Simple Summary
There are diverse reports of the dual role of sirtuin genes and proteins in breast and prostate cancers. This review discusses the current information on the tumor promotion or suppression roles of SIRT1–7 in breast and prostate cancers. Precisely, we highlight that sirtuins regulate various proteins implicated in proliferation, apoptosis, autophagy, chemoresistance, invasion, migration, and metastasis of both breast and prostate cancer. We also provide evidence of the direct regulation of sirtuins by miRNAs, highlighting the consequences of this regulation in breast and prostate cancer. Overall, this review reveals the potential value of sirtuins as biomarkers and/or targets for improved treatment of breast and prostate cancers.
Abstract
Mammalian sirtuins (SIRT1–7) are involved in a myriad of cellular processes, including apoptosis, proliferation, differentiation, epithelial-mesenchymal transition, aging, DNA repair, senescence, viability, survival, and stress response. In this review, we discuss the current information on the mechanistic roles of SIRT1–7 and their downstream effects (tumor promotion or suppression) in cancers of the breast and prostate. Specifically, we highlight the involvement of sirtuins in the regulation of various proteins implicated in proliferation, apoptosis, autophagy, chemoresistance, invasion, migration, and metastasis of breast and prostate cancer. Additionally, we highlight the available information regarding SIRT1–7 regulation by miRNAs, laying much emphasis on the consequences in the progression of breast and prostate cancer.
Keywords:
sirtuins; breast; prostate; cancer; tumor; miRNA; suppression; promotion; progression; metastasis 1. Introduction
Overview of Sirtuins
The silent information regulator 2 (sir2) family of proteins, simply known as sirtuins (sir-two-ins), was first discovered in yeast, where its upregulation increased lifespan [1]. Consistent with this finding, subsequent studies revealed that sirtuins promote longevity in Caenorhabditis elegans [2] and Drosophila melanogaster [3]. As members of the highly conserved class III histone deacetylase, sirtuins, in addition to their initial description as mono-adenosine diphosphate (ADP) transferases, were later found to be a nicotinamide (NAM) adenine dinucleotide (NAD)-dependent group of enzymes [4,5]. By transferring an acetyl group to the ribose moiety of ADP, sirtuins couple the deacetylation of lysine in their substrate protein with the hydrolysis of NAD+, forming 2′-O-acetyl-ADP-ribose (o-AADPR) and releasing NAM, a feedback inhibitor of sirtuins [6,7] (Figure 1).
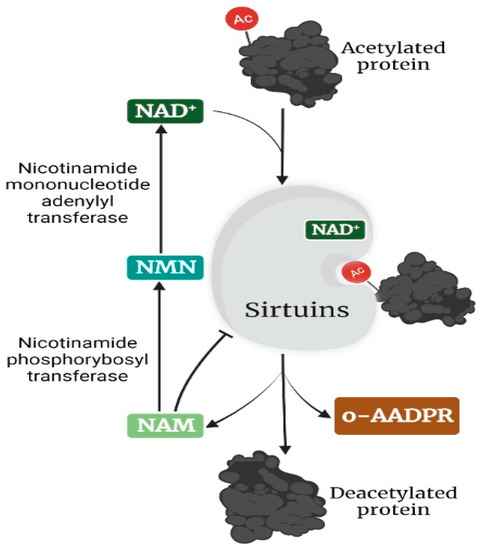
Figure 1.
Mechanism of deacetylation of targets by sirtuins. In the presence of the co-factor NAD+, the acetylated protein target is deacetylated by sirtuins, producing a deacetylated protein, o-AADPR, and NAM, a feedback inhibitor of sirtuins. NAM is reconverted through NMN to NAD+ in two enzyme-catalyzed steps. The image was created with BioRender.com.
In mammals, seven sirtuins (SIRT1–7) have been identified, which primarily function as NAD-dependent deacetylases (SIRT1–3 and SIRT5–7) [8] and ADP-ribosyl transferases (SIRT4 and 6) [9,10]. Additionally, sirtuins have been reported to function as demyristoylases (SIRT1–3 and 6) [11,12], lipoamidases (SIRT4) [13], and desuccinylases/demalonylases/deglutarylases (SIRT5) [14,15]. However, despite these functional differences, sirtuins are classified and distinguished by their subcellular location (Figure 2). SIRT1, 6, and 7 are predominantly located in the nucleus [16,17], whereas SIRT3, 4, and 5 are predominantly located in the mitochondrion [16]. Additionally, SIRT5 has been found expressed in the cytoplasm [18], while SIRT3 has been found to modulate some stress-related and nuclear-encoded mitochondrial gene expression in the nucleus [19] and can translocate from the nucleus to the mitochondrion upon cellular stress [20]. Recently, a study revealed that SIRT4 is also expressed in the cytoplasm and nucleus [21]. SIRT2 is predominantly located in the cytoplasm but can translocate to the nucleus to interact with its nuclear targets [22], especially during mitotic cell division [23]. Additionally, SIRT1 has been found to shuttle between the nucleus and cytoplasm [24,25]. According to the phylogenetic analysis of their core domains, sirtuins are placed into four classes: I (SIRT1, 2, and 3), II (SIRT4), III (SIRT5), and IV (SIRT6 and 7) [26,27].
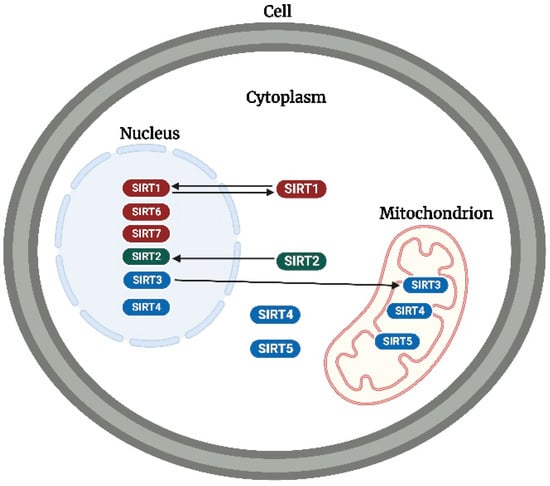
Figure 2.
Subcellular location of sirtuins. SIRT1, 6, and 7 (in red), SIRT2 (in green), and SIRT3, 4, and 5 (in blue) are predominantly located in the nucleus, cytoplasm, and mitochondrion, respectively. SIRT1, 2, and 3 can translocate to the cytoplasm, nucleus, and mitochondrion, respectively. While SIRT 3 and 5 have been detected in the nucleus and cytoplasm, respectively, SIRT4 has been detected in both the cytoplasm and nucleus. The image was created with BioRender.com.
By virtue of the above enzymatic functions, mammalian sirtuins are involved in a myriad of biological processes, including apoptosis, proliferation, differentiation, epithelial-mesenchymal transition (EMT), aging, DNA repair, senescence, viability, survival, and stress response, among other biological functions [27,28,29,30,31,32,33]. Thus, deregulation of sirtuins can be involved in perturbations found in metabolic disorders and cancer. Recently, the role of all mammalian sirtuins in metabolic disorders affecting six human tissues was studied [34]. Furthermore, sirtuins were found to play a dual role in carcinogenesis via tumor promotion and suppression [35].
Given that cancer is an increasing cause of death worldwide, numerous research efforts have been channeled toward understanding the molecular basis of the disease to develop effective treatments. In this regard, several studies have explored the role of sirtuins in the progression, migration, and metastasis of various types of cancer [28,36,37,38], and several anticancer agents that target sirtuins have been developed [39,40]. Additionally, several studies have shown the prognostic potential of sirtuins in various types of cancer [36,41,42]. Globally, cancers of the breast and prostate are among the most diagnosed types of cancer in females and males, respectively [27]. For instance, in Australia, 1 in 7 women and 1 in 6 men will be diagnosed with breast and prostate cancer, respectively, in their lifetime [28]. In the United States, cancers of the breast (31%) and prostate (27%) account for the largest proportion of newly estimated cancer cases in females and males, respectively [43]. Additionally, while the breast and prostate have unique functions, cancers occurring in these tissues share a similarity in both being hormonally driven [44] and typically having high proportions of indolent disease [45]. Thus, investigation of both prostate and breast cancer together can assist in identifying the relevance of specific gene expression differences toward cancer progression in general rather than nuanced differences between cancers arising in different tissues. Thus, in this review, we summarize and discuss the current information on the mechanistic roles of sirtuins (SIRT1–7) in breast and prostate cancer.
2. Mechanistic Roles of Sirtuins in Breast and Prostate Cancer
Sirtuins are reported to play a dual role in the pathogenesis of breast and prostate cancer via the targeting of different proteins, including transcription factors, signaling molecules, and catalytic proteins. Consequently, sirtuins have exhibited both tumor-suppressing and tumor-promoting effects in breast and prostate cancer cells (Figure 3). In this section, we discuss the mechanistic roles of nuclear (SIRT1, 6, and 7), cytoplasmic (SIRT2), and mitochondrial sirtuins (SIRT3, 4, and 5) in breast and prostate cancer (Table 1 and Table 2).
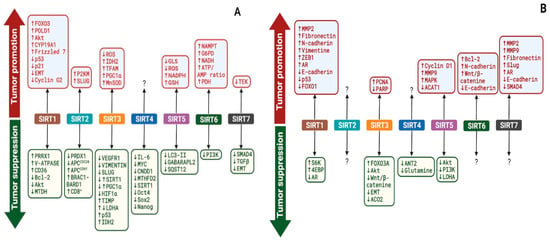
Figure 3.
Outcomes of sirtuins modulation of various targets in breast (A) and prostate (B) cancers. SIRT1–7 regulate the expression and activities of various targets in breast (A) and prostate (B) cancer, resulting in either tumor promotion or suppression. ?—Not Known. The image was created with BioRender.com.

Table 1.
Mechanistic Roles of Sirtuins (SIRT1–7) in Breast Cancer.

Table 2.
Mechanistic Roles of Sirtuins (SIRT1–7) in Prostate Cancer.
2.1. Nuclear Sirtuins in Breast Cancer
2.1.1. SIRT1
Recent studies have revealed that SIRT1 is constitutively upregulated in breast cancer cells [49,50,58]. Additionally, SIRT1 has been shown to modulate different targets in breast cancer, including transcription factors, signaling molecules, and certain enzymes, showing opposing effects by its exhibition of either tumor promotion or tumor suppression. The most prominent transcription factors targeted by SIRT1 in breast cancer include forkhead box O3 (FOXO3), paired related homeobox 1 (PRRX1), and tumor suppressor proteins (p53 and p21). For instance, Mahmud et al. demonstrated that the downstream anti-proliferative activity of acetylated FOXO3, a tumor suppressor whose activity is vulnerable to posttranslational modifications, is activated upon SIRT1 gene silencing in BT474 breast cancer cells, suggesting that SIRT1 deacetylates and inhibits FOXO3 activity to promote breast cancer [48]. They also demonstrated that SIRT1 gene silencing increases BT474 cells’ sensitivity to lapatinib treatment, further suggesting that SIRT1 promotes BT474 cells’ resistance to lapatinib treatment. The protein expression of PRRX1, a transcription factor that induces epithelial-to-mesenchymal transition (EMT) and inhibits cancer stemness, was shown to be downregulated by SIRT1 gene silencing in mouse 4T1 breast cancer cells [46]. Interestingly, SIRT1 gene silencing upregulated the expression of the EMT-related protein E-cadherin and cancer stemness markers kruppel-like factor 4 (KLF4) and aldehyde dehydrogenase 1 family member A1 (ALDH1A1) [46]. Based on their findings, the authors concluded that SIRT1 deacetylates and stabilizes PRRX1 to inhibit breast cancer stemness and metastasis. Importantly, inhibition of SIRT1 by sirtinol, a classic inhibitor of sirtuins, resulted in the activation of p53 and consequently increased p21 protein levels in SK-BR-3 breast cancer cells, thus suggesting a tumor-promoting role of SIRT1 in breast cancer [53]. Moreover, SIRT1 expression has been previously shown to downregulate tumor suppressor genes, such as cyclin G2 and p53, in estrogen-dependent breast cancer cells [59].
SIRT1 has been demonstrated to modulate several intracellular signaling protein molecules, including protein kinase B (PKB), B-cell lymphoma 2 (Bcl-2), metadherin (MTDH), Frizzled 7, EMT-related proteins, and the cluster of differentiation 36 (CD36), in breast cancer cells. In a study by Jin et al., SIRT1 was shown to directly interact with and phosphorylate PKB to promote subsequent downstream proliferative effects in MDA-MB-231 and BT-549 breast cancer cells [50]. To substantiate this finding, the authors further investigated Akt activities in SIRT1-knockdown breast cancer cells and found that proliferation was inhibited both in vitro and in vivo. Conversely, a previous study reported that SIRT1 represses estrogen receptor activation by deactivating Akt activity to suppress the growth of MCF-7 breast cancer cells [55]. These contradictory results may be due to genetic differences between the breast cancer cells used in the studies. However, further investigations are required to illuminate the possible reasons for these contradictions. The expression of Bcl-2, an anti-apoptotic or pro-survival protein, was markedly decreased in MCF-7 and MDA-MB-231 breast cancer cells following the inhibition of SIRT1 with sirtinol [51], which indicates that SIRT1 promotes the growth of breast cancer through the upregulation of the Bcl-2 protein.
Inhibition of SIRT1 with either EX-527 or SIRT1 siRNA resulted in the upregulation of c-Myc (a pro-oncogenic protein) and MTDH (an oncogenic protein) in MDA-MB-231 and BT-549 breast cancer cells. Further, in the presence of c-Myc siRNA, EX-527 could not upregulate MTDH in breast cancer cells [57]. As a mechanism, the authors suggested that the SIRT1-mediated inhibition of the proliferation of the breast cancer cells was due to the downregulation of c-Myc, which resulted in the downregulation of MTDH. Simmons et al. demonstrated that the inhibition of SIRT1 by both inhibitor VII and SIRT1 siRNA markedly downregulated the expression of Frizzled 7, a chronically activated oncogenic receptor in cancers [54]. This finding supports the tumor-promoting role of SIRT1 in breast cancer. Furthermore, another research group investigated the effect of SIRT1 inhibition by SIRT1 siRNA on EMT-related proteins (vimentin, snail-1, and E-cadherin) in MDA-MB-231, MDA-MB-436, and MDA-MB-468 breast cancer cell lines and found that the siRNA inhibition of SIRT1 downregulated the expression of vimentin (a protein required for cell migration, motility, and adhesion) and snail-1 (a repressor of E-cadherin) but upregulated the expression of E-cadherin (a cell-cell adhesion molecule), indicating that SIRT1 induces the invasion of breast cancer cells by modulating the EMT pathway [56]. On the contrary, SIRT1 overexpression by resveratrol (a classical activator of sirtuins) was reported to increase the expression of CD36 (a transmembrane receptor that regulates apoptosis and angiogenesis) to cytotoxic levels, thus inhibiting the proliferation of MCF-7 breast cancer cells [58]. Moreover, there is evidence that resveratrol induces toxicity in breast cancer cells through a SIRT1-dependent mechanism [108,109].
