Node Irradiation on Disease-Free Survival in Women with Node-Positive Breast Cancer: A Randomized Phase 3 Clinical Trial. JAMA Oncol. 2022, 8, 96–105. [Google Scholar] [CrossRef]
- Hindié, E.; Groheux, D.; Brenot-Rossi, I.; Rubello, D.; Moretti, J.L.; Espié, M. The sentinel node procedure in breast cancer: Nuclear medicine as the starting point. J. Nucl. Med. 2011, 52, 405–414. [Google Scholar] [CrossRef]
- Nikolaevich, N.S.; Vasilevich, K.S. Why do we need irradiation of internal mammary lymph nodes in patients with breast cancer: Analysis of lymph flow and radiotherapy studies. Rep. Pract. Oncol. Radiother. 2017, 22, 37–41. [Google Scholar] [CrossRef]
- Cheville, A.L.; Brinkmann, D.H.; Ward, S.B.; Durski, J.; Laack, N.N.; Yan, E.; Schomberg, P.J.; Garces, Y.I.; Suman, V.J.; Petersen, I.A. The addition of SPECT/CT lymphoscintigraphy to breast cancer radiation planning spares lymph nodes critical for arm drainage. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 971–977. [Google Scholar] [CrossRef]
- Wang, W.; Ward, R.; Jia, D.; Ashworth, S.; Estoesta, E.; Moodie, T.; Ahern, V.; Stuart, K.; Ngui, N.; French, J.; et al. Location of arm draining lymph node in relation to breast cancer radiotherapy field and target volume. Radiother. Oncol. 2019, 133, 193–197. [Google Scholar] [CrossRef]
- Waldstein, C.; Moodie, T.; Ashworth, S.; Ahern, V.; Stuart, K.; Wang, W. Feasibility of arm-draining lymph node-sparing radiotherapy of breast cancer: A pilot planning study. J. Med. Imaging Radiat. Oncol. 2021, 65, 951–955. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef]
- Currey, A.; Patten, C.R.; Bergom, C.; Wilson, J.F.; Kong, A.L. Management of the axilla after neo-adjuvant chemotherapy for breast cancer: Sentinel node biopsy and radiotherapy considerations. Breast J. 2018, 24, 902–910. [Google Scholar] [CrossRef]
- Brincat, M.R.; Muscat Baron, Y. Sentinel Lymph Node Biopsy in the Management of Vulvar Carcinoma: An Evidence-Based Insight. Int. J. Gynecol. Cancer 2017, 27, 1769–1773. [Google Scholar] [CrossRef]
- Collarino, A.; Fuoco, V.; Garganese, G.; Pereira Arias-Bouda, L.M.; Perotti, G.; Manca, G.; Vidal-Sicart, S.; Giammarile, F.; de Geus-Oei, L.F.; Scambia, G.; et al. Lymphoscintigraphy and sentinel lymph node biopsy in vulvar carcinoma: Update from a European expert panel. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1261–1274. [Google Scholar] [CrossRef]
- Weinberg, D.; Gomez-Martinez, R.A. Vulvar Cancer. Obstet. Gynecol. Clin. N. Am. 2019, 46, 125–135. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brännström, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of, GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef]
- Woelber, L.; Eulenburg, C.; Grimm, D.; Trillsch, F.; Bohlmann, I.; Burandt, E.; Dieckmann, J.; Klutmann, S.; Schmalfeldt, B.; Mahner, S.; et al. The Risk of Contralateral Non-sentinel Metastasis in Patients with Primary Vulvar Cancer and Unilaterally Positive Sentinel Node. Ann. Surg. Oncol. 2016, 23, 2508–2514. [Google Scholar] [CrossRef]
- Nica, A.; Covens, A.; Vicus, D.; Kupets, R.; Osborne, R.; Cesari, M.; Gien, L.T. Sentinel lymph nodes in vulvar cancer: Management dilemmas in patients with positive nodes and larger tumors. Gynecol. Oncol. 2019, 152, 94–100. [Google Scholar] [CrossRef]
- Winarno, A.S.; Mondal, A.; Martignoni, F.C.; Fehm, T.N.; Hampl, M. The potential risk of contralateral non-sentinel groin node metastasis in women with early primary vulvar cancer following unilateral sentinel node metastasis: A single center evaluation in University Hospital of Düsseldorf. BMC Womens Health 2021, 21, 23. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Bonsang-Kitzis, H.; Deloménie, M.; Ngô, C.; Montero Macias, R.; Koual, M.; Nguyen-Xuan, H.T.; Bats, A.S.; Mathevet, P.; et al. Sentinel lymph node biopsy in early-stage cervical cancer: Current state of art. Bull. Cancer 2020, 107, 696–706. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207. [Google Scholar] [CrossRef]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80. [Google Scholar] [CrossRef]
