Hazard Function Analysis of Recurrence in Patients with Curatively Resected Lung Cancer: Results from the Japanese Lung Cancer Registry in 2010
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Registry
2.2. Patients
2.3. Definition of Recurrence
2.4. Statistical Analysis
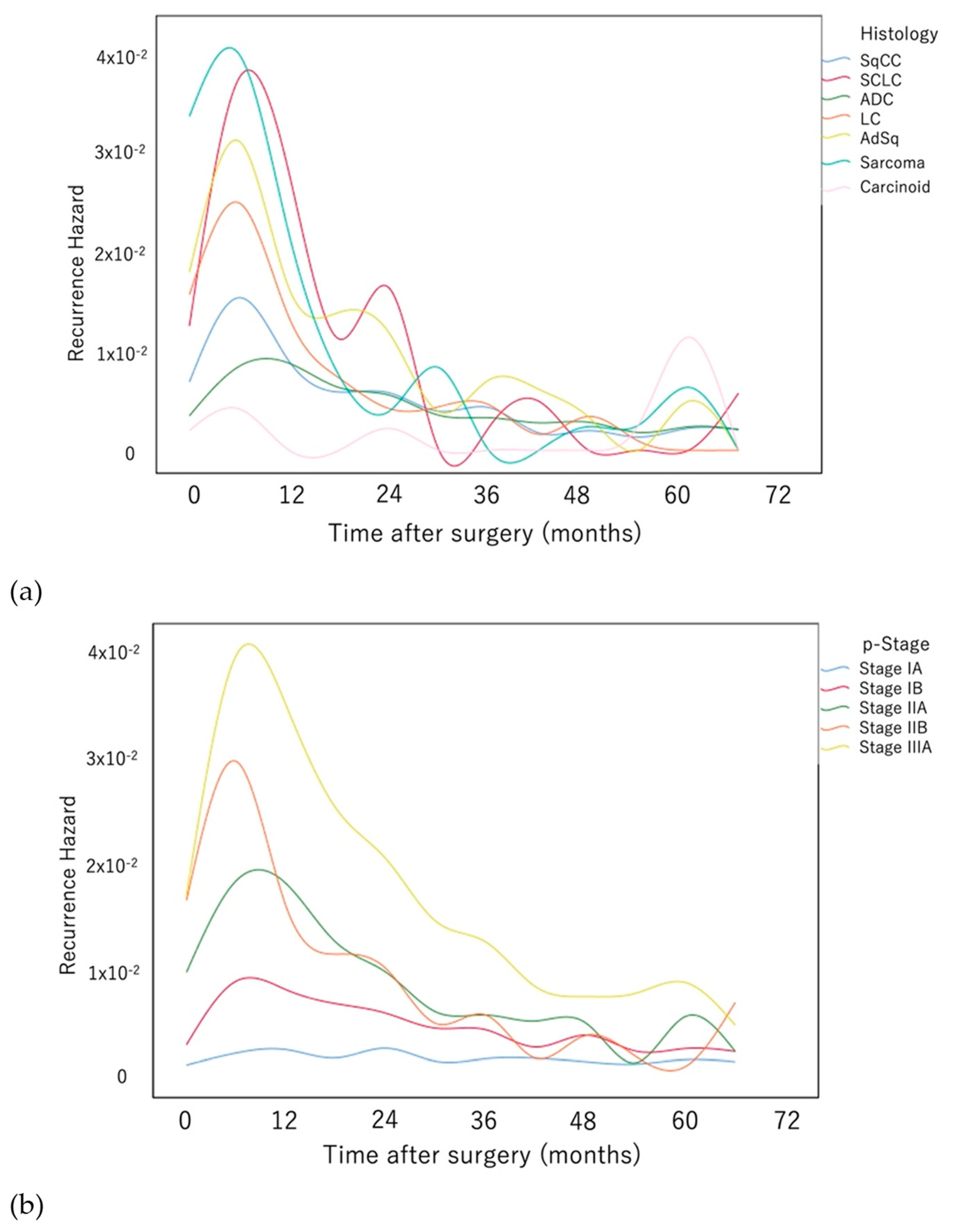
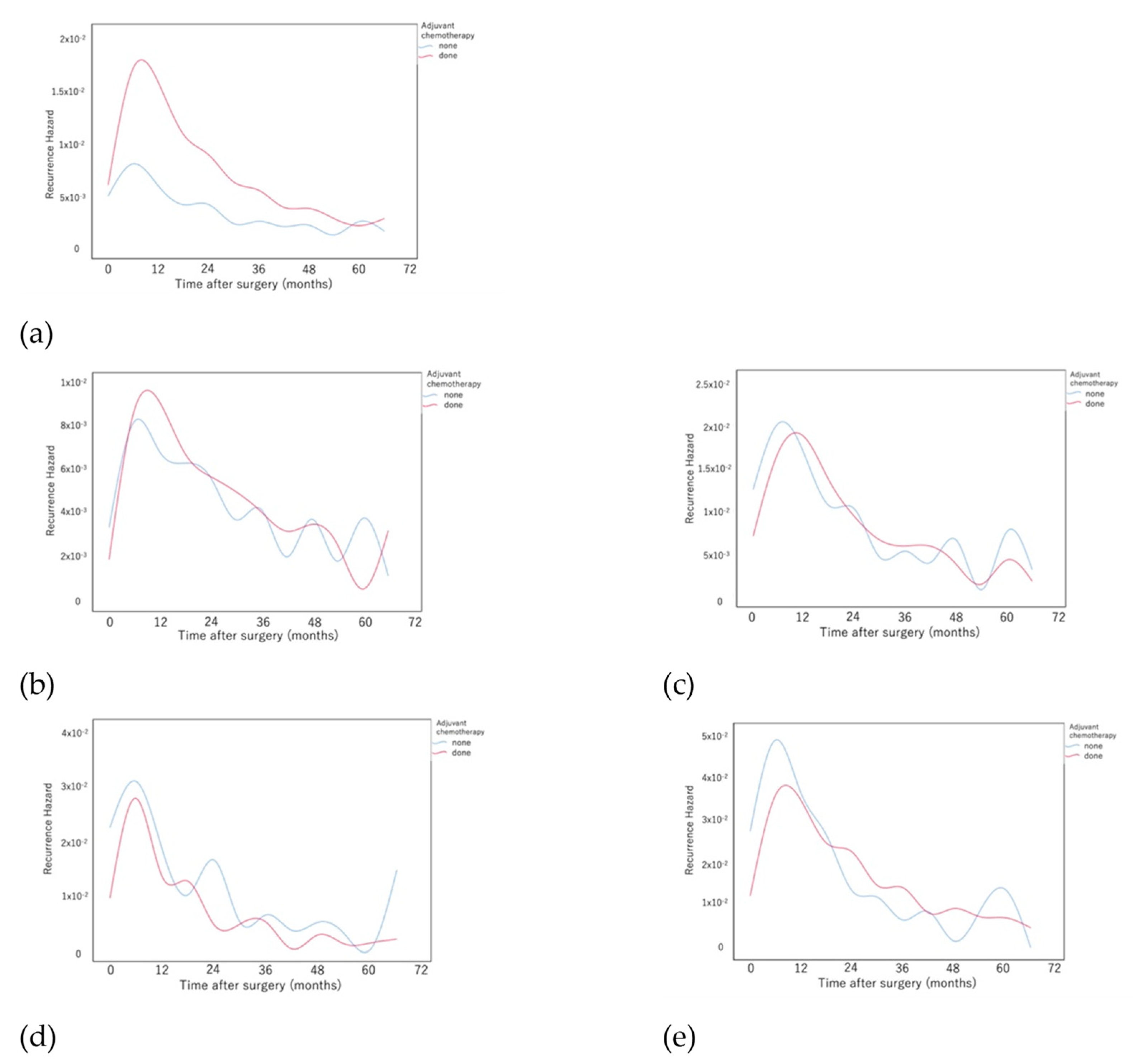
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chansky, K.; Sculier, J.-P.; Crowley, J.J.; Giroux, D.; Van Meerbeeck, J.; Goldstraw, P. The International Association for the Study of Lung Cancer Staging Project: Prognostic Factors and Pathologic TNM Stage in Surgically Managed Non-small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 792–801. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. New Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Goya, T.; Koshiishi, Y.; Sohara, Y.; Eguchi, K.; Mori, M.; Nakanishi, Y.; Tsuchiya, R.; Shimokata, K.; Inoue, H.; et al. A Japanese Lung Cancer Registry Study: Prognosis of 13,010 Resected Lung Cancers. J. Thorac. Oncol. 2008, 3, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Pircher, A.; Augustin, F.; Kocher, F. Evidence-based follow-up in lung cancer? Memo-Mag. Eur. Med. Oncol. 2020, 13, 73–77. [Google Scholar] [CrossRef]
- Westeel, V.; Choma, D.; Clément, F.; Woronoff-Lemsi, M.-C.; Pugin, J.-F.; Dubiez, A.; Depierre, A. Relevance of an intensive postoperative follow-up after surgery for non–small cell lung cancer. Ann. Thorac. Surg. 2000, 70, 1185–1190. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Muley, T.; Safi, S.; Rieken, S.; Bischoff, H.; Kappes, J.; Warth, A.; Herth, F.; Dienemann, H.; Hoffmann, H. The dynamic pattern of recurrence in curatively resected non-small cell lung cancer patients: Experiences at a single institution. Lung Cancer 2015, 90, 224–229. [Google Scholar] [CrossRef]
- Abbas, A.E. Surgical Management of Lung Cancer: History, Evolution, and Modern Advances. Curr. Oncol. Rep. 2018, 20, 98. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Q.; Xu, T.; Deng, W.; Yu, J.; Liao, Z.; Yue, J. Out of the darkness and into the light: New strategies for improving treatments for locally advanced non-small cell lung cancer. Cancer Lett. 2018, 421, 59–62. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer, Version 3. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 7 April 2020).