Interestingly, SIRT1 has also been demonstrated to regulate the expression of certain catalytic proteins in breast cancer. For instance, the transcription of CYP19A1 (a gene encoding the aromatase enzyme) was downregulated in MDA-MB-231 breast cancer cells expressing aromatase following the inhibition of SIRT1 by treatment with both cambinol and inhibitor VII [52]. This finding indicates that SIRT1 promotes the growth of estrogen-dependent breast cancer through the upregulation of CYP19A1 expression. Additionally, another study demonstrated that the knock-out of SIRT1 by shRNA reduced the proliferation, migration, and invasion of MCF-7 breast cancer cells [49]. The shRNA-mediated silencing of SIRT1 downregulated the expression of human DNA polymerase delta 1 (POLD1), a gene that encodes p125 (the catalytic domain of DNA polymerase δ) and regulates cell proliferation and the cell cycle. SIRT1 upregulated the activities of POLD1/p125 to aid the proliferation, migration, and invasion of MCF-7 breast cancer cells. Recently, a study demonstrated that CRISPR-Cas9-mediated deletion of SIRT1 promotes the release of exosomes (containing unique cargoes and hydrolases that degrade the extracellular matrix), impairs lysosomal function, and inhibits lysosomal acidification in MDA-MB-231 breast cancer cells [47]. The authors showed that the deletion of SIRT1 reduces the expression of the vacuolar-type H+ ATPase (V-ATPase) subunit responsible for proper lysosomal acidification and protein degradation. This indicates that SIRT1 upregulated V-ATPase activities to increase lysosomal function, which halted the secretion of exosomes containing hydrolases that degrade the extracellular matrix, thereby ultimately inhibiting the aggressiveness of MDA-MB-231 breast cancer cells.
2.1.2. SIRT6
Although previous studies have demonstrated that SIRT6 expression is downregulated in several human cancers [110], including invasive breast tumors [111], and that SIRT6 expression positively correlates with the survival of breast cancer patients [112], there are few studies demonstrating the mechanistic effects of SIRT6 in breast cancer. Ioris et al. demonstrated that the overexpression of SIRT6 downregulates the transcription of PI3K-controlled genes, independent of its deacetylase activity, to suppress the progression and stem cell-like capacity of PyMT-induced breast cancer in mice, thus indicating a tumor suppressor role of SIRT6 in breast cancer [79]. Conversely, a study recently demonstrated that SIRT6 acts as a tumor promoter, given that its expression promoted MCF-7 cell growth as well as resistance to oxidative stress [78]. These effects were driven by SIRT6 deacetylation and activation of nicotinamide phosphoribosyl transferase and glucose-6-phosphate dehydrogenase, which increase NADH and NADPH levels, respectively, to protect against oxidative stress. Corroborating the tumor-promoting activities of SIRT6, a recent study demonstrated that SIRT6 deletion downregulated the expression and activity of pyruvate dehydrogenase and oxidative phosphorylation-related genes, suppressed the activity of respiratory complexes, and reduced the ATP/AMP ratio in MDA-MB-231 and MCF7 breast cancer cells [80]. The above findings reveal a vital role of SIRT6 in remodeling the metabolic profile to consistently provide energy and nutrients to drive the growth of breast cancer. Moreover, low SIRT6 expression was recently found to be associated with better overall survival of patients with breast cancer [113]. Notwithstanding, more studies are required to investigate the expression level of SIRT6 and its effect on the metabolic profile of breast cancer at various stages of carcinogenesis.
2.1.3. SIRT7
The mRNA expression level of SIRT7 was found to be higher in patients with metastatic breast cancer [114]. More recently, high expression of SIRT7 was associated with poor prognosis of breast cancer [81,115] and positively correlated with increased M1 macrophage infiltration and exhaustion of T-cells in luminal breast cancer [115]. There are conflicting reports on the function of SIRT7 in breast cancer. In BT-549 and MDA-MB-231 breast cancer cells, SIRT7 deacetylated and destabilized SMAD4, resulting in reduced formation of the SMAD2/SMAD3-SMAD4 complex. On translocation to the nucleus, this complex activates EMT-related transcription factors (such as Snail1/2, Twist1/2, and Zeb1/2), thus facilitating the migration and invasion of cancer cells [116]. The authors also demonstrated that the deacetylation and destabilization of SMAD4 promote its degradation by β-TrCP1, a substrate recognition subunit of E3 ubiquitin ligase. Additionally, the authors corroborated their findings with knockout experiments, in which SIRT7 knockout with either shSIRT7 or siSIRT7 activated TGF-β signaling to promote EMT-related gene transcription and lung metastasis of breast cancer. Taken together, the authors demonstrated a tumor-suppressing role of SIRT7 in breast cancer. Furthermore, a recent study demonstrated that SIRT7 antagonizes TGF-β signaling and inhibits metastasis of breast cancer [81]. On the contrary, a more recent study revealed that SIRT7 promotes Adriamycin-induced metastasis in breast cancer by interacting with TIE2 [82], a tyrosine kinase receptor that induced the dormancy of breast cancer cells, resulting in increased resistance to chemotherapy [117]. Given the limited studies conducted to date and contradictory findings, more studies are required to provide insight into the modulation of other signaling proteins (including transcription factors) by SIRT7 causing either tumor-suppressing or tumor-promoting effects in breast cancer.
2.2. Nuclear Sirtuins in Prostate Cancer
2.2.1. SIRT1
Several studies have shown that SIRT1 is highly expressed in prostate cancer [86,87,118,119], suggesting an important role for SIRT1 in its progression. Regardless of its predominant expression in prostate cancer cells, SIRT1 has been demonstrated to either promote or suppress prostate cancer via different mechanisms. Although the mechanisms were not fully elucidated, studies reported that SIRT1 promotes cell growth and chemoresistance in PC3 and DU145 prostate cancer cells [119] as well as the migration and invasion of DU145 prostate cancer cells [120]. A mechanistic study, using PC3 prostate cancer cells with active p53, or PC3 prostate cancer cells regardless of p53 status, demonstrated that SIRT1 inhibition by SIRT1 shRNA or sirtinol downregulates the deacetylation of p53 and FOXO1, thereby activating antiproliferative responses, such as apoptosis and senescence [86]. This suggests that SIRT1 expression may promote the development of prostate cancer by deacetylating and deactivating p53 and FOXO1. Moreover, using DU145 prostate cancer cells, it was previously demonstrated that SIRT1 deacetylates and deactivates FOXO1 transcription activities [121]. To further corroborate these findings, later studies demonstrated that SIRT1 deacetylates FOXO3, a tumor suppressor transcription factor that has been shown to inhibit cell proliferation and promote apoptosis [122], to promote its poly-ubiquitination and subsequent proteasomal degradation [123]. These findings suggest that SIRT1 may mediate its tumor-promoting activities in prostate cancer by preventing the tumor-suppressing function of FOXOs. Nonetheless, animal studies are required to investigate how the tumor microenvironment of prostate cancer influences the outcome of SIRT1 activities in this regard.
The tumor-promoting effects of SIRT1 in prostate cancer have been demonstrated through the regulation of EMT-related protein expression. A 2012 study revealed that SIRT1 promotes the migration and metastasis of DU145 and PC3 prostate cancer cells by downregulating the expression of the epithelial marker E-cadherin and upregulating the expression of mesenchymal markers (N-cadherin and fibronectin) and the EMT-inducing transcription factor ZEB1 [85]. Similarly, Cui et al. [84] showed that siRNA-mediated silencing of SIRT1 expression suppressed the migration and invasion of PC3 prostate cancer cells by upregulating E-cadherin and downregulating N-cadherin and vimentin expression, thus suggesting that SIRT1 activates EMT to promote the migration and metastasis of prostate cancer. Additionally, SIRT1 can promote the migration and invasion of PC3 prostate cancer cells by antagonizing p300/CBP-associated protein (PCAF)-catalyzed MPP8-K439 acetylation, thereby preventing ubiquitin-proteasome-mediated proteolysis of M-phase phosphoprotein 8 [88], a methyl-H3K9 binding protein that promotes E-cadherin gene silencing and EMT of tumor cells.
It is well-established that androgen receptors (AR) play a pivotal role in prostate carcinogenesis [124]. In this regard, studies have investigated the effect of SIRT1 expression on AR, as a mechanism of SIRT1 in the development and progression of prostate cancer. For instance, SIRT1 was demonstrated to deacetylate AR and suppress coactivator-induced interactions between AR amino and carboxyl termini to inhibit ligand-induced (dihydrotestosterone) AR transcription activity, thereby inhibiting the growth of AR-expressing LNCaP (lymph node carcinoma of the prostate) cells [90]. Similarly, it was also demonstrated that SIRT1 deacetylates AR and histone H3 at the promoter region of the prostate-specific antigen gene to suppress AR-mediated gene transcription, thereby inhibiting the proliferation of androgen-responsive LNCaP prostate cancer cells [89]. These findings suggest that low expression of SIRT1 is particularly beneficial for the growth of androgen-responsive prostate cancer. Conversely, androgen-independent prostate cancer cells, such as PC3 and DU145, have been shown to express higher levels of SIRT1 than androgen-dependent LNCaP prostate cancer cells [87]. This may suggest a differential role of SIRT1 in various sub-types of prostate cancer. In contrast with the above-mentioned studies, a more recent study revealed that SIRT1 promotes the progression of LNCaP prostate cancer cells by upregulating AR signaling [91]. Although this contradiction may be influenced by the cancer stage or context [125], it provides opportunities for future studies to ascertain the molecules or intermediates in AR signaling that are modulated by SIRT1 in favor of the progression of prostate cancer.
SIRT1 has been shown to modulate catalytic protein activity as a part of its mechanisms of action in prostate cancer. Notably, it has been revealed that SIRT1 deacetylates and upregulates the expression of matrix metalloproteinase-2 (MMP2) [83], a zinc-dependent endopeptidase that degrades the extracellular matrix to promote the invasion of cancer cells [126]. To corroborate their finding, the authors further demonstrated that siRNA-mediated knockdown of SIRT1 reduced MMP2 protein stability and zymographic activity and consequently inhibited the invasion of PC3 prostate cancer cells. This suggests that SIRT1 upregulates MMP2 activity to promote the progression of prostate cancer. Additionally, shRNA-mediated inhibition of SIRT1 expression induces proliferation and inhibits autophagy of DU145 and PC3 prostate cancer cells by repressing the phosphorylation of p70 ribosomal S6 kinases (S6K) and 4E-binding protein 1 [87], which are downstream effectors of the mammalian target of rapamycin 1 (mTORC1)-mediated protein synthesis regulation [127]. The authors further suggested that SIRT1 promotes the tumorigenesis of prostate cancer via SIRT1/S6K-mediated inhibition of autophagy. In other words, they speculated that the deacetylation of S6K by SIRT1 may trigger its dephosphorylation, leading to a halt in the synthesis of apoptotic or autophagic proteins. To elucidate this mechanism, further studies are required to clarify/verify their claims and ascertain the specific apoptotic or autophagic proteins regulated through this axis.
2.2.2. SIRT6
Unlike in other types of cancer, including breast cancer, SIRT6 is overexpressed in prostate tumor tissues and cells (PC3, DU145, 22RV1, and LNCaP), with high SIRT6 expression correlating with poor overall survival of prostate cancer patients [96,102,103,104]. This indicates an important role of SIRT6 in prostate cancer, which needs to be fully explored. Liu et al. demonstrated that siRNA-mediated inhibition of SIRT6 induces cell cycle arrest at the sub-G1 phase, decreases Bcl-2 expression, induces apoptosis, increases DNA damage, and enhances chemotherapeutic sensitivity of PC3 and DU145 prostate cancer cells [102]. This indicates that SIRT6 promotes the survival and proliferation of prostate cancer by increasing Bcl-2 expression and preventing cell cycle arrest. To corroborate these findings, Xie et al. demonstrated that siRNA-mediated inhibition of SIRT6 reduced the EMT-protein N-cadherin and anti-EMT protein E-cadherin to promote the migration and invasion of PC3 and DU145 prostate cancer cells. Furthermore, a recent study reported that SIRT6 promotes the progression of prostate cancer by abrogating necroptosis-facilitated innate immunity [96]. Another recent study also demonstrated that SIRT6 promotes the progression of prostate cancer by negatively regulating the Wnt/β-Catenin signaling pathway, as shown by a decrease in the expression levels of c-Myc, cyclin D1, and β-Catenin [104]. To date, all studies unequivocally portray SIRT6 as a promoter of prostate cancer.
2.2.3. SIRT7
SIRT7 is overexpressed in various human cancers [128], including prostate cancer [105,106], suggesting a pivotal role of SIRT7 in the progression of prostate cancer. Malik et al. observed an inverse relationship between SIRT7 and the expression of E-cadherin in highly aggressive PC3 prostate cancer cells; that is, there was a low expression of E-cadherin and a high expression of SIRT7 [107]. To verify their findings, using SIRT7-deficient PC3 prostate cancer cells, they demonstrated that the E-cadherin protein level was upregulated, while the mesenchymal marker vimentin and EMT-inducing transcription factor slug were both downregulated [107]. Taken together, these findings indicate that SIRT7 promotes the migration and metastasis of PC3 prostate cancer cells by upregulating EMT. Similarly, corroborating the above-mentioned findings, Haider et al. demonstrated that SIRT7-knockdown reduced the migration of DU145 prostate cancer cells and that SIRT7 overexpression promoted the aggressiveness and docetaxel resistance of LNCaP prostate cancer cells by triggering EMT as demonstrated by an increase in fibronectin [105]. Overall, these findings indicate that SIRT7 overexpression induces EMT to promote the aggressiveness of both androgen-dependent (LNCaP) and androgen-independent (DU145) prostate cancer cells.