- Mathevet, P.; Lécuru, F.; Uzan, C.; Boutitie, F.; Magaud, L.; Guyon, F.; Querleu, D.; Fourchotte, V.; Baron, M.; Bats, A.S.; et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). Eur. J. Cancer 2021, 148, 307–315. [Google Scholar] [CrossRef]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef]
- Tu, H.; Huang, H.; Xian, B.; Li, J.; Wang, P.; Zhao, W.; Chen, X.; Xie, X.; Wang, C.; Kong, B.; et al. Sentinel lymph node biopsy versus pelvic lymphadenectomy in early-stage cervical cancer: A multi-center randomized trial (PHENIX/CSEM 010). Int. J. Gynecol. Cancer 2020, 30, 1829–1833. [Google Scholar] [CrossRef]
- Lavoué, V.; Bats, A.S.; Rouzier, R.; Coutant, C.; Barranger, E.; Daraï, E. Sentinel lymph node procedure followed by laparoscopic pelvic and paraaortic lymphadenectomy in women with IB2-II cervical cancer. Ann. Surg. Oncol. 2007, 14, 2654–2661. [Google Scholar] [CrossRef]
- Chéreau, E.; Feron, J.G.; Ballester, M.; Coutant, C.; Bezu, C.; Rouzier, R.; Touboul, E.; Daraï, E. Contribution of pelvic and para-aortic lymphadenectomy with sentinel node biopsy in patients with IB2-IIB cervical cancer. Br. J. Cancer 2012, 106, 39–44. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Bogani, G.; Palaia, I.; Perniola, G.; Fracassi, A.; Cuccu, I.; Golia D’Auge, T.; Casorelli, A.; Santangelo, G.; Fischetti, M.; Muzii, L.; et al. Assessing the role of low volume disease in endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 68–72. [Google Scholar] [CrossRef]
- Nagar, H.; Wietek, N.; Goodall, R.J.; Hughes, W.; Schmidt-Hansen, M.; Morrison, J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst. Rev. 2021, 6, CD013021. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Burg, L.C.; Verheijen, S.; Bekkers, R.L.M.; IntHout, J.; Holloway, R.W.; Taskin, S.; Ferguson, S.E.; Xue, Y.; Ditto, A.; Baiocchi, G.; et al. The added value of SLN mapping with indocyanine green in low- and intermediate-risk endometrial cancer management: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e66. [Google Scholar] [CrossRef]
- Yu, R.; Matthews, B.J.; Beavis, A.L. The Role of Sentinel Lymph Node Mapping in High-grade Endometrial Cancer. Curr. Treat. Options Oncol. 2022, 23, 1339–1352. [Google Scholar] [CrossRef]
- Bogani, G.; Papadia, A.; Buda, A.; Casarin, J.; Di Donato, V.; Gasparri, M.L.; Plotti, F.; Pinelli, C.; Paderno, M.C.; Lopez, S.; et al. Sentinel node mapping vs. sentinel node mapping plus back-up lymphadenectomy in high-risk endometrial cancer patients: Results from a multi-institutional study. Gynecol. Oncol. 2021, 161, 122–129. [Google Scholar] [CrossRef]
- How, J.A.; Frumovitz, M.; Stewart, K.I.; Soliman, P.T. Lymphatic Mapping and Sentinel Node Biopsy in High-Grade Uterine Cancers. Curr. Oncol. Rep. 2022, 24, 1521–1529. [Google Scholar] [CrossRef]
- Bogani, G.; Di Donato, V.; Papadia, A.; Buda, A.; Casarin, J.; Multinu, F.; Plotti, F.; Gasparri, M.L.; Pinelli, C.; Perrone, A.M.; et al. Hysterectomy alone vs. hysterectomy plus sentinel node mapping in endometrial cancer: Perioperative and long-term results from a propensity-score based study. Eur. J. Surg. Oncol. 2023, 49, 1037–1043. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Graafland, N.M.; van Werkhoven, E.; Meinhardt, W.; Bex, A.; van der Poel, H.G.; van Boven, H.H.; Valdés Olmos, R.A.; Horenblas, S. Contemporary management of regional nodes in penile cancer-improvement of survival? J. Urol. 2014, 191, 68–73. [Google Scholar] [CrossRef]
