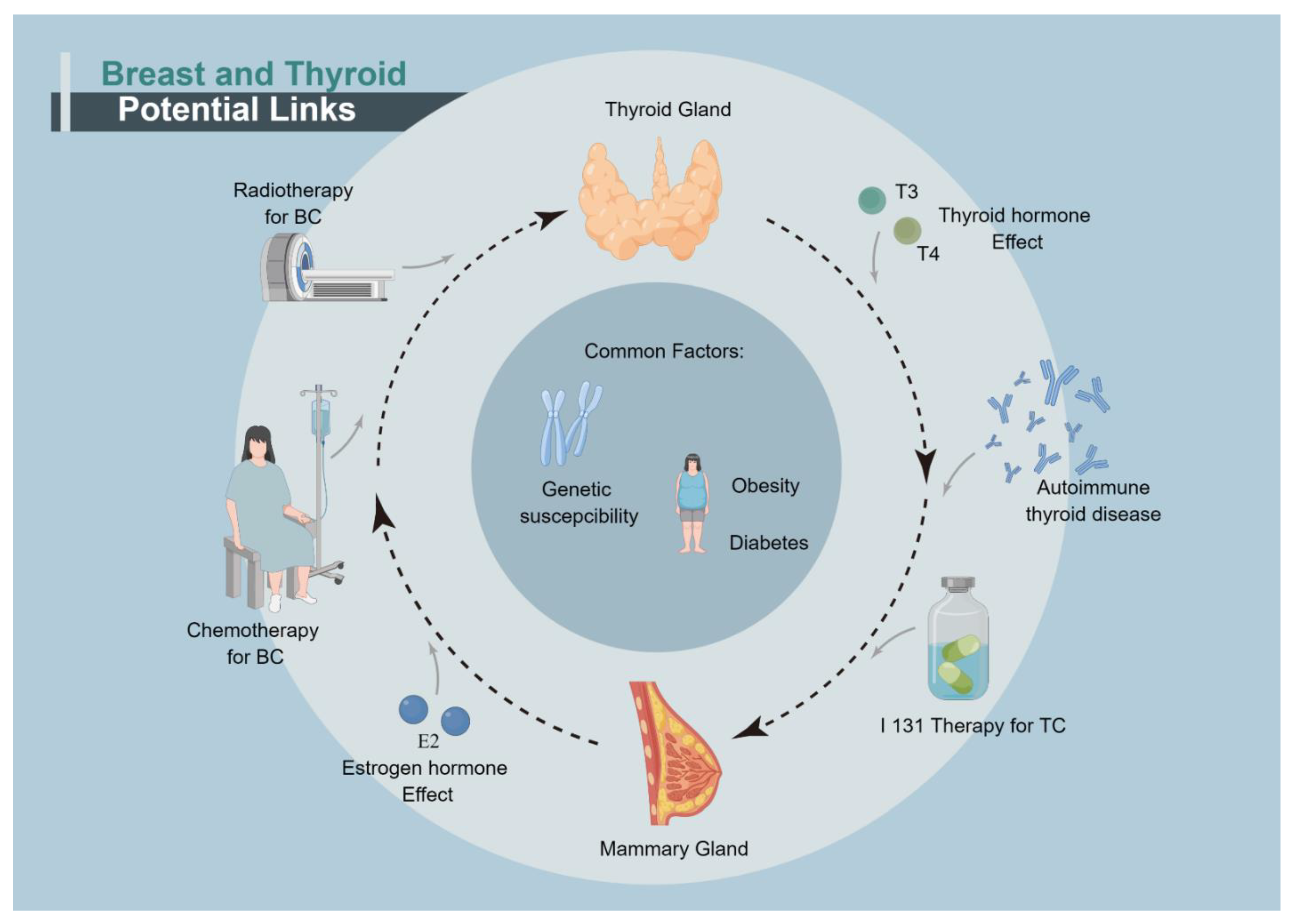
Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions
Abstract
Simple Summary
Abstract
1. Introduction
2. Hormones and Their Receptors
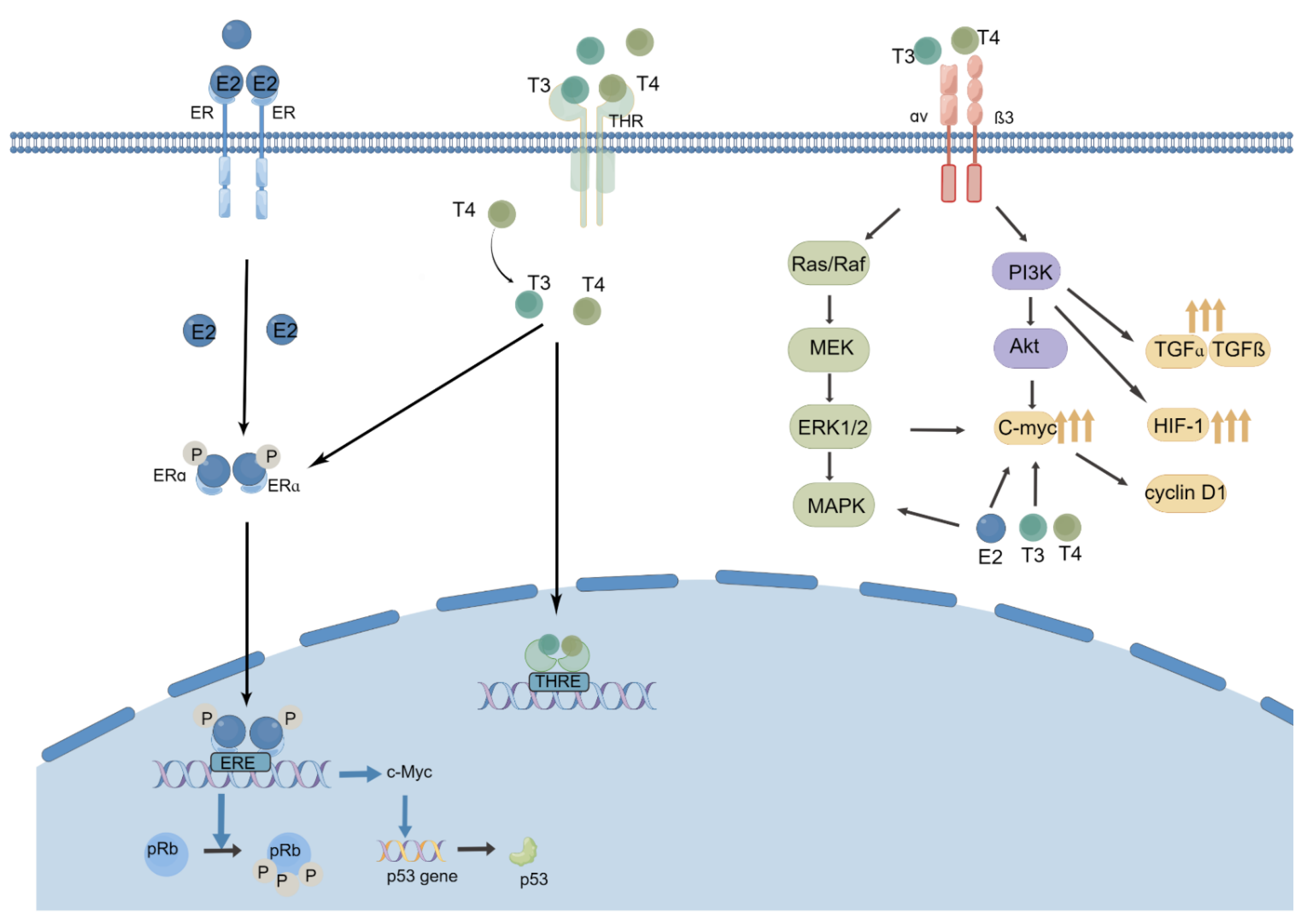
2.1. Thyroid Hormones, Thyroid Hormone Receptor and BC
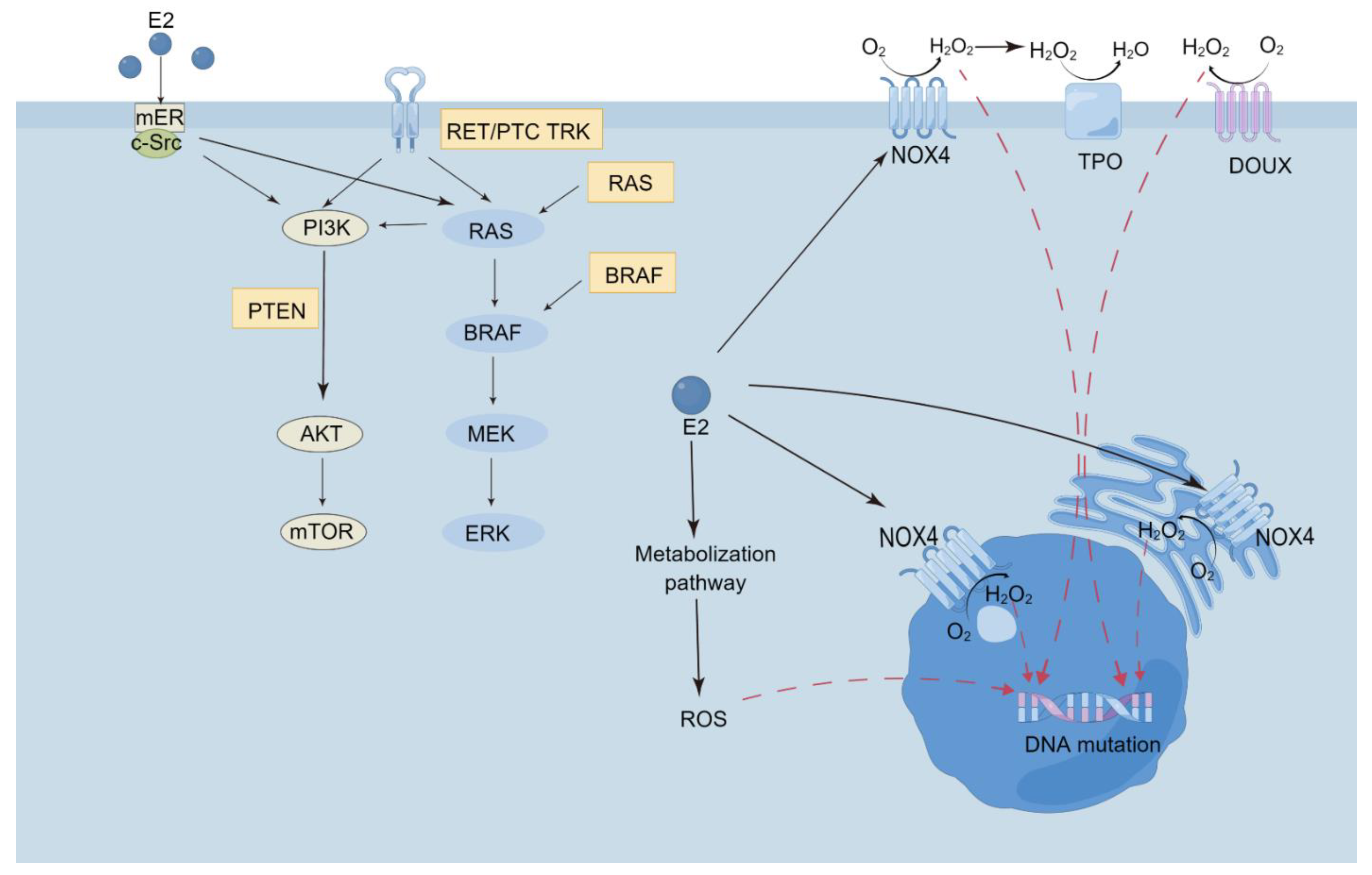
2.2. Estrogen, Progestin, and Its Receptors: ER/PR/HER-2 and TC
3. Autoimmune Thyroid Disease and BC
4. Iodine, Sodium Iodide Symporter and BC
5. Oncogenic Effects of the Therapies for Primary Cancer
5.1. Radioactive Iodine Therapy and Breast Cancer
5.2. Chemotherapy and Thyroid Cancer
5.3. External Beam Radiation, Mammography and Thyroid Cancer
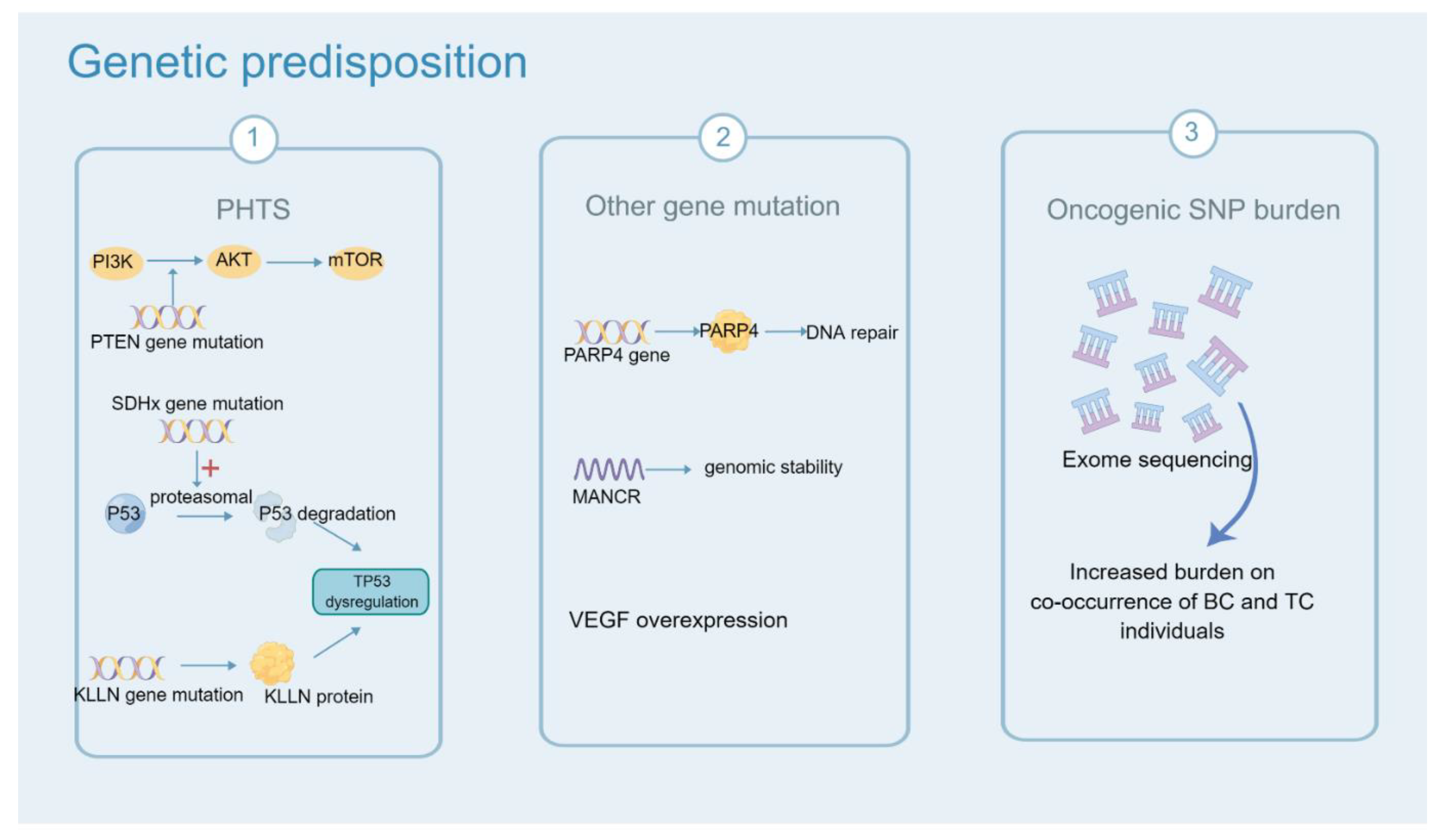
6. Genetic Predisposition
7. Other Factors
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef]
- Beatson, G.T. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Trans. Med. Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar]
- Søgaard, M.; Farkas, D.K.; Ehrenstein, V.; Jørgensen, J.O.L.; Dekkers, O.M.; Sørensen, H.T. Hypothyroidism and hyperthyroidism and breast cancer risk: A nationwide cohort study. Eur. J. Endocrinol. 2016, 174, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Curtis, R.; Hoffman, D.A.; Flannery, J.T. Multiple primary breast and thyroid cancer. Br. J. Cancer 1984, 49, 87–92. [Google Scholar] [CrossRef]
- Osterlind, A.; Olsen, J.H.; Lynge, E.; Ewertz, M. Second cancer following cutaneous melanoma and cancers of the brain, thyroid, connective tissue, bone, and eye in Denmark, 1943–1980. Natl. Cancer Inst. Monogr. 1985, 68, 361–388. [Google Scholar]
- Edhemović, I.; Volk, N.; Auersperg, M. Second primary cancers following thyroid cancer in Slovenia. A population-based cohort study. Eur. J. Cancer 1998, 34, 1813–1814. [Google Scholar]
- Evans, H.S.; Lewis, C.M.; Robinson, D.; Bell, C.M.; Møller, H.; Hodgson, S.V. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br. J. Cancer 2001, 84, 435–440. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Louwman, W.J.; de Vries, E.; Lemmens, V.E.P.P.; Klokman, W.J.; Coebergh, J.W.W. Primary malignancy after primary female breast cancer in the South of the Netherlands, 1972–2001. Breast Cancer Res. Treat. 2005, 93, 91–95. [Google Scholar] [CrossRef]
- Mellemkjær, L.; Christensen, J.; Frederiksen, K.; Pukkala, E.; Weiderpass, E.; Bray, F.; Friis, S.; Andersson, M.; Olsen, J.H. Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1784–1792. [Google Scholar] [CrossRef][Green Version]
- Mellemkjaer, L.; Friis, S.; Olsen, J.H.; Scélo, G.; Hemminki, K.; Tracey, E.; Andersen, A.; Brewster, D.H.; Pukkala, E.; McBride, M.L.; et al. Risk of second cancer among women with breast cancer. Int. J. Cancer 2006, 118, 2285–2292. [Google Scholar] [CrossRef]
- Kim, C.; Bi, X.; Pan, D.; Chen, Y.; Carling, T.; Ma, S.; Udelsman, R.; Zhang, Y. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 2013, 23, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; White, M.G.; Hong, S.; Aschebrook-Kilfoy, B.; Kaplan, E.L.; Angelos, P.; Kulkarni, S.A.; Olopade, O.I.; Grogan, R.H. The Breast-Thyroid Cancer Link: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M.; Nassir, R.; Cheng, T.-Y.D.; Lane, D.; Margolis, K.L. Benign breast disease and risk of thyroid cancer. Cancer Causes Control 2017, 28, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-M.; Chung, L.-M.; Lin, C.-L.; Kao, C.-H. Uterine Fibroids Increase the Risk of Thyroid Cancer. Int. J. Environ. Res. Public Health 2020, 17, 3821. [Google Scholar] [CrossRef]
