Simple Summary
According to the Society for Immunotherapy of Cancer, primary resistance to immune checkpoint inhibitor (ICI) treatment is defined as progression of disease within 6 months of ICI treatment with patients receiving at least 6 weeks of ICI monotherapy. We evaluated factors predictive of primary resistance to ICI monotherapy in 108 advanced non-small-cell lung cancer patients. The prevalence of primary resistance was 54.6%. The majority of patients were male, smokers, received pembrolizumab and had adenocarcinoma histology. We found that female gender, an elevated neutrophil-to-lymphocyte ratio of ≥3 at 6 weeks and a later line of immunotherapy treatment (≥2 lines) were key factors in predicting primary resistance to ICI monotherapy in advanced NSCLC.
Abstract
Introduction: Primary resistance to immune checkpoint inhibitors (ICI) is observed in routine clinical practice. We sought to determine factors predictive of primary resistance to ICI monotherapy, defined by the Society for Immunotherapy of Cancer (SITC) as progression within 6 months of ICI treatment with patients receiving at least 6 weeks of ICI monotherapy, in patients with advanced non-small-cell lung cancer (NSCLC). Method: Patients with stage IV NSCLC treated with at least 6 weeks of single-agent ICI at two tertiary hospitals in Singapore were included. A multivariate logistic regression model was utilised to elucidate factors predictive of primary resistance to ICI. Results: Of the 108 eligible patients, 59 (54.6%) experienced primary resistance. The majority were male (65.7%), smokers (66.3%), Chinese (79.6%), had adenocarcinoma (76.9%), received Pembrolizumab (55.6%) and received immunotherapy treatment in the later line setting (≥2 lines) (61.1%). Female gender (aOR = 3.16, p = 0.041), a sixth-week neutrophil-to-lymphocyte ratio (NLR) of ≥3) (aOR = 3.454, p = 0.037) and a later line of immunotherapy treatment (≥2 lines) (aOR = 2.676, p = 0.040) were factors predictive of primary resistance to ICI monotherapy in patients with advanced NSCLC. Conclusions: Using SITC criteria, an elevated NLR (≥3) at 6 weeks, female gender and a later line of immunotherapy treatment (≥2 lines) were predictive factors of developing primary resistance to ICI monotherapy in patients with advanced NSCLC.
1. Introduction
The field of immune checkpoint inhibitors (ICIs) has evolved rapidly in the last decade for lung cancer. In 2016, Reck and colleagues first reported the superiority of pembrolizumab versus platinum-doublet chemotherapy in treatment-naïve metastatic NSCLC with a programmed death ligand-1 (PDL-1) tumour proportion score (TPS) of ≥50%, with an improved objective response rate (ORR), improved overall survival (OS) and progression-free survival (PFS) being found [1,2]. More recently, atezolizumab and cemiplimab both showed an OS and PFS benefit compared with chemotherapy in treatment-naïve advanced NSCLC with high PD-L1 expression [3,4]. The safety profile favoured ICI compared to platinum-doublet chemotherapy, with improved quality of life and a delay in the deterioration of symptoms being found [5]. However, despite the initial impressive results from these studies, disease progression as the best response remains a major clinical problem in patients with advanced NSCLC, with a reported frequency of 21–27% amongst advanced NSCLC with first-line ICI [1,6,7], 7–18% amongst those with first-line ICI in combination with chemotherapy [8,9,10,11], and 20–44% in the pre-treated setting with ICI monotherapy [12,13,14,15,16].
Clinical definitions of primary resistance to ICI lack consistency across the field. An earlier study has defined it as disease progression using RECIST criteria upon the first CT evaluation or death prior to the first CT evaluation [17], whilst another paper defined it as a failure to ever respond [18]. The Immunotherapy Resistance Taskforce was formed by the Society for Immunotherapy of Cancer (SITC) to develop a consensus on the definition of resistance to ICIs. Primary resistance, as established by the SITC, is defined as progression within 6 months of ICI therapy. Patients must have received at least 6 weeks of ICI monotherapy with the best response of progressive disease or stable disease [19].