- Brouwer, O.R.; van der Poel, H.G.; Bevers, R.F.; van Gennep, E.J.; Horenblas, S. Beyond penile cancer, is there a role for sentinel node biopsy in urological malignancies? Clin. Transl. Imaging 2016, 4, 395–410. [Google Scholar] [CrossRef]
- Mehralivand, S.; van der Poel, H.; Winter, A.; Choyke, P.L.; Pinto, P.A.; Turkbey, B. Sentinel lymph node imaging in urologic oncology. Transl. Androl. Urol. 2018, 7, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Tobias-Machado, M.; Ficarra, V.; Wroclawski, M.L.; Amarante, R.D.M.; Pompeo, A.C.L.; Del Giglio, A. Dynamic sentinel node biopsy for inguinal lymph node staging in patients with penile cancer: A systematic review and cumulative analysis of the literature. Ann. Surg. Oncol. 2011, 18, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Gholami, H.; Zakavi, S.R.; Kakhki, V.R.D.; Tabasi, K.T.; Horenblas, S. Accuracy of sentinel lymph node biopsy for inguinal lymph node staging of penile squamous cell carcinoma: Systematic review and meta-analysis of the literature. J. Urol. 2012, 187, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Saad, Z.Z.; Omorphos, S.; Michopoulou, S.; Gacinovic, S.; Malone, P.; Nigam, R.; Muneer, A.; Bomanji, J. Investigating the role of SPECT/CT in dynamic sentinel lymph node biopsy for penile cancers. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1176–1184. [Google Scholar] [CrossRef]
- Naumann, C.M.; Colberg, C.; Jüptner, M.; Marx, M.; Zhao, Y.; Jiang, P.; Hamann, M.F.; Jünemann, K.P.; Zuhayra, M.; Lützen, U. Evaluation of the diagnostic value of preoperative sentinel lymph node (SLN) imaging in penile carcinoma patients without palpable inguinal lymph nodes via single photon emission computed tomography/computed tomography (SPECT/CT) as compared to planar scintigraphy. Urol. Oncol. 2018, 36, 92.e17–92.e24. [Google Scholar] [CrossRef]
- Leone, A.; Diorio, G.J.; Pettaway, C.; Master, V.; Spiess, P.E. Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 2017, 14, 335–347. [Google Scholar] [CrossRef]
- Robinson, R.; Marconi, L.; MacPepple, E.; Hakenberg, O.W.; Watkin, N.; Yuan, Y.; Lam, T.; MacLennan, S.; Adewuyi, T.E.; Coscione, A.; et al. Risks and Benefits of Adjuvant Radiotherapy After Inguinal Lymphadenectomy in Node-positive Penile Cancer: A Systematic Review by the European Association of Urology Penile Cancer Guidelines Panel. Eur. Urol. 2018, 74, 76–83. [Google Scholar] [CrossRef]
- Kiss, B.; Thoeny, H.C.; Studer, U.E. Current Status of Lymph Node Imaging in Bladder and Prostate Cancer. Urology 2016, 96, 1–7. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M.; Sadeghi, R. Sentinel Node Biopsy in Urothelial Carcinoma of the Bladder: Systematic Review and Meta-Analysis. Urol. Int. 2019, 103, 373–382. [Google Scholar] [CrossRef]
- Blok, J.M.; Kerst, J.M.; Vegt, E.; Brouwer, O.R.; Meijer, R.P.; Bosch, J.L.H.R.; Bex, A.; van der Poel, H.G.; Horenblas, S. Sentinel node biopsy in clinical stage I testicular cancer enables early detection of occult metastatic disease. BJU Int. 2019, 124, 424–430. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadeghi, R.; Sadri, K.; Soltani, S.; Aghaee, A. Sentinel lymph node mapping in post chemotherapy nonseminoma testicular cancer patients undergoing retroperitoneal lymph node dissection: A series of nine cases. Asia Ocean J. Nucl. Med. Biol. 2022, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Vermeeren, L.; Meinhardt, W.; Prevoo, W.; Horenblas, S.; Valdés Olmos, R.A. Intraoperative sentinel node identification and sampling in clinically node-negative renal cell carcinoma: Initial experience in 20 patients. World J. Urol. 2011, 29, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Feng, D.; Li, D.; Zhang, F.; Wei, W. The Role of Lymph Node Dissection for Non-Metastatic Renal Cell Carcinoma: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 790381. [Google Scholar] [CrossRef] [PubMed]