- Tosovic, A.; Becker, C.; Bondeson, A.-G.; Bondeson, L.; Ericsson, U.-B.; Malm, J.; Manjer, J. Prospectively measured thyroid hormones and thyroid peroxidase antibodies in relation to breast cancer risk. Int. J. Cancer 2012, 131, 2126–2133. [Google Scholar] [CrossRef]
- Kuijpens, J.L.P.; Nyklíctek, I.; Louwman, M.W.J.; Weetman, T.A.P.; Pop, V.J.M.; Coebergh, J.-W.W. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid 2005, 15, 1253–1259. [Google Scholar] [CrossRef]
- Muller, I.; Barrett-Lee, P.J. The antigenic link between thyroid autoimmunity and breast cancer. Semin. Cancer Biol. 2020, 64, 122–134. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.S.; Edgerton, S.M.; Salem, H.; Kim, H.M.; Tan, A.C.; Finlay-Schultz, J.; Wellberg, E.A.; Sartorius, C.A.; Jacobsen, B.M.; Haugen, B.R.; et al. Exogenous Thyroid Hormone Is Associated with Shortened Survival and Upregulation of High-Risk Gene Expression Profiles in Steroid Receptor-Positive Breast Cancers. Clin. Cancer Res. 2021, 27, 585–597. [Google Scholar] [CrossRef]
- Ngeow, J.; Sesock, K.; Eng, C. Clinical Implications for Germline PTEN Spectrum Disorders. Endocrinol. Metab. Clin. N. Am. 2017, 46, 503–517. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Yu, Q.; Eng, C. Androgen receptor-induced tumor suppressor, KLLN, inhibits breast cancer growth and transcriptionally activates p53/p73-mediated apoptosis in breast carcinomas. Hum. Mol. Genet. 2013, 22, 2263–2272. [Google Scholar] [CrossRef]
- Ni, Y.; He, X.; Chen, J.; Moline, J.; Mester, J.; Orloff, M.S.; Ringel, M.D.; Eng, C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum. Mol. Genet. 2012, 21, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Kiyotani, K.; Yew, P.Y.; Kato, T.; Tamura, K.; Yap, K.L.; Nielsen, S.M.; Mester, J.L.; Eng, C.; Nakamura, Y.; et al. Germline PARP4 mutations in patients with primary thyroid and breast cancers. Endocr. Relat. Cancer 2016, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, Y.; Xu, J.; Wang, Z.; Ye, G. Identification of differential expressed lncRNAs in human thyroid cancer by a genome-wide analyses. Cancer Med. 2018, 7, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Tracy, K.M.; Tye, C.E.; Ghule, P.N.; Malaby, H.L.H.; Stumpff, J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Mitotically-Associated lncRNA (MANCR) Affects Genomic Stability and Cell Division in Aggressive Breast Cancer. Mol. Cancer Res. 2018, 16, 587–598. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Zhang, S.; Ming, G. Evaluation of thyroid cancer in Chinese females with breast cancer by vascular endothelial growth factor (VEGF), microvessel density, and contrast-enhanced ultrasound (CEUS). Tumour Biol. 2014, 35, 6521–6529. [Google Scholar] [CrossRef]
- Yuan, S.; Kar, S.; Vithayathil, M.; Carter, P.; Mason, A.M.; Burgess, S.; Larsson, S.C. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: A two-sample Mendelian randomization study. Int. J. Cancer 2020, 147, 1895–1903. [Google Scholar] [CrossRef]
- Bakos, B.; Kiss, A.; Árvai, K.; Szili, B.; Deák-Kocsis, B.; Tobiás, B.; Putz, Z.; Ármós, R.; Balla, B.; Kósa, J.; et al. Co-occurrence of thyroid and breast cancer is associated with an increased oncogenic SNP burden. BMC Cancer 2021, 21, 706. [Google Scholar] [CrossRef]