Using the above definition, we performed a retrospective analysis of advanced NSCLC patients who received ICI monotherapy across two tertiary institutions in Singapore to identify factors predictive of primary resistance to ICI. While the molecular mechanisms of resistance to ICIs have been studied and described extensively [12,20,21], studies on clinical factors predictive of primary resistance are lacking. In this study, we included advanced NSCLC patients on ICI monotherapy only to minimize treatment heterogeneity in the study population, and remained consistent with the SITC definition of primary resistance, where only patients treated with systemic anti-PD1 or anti-PD-L1 monotherapy were included [19].
2. Methodology
2.1. Study Design and Participants
Patients with stage IV non-small-cell lung cancer, treated with at least 6 weeks of a single-agent immune checkpoint inhibitor at National University Cancer Institute Singapore and Tan Tock Seng Hospital were included and retrospectively analysed. We defined progression of disease using RECIST criteria based on the first surveillance scan. The study was approved by the National Healthcare Group Domain Specific Review Board (reference number: 2017/01254). Informed consent was obtained from all patients who were alive whereas a waiver of consent was obtained for patients who had deceased prior to 29 February 2020. The authors declare that the study was conducted in accordance with the Helsinki Declaration as revised in 2013. Primary resistance was defined as progression within 6 months of ICI therapy. Patients must have had progressive disease or stable disease as the best response per the SITC definition [19]. Patients with a dual ICI combination, ICI–chemotherapy combination and those who discontinued treatment early due to toxicities were excluded.
2.2. Data Collection
Demographic, clinical, laboratory, treatment and outcome data were extracted from the electronic health records using a standardised data collection form. Demographic data including age, gender, ethnicity and clinical data, such as Eastern Cooperative Oncology Group (ECOG) performance status, body mass index (BMI), histology, EGFR mutation status, PD-L1 tumour proportion score (TPS), smoking status, and presence of brain metastases, were collected. Treatment data on the type, dose, duration and number of cycles of ICI, the line of treatment and other cancer treatment such as chemotherapy, radiotherapy or targeted therapy prior to or after ICI monotherapy were also collected. Laboratory data on the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) at the baseline and at the 6th week were calculated using the formula absolute neutrophil count/absolute lymphocyte count and platelet count/absolute lymphocyte count, respectively. NLR data were analysed as a continuous variable or dichotomised into prespecified cut-offs for ≥3 or <3 [22]. PLR data were analysed as a continuous variable or dichotomised into prespecified cut-offs of ≥180 or <180 [22]. Outcome data of the objective response rate and overall survival were analysed. The objective response rate was assessed, per the criteria used by the SITC taskforce, the RECIST 1.1 criteria [19]. While immune RECIST (iRECIST) has been specifically developed to address issues of mixed responses or pseudo-progression while on immune checkpoint inhibitors, it requires additional validation in assessing the efficacy of anti-PD(L)1 therapy in registration trials [23]. Overall survival was defined as the time of ICI initiation to death from any cause.
2.3. Outcomes
The primary outcome is the prevalence of primary resistance to immune checkpoint inhibitors and factors predictive of primary resistance. The secondary outcome includes OS. Pre-identified variables of interest include age, gender, ECOG performance status, ethnicity, BMI, histology type, line of treatment, epidermal growth factor receptor (EGFR) mutation status, PD-L1 TPS, brain metastases, baseline and 6th week NLR and PLR and the presence of primary resistance.
2.4. Statistical Analysis
Baseline differences between patients were compared using the Mann–Whitney U test for continuous variables and Fisher’s exact test or Pearson’s χ2 test for categorical variables.
A multivariable logistic regression model was utilised to determine the risk factors predictive of primary resistance to ICI. Variables with a p-value of <0.1 upon univariable logistic regression and with less than 10% missing data were included in the multivariable logistic regression model. The strength of fit (discrimination) and goodness-of-fit (calibration) of the logistic model were assessed using methods described by Lemeshow and Hosmer and with confidence intervals derived from bootstrap validation.
Overall survival was defined as the start date of ICI to the date of death from any cause. The difference in overall survival between patients with primary resistance and no primary resistance was analysed using the Kaplan–Meier method, log-rank test and univariable Cox proportional hazard regression model. Follow-up time was evaluated using the reverse Kaplan–Meier method.
All analyses were conducted in R-4.1.0, and a two-sided p-value of <0.05 was considered statistically significant.