- Wit, E.M.K.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Valdes Olmos, R.A.; van Leeuwen, F.W.B.; van den Berg, N.S.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef]
- Hinsenveld, F.J.; Wit, E.M.K.; van Leeuwen, P.J.; Brouwer, O.R.; Donswijk, M.L.; Tillier, C.N.; Vegt, E.; van Muilekom, E.; van Oosterom, M.N.; van Leeuwen, F.W.B.; et al. Prostate-Specific Membrane Antigen PET/CT Combined with Sentinel Node Biopsy for Primary Lymph Node Staging in Prostate Cancer. J. Nucl. Med. 2020, 61, 540–545. [Google Scholar] [CrossRef]
- Narayanan, R.; Wilson, T.G. Sentinel node evaluation in prostate cancer. Clin. Exp. Metastasis 2018, 35, 471–485. [Google Scholar] [CrossRef]
- Rousseau, C.; Rousseau, T.; Campion, L.; Lacoste, J.; Aillet, G.; Potiron, E.; Lacombe, M.; Le Coguic, G.; Mathieu, C.; Kraeber-Bodéré, F. Laparoscopic sentinel lymph node versus hyperextensive pelvic dissection for staging clinically localized prostate carcinoma: A prospective study of 200 patients. J. Nucl. Med. 2014, 55, 753–758. [Google Scholar] [CrossRef]
- Grivas, N.; Wit, E.; Pos, F.; de Jong, J.; Vegt, E.; Bex, A.; Hendricksen, K.; Horenblas, S.; KleinJan, G.; van Rhijn, B.; et al. Sentinel Lymph Node Dissection to Select Clinically Node-negative Prostate Cancer Patients for Pelvic Radiation Therapy: Effect on Biochemical Recurrence and Systemic Progression. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 347–354. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Spratt, D.E.; Pei, I.; Zhang, Z.; Yamada, Y.; Kollmeier, M.; Zelefsky, M.J. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur. Urol. 2013, 64, 895–902. [Google Scholar] [CrossRef]
- De Meerleer, G.; Berghen, C.; Briganti, A.; Vulsteke, C.; Murray, J.; Joniau, S.; Leliveld, A.M.; Cozzarini, C.; Decaestecker, K.; Rans, K.; et al. Elective nodal radiotherapy in prostate cancer. Lancet Oncol. 2021, 22, e348–e357. [Google Scholar] [CrossRef]
- Murthy, V.; Maitre, P.; Kannan, S.; Panigrahi, G.; Krishnatry, R.; Bakshi, G.; Prakash, G.; Pal, M.; Menon, S.; Phurailatpam, R.; et al. Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT): Outcomes From Phase III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Paulson, E.; Davis, B.J.; Spratt, D.E.; Morgan, T.M.; Dearnaley, D.; Tree, A.C.; Efstathiou, J.A.; Harisinghani, M.; Jani, A.B.; et al. NRG Oncology Updated International Consensus Atlas on Pelvic Lymph Node Volumes for Intact and Postoperative Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Sargos, P.; Guerif, S.; Latorzeff, I.; Hennequin, C.; Pommier, P.; Lagrange, J.L.; Créhange, G.; Chapet, O.; de Crevoisier, R.; Azria, D.; et al. Definition of lymph node areas for radiotherapy of prostate cancer: A critical literature review by the French Genito-Urinary Group and the French Association of Urology (GETUG-AFU). Cancer Treat. Rev. 2015, 41, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, U.; Schilling, D.; Müller, A.C.; Bares, R.; Bartenstein, P.; Belka, C. Distribution of prostate sentinel nodes: A SPECT-derived anatomic atlas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Doughton, J.A.; Hofman, M.S.; Eu, P.; Hicks, R.J.; Williams, S. A First-in-Human Study of 68Ga-Nanocolloid PET/CT Sentinel Lymph Node Imaging in Prostate Cancer Demonstrates Aberrant Lymphatic Drainage Pathways. J. Nucl. Med. 2018, 59, 1837–1842. [Google Scholar] [CrossRef]