- Marcheselli, R.; Marcheselli, L.; Cortesi, L.; Bari, A.; Cirilli, C.; Pozzi, S.; Ferri, P.; Napolitano, M.; Federico, M.; Sacchi, S. Risk of Second Primary Malignancy in Breast Cancer Survivors: A Nested Population-Based Case-Control Study. J. Breast Cancer 2015, 18, 378–385. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, C.-L.; Huang, W.-S.; Kao, C.-H. Risk of Breast Cancer in Patients with Thyroid Cancer Receiving or Not Receiving 131I Treatment: A Nationwide Population-Based Cohort Study. J. Nucl. Med. 2016, 57, 685–690. [Google Scholar] [CrossRef]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 2016, 121, 402–413. [Google Scholar] [CrossRef]
- Planck, T.; Hedberg, F.; Calissendorff, J.; Nilsson, A. Liothyronine Use in Hypothyroidism and its Effects on Cancer and Mortality. Thyroid 2021, 31, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-V.-T.; Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N. Thyroid dysfunction and cancer incidence: A systematic review and meta-analysis. Endocr. Relat. Cancer 2020, 27, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Kapdi, C.C.; Wolfe, J.N. Breast cancer. Relationship to thyroid supplements for hypothyroidism. JAMA 1976, 236, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Hellevik, A.I.; Asvold, B.O.; Bjøro, T.; Romundstad, P.R.; Nilsen, T.I.L.; Vatten, L.J. Thyroid function and cancer risk: A prospective population study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Journy, N.M.Y.; Bernier, M.-O.; Doody, M.M.; Alexander, B.H.; Linet, M.S.; Kitahara, C.M. Hyperthyroidism, Hypothyroidism, and Cause-Specific Mortality in a Large Cohort of Women. Thyroid 2017, 27, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Chang, Y.; Lee, K.H.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Ahn, J.; Shin, H.; Ryu, S. Serum concentration of thyroid hormones in abnormal and euthyroid ranges and breast cancer risk: A cohort study. Int. J. Cancer 2019, 145, 3257–3266. [Google Scholar] [CrossRef]
- Brandt, J.; Borgquist, S.; Almquist, M.; Manjer, J. Thyroid function and survival following breast cancer. Br. J. Surg. 2016, 103, 1649–1657. [Google Scholar] [CrossRef]
- Ortega-Olvera, C.; Ulloa-Aguirre, A.; Ángeles-Llerenas, A.; Mainero-Ratchelous, F.E.; González-Acevedo, C.E.; Hernández-Blanco, M.d.L.; Ziv, E.; Avilés-Santa, L.; Pérez-Rodríguez, E.; Torres-Mejía, G. Thyroid hormones and breast cancer association according to menopausal status and body mass index. Breast Cancer Res. 2018, 20, 94. [Google Scholar] [CrossRef]
- Bach, L.; Kostev, K.; Schiffmann, L.; Kalder, M. Association between thyroid gland diseases and breast cancer: A case-control study. Breast Cancer Res. Treat. 2020, 182, 207–213. [Google Scholar] [CrossRef]
- Freitas, P.A.V.C.J.; Vissoci, G.M.; Pinto, R.M.; Lajolo, P.P.; Jorge, P.T. Study Of the Prevalence Of Autoimmune Thyroid Disease In Women With Breast Cancer. Endocr. Pract. 2016, 22, 16–21. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Hai, R.; You, Q.; Xie, L.; Shu, L.; Zhou, X. Thyroid disease is associated with an increased risk of breast cancer: A systematic review and meta-analysis. Gland Surg. 2021, 10, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Zyla, L.E.; Cano, R.; Gómez, S.; Escudero, A.; Rey, L.; Santiano, F.E.; Bruna, F.A.; Creydt, V.P.; Carón, R.W.; Fontana, C.L. Effects of thyroxine on apoptosis and proliferation of mammary tumors. Mol. Cell. Endocrinol. 2021, 538, 111454. [Google Scholar] [CrossRef] [PubMed]