3. Results
Between June 2014 to October 2021, we recruited 222 advanced NSCLC patients treated with immune checkpoint inhibitors at the National University Cancer Institute Singapore and Tan Tock Seng Hospital. Sixty-seven patients discontinued treatment early (<6 weeks) due to adverse events. Forty-three patients received the immunotherapy and chemotherapy combination, while 4 patients had dual immune checkpoint inhibitors. There were one hundred and eight patients who were treated with ICI monotherapy for at least 6 weeks and these patients were included for analysis. All patients did not have a prior immune checkpoint inhibitor in their treatment course. Amongst them, 59 (54.6%) encountered primary resistance (Figure 1).
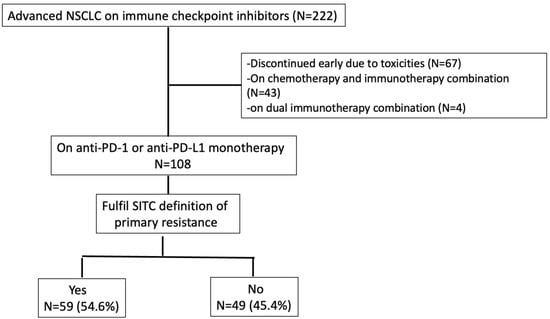
Figure 1.
Consort diagram.
The baseline characteristics of 108 patients included are summarised in Table 1. The median age was 64. Notably, the majority of patients were male (n = 71 [65.7%]), Chinese (n = 86 [79.6%]), smokers (n = 69 [66.3%]), had an ECOG performance status of ≥1 (n = 50 [65.8%]) had an adenocarcinoma histology (n = 83 [76.9%]), were treated with pembrolizumab (n = 60 [55.6%]), and had received immunotherapy treatment in the later line setting (≥2 lines) (n = 66 [61.1%]) (Table 1). Amongst those with primary resistance, 76.3% (n = 45) had progression of disease as the best response, whilst 23.7% (n = 14) had stable disease of less than a 6-month duration. Compared to patients without primary resistance, patients with primary resistance had a higher frequency of progressive disease as the best response (p < 0.001).

Table 1.
Patient demographics.
Compared to patients without primary resistance, more females (p = 0.018), patients treated at later lines (p = 0.034) and patients with EGFR+ tumours (p = 0.018) encountered primary resistance. Patients with primary resistance were also noted to have a significantly lower sixth-week lymphocyte count, a higher sixth-week neutrophil-to-lymphocyte ratio (NLR) when dichotomised at 3 and 5, and a sixth-week platelet to lymphocyte ratio (PLR) when dichotomised at 180 (Table 1).
Upon the univariable logistic regression, female gender (p = 0.020), line of treatment (p = 0.007), the presence of EGFR+ tumours (p = 0.019), pre-treatment lymphocyte count (p = 0.017), sixth-week lymphocyte count (p = 0.017), and sixth-week NLRs of ≥3 (p = 0.003) and ≥5 (p = 0.028) were significantly associated with development of primary resistance (Table 2).

Table 2.
Univariate and multivariate logistic regression.
The multivariable logistic regression model exhibited acceptable discrimination (AUC = 0.749, 95% confidence interval (CI) = 0.643–0.84 computed with 2000 stratified bootstrap replicates; Figure 2). There was no evidence to reject the null hypothesis that the model fit the data (Hosmer–Lemeshow test: χ2 = 1.309, p = 0.995) (Figure 2). Upon the multivariable logistic regression, female gender (aOR = 3.165, 95% CI = 1.078–10.101, p = 0.041), a sixth-week NLR of ≥ 3 (aOR = 3.454, 95% CI = 1.102–11.643, p = 0.037), and a later line of immunotherapy treatment (≥2 lines) (aOR = 2.676, 95% CI = 1.056–7.008, p = 0.040) remained predictive factors of primary resistance to ICI monotherapy in patients with advanced NSCLC. While lymphocyte counts at pre-treatment and at the sixth week were both significantly associated with primary resistance upon univariate analysis, only the sixth-week lymphocyte count was included in the multivariate logistic regression model in view of a larger magnitude of treatment effect. EGFR mutation was also not included in the multivariate analysis in view of the high proportion of missing data, where 29 patients had an unknown EGFR mutation status.