- Onal, C.; Ozyigit, G.; Guler, O.C.; Hurmuz, P.; Torun, N.; Tuncel, M.; Dolek, Y.; Yedekci, Y.; Oymak, E.; Tilki, B.; et al. Role of 68-Ga-PSMA-PET/CT in pelvic radiotherapy field definitions for lymph node coverage in prostate cancer patients. Radiother. Oncol. 2020, 151, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Schiller, K.; Stöhrer, L.; Düsberg, M.; Borm, K.; Devecka, M.; Vogel, M.M.E.; Tauber, R.; Heck, M.M.; Rauscher, I.; Eiber, M.; et al. PSMA-PET/CT-based Lymph Node Atlas for Prostate Cancer Patients Recurring After Primary Treatment: Clinical Implications for Salvage Radiation Therapy. Eur. Urol. Oncol. 2021, 4, 73–83. [Google Scholar] [CrossRef]
- Spratt, D.E.; Vargas, H.A.; Zumsteg, Z.S.; Golia Pernicka, J.S.; Osborne, J.R.; Pei, X.; Zelefsky, M.J. Patterns of Lymph Node Failure after Dose-escalated Radiotherapy: Implications for Extended Pelvic Lymph Node Coverage. Eur. Urol. 2017, 71, 37–43. [Google Scholar] [CrossRef] [PubMed]
- De Bruycker, A.; De Bleser, E.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Visschere, P.; De Man, K.; Delrue, L.; Lambert, B.; Ost, P. Nodal Oligorecurrent Prostate Cancer: Anatomic Pattern of Possible Treatment Failure in Relation to Elective Surgical and Radiotherapy Treatment Templates. Eur. Urol. 2019, 75, 826–833. [Google Scholar] [CrossRef]
- Liskamp, C.P.; Donswijk, M.L.; van der Poel, H.G.; Schaake, E.E.; Vogel, W.V. Nodal recurrence patterns on PET/CT after RTOG-based nodal radiotherapy for prostate cancer. Clin. Transl. Radiat. Oncol. 2020, 22, 9–14. [Google Scholar] [CrossRef]
- de Korne, C.M.; Wit, E.M.; de Jong, J.; Valdés Olmos, R.A.; Buckle, T.; van Leeuwen, F.W.B.; van der Poel, H.G. Anatomical localization of radiocolloid tracer deposition affects outcome of sentinel node procedures in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2558–2568. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, U.; Paulsen, F.; Corvin, S.; Eichhorn, K.; Glocker, S.; Hundt, I.; Birkner, M.; Alber, M.; Anastasiadis, A.; Stenzl, A.; et al. Intensity modulated radiotherapy for high risk prostate cancer based on sentinel node SPECT imaging for target volume definition. BMC Cancer 2005, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.C.; Eckert, F.; Paulsen, F.; Zips, D.; Stenzl, A.; Schilling, D.; Alber, M.; Bares, R.; Martus, P.; Weckermann, D.; et al. Nodal Clearance Rate and Long-Term Efficacy of Individualized Sentinel Node-Based Pelvic Intensity Modulated Radiation Therapy for High-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 263–271. [Google Scholar] [CrossRef]
- Michaud, A.V.; Samain, B.; Ferrer, L.; Fleury, V.; Doré, M.; Colombié, M.; Dupuy, C.; Rio, E.; Guimas, V.; Rousseau, T.; et al. Haute Couture or Ready-to-Wear? Tailored Pelvic Radiotherapy for Prostate Cancer Based on Individualized Sentinel Lymph Node Detection. Cancers 2020, 12, 944. [Google Scholar] [CrossRef]
- Mistrangelo, M.; Pelosi, E.; Bellò, M.; Castellano, I.; Cassoni, P.; Ricardi, U.; Munoz, F.; Racca, P.; Contu, V.; Beltramo, G.; et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of inguinal node metastases in patients with anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 73–78. [Google Scholar] [CrossRef]