- Zehni, A.Z.; Batz, F.; Vattai, A.; Kaltofen, T.; Schrader, S.; Jacob, S.-N.; Mumm, J.-N.; Heidegger, H.H.; Ditsch, N.; Mahner, S.; et al. The Prognostic Impact of Retinoid X Receptor and Thyroid Hormone Receptor alpha in Unifocal vs. Multifocal/Multicentric Breast Cancer. Int. J. Mol. Sci. 2021, 22, 957. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Cockburn, J.G.; Dhesy-Thind, S.K.; Pond, G.R.; Pritchard, K.I.; Nofech-Mozes, S.; Sun, P.; Narod, S.A.; Bane, A. Thyroid hormone receptor beta-1 expression in early breast cancer: A validation study. Breast Cancer Res. Treat. 2018, 171, 709–717. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Cockburn, J.; Pond, G.R.; Pritchard, K.I.; Narod, S.A.; Dhesy-Thind, S.K.; Bane, A. Thyroid hormone receptor α in breast cancer: Prognostic and therapeutic implications. Breast Cancer Res. Treat. 2015, 149, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Moretto, F.C.F.; De Sibio, M.T.; Luvizon, A.C.; Olimpio, R.M.C.; de Oliveira, M.; Alves, C.A.B.; Conde, S.J.; Nogueira, C.R. Triiodothyronine (T3) induces HIF1A and TGFA expression in MCF7 cells by activating PI3K. Life Sci. 2016, 154, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef]
- Dinda, S.; Sanchez, A.; Moudgil, V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene 2002, 21, 761–768. [Google Scholar] [CrossRef]
- Suhane, S.; Ramanujan, V.K. Thyroid hormone differentially modulates Warburg phenotype in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 414, 73–78. [Google Scholar] [CrossRef]
- Flamini, M.I.; Uzair, I.D.; Pennacchio, G.E.; Neira, F.J.; Mondaca, J.M.; Cuello-Carrión, F.D.; Jahn, G.A.; Simoncini, T.; Sanchez, A.M. Thyroid Hormone Controls Breast Cancer Cell Movement via Integrin αV/β3/SRC/FAK/PI3-Kinases. Horm. Cancer 2017, 8, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Chin, Y.-T.; Nana, A.W.; Shih, Y.-J.; Lai, H.-Y.; Tang, H.-Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Mayr, D.; Meindl, A.; Angele, M.; Gallwas, J.; Jeschke, U.; Ditsch, N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PLoS ONE 2015, 10, e0127072. [Google Scholar] [CrossRef]
- Li, N.; Du, X.L.; Reitzel, L.R.; Xu, L.; Sturgis, E.M. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: Review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid 2013, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Borson-Chazot, F.; Delafosse, P.; Schvartz, C.; Guizard, A.-V. Progression of incidence and estimate of net survival from papillary thyroid cancers diagnosed between 2008 and 2016 in France. Ann. D’Endocrinologie 2020, 81, 530–538. [Google Scholar] [CrossRef]
- Messuti, I.; Corvisieri, S.; Bardesono, F.; Rapa, I.; Giorcelli, J.; Pellerito, R.; Volante, M.; Orlandi, F. Impact of pregnancy on prognosis of differentiated thyroid cancer: Clinical and molecular features. Eur. J. Endocrinol. 2014, 170, 659–666. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.H.; Lee, H.; Nam, H.; Park, S.; Jang, M.H.; Kim, J.M.; Kim, E.H.; Jeon, Y.K.; Kim, S.S.; et al. Thyroid cancer after hysterectomy and oophorectomy: A nationwide cohort study. Eur. J. Endocrinol. 2021, 184, 143–151. [Google Scholar] [CrossRef]
- Rosenblatt, K.A.; Gao, D.L.; Ray, R.M.; Nelson, Z.C.; Wernli, K.J.; Li, W.; Thomas, D.B. Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control 2009, 20, 27–34. [Google Scholar] [CrossRef]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Derwahl, M. Linking stem cells to thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 610–613. [Google Scholar] [CrossRef]