More recently, Ding et al. demonstrated that SIRT7 depletion in LNCaP and 22RV1 prostate cancer cells significantly reduces the conversion of LC3B-I to LC3B-II, a vital process that results in autophagy, and that SIRT7 knockdown downregulates vimentin, slug, MMP2, and MMP9 in 22Rv1 prostate cancer cells [106]. They further demonstrated that SIRT7 expression is positively correlated with AR signaling in both prostate cancer tissues and cells (LNCaP and 22Rv1) and that SIRT7 knockdown upregulates SMAD4, a tumor suppressor protein [129], which interacts with AR to control its signaling in prostate cancer [130]. Taken together, these findings indicate that SIRT7 expression promotes the proliferation, metastasis, and androgen-induced autophagy of prostate cancer via induction of AR signaling. Moreover, it has been previously demonstrated that androgens promote the growth of prostate cancer cells via the induction of autophagy [131], thus indicating a favorable effect of autophagy in androgen-responsive prostate cancer cells.
2.3. Cytoplasmic Sirtuins in Breast Cancer
SIRT2
Studies have shown that SIRT2 expression is downregulated in breast cancer tissues compared to normal breast tissues [62,132,133]. Furthermore, SIRT2 was shown to be predominantly involved in the tumorigenesis and prognosis of breast cancer and that, depending on the tumor grade, SIRT2 acts as either a tumor suppressor or tumor promoter in breast tumors [132]. In this regard, studies have investigated the tumor-suppressing or tumor-promoting mechanisms of SIRT2 in breast cancer. Given that SIRT2 translocates to the nucleus during mitotic cell division, Kim et al. demonstrated that SIRT2 deacetylates CDH1 and CDC20 to positively regulate the activity of the anaphase-promoting complex/cyclosome (APC/C) [62], a multi-subunit protein that mediates ubiquitination of distinctly functional substrates, including Aurora-A and -B, survivin Plk1, Nek2A, securin, and cyclins-A and -B [134]. However, some of these APC/C substrates, such as Aurora A, cyclins, and Plk1, are overexpressed in human cancers and promote tumorigenesis [135,136,137]. Hypothetically, the overexpression of the APC/C substrates in human cancers may be due to the downregulated expression of SIRT2, which may result in aberrant mitotic cell division. To verify this hypothesis, Kim et al. demonstrated that SIRT2 deficiency impairs the activity of APC/C, leading to the accumulation of mitotic regulatory proteins (including Aurora A and Plk1), mitotic catastrophe, genetic instability, and, consequently, tumorigenesis [62]. Overall, their findings indicate a tumor-suppressing role of SIRT2 in breast cancer through the promotion of normal mitosis. To further support the tumor-suppressing role of SIRT2 in breast cancer, Fiskus et al. demonstrated that SIRT2 deacetylates and inhibits the peroxidase activity of peroxiredoxin-1 (an antioxidant), thereby sensitizing MCF-7 and MDA-MB-231 breast cancer cells to the cytotoxic effects of increased reactive oxygen species and DNA damage [60].
Conversely, Park et al. demonstrated that SIRT2 deacetylates the lysine 305 (K305) residue of the pyruvate kinase M2 (PKM2) isoform to direct glycolysis and promote tumor growth [63]. To corroborate their findings, they demonstrated that SIRT2 knockout by shSIRT2 RNA altered the PKM2 activity and glycolytic metabolism, thus indicating that SIRT2 ensures the growth and survival of breast cancer through the regulation of glycolysis and PKM2 activity. Additionally, using MCF10A breast cancer cells, Zhou et al. demonstrated that SIRT2 promotes the growth and aggressiveness of basal-like breast cancer by deacetylating the K116 domain of slug [61], a tumor promoter protein that, upon stabilization, facilitates tumor progression and metastasis through EMT, leading to loss of cell adhesion, as well as the enhancement of migratory and invasive properties of tumors [138]. As a mechanism, the authors demonstrated that the protein slug is stabilized by the deacetylation of its K116 domain. Moreover, the stabilization of slug has been shown to prevent its proteasomal degradation, facilitate its transcriptional repression of E-cadherin, and potentiate its anti-apoptotic and cell-invasive functions [139,140]. To corroborate their findings, Zhou et al. further demonstrated that silencing of SIRT2 with shSIRT2 causes the loss of aggressive basal-like breast cancer features in SUM149 and SUM1315 cancer cells [61]. Furthermore, a recent study demonstrated that SIRT2 deacetylase activity promotes the heterodimerization, nuclear retention, and stability of the breast cancer type I susceptibility protein (BRCA1) and BRCA1-associated RING domain protein I [64], a tumor suppressor protein involved in DNA repair and genome stability [141]. Another recent study revealed that SIRT2 positively regulates T cell differentiation, specifically the CD8+ T cells, to facilitate tumor immune response during breast cancer progression [65]. In summary, SIRT2 is highly involved in the initiation and progression of breast cancer, playing either a tumor-promoting or tumor-suppressing role. This opens further opportunities to explore the distinct roles of SIRT2 in breast cancer, particularly in the initiation processes of breast cancer, such as DNA damage, genetic instability, and mitotic catastrophe, as this could provide insights into the early detection and treatment of breast cancers.
2.4. Cytoplasmic Sirtuins in Prostate Cancer
SIRT2
Unlike in breast cancer, SIRT2 was previously shown to be highly expressed in prostate cancer cells compared with normal prostate cells [86]. However, in a recent study, SIRT2 expression was shown to decline from benign to malignant and then metastatic prostate cancer [142]. To corroborate this finding, Lee et al. showed that SIRT2 protein levels are reduced in castrate-resistant prostate cancer. These findings suggest a tumor stage-dependent expression of SIRT2 in prostate cancer, such that the SIRT2 protein level reduces as the prostate tumor progresses. Unfortunately, to date, there are no studies that have investigated the mechanistic role of SIRT2 in prostate cancer. In this regard, future studies are required to determine the oncogenic or tumor-suppressing effect of SIRT2 in prostate cancer.
2.5. Mitochondrial Sirtuins in Breast Cancer
2.5.1. SIRT3
The primary mitochondrial-localized deacetylase SIRT3 is known to play a vital role in mitochondrial metabolism [143]. Loss of SIRT3 culminates in hyperacetylation of mitochondrial proteins, which subsequently leads to the reduction of mitochondrial ability to produce ATP and induction of oxidative stress [144]. By deacetylating several enzymes involved in mitochondrial metabolism, such as isocitrate dehydrogenase and manganese superoxide dismutase, SIRT3 protects against pathological conditions, including aging-associated pathophysiologies [145,146]. Furthermore, the dual roles of SIRT3 in various types of cancers have been extensively reviewed [147], and their role in breast cancer is discussed below.
The expression of SIRT3 is decreased in human breast cancers [148,149]. To substantiate the decreased expression of SIRT3 in breast cancer, further investigations revealed that the SIRT3 gene is deleted in approximately 20 percent of human cancers and 40 percent of breast cancers [149]. Corroborating this, the SIRT3 gene is deleted in breast cancer more than in other cancers [148]. Armed with these findings, several researchers have investigated the tumor-suppressive role of SIRT3 in breast cancer. Finley et al. demonstrated that SIRT3 suppresses the Warburg effect and proliferation of human breast cancers [149]. Mechanistically, the authors demonstrated that SIRT3 opposes the Warburg effect by destabilizing hypoxia-inducible factor 1α (HIF1α), the main facilitator of increased glycolysis and lactate production during hypoxic conditions [149]. The authors also revealed that SIRT3 overexpression suppresses the proliferation of CAMA1 breast cancer cells in the presence of high glucose [149]. To corroborate their findings, the authors demonstrated that the knockout of SIRT3 promoted the Warburg effect in human breast cancer cells, thus promoting the survival of the breast cancer cells [149].
Additionally, Zou et al. demonstrated that the loss of SIRT3 (following treatment with SIRT3 shRNA) in MCF-7 breast cancer cells resulted in increased acetylation at lysine 413 of isocitrate dehydrogenase 2 (IDH2), a key enzyme in the Krebs cycle that oxidizes and decarboxylates isocitrate into α-ketoglutarate, and that SIRT3 loss decreases the level of IDH2 dimerization [67]. The authors further demonstrated that SIRT3 loss reduced IDH2 activities by decreasing IDH2 dimerization and that IDH2 acetylation at lysine 413 impairs mitochondrial respiration and detoxification, increases ROS production, and correlates with Luminal B breast cancer risk [67]. These findings suggest that SIRT3 expression may reverse the tumorigenic phenotype in breast cancer cells. Pinteric et al. demonstrated that de novo overexpression of SIRT3 downregulates the expression of vegfr1 (a proangiogenic protein involved in cell proliferation), EMT markers (vimentin and slug), lactate dehydrogenase A (LDHA; a glycolytic marker), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), SIRT1, superoxide dismutase 2 (SOD2), and catalase (CAT) in MCF-7 breast cancer cells, thus indicating a tumor suppressor effect of SIRT3 in breast cancer [68]. Supporting this finding, a recent study demonstrated that the overexpression of SIRT3 promoted apoptosis and inhibited the proliferation of MCF-7 and MDA-MB-231 breast cancer cells [71].
Intriguingly, another recent study showed that while SIRT3 expression improved mitochondrial mass and potential metabolism (Warburg effect) and increased SOD2 and CAT expression, it also increased mitochondrial ROS, DNA damage, and expression of tissue inhibitor of metalloproteinase 1 (a major inhibitor of MMP9), which have been shown to promote malignant progression and metastasis [150]. Further, SIRT3 expression also induced the formation of multinucleated cells and apoptosis and inhibited the proliferation of MDA-MB-231 breast cancer cells [69].
Further supporting the tumor suppressor role of SIRT3 in breast cancer, SIRT3 suppresses estrogen-induced cytosolic and mitochondrial ROS production, diminishes estrogen-induced DNA synthesis, and upregulates p53 expression in MCF-7 breast cancer cells [70], despite its increasing antioxidant activities and cytosolic ROS. From the above-mentioned studies, it is evident that there is a well-established tumor suppressor role of SIRT3 in breast cancer.
To date, only one study has shown a possible tumor-promoting effect of SIRT3 in breast cancer. SIRT3 knockdown in MCF-7 cells by siRNA increased ROS production (196%), formation of autophagic bodies (47%), and apoptosis (23%); it also decreased MnSOD (20%), IDH2 (50%), PGC1α (12%), and mitochondrial transcription factor A (27%) levels and reduced cell viability (20%) [66]. These findings indicate that SIRT3 expression prevents ROS production, autophagy, and apoptosis, as well as increases cell viability and survival of breast cancer cells, thus demonstrating a tumor-promoting effect. However, given the limited information in this research area, further investigations are required to fully establish the tumor-promoting effect of SIRT3 before a conclusion can be reached.
2.5.2. SIRT4
Like other mitochondrial sirtuins, SIRT4 is a mitochondrial deacetylase involved in energy metabolism [151]. However, in addition to its deacetylase activity, SIRT4 possesses ADP-ribosyl transferase activity, which catalyzes the transfer of ADP-ribosyl units to targets, such as glutamate dehydrogenase [9]. SIRT4 has been reported to be a therapeutic target that has both oncogenic and tumor suppressor effects in cancers [152]; however, the underlying mechanism is still a matter of debate. So far, most studies have demonstrated that SIRT4 regulates glutamine metabolism [153]. Notably, it is through the inhibition of glutamine metabolism that SIRT4 exhibits tumor suppressor effects in B Cell Lymphoma [154], 2014), colorectal cancer [155], and thyroid cancer [156].
In breast cancer, the expression pattern of SIRT4 is controversial. While some studies report that SIRT4 expression is upregulated in breast cancer [157,158], others report downregulation [97,129,153,159]. The differences in the expression patterns of SIRT4 in breast cancer cells may be attributed to differences in the breast cancer cell types and quantitation methods for SIRT4 used in these studies. Given its differential pattern of expression, it can be speculated that SIRT4 exhibits both tumor-suppressing and tumor-promoting effects. For instance, although the underlying mechanism was not investigated, a 2017 study demonstrated that SIRT4 upregulation promotes the proliferation, migration, and invasion of MDA-MB-435S breast cancer cells [158], although it should be noted that there is also controversy over whether this cell line is of breast origin [160]. Additionally, a recent study demonstrated that SIRT4 deacetylates methylenetetrahydrofolate dehydrogenase/methylenetetrahydrofolate cyclohydrolase 2 (MTHFD2), a vital bifunctional enzyme in folate metabolism, to remodel folate metabolism against breast cancer cells. As a mechanism, the authors revealed that SIRT4 deacetylation of MTHFD2 destabilizes MTHFD2 and causes its proteasomal degradation, leading to NADPH reduction and intracellular ROS accumulation in the breast tumors which, in turn, inhibits the proliferation of the breast cancer cells [73].
On the contrary, another study demonstrated that SIRT4 overexpression downregulates IL-6 expression and STAT3 Y705 phosphorylation, as well as the transcription and translation of STAT3 target genes (MYC and CNDD1), to enhance the sensitivity of MCF7 and T47D breast cancer cells to tamoxifen, thus potentiating the effect of this chemotherapy against breast cancer cells [72]. Supporting this, the downregulation of SIRT4 in breast cancer cell lines resulted in decreased expression of SIRT1 and stem cell markers Oct4, Sox2, and Nanog. Further, the SIRT4-mediated downregulation of SIRT1 led to increased acetylation of H4K16, which caused the downregulation of BRCA1 expression at both mRNA and protein levels [74]. The authors suggested the crosstalk between the mitochondrial and nuclear sirtuins following SIRT1 downregulation by SIRT4 expression culminated in the suppression of breast cancer. This needs to be investigated further, considering that SIRT1 is constitutively upregulated in breast cancer and its inhibition suppresses breast cancer (as discussed in 2.1 above).
2.5.3. SIRT5
In addition to its deacetylase activity, SIRT5 has been shown to exhibit demalonylase, deglutarylase, and succinylase activities [14,161]. As a member of the mitochondrial sirtuins, SIRT5, through its deacetylase activities, participates in several biological processes, such as metabolic regulation, aging, and oxidative stress [162]. SIRT5 expression has been evaluated in various kinds of cancer, particularly in head and neck squamous cell carcinoma [163], endometrial carcinoma [164], basal carcinoma [165], and hepatocellular carcinoma [166]. Although SIRT5 expression was found to be upregulated in breast cancer [76,159], which may be indicative of its role in breast cancer progression, only a few studies have revealed the mechanisms underlying the role of SIRT5 in breast cancer.