- Mistrangelo, M.; Pelosi, E.; Bellò, M.; Ricardi, U.; Milanesi, E.; Cassoni, P.; Baccega, M.; Filippini, C.; Racca, P.; Lesca, A.; et al. Role of positron emission tomography-computed tomography in the management of anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 66–72. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Guarneri, G.; Canevari, C.; Tamburini, A.; Slim, N.; Passoni, P.; Rosati, R. Prognostic value of fluorodeoxyglucose positron emission tomography/computed tomography and inguinal sentinel lymph node biopsy in patients with anal cancer. Colorectal Dis. 2019, 21, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Mistrangelo, D.M.; Bellò, M.; Cassoni, P.; Milanesi, E.; Racca, P.; Munoz, F.; Fora, G.; Rondi, N.; Gilbo, N.; Senetta, R.; et al. Value of staging squamous cell carcinoma of the anal margin and canal using the sentinel lymph node procedure: An update of the series and a review of the literature. Br. J. Cancer 2013, 108, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Noorani, A.; Rabey, N.; Durrani, A.; Walsh, S.R.; Davies, R.J. Systematic review of sentinel lymph node biopsy in anal squamous cell carcinoma. Int. J. Surg. 2013, 11, 762–766. [Google Scholar] [CrossRef]
- Tehranian, S.; Treglia, G.; Krag, D.N.; Dabbagh Kakhki, V.R.; Zakavi, S.R.; Sadeghi, R.; Keshtgar, M. Sentinel node mapping in anal canal cancer: Systematic review and meta-analysis. J. Gastrointestin Liver Dis. 2013, 22, 321–328. [Google Scholar]
- De Nardi, P.; Carvello, M.; Staudacher, C. New approach to anal cancer: Individualized therapy based on sentinel lymph node biopsy. World J. Gastroenterol. 2012, 18, 6349–6356. [Google Scholar] [CrossRef] [PubMed]
- Gretschel, S.; Warnick, P.; Bembenek, A.; Dresel, S.; Koswig, S.; String, A.; Hünerbein, M.; Schlag, P.M. Lymphatic mapping and sentinel lymph node biopsy in epidermoid carcinoma of the anal canal. Eur. J. Surg. Oncol. 2008, 34, 890–894. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.S.; Beukema, J.C.; van Dam, G.M.; Slart, R.; Lemstra, C.; Wiggers, T. Limited value of staging squamous cell carcinoma of the anal margin and canal using the sentinel lymph node procedure: A prospective study with long-term follow-up. Ann. Surg. Oncol. 2010, 17, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Slim, N.; Passoni, P.; Incerti, E.; Tummineri, R.; Gumina, C.; Cattaneo, G.M.; De Nardi, P.; Canevari, C.; Fiorino, C.; Ronzoni, M.; et al. Impact of sentinel lymph-node biopsy and FDG-PET in staging and radiation treatment of anal cancer patients. Sci. Rep. 2020, 10, 14613. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Mistrangelo, M.; Burtulo, G.; Passoni, P.; Slim, N.; Ronzoni, M.; Canevari, C.; Parolini, D.; Massimino, L.; Franco, P.; et al. Tailoring the radiotherapy approach in patients with anal squamous cell carcinoma based on inguinal sentinel lymph node biopsy. J. Surg. Oncol. 2021, 123, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 2011, 33, 1260–1264. [Google Scholar] [CrossRef]
- Thompson, C.F.; St John, M.A.; Lawson, G.; Grogan, T.; Elashoff, D.; Mendelsohn, A.H. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2013, 270, 2115–2122. [Google Scholar] [CrossRef]
- Garrel, R.; Poissonnet, G.; Moyà Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy Versus Neck Node Dissection in Operable T1-T2N0 Oral and Oropharyngeal Cancer. JCO 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Mahieu, R.; den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; et al. Contralateral Regional Recurrence in Lateralized or Paramedian Early-Stage Oral Cancer Undergoing Sentinel Lymph Node Biopsy-Comparison to a Historic Elective Neck Dissection Cohort. Front. Oncol. 2021, 11, 644306. [Google Scholar] [CrossRef]
- Grégoire, V.; Coche, E.; Cosnard, G.; Hamoir, M.; Reychler, H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother. Oncol. 2000, 56, 135–150. [Google Scholar] [CrossRef]