- Xu, S.; Chen, G.; Peng, W.; Renko, K.; Derwahl, M. Oestrogen action on thyroid progenitor cells: Relevant for the pathogenesis of thyroid nodules? J. Endocrinol. 2013, 218, 125–133. [Google Scholar] [CrossRef]
- Faria, C.C.; Peixoto, M.S.; Carvalho, D.P.; Fortunato, R.S. The Emerging Role of Estrogens in Thyroid Redox Homeostasis and Carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 2514312. [Google Scholar] [CrossRef]
- Arain, S.A.; Shah, M.H.; Meo, S.A.; Jamal, Q. Estrogen receptors in human thyroid gland. An immunohistochemical study. Saudi Med. J. 2003, 24, 174–178. [Google Scholar]
- Jaklic, B.R.; Rushin, J.; Ghosh, B.C. Estrogen and progesterone receptors in thyroid lesions. Ann. Surg. Oncol. 1995, 2, 429–434. [Google Scholar] [CrossRef]
- Tavangar, S.M.; Monajemzadeh, M.; Larijani, B.; Haghpanah, V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singap. Med. J. 2007, 48, 744–747. [Google Scholar]
- Vannucchi, G.; De Leo, S.; Perrino, M.; Rossi, S.; Tosi, D.; Cirello, V.; Colombo, C.; Bulfamante, G.; Vicentini, L.; Fugazzola, L. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur. J. Endocrinol. 2015, 173, 29–36. [Google Scholar] [CrossRef]
- Di Vito, M.; De Santis, E.; Perrone, G.A.; Mari, E.; Giordano, M.C.; De Antoni, E.; Coppola, L.; Fadda, G.; Tafani, M.; Carpi, A.; et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser- capture microdissection and molecular biology. Cancer Sci. 2011, 102, 1921–1927. [Google Scholar] [CrossRef]
- Heikkilä, A.; Hagström, J.; Mäenpää, H.; Louhimo, J.; Siironen, P.; Heiskanen, I.; Haglund, C.; Arola, J. Loss of estrogen receptor Beta expression in follicular thyroid carcinoma predicts poor outcome. Thyroid 2013, 23, 456–465. [Google Scholar] [CrossRef]
- Thomas, C.; Gustafsson, J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef]
- Tafani, M.; Pucci, B.; Russo, A.; Schito, L.; Pellegrini, L.; Perrone, G.A.; Villanova, L.; Salvatori, L.; Ravenna, L.; Petrangeli, E.; et al. Modulators of HIF1α and NFkB in Cancer Treatment: Is it a Rational Approach for Controlling Malignant Progression? Front. Pharmacol. 2013, 4, 13. [Google Scholar] [CrossRef]
- Tafani, M.; Schito, L.; Pellegrini, L.; Villanova, L.; Marfe, G.; Anwar, T.; Rosa, R.; Indelicato, M.; Fini, M.; Pucci, B.; et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis 2011, 32, 1167–1175. [Google Scholar] [CrossRef]
- Kim, J.S.; Bae, J.S.; Kim, K.H.; Ahn, C.H.; Oh, S.J.; Jeon, H.M.; Lim, K.W.; Chun, C.S. Clinical Analysis of PTEN, p53 and Her-2/neu Expressions in Thyroid Cancers. Cancer Res. Treat. 2001, 33, 433–437. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrè, G.; Giovanella, L.; Siracusa, M.; Simone, A.; Branca, G.; Scarfì, R.; Trimarchi, F.; Ieni, A.; et al. HER2 Analysis in Sporadic Thyroid Cancer of Follicular Cell Origin. Int. J. Mol. Sci. 2016, 17, 2040. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, D.-L. Study on extracellular matrix metalloproteinase inducer and human epidermal growth factor receptor-2 protein expression in papillary thyroid carcinoma using a quantum dot-based immunofluorescence technique. Exp. Ther. Med. 2015, 9, 1331–1335. [Google Scholar] [CrossRef][Green Version]
- Apostol, D.C.; Caruntu, I.-D.; Lozneanu, L.; Andriescu, E.C.; Giusca, S.E. HER-2/neu expression in different histological subtypes of papillary thyroid carcinoma. Rom. J. Morphol. Embryol. 2017, 58, 439–444. [Google Scholar]