Polletta et al. demonstrated that ammonia production decreased in SIRT5 overexpressing MDA-MB-231 breast cancer cells, which was corroborated by the increase in ammonia production in SIRT5-silenced cells or cells treated with a specific inhibitor of SIRT5 (MC3482) [75]. The authors further demonstrated that SIRT5 inhibition leads to increased succinylation of glutaminase (GLS), an enzyme that catalyzes the conversion of glutamine to glutamate, and that ammonia increases the levels of autophagy markers (MAP1LC3B, GABARAP, and GABARAPL2) and mitophagy markers (BNIP3 and PINK1-PARK2 system). Taken together, the authors concluded that SIRT5 expression regulates ammonia-induced autophagy and mitophagy of MDA-MB-231 breast cancer cells by inhibiting GLS activity. On the contrary, a recent study demonstrated that SIRT5 desuccinylates GLS at residue K164, stabilizing and protecting it from ubiquitin-mediated degradation and that SIRT5 expression promotes the proliferation and tumorigenesis of MDA-MB-231 breast cancer cells [76]. Given the disagreement between these two studies regarding the effect of SIRT5 on GLS activities, further clarification by future studies is required before a conclusion can be reached.
Importantly, an in vivo study of SIRT5 knockout MMTV-PyMT mice supported the tumor-promoting effect of SIRT5 in breast cancer. SIRT5 knockout MMTV-PyMT mice had higher survival and reduced tumor size and lung metastases compared to the SIRT5 wild-type MMTV-PyMT mice [77]. The authors further demonstrated that SIRT5 knockout cancer cells had increased levels of ROS and reduced levels of important antioxidants NADPH and GSH. As such, the authors concluded that SIRT5 may promote breast cancer by mitigating ROS via one or several of its targets [77]. To date, most studies suggest that SIRT5 acts as a tumor promoter in breast cancer. Consistent with this deduction, a recent study established SIRT5 inhibition as a crucial promising therapeutic approach against breast cancer in vivo and in vitro [167].
2.6. Mitochondrial Sirtuins in Prostate Cancer
2.6.1. SIRT3
The expression of SIRT3 in prostate cancer remains controversial. SIRT3 expression has been reported to be decreased in prostate cancer by two studies: Quan et al. [92] found that SIRT3 is moderately downregulated in prostate carcinoma tissues and Li et al. [93] detected a decrease in metastatic tissues compared to prostate tumor tissues. Singh et al. [94] found that SIRT3 was upregulated in cancerous prostatic tissues and cell lines compared to normal tissues and prostatic epithelia cells, respectively. These conflicting findings may suggest a differential pattern of SIRT3 expression in prostate cancer tissues and cells, which may depend on the cell type, tumor stage or grade, and genetic differences in the prostate cancer tissues or cells. In this regard, further studies are needed to clarify the expression pattern of SIRT3 in prostate cancer tissues and cells. Similarly, mechanistic studies have been conducted in prostate cancer, which indicated that SIRT3 has a dual role in prostate carcinogenesis.
Supporting a tumor suppressive function, SIRT3 expression in C42B and PC3 prostate cancer cells downregulated the expression of c-Myc [92], a well-established oncogenic protein [168]. The same authors further demonstrated that SIRT3 expression destabilizes c-Myc by inhibiting the PI3k/Akt pathway (in vitro and in vivo) and that SIRT3 expression suppresses the PI3K/Akt pathway by downregulating ROS levels. Therefore, the authors concluded that SIRT3 expression inhibits the proliferation of prostate cancer via the above-mentioned mechanisms. Corroborating this finding, Li et al. found that SIRT3 expression suppressed EMT (via upregulation of E-cadherin and downregulation of N-cadherin and vimentin expression) in C42B prostate cancer cells by upregulating FOXO3A [93], a transcription factor that has been shown to suppress EMT and metastasis of prostate cancer [169]. They also found that SIRT3 overexpression downregulates the expression of β-catenin in C42B prostate cancer cells, thereby suppressing the Wnt/β-catenin signaling, a pathway that has been shown to inhibit the nuclear localization of FOXO3A and the expression of FOXO3A target genes [170]. Put together, the authors concluded that SIRT3 inhibits the metastasis of prostate cancer via the Wnt/β-catenin/FOXO3A signaling axis. Consistent with this tumor suppressing effect of SIRT3, a recent study demonstrated that SIRT3 expression reduces the acetylation level of mitochondrial aconitase, leading to the reduction of prostate cancer growth [95].
In contrast with the above-mentioned studies indicating the tumor suppressor function of SIRT3 in prostate cancer, Singh et al. demonstrated that SIRT3 expression inhibits the cleavage of poly (ADP-ribose) polymerase [94], a known marker of apoptosis, and upregulates the expression of proliferating cell nuclear antigen, a known marker of cell proliferation, in DU145 and 22Rν1 prostate cancer cells. The authors concluded that SIRT3 has a pro-proliferative role in prostate cancers. Furthermore, a recent study revealed that SIRT3 suppresses necroptosis-induced innate immunity to promote the progression of prostate cancer [96]. To validate this finding, the authors demonstrated that the knockdown of SIRT3 led to the recruitment of macrophages and neutrophils and induced necroptosis in prostate cancer cells. Notwithstanding, future mechanistic studies should be channeled toward validating these findings.
2.6.2. SIRT4
Unlike in other human cancers, including breast cancer, the expression pattern of SIRT4 and its mechanistic roles in prostate cancer have been sparsely investigated. Nevertheless, a study found that SIRT4 suppresses the proliferation of DU145 prostate cancer cells by inhibiting the uptake of glutamine [97], a metabolite vital for proliferating and cancerous cells [171]. Furthermore, Li et al. demonstrated that SIRT4 promotes mitochondrial-mediated apoptosis in PC3 prostate cancer cells by deacetylating and promoting the ubiquitination and degradation of adenine nucleotide translocase-2 [98], a mitochondrial inner membrane protein that is highly expressed in cancer cells [172]. Notwithstanding, further studies are required to clarify the expression pattern of SIRT4 and its mechanistic roles in prostate cancer.
2.6.3. SIRT5
As in breast cancer, SIRT5 was found to be highly expressed in prostate tumor tissues compared to normal prostate tissues [99]. However, in contrast, recent studies have revealed that SIRT5 expression is downregulated in advanced prostate cancer [100,101], suggesting that SIRT5 downregulation is dependent on the progression or stage of the prostate cancer cells. These findings may indicate a tumor-stage-dependent role of SIRT5 in prostate carcinogenesis. There are few studies on the mechanistic roles of SIRT5 in prostate cancer. A recent study demonstrated that SIRT5 promotes the proliferation and migration of LNCaP and PC3 prostate cancer cells [99]. This was shown to occur through increased expression of SIRT5, which resulted in the upregulation of cyclin D1, MMP9, and MAPK signaling-related proteins. The authors further demonstrated that SIRT5 decreased the protein levels of acetyl-CoA acetyltransferase 1 (ACAT1), a promoter of prostate cancer [173]. Since ACAT1 negatively regulates the MAPK signaling pathway [99], Peck and Shulze [173] concluded that SIRT5 promotes the proliferation, invasion, and migration of prostate cancer via the downregulation of ACAT1 protein levels.
Conversely, a more recent study demonstrated that SIRT5 expression reduces the migration and invasion of PC3 prostate cancer cells [101]. This was found to occur via SIRT5 desuccinylation of LDHA to reduce its activity, culminating in the growth reduction and survival of prostate cancer cells [101]. Consistent with this finding, another recent study demonstrated that SIRT5 suppressed the progression of PC3 prostate cancer cells by downregulating the PI3K/AKT/NK-kB pathway [100]. Interestingly, the authors further revealed that SIRT5-facilitated downregulation of the PI3K/AKT/NK-kB pathway reduced as the prostate cancer metastasized from the bone to other tissues [100]. The findings of these two recent studies indicate that the tumor-suppressing effect of SIRT5 in prostate cancer increases as the prostate cancer progresses but reduces at the point of secondary metastasis (from the bone to other tissues). Nevertheless, further studies are required to corroborate the current findings and investigate the consequences of the modulation of other targets by SIRT5 in prostate cancer.
3. Mechanistic Role of Sirtuins Regulation by microRNAs (miRNAs) in Breast and Prostate Cancers
3.1. Overview of miRNA
In 1993, the first miRNA lin-4 was reported to exist in Caenorhabditis elegans [174,175]. After a series of intensive scientific investigations, the same groups found that lin-4 was not an RNA-encoded protein but a non-coding RNA [176,177]. Furthermore, they found that lin-4 post-transcriptionally downregulates lin-14 (a gene found in Caenorhabditis elegans) through its 3′ untranslated region (UTR) and that the 3′UTR of lin-14 was complementary to that of lin-4 [175]. Accordingly, they proposed that lin-4 post-transcriptionally regulates lin-14 [174]. Following this landmark discovery, over 1,000 miRNAs have been detected in various species [178].
miRNAs are small non-coding RNAs composed of an average of 22 nucleotides that suppress gene expression by interacting with the 3′ UTR of target mRNAs. Moreover, several studies have demonstrated that miRNAs repress mRNA translation and induce mRNA deadenylation and decapping by binding to the 3′ UTR of their target mRNAs [179,180]. In addition, other regions in the target mRNA, such as the promoter region, 5′ UTR, and coding region have been shown to have miRNA binding sites [181]. According to scientific findings, when miRNAs bind to the 5′ UTR and coding region of their target mRNA, they induce the silencing of gene expression [182]; whereas, when they interact with the promoter region of their target mRNA, they induce gene transcription [183].
miRNAs have been implicated in normal biological processes [184,185,186], as well as in various human diseases [187,188]. Based on this fact, several studies have explored the diagnostic and prognostic potential of miRNAs in various human diseases [189,190,191,192]. Furthermore, in various cancer diseases, miRNAs have been demonstrated to confer either oncogenic or tumor suppressor effects [193,194,195,196]. Most importantly, different roles of miRNA, including tumorigenesis, progression, metastasis, diagnosis, and prognosis, in breast [197,198,199,200] and prostate [201,202,203,204] cancer have been reported in the literature. The current knowledge on the regulation of SIRT1–7 by miRNAs will be discussed in a subsequent section of this review, highlighting the consequences of this regulation in breast and prostate cancer.
Reportedly, approximately 30% of genes are regulated by miRNAs. Consistent with this report, the regulatory functions of miRNAs in the progression, metastasis, and drug-resistance of breast tumors and other cancers have been documented [198,205]. Although miRNAs are well-established as regulators of various proteins involved in signaling pathways in breast and prostate cancer, there is ongoing research on the regulation of sirtuins by miRNAs in breast and prostate cancer. Given the limited information in the literature to date, we discuss the current information on the mechanistic role of miRNA-mediated regulation of SIRT1 and 7 in breast cancer (Table 3) and SIRT1 in prostate cancer (Table 4) and elucidate a common mechanism for this regulation (Figure 4).

Table 3.
Mechanistic Roles of Sirtuins (SIRT1–7) Regulation by miRNAs in Breast Cancer.

Table 4.
Mechanistic Roles of Sirtuins (SIRT1–7) Regulation by miRNAs in Prostate Cancer.
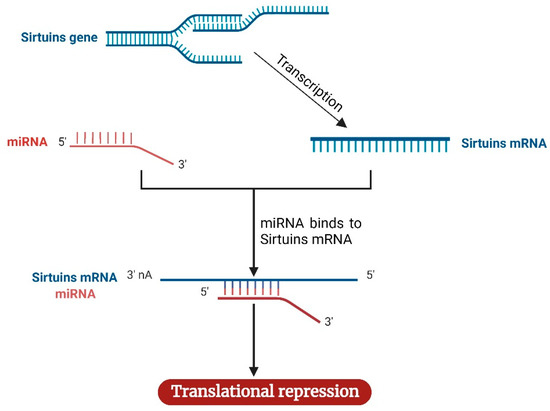
Figure 4.
Mechanism of sirtuins regulation by miRNAs. Following the transcription of the sirtuins gene to mRNA, miRNA complementary to sirtuins binds the sirtuins mRNA, causing the repression of sirtuins mRNA translation. The image was created with BioRender.com.
3.2. SIRT1 Regulation by miRNAs in Breast Cancer
Several miRNAs have been found to regulate SIRT1 expression in breast cancer, serving as either tumor suppressors or tumor promoters through an indirect mechanism, depending on their effect on SIRT1 expression. For instance, Eades et al. demonstrated that miR-200a epigenetic silencing contributes, at least in part, to the overexpression of SIRT1, and the SIRT1 transcript is subject to regulation by miR-200a via SIRT1 3′-UTR [211]. They further demonstrated that miR-200a expression or SIRT1 knockdown prevents the transformation of normal mammary epithelial cells and that overexpression of SIRT1 is associated with decreased miR-200a in breast cancer patients. Furthermore, Li et al. (2013b) found that miR-34a is downregulated in BT474, MDA-MB-231, MDA-MB-435 (although this may not be relevant due to a postulation that it is a melanoma cell line), MDA-MB-468, and SK-BR-3 breast cancer cells. The same investigators also found that miR-34a expression (ectopic) suppressed the proliferation, invasion, and induced apoptosis of these breast cancer cells. They further demonstrated that Bcl-2 and SIRT1 are targets of miR-34a and are negatively correlated with the ectopic expression of miR-34a. Moreover, miR-34a has been well-established as a tumor suppressor [220,221].
Furthermore, a study demonstrated that miR-22 is downregulated in MCF-7 and MDA-MB-231 breast cancer cells, and the overexpression of miR-22 or SIRT1 knockdown induces apoptosis and decreases the survival of breast cancer cells [209]. The authors further demonstrated that miR-22 negatively regulates the expression of SIRT1, and SIRT1 ectopic expression reversed the inductive effect of miR-22 on apoptosis and the radiosensitivity of breast cancer cells. This indicates a tumor suppressor role of miR-22 through the negative regulation of SIRT1. Similarly, Liang et al. reported that miR-4766-5p negatively regulates SIRT1 expression to suppress cell proliferation, metastasis, and chemoresistance in breast cancer cells [208]. Another study found that miR-590-3p expression is downregulated in MCF-7 and MDA-MB-231 breast cancer cells, and miR-590-3p exerts its antitumor effects (induction of apoptosis and reduction of cell survival) by targeting SIRT1 in breast cancer cells [207]. Another study demonstrated that SIRT1 expression is inhibited by miR-211-5p by targeting SIRT1 via its 3′-UTR, and miR-211-5p expression culminated in decreased acetylation of p53, which was associated with reduced cell viability and apoptosis induction in breast cancer cells [206]. The authors concluded that the negative regulation of SIRT1 by miR-211-5p reduces survival and induces cell death of breast cancer. So far, the information provided above reveals that most miRNAs targeting SIRT1 act as tumor suppressors in breast cancer; however, studies are needed to clarify and verify the possible tumor-promoting effects of SIRT1 negative regulation by miRNAs in breast cancer. Additionally, future studies should also attempt to match the effects of the miRNA with the SIRT1 functions in breast cancer discussed above.