- Rose, S. International Ethical Guidelines for Epidemiological Studies. Am. J. Epidemiol. 2009, 170, 1451–1452. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Okami, J.; Shintani, Y.; Okumura, M.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.-I.; Date, H.; Yokoi, K.; et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J. Thorac. Oncol. 2019, 14, 212–222. [Google Scholar] [CrossRef]
- International Union against Cancer (UICC). TNM Classification of Malignant Tumours, 7th ed.; Sobin, L.H., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Fink, S.A.; Brown Jr, R.S. Survival Analysis. Gastroenterol Hepatol 2006, 2, 380–383. [Google Scholar]
- Gasser, T.; Müller, H.-G. Kernel Estimation of Regression Functions; Springer: Berlin/Heidelberg, Germany, 1979; pp. 23–68. [Google Scholar] [CrossRef]
- Zhu, J.-F.; Feng, X.-Y.; Zhang, X.-W.; Wen, Y.-S.; Lin, P.; Rong, T.-H.; Cai, L.; Zhang, L.-J. Time-Varying Pattern of Postoperative Recurrence Risk of Early-Stage (T1a-T2bN0M0) Non-Small Cell Lung Cancer (NSCLC): Results of a Single-Center Study of 994 Chinese Patients. PLoS ONE 2014, 9, e106668. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tsuboi, M.; Sakamaki, K.; Nishii, T.; Yamamoto, T.; Nagashima, T.; Ando, K.; Ishikawa, Y.; Woo, T.; Adachi, H.; et al. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2016, 49, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, C.R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Demicheli, R. Metastasis Dynamics for Non–Small-Cell Lung Cancer: Effect of Patient and Tumor-Related Factors. Clin. Lung Cancer 2013, 14, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Kelsey, C. Recurrence Dynamics for Non–Small-Cell Lung Cancer: Effect of Surgery on the Development of Metastases. J. Thorac. Oncol. 2012, 7, 723–730. [Google Scholar] [CrossRef]
- Yun, J.K.; Lee, G.D.; Choi, S.; Kim, Y.-H.; Kim, D.K.; Park, S.-I.; Kim, H.R. Various recurrence dynamics for non-small cell lung cancer depending on pathological stage and histology after surgical resection. Transl. Lung Cancer Res. 2022, 11, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Steslicke, W.E. Development of Health Insurance Policy in Japan. J. Heal Politi-Policy Law 1982, 7, 197–226. [Google Scholar] [CrossRef]
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int. J. Clin. Oncol. 2019, 24, 731–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Z.; Huang, Y.-L.; Wu, C.C.; Tang, E.-K.; Chen, C.-S.; Mar, G.-Y.; Yen, Y.; Wu, M.-T. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin. Lung Cancer 2016, 17, e45–e56. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Qu, X.; Huang, X.; Yan, W.; Wu, L.; Dai, K. A meta-analysis of 18FDG-PET–CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur. J. Radiol. 2012, 81, 1007–1015. [Google Scholar] [CrossRef]
- Manjili, M.H. Tumor Dormancy and Relapse: From a Natural Byproduct of Evolution to a Disease State. Cancer Res. 2017, 77, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Cole, K.; Pravoverov, K.; Talmadge, J.E. Role of myeloid-derived suppressor cells in metastasis. Cancer Metastasis Rev. 2021, 40, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Safi, S.; Blattner, C.; Rathinasamy, A.; Umansky, L.; Juenger, S.; Warth, A.; Eichhorn, M.; Muley, T.; Herth, F.J.F.; et al. Circulating and Tumor Myeloid-derived Suppressor Cells in Resectable Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 777–787. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2009, 11, 121–128. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; Obyrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.-K.; Sriuranpong, V.; Ho, J.C.; Ong, C.K.; Tsai, C.-M.; Chung, C.-H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]