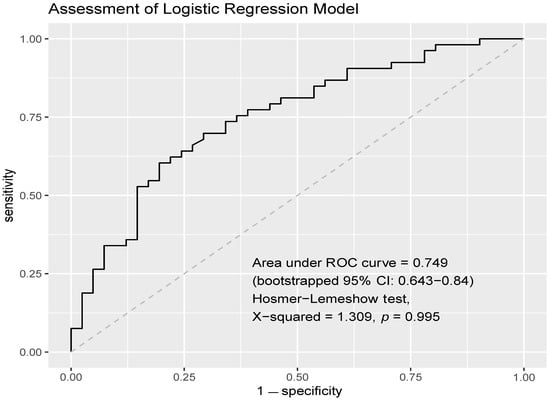
Figure 2.
AUROC of multivariate logistic regression model. The multivariable logistic regression model exhibited acceptable discrimination (AUC = 0.749, 95% CI = 0.643–0.84). There was no evidence to reject the null hypothesis that the model fits the data (Hosmer–Lemeshow test: χ2 = 1.309, p = 0.995).
The median duration of ICI therapy was 2.7 months (82 days) for patients with primary resistance compared to 11.7 months (352 days) for those without primary resistance (p < 0.001). The median duration of follow-up was 49.2 months. Patients with primary resistance had a significantly shorter survival duration compared to those without primary resistance (unadjusted HR = 0.264, 95% CI = 0.163–0.427, p < 0.0001; median survival time = 9.76 vs. 19.33 months) (Supplementary Figure S1).
4. Discussion
Reports on the clinical characteristics of primary resistance to ICIs among NSCLC patients have been sparse. In a study of 93 pre-treated advanced NSCLC patients in the United States who received ICI monotherapy, never-smokers or those who smoked fewer pack years, more involved metastatic sites, more prior therapies and a lower mean albumin level were reported as factors predictive of primary resistance to ICI therapy [17]. The authors reported a primary resistance in 38.7% of patients and defined primary resistance as progressive disease upon the first radiological evaluation or death prior to the first CT evaluation [17]. A retrospective study in France conducted by Bernichon and colleagues involving 96 NSCLC patients treated with nivolumab did not identify any clinical factors that were predictive of primary or secondary resistance [24]. Factors associated with the response to ICIs have been described amongst patients with advanced NSCLC. An exploratory analysis of 268 NSCLC patients treated with anti-PD-1 therapy at the Princess Margaret Cancer Centre in Canada found that current and former smokers had significantly higher response rates compared to non-smokers [25]. Similarly, the increase in smoking years was also associated with positive anti-PD-1 therapy response [26]. A high Patras Immunotherapy Score (PIOS) (calculated using the formula performance status x body mass index/lines of treatment x age) was also reported to be associated with better response with anti-PD-1 treatment in advanced NSCLC [27]. Our study is one of the largest retrospective studies involving 108 advanced NSCLC patients treated in Asia. We had a homogenous population, with all patients being treated with anti-PD1 or anti-PD-L1 monotherapy. In addition, we adhered to a standardised definition of primary resistance per the SITC, a key feature absent in earlier studies. While there have been several definitions of primary resistance, we chose the SITC definition as it was generated by a multistakeholder taskforce comprising experts in cancer immunotherapy from academia, industry and the US government, with the goal to provide guidance for clinical trial design and analyses surrounding mechanisms of resistance to immune checkpoint inhibitors [19]. To our knowledge, this is the first study evaluating ICI resistance using the SITC definition. In our study, clinical factors were studied in detail, along with peripheral blood markers. We found that female gender, elevated NLR at six weeks and line of treatment were key factors in predicting primary resistance to ICI.
Progression of disease as the best response has been reported at a frequency of 21–27% amongst advanced NSCLC upon first-line ICI monotherapy [1,6,7] and at a frequency of 20–44% in the second-line setting with ICI monotherapy [12,13,14,15,16]. In our study, the frequencies of primary resistance amongst those treated in the first- and second-line settings were 27.1% and 32.2%, respectively—a finding similar to that reported in the literature. Overall, more than half (54.6%) of the patients encountered primary resistance. This frequency is higher than that reported in the literature. One reason that could explain this is the relatively high proportion of heavily pre-treated patients in our cohort. Approximately 30% of patients had received ICI in the third line setting and beyond for their advanced cancer.