3.3. SIRT7 Regulation by miRNAs in Breast Cancer
Based on our literature search, there is limited information on the regulation of SIRT7 by miRNAs in breast cancer. Being the only available data at the time of our literature search, Li and Li [212] demonstrated that miR-3666 expression is reduced in MCF-7, BT474, MDA-MB-231, and MDA-MB-468 breast cancer cells and that miR-3666 overexpression inhibits the proliferation of breast cancer cells. The authors further demonstrated that miR-3666 targets the SIRT7 3′-UTR, and miR-3666 overexpression reduces the expression of SIRT7, resulting in increased proliferation and reduced apoptosis of breast cancer cells [212]. The reduced miR-3666 expression and increased SIRT7 expression in MDA-MB-231 breast cancer cells, as mentioned above, suggest their prognostic and diagnostic relevance in breast cancer. Surprisingly, miR-3666 was recently established as a tumor suppressor in different cancers, such as head and neck squamous cell carcinoma (HNSCC) [222] and glioblastomas (GMB) [223]. However, it is worth noting that SIRT7 expression is downregulated in human HNSCC tissues [163] but upregulated in human glioma tissues [224], and >50% of gliomas are reported to be GMB [225]. Therefore, it may be hypothesized that a reduced expression of miR-3666 is corroborated by an increased expression of SIRT7 in GMB but not in HNSCC. Given these scenarios, further investigations are required to corroborate the present findings, ascertain the tumor-promoting roles of miRNAs via regulation of SIRT7 in breast cancer, as well as determine how miRNAs-induced effects match with the SIRT7 functions in breast cancer discussed above.
3.4. SIRT1 Regulation by miRNAs in Prostate Cancer
Although several studies have investigated the role of miRNAs, including miR-1, -21, -106b, -141, -145, -182, -187, -205, -221, -375, -490-3p, and -Let-7c in the prognosis, diagnosis, and progression of prostate cancer [226,227,228,229], there is limited information on the modulation of sirtuins by miRNAs in prostate cancer. Given the tumor-promoting role of SIRT1 in prostate cancer, which is evident in its high expression in prostate cancer [87], several studies have investigated the modulatory roles of various miRNAs on SIRT1 in prostate cancer, suggesting a tumor suppressor role of miRNAs through the inhibition of SIRT1 expression. For instance, Fujita et al. (2008) demonstrated that miR-34a expression is downregulated in PC3 and DU145 prostate cancer cells, and ectopic expression of miR-34a downregulates SIRT1 protein expression in PC3 prostate cancer cells at the transcriptional level (that is, via reduction of SIRT1 promoter activity) but not at the post-transcriptional level (that is, via interaction with SIRT1 3′-UTR). The authors further demonstrated that the ectopic expression of miR-34a induces cell cycle arrest, inhibits cell growth, and abrogates camptothecin resistance of PC3 prostate cancer cells. Taken together, these findings indicate a tumor suppressor role of miR-34a through the negative regulation of SIRT1 in prostate cancer. Moreover, a subsequent study confirmed that miR-34a expression is downregulated in prostate cancer tissues compared to normal prostate tissues, and miR-34a expression inhibits the proliferation of PC3 prostate cancer cells by downregulating SIRT1 expression [219].
miR-212 was found to be downregulated in prostate cancer tissues compared with normal prostate tissues [218]. Its expression inhibits SIRT1 expression (via interaction with SIRT1 3’UTR), starvation- and SIRT1-induced autophagy, and angiogenesis of LNCaP and PC3 prostate cancer cells [218]. These findings suggest a tumor suppressor role of miR-212 through the negative regulation of SIRT1 in prostate cancer. Other subsequent studies have also shown that certain miRNAs, whose expressions are downregulated in prostate cancer, act as tumor suppressors by negatively regulating SIRT1 via its 3′UTR. For instance, by inhibiting SIRT1 protein expression, researchers demonstrated that miR-449a ectopic expression suppresses the invasiveness of prostate cancer [217]; miR-204 enhances both docetaxel-induced apoptosis [214] and doxorubicin-induced mitochondrial-mediated apoptosis [216] of prostate cancer, and miR-138-5p inhibits the proliferation and lipid metabolism of prostate cancer [215].
So far, most of the above-mentioned studies suggest that miRNAs directly interact with SIRT1 to potentiate their tumor-suppressing effect in prostate cancer. On the contrary, Yang et al. demonstrated that miR-221 and miR-222 are highly expressed in PC3 compared to LNCaP prostate cancer cells and do not target or directly interact with SIRT1 mRNA via its 3′UTR [230]. Interestingly, the authors further showed that the inhibition of these miRNAs reduces proliferation, increases apoptosis, and reduces migration of PC3 prostate cancer cells, which were abrogated by SIRT1 expression. This suggests interesting crosstalks between these miRNAs and SIRT1 in prostate cancer, which needs to be explored in future studies. As in breast cancer, there is currently no study demonstrating the tumor-promoting effect of miRNAs via the SIRT1 axis in prostate cancer.
4. Conclusions
This extensive review of the current knowledge on the mechanistic roles of nuclear sirtuins (SIRT1, 6, and 7), cytoplasmic sirtuins (SIRT2), and mitochondrial sirtuins (SIRT3, 4, and 5) has shown that breast and prostate cancer cells or tissues portray different expression patterns of the various types of sirtuins to aid their tumorigenic phenotypes and potentiate their progression through various carcinogenic stages. Furthermore, we have also shown that sirtuins regulate various proteins to promote or suppress breast and prostate cancer. Specifically, we highlighted that sirtuins regulate various proteins implicated in proliferation, apoptosis, autophagy, chemoresistance, invasion, migration, and metastasis of both breast and prostate cancer. To further illustrate the relevance of sirtuins expression in breast and prostate cancer, we have provided available evidence of the direct regulation of sirtuins by miRNAs (via direct interaction with sirtuins mRNAs), highlighting the consequences of this regulation in breast and prostate cancer. While many studies have begun to investigate the specific mechanisms by which sirtuins influence either breast or prostate cancer, no study has investigated both in the same study. As a result, the specific investigation of either of these cancers has focused on different key genes and/or proteins involved in various molecular pathways. Comparing Figure 3a and Figure 3b, it is evident that similar functions of sirtuins are relied upon by both cancers; however, the specific genes driving these functions may be different. Future studies verifying these genetic mechanisms in both cancers will be imperative to determine if sirtuins can be targeted in the same way as novel treatments for these cancers. In general, this review highlights that sirtuins show similar differences in expression in both breast and prostate cancer, which then drive similar molecular mechanisms to promote the progression of cancer and thus represent new therapeutic targets. Notwithstanding, research on the mechanistic role of sirtuins and consequences of their regulation by miRNAs in breast and prostate carcinogenesis is ongoing. Future studies are required to illuminate the grey areas and validate the current knowledge. Nonetheless, this review has highlighted the potential value of sirtuins as biomarkers and/or targets for improved treatment of breast and prostate cancers.
Author Contributions
Conceptualization, C.I.O. & J.W.; writing—original draft preparation, C.I.O.; writing—review and editing, C.I.O., J.W. & C.J.S.; supervision, J.W. & C.J.S.; funding acquisition, C.I.O., J.W. & C.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a University of Newcastle (Australia) Vice Chancellors Post-Graduate Scholarship awarded to Cosmos Ifeanyi Onyiba.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L.C. Elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 2006, 125, 1165–1177. [Google Scholar] [PubMed]
- Bauer, J.H.; Morris, S.N.S.; Chang, C.; Flatt, T.; Wood, J.G.; Helfand, S.L. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging 2009, 1, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.-I.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell. 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Denu, J.M. The Sir 2 family of protein deacetylases. Curr. Opin. Chem. Biol. 2005, 9, 431–440. [Google Scholar] [CrossRef]
- Sosnowska, B.; Mazidi, M.; Penson, P.; Gluba-Brzózka, A.; Rysz, J.; Banach, M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis 2017, 265, 275–282. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The human sirtuin family: Evolutionary divergences and functions. Hum Genom. 2011, 5, 485–496. [Google Scholar] [CrossRef]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef]
- Liszt, G.; Ford, E.; Kurtev, M.; Guarente, L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 2005, 280, 21313–21320. [Google Scholar] [CrossRef]
- Rauh, D.; Fischer, F.; Gertz, M.; Lakshminarasimhan, M.; Bergbrede, T.; Aladini, F.; Kambach, C.; Becker, C.F.W.; Zerweck, J.; Schutkowski, M.; et al. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 2014, 159, 1615–16125. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Therapeutic Potential and Activity Modulation of the Protein Lysine Deacylase Sirtuin 5. J. Med. Chem. 2022, 65, 9580–9606. [Google Scholar] [CrossRef] [PubMed]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Sacconnay, L.; Carrupt, P.-A.; Nurisso, A. Human sirtuins: Structures and flexibility. J. Struct. Biol. 2016, 196, 534–542. [Google Scholar] [CrossRef]
- Du, Y.; Hu, H.; Hua, C.; Du, K.; Wei, T. Tissue distribution, subcellular localization, and enzymatic activity analysis of human SIRT5 isoforms. Biochem. Biophys. Res. Commun. 2018, 503, 763–769. [Google Scholar] [CrossRef]
- Iwahara, T.; Bonasio, R.; Narendra, V.; Reinberg, D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol. Cell. Biol. 2012, 32, 5022–5034. [Google Scholar] [CrossRef]
- Scher, M.B.; Vaquero, A.; Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007, 21, 920–928. [Google Scholar] [CrossRef]
- Ramadani-Muja, J.; Gottschalk, B.; Pfeil, K.; Burgstaller, S.; Rauter, T.; Bischof, H.; Waldeck-Weiermair, M.; Bugger, H.; Graier, W.F.; Malli, R. Visualization of Sirtuin 4 Distribution between Mitochondria and the Nucleus, Based on Bimolecular Fluorescence Self-Complementation. Cells 2019, 8, 1583. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, M.J.G.; Pereira, J.M.; Impens, F.; Hamon, M.A. Active nuclear import of the deacetylase Sirtuin-2 is controlled by its C-terminus and importins. Sci. Rep. 2020, 10, 2034. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, A.; Scher, M.B.; Lee, D.H.; Sutton, A.; Cheng, H.-L.; Alt, F.W.; Serrano, L.; Sternglanz, R.; Reinberg, D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, X. Nucleus or cytoplasm? The mysterious case of SIRT1’s subcellular localization. Cell Cycle 2016, 15, 3337–3338. [Google Scholar] [CrossRef]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef]
- Frye, R.A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef]
- George, J.; Ahmad, N. Mitochondrial Sirtuins in Cancer: Emerging Roles and Therapeutic Potential. Cancer Res. 2016, 76, 2500–2506. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Cui, Y.; Wei, Q.; Chen, S.; Wang, X. Effects of SIRT1 silencing on viability, invasion and metastasis of human glioma cell lines. Oncol. Lett. 2019, 17, 3701–3708. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, X.; Yi, J.; Huang, J.; He, J.; Tao, Y. Sirtuins in metabolism, DNA repair and cancer. J. Exp. Clin. Cancer Res. 2016, 35, 182. [Google Scholar] [CrossRef]
- Nagarajan, P.; Parthun, M.R. The flip side of sirtuins: The emerging roles of protein acetyltransferases in aging. Aging 2020, 12, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Germain, D. SirT3 Regulates a Novel Arm of the Mitochondrial Unfolded Protein Response. Mol. Cell. Biol. 2014, 34, 699–710, Erratum in Mol. Cell. Biol. 2017, 37, e00191–e00217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef] [PubMed]
- Maissan, P.; Mooij, E.; Barberis, M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Biology 2021, 10, 194. [Google Scholar] [CrossRef]
- Bosch-Presegué, L.; Vaquero, A. The dual role of sirtuins in cancer. Genes Cancer 2011, 2, 648–662. [Google Scholar] [CrossRef]
- Bai, L.; Lin, G.; Sun, L.; Liu, Y.; Huang, X.; Cao, C.; Guo, Y.; Xie, C. Upregulation of SIRT6 predicts poor prognosis and promotes metastasis of non-small cell lung cancer via the ERK1/2/MMP9 pathway. Oncotarget 2016, 7, 40377–40386. [Google Scholar] [CrossRef]
- Lin, H.; Hao, Y.; Zhao, Z.; Tong, Y. Sirtuin 6 contributes to migration and invasion of osteosarcoma cells via the ERK1/2/MMP9 pathway. FEBS Open Bio 2017, 7, 1291–1301. [Google Scholar] [CrossRef]