- Biau, J.; Lapeyre, M.; Troussier, I.; Budach, W.; Giralt, J.; Grau, C.; Kazmierska, J.; Langendijk, J.A.; Ozsahin, M.; O’Sullivan, B.; et al. Selection of lymph node target volumes for definitive head and neck radiation therapy: A 2019 Update. Radiother. Oncol. 2019, 134, 1–9. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; Verheij, M.; van den Brekel, M.W.M. Elective unilateral nodal irradiation in head and neck squamous cell carcinoma: A paradigm shift. Eur. J. Cancer 2017, 82, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; van Werkhoven, E.; Navran, A.; Karakullukcu, B.; Hamming-Vrieze, O.; Machiels, M.; van der Velden, L.A.; Vogel, W.V.; Klop, W.M. Contralateral regional recurrence after elective unilateral neck irradiation in oropharyngeal carcinoma: A literature-based critical review. Cancer Treat. Rev. 2017, 59, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Overgaard, M.; Grau, C. Morbidity after ipsilateral radiotherapy for oropharyngeal cancer. Radiother. Oncol. 2007, 85, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Jellema, A.P.; Slotman, B.J.; Doornaert, P.; Leemans, C.R.; Langendijk, J.A. Unilateral versus bilateral irradiation in squamous cell head and neck cancer in relation to patient-rated xerostomia and sticky saliva. Radiother. Oncol. 2007, 85, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Manikantan, K.; Khode, S.; Sayed, S.I.; Roe, J.; Nutting, C.M.; Rhys-Evans, P.; Harrington, K.J.; Kazi, R. Dysphagia in head and neck cancer. Cancer Treat. Rev. 2009, 35, 724–732. [Google Scholar] [CrossRef]
- Spencer, C.R.; Gay, H.A.; Haughey, B.H.; Nussenbaum, B.; Adkins, D.R.; Wildes, T.M.; DeWees, T.A.; Lewis, J.S.; Thorstad, W.L. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer 2014, 120, 3994–4002. [Google Scholar] [CrossRef]
- de Veij Mestdagh, P.D.; Jonker, M.C.J.; Vogel, W.V.; Schreuder, W.H.; Donswijk, M.L.; Klop, W.M.C.; Al-Mamgani, A. SPECT/CT-guided lymph drainage mapping for the planning of unilateral elective nodal irradiation in head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2018, 275, 2135–2144. [Google Scholar] [CrossRef]
- de Veij Mestdagh, P.D.; Janssen, T.; Lamers, E.; Carbaat, C.; Hamming-Vrieze, O.; Vogel, W.V.; Sonke, J.J.; Al-Mamgani, A. SPECT/CT-guided elective nodal irradiation for head and neck cancer: Estimation of clinical benefits using NTCP models. Radiother. Oncol. 2019, 130, 18–24. [Google Scholar] [CrossRef]
- de Veij Mestdagh, P.D.; Walraven, I.; Vogel, W.V.; Schreuder, W.H.; van Werkhoven, E.; Carbaat, C.; Donswijk, M.L.; van den Brekel, M.W.M.; Al-Mamgani, A. SPECT/CT-guided elective nodal irradiation for head and neck cancer is oncologically safe and less toxic: A potentially practice-changing approach. Radiother. Oncol. 2020, 147, 56–63. [Google Scholar] [CrossRef]
- de Veij Mestdagh, P.D.; Schreuder, W.H.; Vogel, W.V.; Donswijk, M.L.; van Werkhoven, E.; van der Wal, J.E.; Dirven, R.; Karakullukcu, B.; Sonke, J.J.; van den Brekel, M.W.M.; et al. Mapping of sentinel lymph node drainage using SPECT/CT to tailor elective nodal irradiation in head and neck cancer patients (SUSPECT-2): A single-center prospective trial. BMC Cancer 2019, 19, 1110. [Google Scholar] [CrossRef] [PubMed]
- Novikov, S.N.; Krzhivitskii, P.I.; Radgabova, Z.A.; Kotov, M.A.; Girshovich, M.M.; Artemyeva, A.S.; Melnik, Y.S.; Kanaev, S.V. Single photon emission computed tomography-computed tomography visualization of sentinel lymph nodes for lymph flow guided nodal irradiation in oral tongue cancer. Radiat. Oncol. J. 2021, 39, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Longton, E.; Lawson, G.; Bihin, B.; Mathieu, I.; Hanin, F.X.; Deheneffe, S.; Vander Borght, T.; Laloux, M.; Daisne, J.F. Individualized Prophylactic Neck Irradiation in Patients with cN0 Head and Neck Cancer Based on Sentinel Lymph Node(s) Identification: Definitive Results of a Prospective Phase 1-2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 652–661. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).