Female gender predicted for primary resistance to ICI monotherapy in NSCLC patients in our study. Whether or not gender difference confers a survival difference to ICI therapy remains unclear. Some studies report that females have an increased survival benefit [28], while others have supported favourable outcomes in men compared to women [29,30]. A meta-analysis by Conforti and colleagues involving 1672 advanced NSCLC patients found that anti-PD1 or anti-PD-L1 monotherapy was highly effective in men but not in women, even in patients expressing high PD-L1 levels [31]. A recent study of 9000 NSCLC patients, however, found that ICI confers a similar survival benefit regardless of gender [32]. In contrast, a pooled analysis reported that women with advanced NSCLC derived a larger benefit from the addition of chemotherapy to anti-PD-1/anti-PD-L1 compared to men [33]. From the biological perspective, several mechanisms have been postulated to explain the gender differences. Firstly, immune responses between men and women differ, with women exhibiting a higher efficiency of antigen-presenting cells and macrophage activation, and higher levels of B cells, antibody production, CD4+ T-cells and T helper 2 cell response, while men expressed higher levels of CD8+ T-cells, regulatory T-cells and Th1 cell response [34]. Secondly, immune response can be modulated by hormones including oestrogen, progesterone and testosterone [35,36]. Gender differences in gut microbiome composition may also impact immune competency [37]. Lastly, the difference in expression of X-linked immune-related genes such as TLR7, TLR9, IL-2, IL-4 and IL-15 has been reported to drive the difference in response to ICI between the two genders [38]. As there is a lower representation of women in clinical trials [32], future studies involving a larger number of female patients can help shed light on the impact of gender as a predictor of ICI response in NSCLC.
Elevated NLR at the sixth week was also a predictor of primary resistance to ICI monotherapy in advanced NSCLC. Systemic inflammation plays a crucial role in tumour development and has been associated with prognosis in solid tumours due to its effect on the immune response to the disease [39,40]. Neutrophil-to-lymphocyte ratio (NLR) has been described as a marker of the general immune response to stress stimuli [41,42]. Earlier studies have reported that an elevated neutrophil-to-lymphocyte ratio (NLR) was associated with poorer outcomes in NSCLC treated with ICIs [22,43,44,45,46]. In a multi-centre retrospective study of 466 NSCLC patients across Europe, Mezquita and colleagues reported that a pre-treatment NLR of > 3 was correlated with a worse outcome for ICI, but not for chemotherapy [46]. Similarly, a meta-analysis of 21 studies involving 1845 NSCLC patients demonstrated that a high pre-treatment NLR and platelet-to-lymphocyte ratio (PLR) were associated with poorer outcomes in patients treated with ICIs [47]. In our study, whilst baseline NLR was not a predictive factor, elevated NLR at the sixth week was predictive of primary resistance to ICI therapy. This finding is consistent with earlier reports. A retrospective study in Korea found that a high post-treatment NLR of ≥5 was associated with poor prognosis in advanced NSCLC patients receiving an anti-PD1 inhibitor [48]. Similarly, a retrospective study of 41 small-cell lung cancer patients in China found that a post treatment NLR of ≥5 was associated with a shorter PFS with ICI therapy in the second line or later-line setting [49]. Taken together, NLR is a simple blood-based biomarker that can help predict resistance to ICI therapy amongst lung cancer patients. Future prospective studies are warranted to validate its use in the clinical setting.
In this study, lower pre-treatment and sixth-week lymphocyte counts were significantly associated with a risk of primary resistance to ICI upon univariate analysis. The sixth-week lymphocyte count was eventually included in the multivariate logistic regression model in view of a larger magnitude of treatment effect. An earlier retrospective study conducted at Johns Hopkins hospital demonstrated that a lower absolute pre-treatment lymphocyte count was associated with less clinical benefits with anti-PD-1 therapy in recurrent or metastatic head and neck squamous cell carcinomas [50]. Although the sixth-week lymphocyte count did not reach statistical significance upon multivariate logistic regression analysis in our study, both pre-treatment and sixth-week lymphocyte count remain potential biomarkers that can be explored in future immunotherapy trials.
Thirty-nine percent of patients received ICI monotherapy in the first line in this study, while the rest of the patients were treated in the second-line setting and beyond. Notably, 32.4% of the patients received ICI in the third-line setting and beyond. As this study recruited patients treated with ICI monotherapy between June 2014 and October 2021, and many of the heavily pre-treated patients were recruited before ICI was established as a standard first- or second-line treatment in advanced NSCLC. We reported that a later line of immunotherapy treatment (≥2 lines) was one of the key predictors of primary resistance to ICIs. This finding is consistent with that of prior studies, showing poorer response rates in subsequent lines of therapies in advanced NSCLC. An earlier study on advanced NSCLC using ICI monotherapy also confirmed the same finding [17].