- Garcia-Peterson, L.M.; Wilking-Busch, M.J.; Ndiaye, M.A.; Philippe, C.G.A.; Setaluri, V.; Ahmad, N. Sirtuins in Skin and Skin Cancers. Ski. Pharm. Physiol. 2017, 30, 216–224. [Google Scholar] [CrossRef]
- Hu, J.; Jing, H.; Lin, H. Sirtuin inhibitors as anticancer agents. Future Med. Chem. 2014, 6, 945–966. [Google Scholar] [CrossRef]
- Kozako, T.; Suzuki, T.; Yoshimitsu, M.; Arima, N.; Honda, S.-I.; Soeda, S. Anticancer agents targeted to sirtuins. Molecules 2014, 19, 20295–20313. [Google Scholar] [CrossRef]
- McGlynn, L.M.; McCluney, S.; Jamieson, N.; Thomson, J.; MacDonald, A.I.; Oien, K.; Dickson, E.J.; Carter, C.R.; McKay, C.J.; Shiels, P.G. SIRT3 & SIRT7: Potential Novel Biomarkers for Determining Outcome in Pancreatic Cancer Patients. PLoS ONE 2015, 10, e0131344. [Google Scholar]
- Tan, Y.; Li, B.; Peng, F.; Gong, G.; Li, N. Integrative Analysis of Sirtuins and Their Prognostic Significance in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar]
- Risbridger, G.P.; Davis, I.D.; Birrell, S.N.; Tilley, W. Breast and prostate cancer: More similar than different. Nat. Rev. Cancer 2010, 10, 205–212. [Google Scholar] [CrossRef]
- Jørgensen, K.J.; Gøtzsche, P.C.; Kalager, M.; Zahl, P.-H. Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis. Ann. Intern. Med. 2017, 166, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.-G.; Huang, J.-D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef]
- Latifkar, A.; Ling, L.; Hingorani, A.; Johansen, E.; Clement, A.; Zhang, X.; Hartman, J.; Fischbach, C.; Lin, H.; Cerione, R.A.; et al. Loss of Sirtuin 1 Alters the Secretome of Breast Cancer Cells by Impairing Lysosomal Integrity. Dev. Cell 2019, 49, 393–408.e7. [Google Scholar] [CrossRef]
- Mahmud, Z.; Gomes, A.R.; Lee, H.J.; Aimjongjun, S.; Jiramongkol, Y.; Yao, S.; Zona, S.; Alasiri, G.; Gong, G.; Yagüe, E.; et al. EP300 and SIRT1/6 Co-Regulate Lapatinib Sensitivity Via Modulating FOXO3-Acetylation and Activity in Breast Cancer. Cancers 2019, 11, 1067. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Q.; Chen, R.; Wei, C.; Mo, Q. SIRT1 promotes proliferation, migration, and invasion of breast cancer cell line MCF-7 by upregulating DNA polymerase delta1 (POLD1). Biochem. Biophys. Res. Commun. 2018, 502, 351–357. [Google Scholar] [CrossRef]
- Jin, X.; Wei, Y.; Xu, F.; Zhao, M.; Dai, K.; Shen, R.; Yang, S.; Zhang, N. SIRT1 promotes formation of breast cancer through modulating Akt activity. J. Cancer 2018, 9, 2012–2023. [Google Scholar] [CrossRef]
- Kuo, S.J.; Lin, H.-Y.; Chien, S.-Y.; Chen, D.-R. SIRT1 suppresses breast cancer growth through downregulation of the Bcl-2 protein. Oncol. Rep. 2013, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Holloway, K.R.; Barbieri, A.; Malyarchuk, S.; Saxena, M.; Nedeljkovic-Kurepa, A.; Mehl, M.C.; Wang, A.; Gu, X.; Pruitt, K. SIRT1 positively regulates breast cancer associated human aromatase (CYP19A1) expression. Mol. Endocrinol. 2013, 27, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Avino, S.; A Pellegrino, M.; De Francesco, E.M.; De Marco, P.; Lappano, R.; Vivacqua, A.; Cirillo, F.; Rigiracciolo, D.C.; Scarpelli, A.; et al. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis. 2015, 6, e1834. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.E.J.; Pandey, S.; Nedeljkovic-Kurepa, A.; Saxena, M.; Wang, A.; Pruitt, K. Frizzled 7 expression is positively regulated by SIRT1 and β-catenin in breast cancer cells. PLoS ONE 2014, 9, e98861. [Google Scholar] [CrossRef]
- Moore, R.L.; Faller, D.V. SIRT1 represses estrogen-signaling, ligand-independent ERα-mediated transcription, and cell proliferation in estrogen-responsive breast cells. J. Endocrinol. 2013, 216, 273–285. [Google Scholar] [CrossRef]
- Jin, M.-S.; Hyun, C.L.; Park, I.A.; Kim, J.Y.; Chung, Y.R.; Im, S.-A.; Lee, K.-H.; Moon, H.-G.; Ryu, H.S. SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer. Tumour Biol. 2016, 37, 4743–4753. [Google Scholar] [CrossRef]
- Gollavilli, N.; Kanugula, A.K.; Koyyada, R.; Karnewar, S.; Neeli, P.K.; Kotamraju, S. AMPK inhibits MTDH expression via GSK3β and SIRT1 activation: Potential role in triple negative breast cancer cell proliferation. FEBS J. 2015, 282, 3971–3985. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, T.; Wang, X.; Zhang, D. The Contrary Effects of Sirt1 on MCF7 Cells Depend on CD36 Expression Level. J. Surg. Res. 2019, 238, 248–254. [Google Scholar] [CrossRef]
- Elangovan, S.; Ramachandran, S.; Venkatesan, N.; Ananth, S.; Gnana-Prakasam, J.P.; Martin, P.M.; Browning, D.D.; Schoenlein, P.V.; Prasad, P.D.; Ganapathy, V.; et al. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor α in breast cancer. Cancer Res. 2011, 71, 6654–6664. [Google Scholar] [CrossRef]
- Fiskus, W.; Coothankandaswamy, V.; Chen, J.; Ma, H.; Ha, K.; Saenz, D.T.; Krieger, S.S.; Mill, C.P.; Sun, B.; Huang, P.; et al. SIRT2 Deacetylates and Inhibits the Peroxidase Activity of Peroxiredoxin-1 to Sensitize Breast Cancer Cells to Oxidant Stress-Inducing Agents. Cancer Res. 2016, 76, 5467–5478. [Google Scholar] [CrossRef]
- Zhou, W.; Ni, T.K.; Wronski, A.; Glass, B.; Skibinski, A.; Beck, A.; Kuperwasser, C. The SIRT2 Deacetylase Stabilizes Slug to Control Malignancy of Basal-like Breast Cancer. Cell Rep. 2016, 17, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Vassilopoulos, A.; Wang, R.-H.; Lahusen, T.; Xiao, Z.; Xu, X.; Li, C.; Veenstra, T.D.; Li, B.; Yu, H.; et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 2011, 20, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ozden, O.; Liu, G.; Song, H.Y.; Zhu, Y.; Yan, Y.; Zou, X.; Kang, H.-J.; Jiang, H.; Principe, D.R.; et al. SIRT2-Mediated Deacetylation and Tetramerization of Pyruvate Kinase Directs Glycolysis and Tumor Growth. Cancer Res. 2016, 76, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Minten, E.V.; Kapoor-Vazirani, P.; Li, C.; Zhang, H.; Balakrishnan, K.; Yu, D.S. SIRT2 promotes BRCA1-BARD1 heterodimerization through deacetylation. Cell Rep. 2021, 34, 108921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, J.; Guo, M.; Gao, X.; Wu, X.; Bai, N.; Guo, W.; Li, N.; Yi, F.; Cheng, R.; et al. The NAD-dependent deacetylase SIRT2 regulates T cell differentiation involved in tumor immune response. Int. J. Biol. Sci. 2020, 16, 3075–3084. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Pons, D.G.; Sastre-Serra, J.; Oliver, J.; Roca, P. SIRT3 Silencing Sensitizes Breast Cancer Cells to Cytotoxic Treatments Through an Increment in ROS Production. J. Cell Biochem. 2017, 118, 397–406. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, Y.; Park, S.-H.; Liu, G.; O’Brien, J.; Jiang, H.; Gius, D. SIRT3-Mediated Dimerization of IDH2 Directs Cancer Cell Metabolism and Tumor Growth. Cancer Res. 2017, 77, 3990–3999. [Google Scholar] [CrossRef]
- Pinterić, M.; Podgorski, I.I.; Sobočanec, S.; Hadžija, M.P.; Paradžik, M.; Dekanić, A.; Marinović, M.; Halasz, M.; Belužić, R.; Davidović, G.; et al. De novo expression of transfected sirtuin 3 enhances susceptibility of human MCF-7 breast cancer cells to hyperoxia treatment. Free Radic Res. 2018, 52, 672–684. [Google Scholar] [CrossRef]
- Podgorski, I.I.; Pinterić, M.; Marčinko, D.; Hadžija, M.P.; Filić, V.; Ciganek, I.; Pleše, D.; Balog, T.; Sobočanec, S. Combination of sirtuin 3 and hyperoxia diminishes tumorigenic properties of MDA-MB-231 cells. Life Sci. 2020, 254, 117812. [Google Scholar] [CrossRef]
- Pinterić, M.; Podgorski, I.I.; Hadžija, M.P.; Filić, V.; Paradžik, M.; Proust, B.L.J.; Dekanić, A.; Ciganek, I.; Pleše, D.; Marčinko, D.; et al. Sirt3 Exerts Its Tumor-Suppressive Role by Increasing p53 and Attenuating Response to Estrogen in MCF-7 Cells. Antioxidants 2020, 9, 294. [Google Scholar] [CrossRef]
- Zu, Y.; Chen, X.-F.; Li, Q.; Zhang, S.-T.; Si, L.-N. PGC-1α activates SIRT3 to modulate cell proliferation and glycolytic metabolism in breast cancer. Neoplasma 2021, 68, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, J.; Fu, L.; Gai, J.; Guan, J.; Li, Q. SIRT4 enhances the sensitivity of ER-positive breast cancer to tamoxifen by inhibiting the IL-6/STAT3 signal pathway. Cancer Med. 2019, 8, 7086–7097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, D.; Li, J.; Su, Y.; Liu, S.; Lei, Q.-Y.; Yin, M. Deacetylation of MTHFD2 by SIRT4 senses stress signal to inhibit cancer cell growth by remodeling folate metabolism. J. Mol. Cell Biol. 2022, 14, mjac020. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, X.; Ren, Y.; Li, J.; Li, P.; Jiao, Q.; Meng, P.; Wang, F.; Wang, Y.; Wang, Y.-S.; et al. Loss of SIRT4 promotes the self-renewal of Breast Cancer Stem Cells. Theranostics 2020, 10, 9458–9476. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef]
- Greene, K.S.; Lukey, M.J.; Wang, X.; Blank, B.; Druso, J.E.; Lin, M.-C.J.; Stalnecker, C.A.; Zhang, C.; Abril, Y.N.; Erickson, J.W.; et al. SIRT5 stabilizes mitochondrial glutaminase and supports breast cancer tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 26625–26632. [Google Scholar] [CrossRef]
- Fernandez, I.; Abril, Y.N.; Hong, J.Y.; Yang, M.; Sadhukhan, S.; Lin, H.; Weiss, R. Abstract 4806: SIRT5 inhibition causes increased oxidative stress and impairs tumor progression and metastasis. Cancer Res. 2020, 80, 4806. [Google Scholar] [CrossRef]
- Sociali, G.; Grozio, A.; Caffa, I.; Schuster, S.; Becherini, P.; Damonte, P.; Sturla, L.; Fresia, C.; Passalacqua, M.; Mazzola, F.; et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)(H) pools in cancer cells. FASEB J. 2019, 33, 3704–3717. [Google Scholar] [CrossRef]
- Ioris, R.M.; Galié, M.; Ramadori, G.; Anderson, J.G.; Charollais, A.; Konstantinidou, G.; Brenachot, X.; Aras, E.; Goga, A.; Ceglia, N.; et al. SIRT6 Suppresses Cancer Stem-like Capacity in Tumors with PI3K Activation Independently of Its Deacetylase Activity. Cell Rep. 2017, 18, 1858–1868. [Google Scholar] [CrossRef]
- Becherini, P.; Caffa, I.; Piacente, F.; Damonte, P.; Vellone, V.G.; Passalacqua, M.; Benzi, A.; Bonfiglio, T.; Reverberi, D.; Khalifa, A.; et al. SIRT6 enhances oxidative phosphorylation in breast cancer and promotes mammary tumorigenesis in mice. Cancer Metab. 2021, 9, 6. [Google Scholar] [CrossRef]
- Tang, X.; Shi, L.; Xie, N.; Liu, Z.; Qian, M.; Meng, F.; Xu, Q.; Zhou, M.; Cao, X.; Zhu, W.-G.; et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat. Commun. 2017, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, Y.; Shao, L.; Zhuang, J.; Huo, Q.; He, S.; Chen, S.; Wang, J.; Xie, N. SIRT7 interacts with TEK (TIE2) to promote adriamycin induced metastasis in breast cancer. Cell Oncol. 2021, 44, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Lovaas, J.D.; Zhu, L.; Chiao, C.Y.; Byles, V.; Faller, D.V.; Dai, Y. SIRT1 enhances matrix metalloproteinase-2 expression and tumor cell invasion in prostate cancer cells. Prostate 2013, 73, 522–530. [Google Scholar] [CrossRef]
- Cui, Y.; Li, J.; Zheng, F.; Ouyang, Y.; Chen, X.; Zhang, L.; Chen, Y.; Wang, L.; Mu, S.; Zhang, H. Effect of SIRT1 Gene on Epithelial-Mesenchymal Transition of Human Prostate Cancer PC-3 Cells. Med. Sci. Monit. 2016, 22, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Zhu, L.; Lovaas, J.D.; Chmilewski, L.K.; Wang, J.; Faller, D.V.; Dai, Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 2012, 31, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Nihal, M.; Zhong, W.; Ahmad, N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J. Biol. Chem. 2009, 284, 3823–3832. [Google Scholar] [CrossRef]
- Li, G.; Rivas, P.; Bedolla, R.; Thapa, D.; Reddick, R.L.; Ghosh, R.; Kumar, A.P. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: Involvement of SIRT1/S6K axis. Cancer Prev. Res. 2013, 6, 27–39. [Google Scholar] [CrossRef]
- Sun, L.; Kokura, K.; Izumi, V.; Koomen, J.M.; Seto, E.; Chen, J.; Fang, J. MPP8 and SIRT1 crosstalk in E-cadherin gene silencing and epithelial-mesenchymal transition. EMBO Rep. 2015, 16, 689–699. [Google Scholar] [CrossRef]
- Dai, Y.; Ngo, D.; Forman, L.W.; Qin, D.C.; Jacob, J.; Faller, D.V. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol. Endocrinol. 2007, 21, 1807–1821. [Google Scholar] [CrossRef]
- Fu, M.; Liu, M.; Sauve, A.A.; Jiao, X.; Zhang, X.; Wu, X.; Powell, M.J.; Yang, T.; Gu, W.; Avantaggiati, M.L.; et al. Hormonal control of androgen receptor function through SIRT1. Mol. Cell Biol. 2006, 26, 8122–8135. [Google Scholar] [CrossRef]
- Huang, S.B.; Thapa, D.; Munoz, A.R.; Hussain, S.S.; Yang, X.; Bedolla, R.G.; Osmulski, P.; Gaczynska, M.E.; Lai, Z.; Chiu, Y.-C.; et al. Androgen deprivation-induced elevated nuclear SIRT1 promotes prostate tumor cell survival by reactivation of AR signaling. Cancer Lett. 2021, 505, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Wang, N.; Chen, Q.; Xu, J.; Cheng, W.; Di, M.; Xia, W.; Gao, W.-Q. SIRT3 inhibits prostate cancer by destabilizing oncoprotein c-MYC through regulation of the PI3K/Akt pathway. Oncotarget 2015, 6, 26494–26507. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Quan, Y.; Xia, W. SIRT3 inhibits prostate cancer metastasis through regulation of FOXO3A by suppressing Wnt/β-catenin pathway. Exp. Cell Res. 2018, 364, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Singh, C.K.; Chhabra, G.; Nihal, M.; Iczkowski, K.A.; Ahmad, N. Abstract 539: Pro-proliferative function of the histone deacetylase SIRT3 in prostate cancer. Cancer Res. 2018, 78, 539. [Google Scholar] [CrossRef]