| Factors | N | (%) | ||
|---|---|---|---|---|
| Age | <75 | 9290 | 72.0 | |
| ≥75 | 3607 | 28.0 | ||
| Sex | Male | 8017 | 62.2 | |
| Female | 4880 | 37.8 | ||
| ECOG-PS | 0 | 10,758 | 83.4 | |
| 1 | 1548 | 12.0 | ||
| >2 | 265 | 2.1 | ||
| Smoking history | non-smoker | 4490 | 34.8 | |
| ex-smoker | 6093 | 47.2 | ||
| current smoker | 1883 | 14.6 | ||
| %VC | (%) | 107 ± 18 | ||
| FEV1/FVC | (%) | 74 ± 10 | ||
| type of surgery | Pneumonectomy | 227 | 1.8 | |
| bi-lobectomy | 335 | 2.6 | ||
| Lobectomy | 12,335 | 95.6 | ||
| operation time | (min) | 222 ± 81 | ||
| pathological T factor | T1a | 3683 | 28.6 | |
| T1b | 2709 | 21.0 | ||
| T2a | 4566 | 35.4 | ||
| T2b | 733 | 5.7 | ||
| T3 | 1066 | 8.3 | ||
| T4 | 116 | 0.9 | ||
| Tumor size | (cm) | 3.0 ± 1.7 | ||
| pathological N factor | 0 | 10,152 | 78.7 | |
| 1 | 1307 | 10.1 | ||
| >2 | 1350 | 10.5 | ||
| pathological stage | IA | 5585 | 43.3 | |
| IB | 3303 | 25.6 | ||
| IIA | 1476 | 11.4 | ||
| IIB | 775 | 6.0 | ||
| IIIA | 1593 | 12.4 | ||
| IIIB | 43 | 0.3 | ||
| Histology | Adenocarcinoma | 9004 | 69.8 | |
| Squamous cell carcinoma | 2691 | 20.9 | ||
| Large cell carcinoma | 398 | 3.1 | ||
| Adenosquamous carcinoma | 270 | 2.1 | ||
| Sarcoma | 191 | 1.5 | ||
| Small cell carcinoma | 179 | 1.4 | ||
| Carcinoid | 89 | 0.7 | ||
| Others | 75 | 0.6 | ||
| pleural infiltration | Negative | 9276 | 71.9 | |
| Positive | 3528 | 27.4 | ||
| adjuvant therapy | Chemotherapy | 4267 | 33.1 | |
| Radiotherapy | 187 | 1.4 | ||
| Histology | Pathological Stage | Search for Intrathoracic Recurrence | Search for Extrathoracic Recurrence | ||
|---|---|---|---|---|---|
| until 24 months | after 24 months | until 24 months | after 24 months | ||
| ADC | IA | occasional | occasional | optional | |
| IB/II | frequent | occasional | |||
| IIIA | frequent | ||||
| SqCC | IA | occasional | |||
| IB/II | frequent | occasional | |||
| IIIA | frequent | occasional | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, Y.; Kawamura, M.; Okami, J.; Shintani, Y.; Ito, H.; Ohtsuka, T.; Toyooka, S.; Mori, T.; Watanabe, S.-i.; Asamura, H.; et al. Hazard Function Analysis of Recurrence in Patients with Curatively Resected Lung Cancer: Results from the Japanese Lung Cancer Registry in 2010. Cancers 2022, 14, 5119. https://doi.org/10.3390/cancers14205119
Yamauchi Y, Kawamura M, Okami J, Shintani Y, Ito H, Ohtsuka T, Toyooka S, Mori T, Watanabe S-i, Asamura H, et al. Hazard Function Analysis of Recurrence in Patients with Curatively Resected Lung Cancer: Results from the Japanese Lung Cancer Registry in 2010. Cancers. 2022; 14(20):5119. https://doi.org/10.3390/cancers14205119
Chicago/Turabian StyleYamauchi, Yoshikane, Masafumi Kawamura, Jiro Okami, Yasushi Shintani, Hiroyuki Ito, Takashi Ohtsuka, Shinichi Toyooka, Takeshi Mori, Shun-ichi Watanabe, Hisao Asamura, and et al. 2022. "Hazard Function Analysis of Recurrence in Patients with Curatively Resected Lung Cancer: Results from the Japanese Lung Cancer Registry in 2010" Cancers 14, no. 20: 5119. https://doi.org/10.3390/cancers14205119
APA StyleYamauchi, Y., Kawamura, M., Okami, J., Shintani, Y., Ito, H., Ohtsuka, T., Toyooka, S., Mori, T., Watanabe, S.-i., Asamura, H., Chida, M., Endo, S., Kadokura, M., Nakanishi, R., Miyaoka, E., Suzuki, H., Yoshino, I., & Date, H. (2022). Hazard Function Analysis of Recurrence in Patients with Curatively Resected Lung Cancer: Results from the Japanese Lung Cancer Registry in 2010. Cancers, 14(20), 5119. https://doi.org/10.3390/cancers14205119