We acknowledge several limitations. Firstly, this was a retrospective study conducted across two institutions. Secondly, the sample size was relatively small at 108 patients. Thirdly, the population studied was heterogeneous; we included patients who received front-line and later-line immunotherapy treatment, patients of all PD-L1 TPS were studied and 19 patients carried an EGFR mutation. Fourthly, while the SITC required a confirmatory CT scan 4 weeks after the initial progression of the disease, none of our patients had a confirmatory second CT scan. Progression of disease was assessed at the first radiological assessment. Lastly, this study also did not include coupling of genomic data in predicting primary resistance to ICI in our patients. Nonetheless, to our knowledge, this is the first study reporting factors predictive of primary resistance among advanced NSCLC patients, while adhering to a uniform SITC definition of primary resistance.
Mechanisms behind primary resistance to ICI have been extensively studied, spanning from tumour factors (intrinsic and extrinsic factors) to host factors [12,20,21,51]. Tumour-intrinsic mechanisms include a lack of tumour immunogenicity (low tumour mutational burden (TMB), heterogeneous antigens, and mutation of certain genes), loss of tumour antigen expression, loss of HLA expression, aberration in signalling pathways such as those of mitogen-activated protein kinase (MAPK), PI3K, WNT, and IFN, and constitutive PD-L1 expression [21,52,53,54,55]. Extrinsic factors, on the other hand, involve components other than tumour cells within the tumour microenvironment. These include the presence of regulatory T-cells which suppress the effector T-cell response [56,57], myeloid-derived suppressor cells which are implicated in promoting tumour angiogenesis, cell invasion and metastases [58] and tumour-associated M2 macrophages which can affect the response to ICI therapy [59,60]. Other extrinsic factors that have been described include T-cell related factors (alternative immune checkpoints, T-cell exhaustion and phenotype alteration, T-cell receptor (TCR) repertoire, and epigenetic modification), cytokines and metabolites (e.g., TGF-B, adenosine) related to the tumour micro-environment [21,61,62]. Host-related characteristics leading to primary resistance including alterations in the gut microbiome, antibiotic use, inflammation state and autoimmunity have also been described [63,64].
Currently, to reduce the rate of primary resistance to ICI, therapeutic strategies have included the addition of chemotherapy to ICI therapy upfront [8,9] or, upon ICI progression (ClinicalTrials.gov identifier: NCT03793179), combining dual ICIs with or without chemotherapy [65,66], and adding novel agents or targeted therapies to ICI [12]. To enhance precision medicine and personalised cancer immunotherapy, advances in biomarker development are currently ongoing together with efforts in understanding the resistance mechanisms against ICIs.
In conclusion, in this retrospective study of 108 advanced NSCLC patients receiving ICI monotherapy, an elevated NLR at the sixth week, female gender and a later line of immunotherapy treatment (≥2 lines) were predictors of primary resistance to ICI therapy. Future larger studies are warranted to validate our key findings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers15102733/s1. Figure S1: Overall survival of patients with and without primary resistance. Patients with primary resistance had shorter overall survival compared to those without primary resistance, with median overall survival of 9.76 months vs. 19.33 months (unadjusted HR = 0.264, 95% CI = 0.163–0.427, p < 0.0001).
Author Contributions
Conceptualisation, Y.H. and R.A.S.; methodology, Y.H., J.J.Z., Y.Y.S. and R.A.S.; formal analysis, J.J.Z. and Y.Y.S.; investigation and resources, Y.H., A.S.C.W., Y.A., A.K., S.H.T., F.A., L.D.B. and B.C.G.; writing, Y.H., J.J.Z., Y.Y.S. and R.A.S.; review and editing, Y.H. and R.A.S.; Supervision, R.A.S.; project administration, Y.H., A.S.C.W., Y.A., A.K., S.H.T., F.A., B.C.G. and R.A.S.; funding acquisition: Y.H. and A.S.C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Research Foundation, Singapore, and National Medical Research Council (NMRC), Singapore, under its NMRC Centre Grant Programme (NMRC/CG/M005/2017_NCIS) and the National University Hospital System Seed Fund (NUHSRO/2021/100/RO5+6/Seed-Sept/03).