- Sawant Dessai, A.; Dominguez, M.P.; Chen, U.-I.; Hasper, J.; Prechtl, C.; Yu, C.; Katsuta, E.; Dai, T.; Zhu, B.; Jung, S.; et al. Transcriptional Repression of SIRT3 Potentiates Mitochondrial Aconitase Activation to Drive Aggressive Prostate Cancer to the Bone. Cancer Res. 2021, 81, 50–63. [Google Scholar] [CrossRef]
- Fu, W.; Li, H.; Fu, H.; Zhao, S.; Shi, W.; Sun, M.; Li, Y. The SIRT3 and SIRT6 Promote Prostate Cancer Progression by Inhibiting Necroptosis-Mediated Innate Immune Response. J. Immunol. Res. 2020, 2020, 8820355. [Google Scholar] [CrossRef]
- Csibi, A.; Fendt, S.-M.; Li, C.; Poulogiannis, G.; Choo, A.Y.; Chapski, D.J.; Jeong, S.M.; Dempsey, J.M.; Parkhitko, A.; Morrison, T.; et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 2013, 153, 840–854. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Liu, T.; Hu, B.; Li, J.; Liu, C.; Liu, T.; Li, F. Mitochondrial PAK6 inhibits prostate cancer cell apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics 2020, 10, 2571–2586. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, X.; Gai, J.; Sun, X.; Zhao, J.; Li, J.; Li, Y.; Cheng, M.; Du, T.; Fu, L.; et al. Sirtuin 5 regulates the proliferation, invasion and migration of prostate cancer cells through acetyl-CoA acetyltransferase 1. J. Cell. Mol. Med. 2020, 24, 14039–14049. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeon, J.M.; Na, A.Y.; Kwon, O.K.; Bang, I.H.; Ha, Y.-S.; Bae, E.J.; Park, B.-H.; Lee, E.H.; Kwon, T.G.; et al. SIRT5 Directly Inhibits the PI3K/AKT Pathway in Prostate Cancer Cell Lines. Cancer Genom. Proteom. 2022, 19, 50–59. [Google Scholar] [CrossRef]
- Kwon, O.K.; Bang, I.H.; Choi, S.Y.; Jeon, J.M.; Na, A.-Y.; Gao, Y.; Cho, S.S.; Ki, S.H.; Choe, Y.; Lee, J.N.; et al. SIRT5 Is the desuccinylase of LDHA as novel cancer metastatic stimulator in aggressive prostate cancer. Genom. Proteom. Bioinform. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Q.R.; Wang, B.; Shao, J.; Zhang, T.; Liu, T.; Huang, G.; Xia, W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein. Cell 2013, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wong, A.S.; Xia, W. Abstract 1151: SIRT6 induces EMT and promotes cancer cell invasion and migration in prostate cancer. Cancer Res. 2014, 74, 1151. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, R.; Song, L.-D.; Zhu, L.-F.; Zhan, J.-F. SIRT6 Promotes the Progression of Prostate Cancer via Regulating the Wnt/β-Catenin Signaling Pathway. J. Oncol. 2022, 2022, 2174758. [Google Scholar] [CrossRef] [PubMed]
- Haider, R.; Massa, F.; Kaminski, L.; Clavel, S.; Djabari, Z.; Robert, G.; Laurent, K.; Michiels, J.-F.; Durand, M.; Ricci, J.-E.; et al. Sirtuin 7: A new marker of aggressiveness in prostate cancer. Oncotarget 2017, 8, 77309–77316. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jiang, C.-Y.; Zhang, Y.; Zhao, J.; Han, B.-M.; Xia, S.-J. SIRT7 depletion inhibits cell proliferation and androgen-induced autophagy by suppressing the AR signaling in prostate cancer. J. Exp. Clin. Cancer Res. 2020, 39, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Villanova, L.; Tanaka, S.; Aonuma, M.; Roy, N.; Berber, E.; Pollack, J.R.; Michishita-Kioi, E.; Chua, K.F. SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors. Sci. Rep. 2015, 5, 9841. [Google Scholar] [CrossRef]
- Deus, C.M.; Serafim, T.L.; Magalhães-Novais, S.; Vilaça, A.; Moreira, A.C.; Sardão, V.A.; Cardoso, S.M.; Oliveira, P.J. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch. Toxicol. 2017, 91, 1261–1278. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Kugel, S.; Feldman, J.L.; Klein, M.A.; Silberman, D.M.; Sebastian, C.; Mermel, C.; Dobersch, S.; Clark, A.R.; Getz, G.; Denu, J.M.; et al. Identification of and Molecular Basis for SIRT6 Loss-of-Function Point Mutations in Cancer. Cell Rep. 2015, 13, 479–488. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Zhang, X. The promoter methylation status and mRNA expression levels of CTCF and SIRT6 in sporadic breast cancer. DNA Cell Biol. 2014, 33, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Thirumurthi, U.; Shen, J.; Xia, W.; LaBaff, A.M.; Wei, Y.; Li, C.-W.; Chang, W.-C.; Chen, C.-H.; Lin, H.-K.; Yu, D.; et al. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci. Signal 2014, 7, ra71. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.; Zhao, X.; Lai, S.; Yuan, Z.; Zhan, Y.; Ni, K.; Liu, Z.; Liu, L.; Xin, R.; et al. Clinicopathological and prognostic value of SIRT6 in patients with solid tumors: A meta-analysis and TCGA data review. Cancer Cell Int. 2022, 22, 84. [Google Scholar] [CrossRef] [PubMed]
- Aljada, A.; Saleh, A.M.; Alkathiri, M.; Shamsa, H.B.; Al-Bawab, A.; Nasr, A. Altered Sirtuin 7 Expression is Associated with Early Stage Breast Cancer. Breast Cancer 2015, 9, 3–8. [Google Scholar] [CrossRef]
- Huo, Q.; Li, Z.; Cheng, L.; Yang, F.; Xie, N. SIRT7 Is a Prognostic Biomarker Associated with Immune Infiltration in Luminal Breast Cancer. Front. Oncol. 2020, 10, 621. [Google Scholar] [CrossRef]
- Thiery, J. Epithelial—Mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Drescher, F.; Juárez, P.; Arellano, D.L.; Serafín-Higuera, N.; Olvera-Rodriguez, F.; Jiménez, S.; Licea-Navarro, A.F.; Fournier, P.G. TIE2 Induces Breast Cancer Cell Dormancy and Inhibits the Development of Osteolytic Bone Metastases. Cancers 2020, 12, 868. [Google Scholar] [CrossRef]
- Hoffmann, M.J.; Engers, R.; Florl, A.R.; Otte, A.P.; Muller, M.; Schulz, W.A. Expression changes in EZH2, but not in BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation changes in prostate cancer. Cancer Biol. Ther. 2007, 6, 1403–1412. [Google Scholar] [CrossRef]
- Kojima, K.; Ohhashi, R.; Fujita, Y.; Hamada, N.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem. Biophys. Res. Commun. 2008, 373, 423–428. [Google Scholar] [CrossRef]
- Nakane, K.; Fujita, Y.; Terazawa, R.; Atsumi, Y.; Kato, T.; Nozawa, Y.; Deguchi, T.; Ito, M. Inhibition of cortactin and SIRT1 expression attenuates migration and invasion of prostate cancer DU145 cells. Int. J. Urol. 2012, 19, 71–79. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, H.; Haller, E.M.; Nicosia, S.V.; Bai, W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005, 24, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chan, C.-H.; Chen, K.; Guan, X.; Lin, H.-K.; Tong, Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene 2012, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.B.; Rivas, P.; Yang, X.; Lai, Z.; Chen, Y.; Schadler, K.L.; Hu, M.; Reddick, R.L.; Ghosh, R.; Kumar, A.P. SIRT1 inhibition-induced senescence as a strategy to prevent prostate cancer progression. Mol. Carcinog. 2022, 61, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Jost, M.; Maquoi, E. Matrix metalloproteinases at cancer tumor-host interface. Semin. Cell Dev. Biol. 2008, 19, 52–60. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef]
- Du, X.; Li, Q.; Yang, L.; Liu, L.; Cao, Q.; Li, Q. SMAD4 activates Wnt signaling pathway to inhibit granulosa cell apoptosis. Cell Death Dis. 2020, 11, 373. [Google Scholar] [CrossRef]
- Zhu, M.L.; Partin, J.V.; Bruckheimer, E.M.; Strup, S.E.; Kyprianou, N. TGF-beta signaling and androgen receptor status determine apoptotic cross-talk in human prostate cancer cells. Prostate 2008, 68, 287–295. [Google Scholar] [CrossRef]
- Shi, Y.; Han, J.J.; Tennakoon, J.B.; Mehta, F.F.; Merchant, F.; Burns, A.R.; Howe, M.K.; McDonnell, D.P.; Frigo, D.E. Androgens promote prostate cancer cell growth through induction of autophagy. Mol. Endocrinol. 2013, 27, 280–295. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, L.M.; Zino, S.; MacDonald, A.I.; Curle, J.; Reilly, J.E.; Mohammed, Z.M.A.; McMillan, D.C.; Mallon, E.; Payne, A.P.; Edwards, J.; et al. SIRT2: Tumour suppressor or tumour promoter in operable breast cancer? Eur. J. Cancer 2014, 50, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhou, M.; Yang, Y. Upregulated tumor sirtuin 2 expression correlates with reduced TNM stage and better overall survival in surgical breast cancer patients. Ir. J. Med. Sci. 2020, 189, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009, 4, 2. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Casimiro, M.C.; Crosariol, M.; Loro, E.; Li, Z.; Pestell, R.G. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012, 3, 649–657. [Google Scholar] [CrossRef]
- Saeki, T.; Ouchi, M.; Ouchi, T. Physiological and oncogenic Aurora-A pathway. Int. J. Biol. Sci. 2009, 5, 758–762. [Google Scholar] [CrossRef]
- Schmit, T.L.; Zhong, W.; Nihal, M.; Ahmad, N. Polo-like kinase 1 (Plk1) in non-melanoma skin cancers. Cell Cycle 2009, 8, 2697–2702. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.-L.; Chang, Y.-L.; Wu, C.-T.; Chao, Y.-C.; Kao, S.-H.; Yuan, A.; Lin, C.-W.; Yang, S.-C.; Chan, W.-K.; et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat. Cell Biol. 2009, 11, 694–704. [Google Scholar] [CrossRef]
- Wu, W.S.; Heinrichs, S.; Xu, D.; Garrison, S.P.; Zambetti, G.P.; Adams, J.M.; Look, A.T. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005, 123, 641–653. [Google Scholar] [CrossRef]
- Tarsounas, M.; Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299. [Google Scholar] [CrossRef]
- Damodaran, S.; Damaschke, N.; Gawdzik, J.; Yang, B.; Shi, C.; Allen, G.O.; Huang, W.; Denu, J.; Jarrard, D. Dysregulation of Sirtuin 2 (SIRT2) and histone H3K18 acetylation pathways associates with adverse prostate cancer outcomes. BMC Cancer 2017, 17, 874. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.J.; Lane, J.D.; Balthasar, N. SIRT3: A Central Regulator of Mitochondrial Adaptation in Health and Disease. Genes Cancer 2013, 4, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.M.; Andrabi, S.A. SIRT3 Regulation Under Cellular Stress: Making Sense of the Ups and Downs. Front. Neurosci. 2018, 12, 799. [Google Scholar] [CrossRef]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef]
- Sidorova-Darmos, E.; Sommer, R.; Eubanks, J.H. The Role of SIRT3 in the Brain Under Physiological and Pathological Conditions. Front. Cell Neurosci. 2018, 12, 196. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. SIRT3: Oncogene and Tumor Suppressor in Cancer. Cancers 2017, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Patel, K.; Muldoon-Jacobs, K.; Bisht, K.S.; Aykin-Burns, N.; Pennington, J.D.; van der Meer, R.; Nguyen, P.; Savage, J.; Owens, K.M.; et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010, 17, 41–52. [Google Scholar] [CrossRef]
- Finley, L.W.; Carracedo, A.; Lee, J.; Souza, A.; Egia, A.; Zhang, J.; Teruya-Feldstein, J.; Moreira, P.; Cardoso, S.M.; Clish, C.; et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 2011, 19, 416–428. [Google Scholar] [CrossRef]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef]
- Nasrin, N.; Wu, X.; Fortier, E.; Feng, Y.; Bare, O.C.; Chen, S.; Ren, X.; Wu, Z.; Streeper, R.S.; Bordone, L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J. Biol. Chem. 2010, 285, 31995–32002. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhu, G. Sirtuin-4 (SIRT4), a therapeutic target with oncogenic and tumor-suppressive activity in cancer. Onco Targets Ther. 2018, 11, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Xiao, C.; Finley, L.W.; Lahusen, T.; Souza, A.L.; Pierce, K.; Li, Y.-H.; Wang, X.; Laurent, G.; German, N.J.; et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 2013, 23, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Lee, A.; Lee, J.; Haigis, M.C. SIRT4 protein suppresses tumor formation in genetic models of Myc-induced B cell lymphoma. J. Biol. Chem. 2014, 289, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Miyo, M.; Yamamoto, H.; Konno, M.; Colvin, H.; Nishida, N.; Koseki, J.; Kawamoto, K.; Ogawa, H.; Hamabe, A.; Uemura, M.; et al. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br. J. Cancer 2015, 113, 492–499. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, J.; Feng, S.; Chen, X.; Huang, H.; Wang, C.; Yu, Y.; He, Y.; Han, S.; Zheng, L. SIRT4 inhibits the proliferation, migration, and invasion abilities of thyroid cancer cells by inhibiting glutamine metabolism. Onco Targets Ther. 2019, 12, 2397–2408. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, T.; Zhang, X.; Geng, J.; He, X.; Nu, M.; Pang, D. Decreased sirtuin 4 expression is associated with poor prognosis in patients with invasive breast cancer. Oncol. Lett. 2016, 12, 2606–2612. [Google Scholar] [CrossRef]
- Huang, G.; Lin, Y.; Zhu, G. SIRT4 is upregulated in breast cancer and promotes the proliferation, migration and invasion of breast cancer cells. Int. J. Clin. Exp. Pathol. 2017, 10, 11849–11856. [Google Scholar]
- Igci, M.; Kalender, M.E.; Borazan, E.; Bozgeyik, I.; Bayraktar, R.; Bozgeyik, E.; Camci, C.; Arslan, A. High-throughput screening of Sirtuin family of genes in breast cancer. Gene 2016, 586, 123–128. [Google Scholar] [CrossRef]