- Mdah, W.; Mzalbat, R.; Gilbey, P.; Stein, M.; Sharabi, A.; Zidan, J. Lack of HER-2 gene amplification and association with pathological and clinical characteristics of differentiated thyroid cancer. Mol. Clin. Oncol. 2014, 2, 1107–1110. [Google Scholar] [CrossRef]
- Medici, M.; Porcu, E.; Pistis, G.; Teumer, A.; Brown, S.J.; Jensen, R.A.; Rawal, R.; Roef, G.L.; Plantinga, T.S.; Vermeulen, S.H.; et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014, 10, e1004123. [Google Scholar] [CrossRef]
- Abu-Shakra, M.; Buskila, D.; Ehrenfeld, M.; Conrad, K.; Shoenfeld, Y. Cancer and autoimmunity: Autoimmune and rheumatic features in patients with malignancies. Ann. Rheum. Dis. 2001, 60, 433–441. [Google Scholar] [CrossRef]
- Ito, K.; Maruchi, N. Breast cancer in patients with Hashimoto’s thyroiditis. Lancet 1975, 2, 1119–1121. [Google Scholar] [CrossRef]
- Muller, I.; Giani, C.; Zhang, L.; Grennan-Jones, F.A.; Fiore, E.; Belardi, V.; Rosellini, V.; Funel, N.; Campani, D.; Giustarini, E.; et al. Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int. J. Cancer 2014, 134, 1706–1714. [Google Scholar] [CrossRef]
- Godlewska, M.; Arczewska, K.D.; Rudzińska, M.; Łyczkowska, A.; Krasuska, W.; Hanusek, K.; Ruf, J.; Kiedrowski, M.; Czarnocka, B. Thyroid peroxidase (TPO) expressed in thyroid and breast tissues shows similar antigenic properties. PLoS ONE 2017, 12, e0179066. [Google Scholar] [CrossRef]
- Muller, I.; Zhang, L.; Giani, C.; Dayan, C.M.; Ludgate, M.E.; Grennan-Jones, F. The sodium iodide symporter is unlikely to be a thyroid/breast shared antigen. J. Endocrinol. Investig. 2016, 39, 323–331. [Google Scholar] [CrossRef]
- Giani, C.; Fierabracci, P.; Bonacci, R.; Gigliotti, A.; Campani, D.; De Negri, F.; Cecchetti, D.; Martino, E.; Pinchera, A. Relationship between breast cancer and thyroid disease: Relevance of autoimmune thyroid disorders in breast malignancy. J. Clin. Endocrinol. Metab. 1996, 81, 990–994. [Google Scholar]
- Smyth, P.P.; Shering, S.G.; Kilbane, M.T.; Murray, M.J.; McDermott, E.W.; Smith, D.F.; O’Higgins, N.J. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J. Clin. Endocrinol. Metab. 1998, 83, 2711–2716. [Google Scholar] [CrossRef]
- Turken, O.; NarIn, Y.; DemIrbas, S.; Onde, M.E.; Sayan, O.; KandemIr, E.G.; YaylacI, M.; Ozturk, A. Breast cancer in association with thyroid disorders. Breast Cancer Res. 2003, 5, R110–R113. [Google Scholar] [CrossRef]
- Giustarini, E.; Pinchera, A.; Fierabracci, P.; Roncella, M.; Fustaino, L.; Mammoli, C.; Giani, C. Thyroid autoimmunity in patients with malignant and benign breast diseases before surgery. Eur. J. Endocrinol. 2006, 154, 645–649. [Google Scholar] [CrossRef][Green Version]
- Jiskra, J.; Barkmanova, J.; Limanova, Z.; Lánská, V.; Smutek, D.; Potlukova, E.; Antosova, M. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol. Rep. 2007, 18, 1603–1611. [Google Scholar] [CrossRef]
- Graceffa, G.; Scerrino, G.; Militello, G.; Laise, I.; Randisi, B.; Melfa, G.; Orlando, G.; Mazzola, S.; Cipolla, C.; Cocorullo, G. Breast cancer in previously thyroidectomized patients: Which thyroid disorders are a risk factor? Future Sci. OA 2021, 7, FSO699. [Google Scholar] [CrossRef]
- Hedley, A.J.; Jones, S.J.; Spiegelhalter, D.J.; Clements, P.; Bewsher, P.D.; Simpson, J.G.; Weir, R.D. Breast cancer in thyroid disease: Fact or fallacy? Lancet 1981, 1, 131–133. [Google Scholar] [CrossRef]