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and approved by the National Healthcare Group Domain Specific Review Board (reference number: 2017/01254).
Informed Consent Statement
Informed consent was obtained from all patients who were alive whereas a waiver of consent was obtained for patients who had deceased prior to 29 February 2020.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to thank all patients who have participated in the study, and Felly Ng, Lena Kwok, Priscilla Chong and Xu Xiaoying for the administrative support of the study.
Conflicts of Interest
All authors have no conflict of interest to declare except BC Goh and Ross Soo. Ross Soo received research funding from AstraZeneca and Boehringer Ingelheim, and received an honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Merck, Roche, Takeda, Yuhan, Novartis, Lilly, Amgen and Puma. BC Goh received an honorarium from Novartis, MSD, and Bayer Healthcare and provided consultancy for Bayer Healthcare, MSD and Adagene. He also has stock ownership in Gilead Sciences and Merus Therapeutics.
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-Related Quality-of-Life Results for Pembrolizumab versus Chemotherapy in Advanced, PD-L1-Positive NSCLC (KEYNOTE-024): A Multicentre, International, Randomised, Open-Label Phase 3 Trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in Combination with Carboplatin plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Soo, R.A. Resistance to Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Biomarkers and Therapeutic Strategies. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937902. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Shah, S.; Wood, K.; Labadie, B.; Won, B.; Brisson, R.; Karrison, T.; Hensing, T.; Kozloff, M.; Bao, R.; Patel, J.D.; et al. Clinical and Molecular Features of Innate and Acquired Resistance to Anti-PD-1/PD-L1 Therapy in Lung Cancer. Oncotarget 2018, 9, 4375–4384. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining Tumor Resistance to PD-1 Pathway Blockade: Recommendations from the First Meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Zhou, Q. The Resistance Mechanisms of Lung Cancer Immunotherapy. Front. Oncol. 2020, 10, 568059. [Google Scholar] [CrossRef]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. IRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Bernichon, E.; Tissot, C.; Bayle-Bleuez, S.; Rivoirard, R.; Bouleftour, W.; Forest, F.; Tinquaut, F.; Mery, B.; Fournel, P. Predictive Resistance Factors in Lung Cancer Patients Treated with Nivolumab. Retrospective Study. Bull. Cancer 2021, 108, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.N.; Karim, K.; Sung, M.; Le, L.W.; Lau, S.C.M.; Sacher, A.; Leighl, N.B. Tobacco Exposure and Immunotherapy Response in PD-L1 Positive Lung Cancer Patients. Lung Cancer 2020, 150, 159–163. [Google Scholar] [CrossRef]
- Chiu, M.; Lipka, M.B.; Bhateja, P.; Fu, P.; Dowlati, A. A Detailed Smoking History and Determination of MYC Status Predict Response to Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 55–60. [Google Scholar] [CrossRef]
- Dimitrakopoulos, F.-I.; Nikolakopoulos, A.; Kottorou, A.; Kalofonou, F.; Liolis, E.; Frantzi, T.; Pyrousis, I.; Koutras, A.; Makatsoris, T.; Kalofonos, H. PIOS (Patras Immunotherapy Score) Score Is Associated with Best Overall Response, Progression-Free Survival, and Post-Immunotherapy Overall Survival in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC) Treated with Anti-Program Cell Death-1 (PD-1) Inhibitors. Cancers 2020, 12, 1257. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between Sex and Efficacy of Immune Checkpoint Inhibitors (PD-1 and CTLA-4 Inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer Immunotherapy Efficacy and Patients’ Sex: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Corti, C.; Bagnardi, V.; Queirolo, P.; Catania, C.; De Pas, T.; Giaccone, G. Sex-Based Differences in Response to Anti-PD-1 or PD-L1 Treatment in Patients with Non-Small-Cell Lung Cancer Expressing High PD-L1 Levels. A Systematic Review and Meta-Analysis of Randomized Clinical Trials. ESMO Open 2021, 6, 100251. [Google Scholar] [CrossRef] [PubMed]