- Prasad, V.V.; Gopalan, R.O. Continued use of MDA-MB-435, a melanoma cell line, as a model for human breast cancer, even in year, 2014. NPJ Breast Cancer 2015, 1, 15002. [Google Scholar] [CrossRef]
- Tan, M.; Peng, C.; Anderson, K.A.; Chhoy, P.; Xie, Z.; Dai, L.; Park, J.; Chen, Y.; Huang, H.; Zhang, Y.; et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014, 19, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, X.; He, Y.; Yuan, C.; Chen, Q.; Li, G.; Chen, X. Sirtuin 5: A review of structure, known inhibitors and clues for developing new inhibitors. Sci. China Life Sci. 2017, 60, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Lin, P.-M.; Lin, S.-F.; Hsu, C.-H.; Lin, H.-C.; Hu, M.-L.; Yang, M.-Y. Altered expression of SIRT gene family in head and neck squamous cell carcinoma. Tumour. Biol. 2013, 34, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Monteiro-Reis, S.; Almeida-Rios, D.; Vieira, R.; Castro, A.; Moutinho, M.; Rodrigues, M.; Graça, I.; Lopes, J.M.; Jerónimo, C. Assessing sirtuin expression in endometrial carcinoma and non-neoplastic endometrium. Oncotarget 2016, 7, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Temel, M.; Koç, M.N.; Ulutaş, S.; Göğebakan, B. The expression levels of the sirtuins in patients with BCC. Tumour Biol. 2016, 37, 6429–6435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, C.; Tian, Y.; Yao, Y.; Mao, J.; Wang, H.; Li, Z.; Xu, Y.; Ye, M.; Wang, L. SIRT5 Promotes Hepatocellular Carcinoma Progression by Regulating Mitochondrial Apoptosis. J. Cancer 2019, 10, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Abril, Y.L.N.; Fernandez, I.R.; Hong, J.Y.; Chiang, Y.-L.; Kutateladze, D.A.; Zhao, Q.; Yang, M.; Hu, J.; Sadhukhan, S.; Li, B.; et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 2021, 40, 1644–1658. [Google Scholar] [CrossRef]
- Garte, S.J. The c-myc oncogene in tumor progression. Crit. Rev. Oncog. 1993, 4, 435–449. [Google Scholar]
- Chou, C.C.; Lee, K.-H.; Lai, I.-L.; Wang, D.; Mo, X.; Kulp, S.K.; Shapiro, C.L.; Chen, C.-S. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014, 74, 4783–4795. [Google Scholar] [CrossRef]
- Dehner, M.; Hadjihannas, M.; Weiske, J.; Huber, O.; Behrens, J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 2008, 283, 19201–19210. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Chevrollier, A.; Loiseau, D.; Chabi, B.; Renier, G.; Douay, O.; Malthièry, Y.; Stepien, G. ANT2 Isoform Required for Cancer Cell Glycolysis. J. Bioenerg. Biomembr. 2005, 37, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.; Schulze, A. Cholesteryl Esters: Fueling the Fury of Prostate Cancer. Cell Metabolism 2014, 19, 350–352. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Lee, R.; Feinbaum, R.; Ambros, V. A short history of a short RNA. Cell 2004, 116, S89–S92. [Google Scholar] [CrossRef]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. Calin, MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. 2011, 717, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Lizano, E.; Houben, A.J.S.; Bezdan, D.; Báñez-Coronel, M.; Kudla, G.; Mateu-Huertas, E.; Kagerbauer, B.; González, J.; Chen, K.C.; et al. Evidence for the biogenesis of more than 1000 novel human microRNAs. Genome. Biol. 2014, 15, R57. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Ipsaro, J.J.; Joshua-Tor, L. Joshua-Tor, From guide to target: Molecular insights into eukaryotic RNA-interference machinery. Nat. Struct. Mol. Biol. 2015, 22, 20–28. [Google Scholar] [CrossRef]
- Xu, W.; Lucas, A.S.; Wang, Z.; Liu, Y. Identifying microRNA targets in different gene regions. BMC Bioinform. 2014, 15, S4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, W.; Liu, Y.; Liu, T.; Li, C.; Wang, L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5’UTR of RUNX3. Oncol. Lett. 2018, 15, 7215–7220. [Google Scholar] [CrossRef] [PubMed]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Pandi, G.; Vemuganti, R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS ONE 2013, 8, e79467. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Brkić, J.; Hayder, H.; Peng, C. MicroRNAs in Human Placental Development and Pregnancy Complications. Int. J. Mol. Sci. 2013, 14, 5519–5544. [Google Scholar] [CrossRef] [PubMed]
- Rasko, J.E.; Wong, J.J. Nuclear microRNAs in normal hemopoiesis and cancer. J. Hematol. Oncol. 2017, 10, 8. [Google Scholar] [CrossRef]
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Smith, M.A.; Wehrkamp, C.J.; Mohr, A.M.; Mott, J.L. MicroRNA Function in Human Diseases. Med. Epigenetics 2013, 1, 106–115. [Google Scholar] [CrossRef]
- Sumathipala, M.; Weiss, S.T. Predicting miRNA-based disease-disease relationships through network diffusion on multi-omics biological data. Sci. Rep. 2020, 10, 8705. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Valis, M.; Kuca, K.; Zhang, B.; Hort, J. MicroRNAs in Alzheimer’s Disease: Diagnostic Markers or Therapeutic Agents? Front. Pharm. 2019, 10, 665. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Dwivedi, S.; Purohit, P.; Sharma, P. MicroRNAs and Diseases: Promising Biomarkers for Diagnosis and Therapeutics. Indian J. Clin. Biochem. 2019, 34, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Frixa, T.; Donzelli, S.; Blandino, G. Oncogenic MicroRNAs: Key Players in Malignant Transformation. Cancers 2015, 7, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Chen, F.; Hu, T.; Peng, W.; Gu, Q.; Sun, Y. The Diverse Oncogenic and Tumor Suppressor Roles of microRNA-105 in Cancer. Front. Oncol. 2019, 9, 518. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, Y.; Sun, D.; Wang, S.; Ding, K.; Liu, M.; Zhang, Y.; Miao, Y.; Liu, H.; Zhou, F. MicroRNA-181a Functions as an Oncogene in Gastric Cancer by Targeting Caprin-1. Front. Pharm. 2018, 9, 1565. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Effect of miRNAs in lung cancer suppression and oncogenesis. Open Life Sci. 2016, 11, 441–446. [Google Scholar] [CrossRef][Green Version]
- Hannafon, B.N.; Cai, A.; Calloway, C.L.; Xu, Y.-F.; Zhang, R.; Fung, K.-M.; Ding, W.-Q. miR-23b and miR-27b are oncogenic microRNAs in breast cancer: Evidence from a CRISPR/Cas9 deletion study. BMC Cancer 2019, 19, 642. [Google Scholar]
- Kanchan, R.K.; Siddiqui, J.A.; Mahapatra, S.; Batra, S.K.; Nasser, M.W. microRNAs Orchestrate Pathophysiology of Breast Cancer Brain Metastasis: Advances in Therapy. Mol. Cancer 2020, 19, 29. [Google Scholar] [CrossRef]
- Loh, H.Y.; Norman, B.P.; Lai, K.-S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef]
- Maskey, N.; Li, D.; Xu, H.; Song, H.; Wu, C.; Hua, K.; Song, J.; Fang, L. MicroRNA-340 inhibits invasion and metastasis by downregulating ROCK1 in breast cancer cells. Oncol. Lett. 2017, 14, 2261–2267. [Google Scholar] [CrossRef]
- Hoey, C.; Ahmed, M.; Ghiam, A.F.; Vesprini, D.; Huang, X.; Commisso, K.; Commisso, A.; Ray, J.; Fokas, E.; Loblaw, D.A.; et al. Circulating miRNAs as non-invasive biomarkers to predict aggressive prostate cancer after radical prostatectomy. J. Transl. Med. 2019, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Bidarra, D.; Constâncio, V.; Barros-Silva, D.; Ramalho-Carvalho, J.; Moreira-Barbosa, C.; Antunes, L.; Maurício, J.; Oliveira, J.; Henrique, R.; Jerónimo, C.; et al. Circulating MicroRNAs as Biomarkers for Prostate Cancer Detection and Metastasis Development Prediction. Front. Oncol. 2019, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Lo, U.-G.; Yang, D.; Hsieh, J.-T. The role of microRNAs in prostate cancer progression. Transl. Androl. Urol. 2013, 2, 228–241. [Google Scholar] [PubMed]
- Richardsen, E.; Andersen, S.; Melbø-Jørgensen, C.; Rakaee, M.; Ness, N.; Al-Saad, S.; Nordby, Y.; Pedersen, M.I.; Dønnem, T.; Bremnes, R.M.; et al. MicroRNA 141 is associated to outcome and aggressive tumor characteristics in prostate cancer. Sci. Rep. 2019, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.K.; Alahari, S.K. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009, 28, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.; Abdolvahabi, Z.; Hesari, Z.; Tavakoli-Yaraki, M.; Yousefi, Z.; Seiri, P.; Hosseinkhani, S.; Nourbakhsh, M. Inhibition of sirtuin 1 deacetylase by miR-211-5p provides a mechanism for the induction of cell death in breast cancer cells. Gene 2019, 711, 143939. [Google Scholar] [CrossRef]
- Abdolvahabi, Z.; Nourbakhsh, M.; Hosseinkhani, S.; Hesari, Z.; Alipour, M.; Jafarzadeh, M.; Ghorbanhosseini, S.S.; Seiri, P.; Yousefi, Z.; Yarahmadi, S.; et al. MicroRNA-590-3P suppresses cell survival and triggers breast cancer cell apoptosis via targeting sirtuin-1 and deacetylation of p53. J. Cell Biochem. 2019, 120, 9356–9368. [Google Scholar] [CrossRef]
- Liang, Y.; Song, X.; Li, Y.; Sang, Y.; Zhang, N.; Zhang, H.; Liu, Y.; Duan, Y.; Chen, B.; Guo, R.; et al. A novel long non-coding RNA-PRLB acts as a tumor promoter through regulating miR-4766-5p/SIRT1 axis in breast cancer. Cell Death Dis. 2018, 9, 563. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, D.; Wei, X. miR-22 suppresses tumorigenesis and improves radiosensitivity of breast cancer cells by targeting Sirt1. Biol. Res. 2017, 50, 27. [Google Scholar] [CrossRef]
- Li, L.; Yuan, L.; Luo, J.; Gao, J.; Guo, J.; Xie, X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin. Exp. Med. 2013, 13, 109–117. [Google Scholar] [CrossRef]
- Eades, G.; Yao, Y.; Yang, M.; Zhang, Y.; Chumsri, S.; Zhou, Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J. Biol. Chem. 2011, 286, 25992–26002. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, L. MicroRNA-3666 inhibits breast cancer cell proliferation by targeting sirtuin 7. Mol. Med. Rep. 2017, 16, 8493–8500. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kojima, K.; Hamada, N.; Ohhashi, R.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2008, 377, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Ma, B. The UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. Cancer Chemother. Pharm. 2016, 78, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ma, B.; Jiang, X.; Li, X.; Jia, Y. Astragalus Polysaccharides Inhibits Tumorigenesis and Lipid Metabolism Through miR-138-5p/SIRT1/SREBP1 Pathway in Prostate Cancer. Front. Pharmacol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Shu, Y.; Ren, L.; Xie, B.; Liang, Z.; Chen, J. MiR-204 enhances mitochondrial apoptosis in doxorubicin-treated prostate cancer cells by targeting SIRT1/p53 pathway. Oncotarget 2017, 8, 97313–97322. [Google Scholar] [CrossRef]
- Kumar, P.; Sharad, S.; Petrovics, G.; Mohamed, A.; Dobi, A.; Sreenath, T.L.; Srivastava, S.; Biswas, R. Loss of miR-449a in ERG-associated prostate cancer promotes the invasive phenotype by inducing SIRT1. Oncotarget 2016, 7, 22791–22806. [Google Scholar] [CrossRef]
- Ramalinga, M.; Roy, A.; Srivastava, A.; Bhattarai, A.; Harish, V.; Suy, S.; Collins, S.; Kumar, D. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget 2015, 6, 34446–34457. [Google Scholar] [CrossRef]
- Duan, K.; Ge, Y.-C.; Zhang, X.-P.; Wu, S.-Y.; Feng, J.-S.; Chen, S.-L.; Zhang, L.; Yuan, Z.-H.; Fu, C.-H. miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression. Oncol. Lett. 2015, 10, 3223–3227. [Google Scholar] [CrossRef]
- Slabáková, E.; Culig, Z.; Remšík, J.; Souček, K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017, 8, e3100, Erratum in Cell Death Dis. 2018, 9, 783. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, Y.; Wu, B.; Cao, Y. MicroRNA-3666 Suppresses Cell Growth in Head and Neck Squamous Cell Carcinoma Through Inhibition of PFKFB3-Mediated Warburg Effect. Onco. Targets Ther. 2020, 13, 9029–9041. [Google Scholar] [CrossRef] [PubMed]
- Shou, T.; Yang, H.; Lv, J.; Liu, D.; Sun, X. MicroRNA-3666 suppresses the growth and migration of glioblastoma cells by targeting KDM2A. Mol. Med. Rep. 2019, 19, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Liu, K.; Lin, Q.; Yang, W.; Liu, D.; Lin, Z.; Shao, W.; Ji, T. Sirtuin 7 promotes glioma proliferation and invasion through activation of the ERK/STAT3 signaling pathway. Oncol. Lett. 2019, 17, 1445–1452. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.-S. miR-490-3p modulates the progression of prostate cancer through regulating histone deacetylase 2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 539–546. [Google Scholar]
- Luu, H.N.; Lin, H.-Y.; Sørensen, K.D.; Ogunwobi, O.; Kumar, N.; Chornokur, G.; Phelan, C.; Jones, D.; Kidd, L.; Batra, J.; et al. miRNAs associated with prostate cancer risk and progression. BMC Urol. 2017, 17, 18. [Google Scholar]
- Nayak, B.; Khan, N.; Garg, H.; Rustagi, Y.; Singh, P.; Seth, A.; Dinda, A.K.; Kaushal, S. Role of miRNA-182 and miRNA-187 as potential biomarkers in prostate cancer and its correlation with the staging of prostate cancer. Int. Braz. J. Urol. 2020, 46, 614–623. [Google Scholar] [CrossRef]
- Wagner, S.; Ngezahayo, A.; Escobar, H.M.; Nolte, I. Role of miRNA let-7 and its major targets in prostate cancer. Biomed. Res. Int. 2014, 2014, 376326. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Gan, R.; Zhao, L.; Li, W.; Zhou, H.; Wang, X.; Lü, J.; Meng, Q.H. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE 2014, 9, e98833. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).