- Pan, X.-F.; Ma, Y.-J.; Tang, Y.; Yu, M.-M.; Wang, H.; Fan, Y.-R. Breast cancer populations may have an increased prevalence of thyroglobulin antibody and thyroid peroxidase antibody: A systematic review and meta-analysis. Breast Cancer 2020, 27, 828–836. [Google Scholar] [CrossRef]
- Szychta, P.; Szychta, W.; Gesing, A.; Lewiński, A.; Karbownik-Lewińska, M. TSH receptor antibodies have predictive value for breast cancer-retrospective analysis. Thyroid Res. 2013, 6, 8. [Google Scholar] [CrossRef]
- Oh, H.J.; Chung, J.-K.; Kang, J.H.; Kang, W.J.; Noh, D.Y.; Park, I.A.; Jeong, J.M.; Lee, D.S.; Lee, M.C. The relationship between expression of the sodium/iodide symporter gene and the status of hormonal receptors in human breast cancer tissue. Cancer Res. Treat. 2005, 37, 247–250. [Google Scholar] [CrossRef][Green Version]
- Dubenko, M.; Breining, D.; Surks, M.I. Sclerosing lymphocytic lobulitis of the breast in a patient with Graves’ disease. Thyroid 2003, 13, 309–311. [Google Scholar] [CrossRef]
- Soler, N.G.; Khardori, R. Fibrous disease of the breast, thyroiditis, and cheiroarthropathy in type I diabetes mellitus. Lancet 1984, 1, 193–195. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, S.J.; Jung, H.K. Sclerosing lymphocytic lobulitis manifesting as suspicious microcalcifications with Hashimoto’s thyroiditis in a young woman. Breast J. 2013, 19, 539–541. [Google Scholar] [CrossRef]
- Hermsen, B.B.J.; von Mensdorff-Pouilly, S.; Fabry, H.F.J.; Winters, H.A.H.; Kenemans, P.; Verheijen, R.H.M.; van Diest, P.J. Lobulitis is a frequent finding in prophylactically removed breast tissue from women at hereditary high risk of breast cancer. J. Pathol. 2005, 206, 220–223. [Google Scholar] [CrossRef]
- Hieken, T.J.; Chen, J.; Chen, B.; Johnson, S.; Hoskin, T.L.; Degnim, A.C.; Walther-Antonio, M.R.; Chia, N. The breast tissue microbiome, stroma, immune cells and breast cancer. Neoplasia 2022, 27, 100786. [Google Scholar] [CrossRef]
- Smyth, P.P.A. The thyroid, iodine and breast cancer. Breast Cancer Res. 2003, 5, 235–238. [Google Scholar] [CrossRef]
- Pisani, P.; Parkin, D.M.; Bray, F.; Ferlay, J. Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer 1999, 83, 18–29. [Google Scholar] [CrossRef]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef]
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878. [Google Scholar] [CrossRef]
- Ryan, J.; Curran, C.E.; Hennessy, E.; Newell, J.; Morris, J.C.; Kerin, M.J.; Dwyer, R.M. The sodium iodide symporter (NIS) and potential regulators in normal, benign and malignant human breast tissue. PLoS ONE 2011, 6, e16023. [Google Scholar] [CrossRef]
- Berger, F.; Unterholzner, S.; Diebold, J.; Knesewitsch, P.; Hahn, K.; Spitzweg, C. Mammary radioiodine accumulation due to functional sodium iodide symporter expression in a benign fibroadenoma. Biochem. Biophys. Res. Commun. 2006, 349, 1258–1263. [Google Scholar] [CrossRef]
- Beyer, S.J.; Jimenez, R.E.; Shapiro, C.L.; Cho, J.Y.; Jhiang, S.M. Do cell surface trafficking impairments account for variable cell surface sodium iodide symporter levels in breast cancer? Breast Cancer Res. Treat. 2009, 115, 205–212. [Google Scholar] [CrossRef]
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− symporter (NIS): Mechanism and medical impact. Endocr. Rev. 2014, 35, 106–149. [Google Scholar] [CrossRef]
- Dadachova, E.; Carrasco, N. The Na/I symporter (NIS): Imaging and therapeutic applications. Semin. Nucl. Med. 2004, 34, 23–31. [Google Scholar] [CrossRef]