- Madala, S.; Rasul, R.; Singla, K.; Sison, C.P.; Seetharamu, N.; Castellanos, M.R. Gender Differences and Their Effects on Survival Outcomes in Lung Cancer Patients Treated With PD-1/PD-L1 Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Clin. Oncol. 2022, 34, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; Viale, G.; De Pas, T.; Pagan, E.; Pennacchioli, E.; Cocorocchio, E.; Ferrucci, P.F.; De Marinis, F.; et al. Sex-Based Heterogeneity in Response to Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Dinesh, R.K.; Hahn, B.H.; Singh, R.P. PD-1, Gender, and Autoimmunity. Autoimmun. Rev. 2010, 9, 583–587. [Google Scholar] [CrossRef]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Estrogen-Mediated Immunomodulation Involves Reduced Activation of Effector T Cells, Potentiation of Treg Cells, and Enhanced Expression of the PD-1 Costimulatory Pathway. J. Neurosci. Res. 2006, 84, 370–378. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Fish, E.N. The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z.; Jablonska, J. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediators Inflamm. 2015, 2015, 701067. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral Blood Markers Predictive of Outcome and Immune-Related Adverse Events in Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers in Patients with Non-Small Cell Lung Cancer (NSCLC) Treated with Nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, J.; Tang, S.; Sun, G. Predictive Value of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis. Int. Immunopharmacol. 2020, 85, 106677. [Google Scholar] [CrossRef]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.-W.; Heo, D.S.; Lee, J.S. Post-Treatment Neutrophil-to-Lymphocyte Ratio at Week 6 Is Prognostic in Patients with Advanced Non-Small Cell Lung Cancers Treated with Anti-PD-1 Antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef]
- Xiong, Q.; Huang, Z.; Xin, L.; Qin, B.; Zhao, X.; Zhang, J.; Shi, W.; Yang, B.; Zhang, G.; Hu, Y. Post-Treatment Neutrophil-to-Lymphocyte Ratio (NLR) Predicts Response to Anti-PD-1/PD-L1 Antibody in SCLC Patients at Early Phase. Cancer Immunol. Immunother. 2021, 70, 713–720. [Google Scholar] [CrossRef]
- Ho, W.J.; Yarchoan, M.; Hopkins, A.; Mehra, R.; Grossman, S.; Kang, H. Association between Pretreatment Lymphocyte Count and Response to PD1 Inhibitors in Head and Neck Squamous Cell Carcinomas. J. Immunother. Cancer 2018, 6, 84. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-Novo and Acquired Resistance to Immune Checkpoint Targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Morris, L.; Havel, J.J.; Makarov, V.; Desrichard, A.; Chan, T.A. The Role of Neoantigens in Response to Immune Checkpoint Blockade. Int. Immunol. 2016, 28, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Oida, T.; Zhang, X.; Goto, M.; Hachimura, S.; Totsuka, M.; Kaminogawa, S.; Weiner, H.L. CD4+CD25- T Cells That Express Latency-Associated Peptide on the Surface Suppress CD4+CD45RBhigh-Induced Colitis by a TGF-Beta-Dependent Mechanism. J. Immunol. 2003, 170, 2516–2522. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Meyer, C.; Cagnon, L.; Costa-Nunes, C.M.; Baumgaertner, P.; Montandon, N.; Leyvraz, L.; Michielin, O.; Romano, E.; Speiser, D.E. Frequencies of Circulating MDSC Correlate with Clinical Outcome of Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Immunother. 2014, 63, 247–257. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Hu, W.; Li, X.; Zhang, C.; Yang, Y.; Jiang, J.; Wu, C. Tumor-Associated Macrophages in Cancers. Clin. Transl. Oncol. 2016, 18, 251–258. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Zhang, H.; Conrad, D.M.; Butler, J.J.; Zhao, C.; Blay, J.; Hoskin, D.W. Adenosine Acts through A2 Receptors to Inhibit IL-2-Induced Tyrosine Phosphorylation of STAT5 in T Lymphocytes: Role of Cyclic Adenosine 3′,5′-Monophosphate and Phosphatases. J. Immunol. 2004, 173, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Cheng, L.; Wang, Y.; Zhang, Y.-K.; Zhao, M.-F.; Liang, G.-D.; Zhang, M.-C.; Li, Y.-G.; Zhao, J.-B.; Gao, Y.-N.; et al. Dysbiosis of the Gut Microbiome in Lung Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Zang, D.; Zhang, M.; Li, X.; Liu, D.; Gao, B.; Zhou, H.; Sun, J.; Han, X.; et al. The Role of Gut Microbiota in Lung Cancer: From Carcinogenesis to Immunotherapy. Front. Oncol. 2021, 11, 720842. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).