- Ron, E.; Doody, M.M.; Becker, D.V.; Brill, A.B.; Curtis, R.E.; Goldman, M.B.; Harris, B.S.; Hoffman, D.A.; McConahey, W.M.; Maxon, H.R.; et al. Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow-up Study Group. JAMA 1998, 280, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Metso, S.; Auvinen, A.; Huhtala, H.; Salmi, J.; Oksala, H.; Jaatinen, P. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer 2007, 109, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Ryödi, E.; Metso, S.; Jaatinen, P.; Huhtala, H.; Saaristo, R.; Välimäki, M.; Auvinen, A. Cancer Incidence and Mortality in Patients Treated Either With RAI or Thyroidectomy for Hyperthyroidism. J. Clin. Endocrinol. Metab. 2015, 100, 3710–3717. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Berrington de Gonzalez, A.; Bouville, A.; Brill, A.B.; Doody, M.M.; Melo, D.R.; Simon, S.L.; Sosa, J.A.; Tulchinsky, M.; Villoing, D.; et al. Association of Radioactive Iodine Treatment With Cancer Mortality in Patients With Hyperthyroidism. JAMA Intern. Med. 2019, 179, 1034–1042. [Google Scholar] [CrossRef]
- Melo, D.R.; Brill, A.B.; Zanzonico, P.; Vicini, P.; Moroz, B.; Kwon, D.; Lamart, S.; Brenner, A.; Bouville, A.; Simon, S.L. Organ Dose Estimates for Hyperthyroid Patients Treated with (131)I: An Update of the Thyrotoxicosis Follow-Up Study. Radiat. Res. 2015, 184, 595–610. [Google Scholar] [CrossRef]
- Silva-Vieira, M.; Carrilho Vaz, S.; Esteves, S.; Ferreira, T.C.; Limbert, E.; Salgado, L.; Leite, V. Second Primary Cancer in Patients with Differentiated Thyroid Cancer: Does Radioiodine Play a Role? Thyroid 2017, 27, 1068–1076. [Google Scholar] [CrossRef]
- Chen, K.-T.; Hu, Y.-W. Risk of Breast Cancer in Patients with Thyroid Cancer Receiving 131I Treatment: Is There an Immortal Time Bias? J. Nucl. Med. 2016, 57, 1324. [Google Scholar] [CrossRef][Green Version]
- Ko, K.-Y.; Kao, C.-H.; Lin, C.-L.; Huang, W.-S.; Yen, R.-F. (131)I treatment for thyroid cancer and the risk of developing salivary and lacrimal gland dysfunction and a second primary malignancy: A nationwide population-based cohort study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1172–1178. [Google Scholar] [CrossRef]
- Shim, S.R.; Kitahara, C.M.; Cha, E.S.; Kim, S.-J.; Bang, Y.J.; Lee, W.J. Cancer Risk After Radioactive Iodine Treatment for Hyperthyroidism: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2125072. [Google Scholar] [CrossRef]
- Haghi-Aminjan, H.; Farhood, B.; Rahimifard, M.; Didari, T.; Baeeri, M.; Hassani, S.; Hosseini, R.; Abdollahi, M. The protective role of melatonin in chemotherapy-induced nephrotoxicity: A systematic review of non-clinical studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 937–950. [Google Scholar] [CrossRef]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Janssen, L.G.M.; Charehbili, A.; Dijkgraaf, E.M.; Smit, V.T.H.B.M.; Kessels, L.W.; van Bochove, A.; van Laarhoven, H.W.M.; Meershoek-Klein Kranenbarg, E.; van Leeuwen-Stok, A.E.; et al. Thyroid function alters during neoadjuvant chemotherapy in breast cancer patients: Results from the NEOZOTAC trial (BOOG 2010-01). Breast Cancer Res. Treat. 2015, 149, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kailajärvi, M.; Ahokoski, O.; Virtanen, A.; Salminen, E.; Irjala, K. Alterations in laboratory test results during adjuvant breast cancer treatment. Clin. Chem. Lab. Med. 2000, 38, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Allen, K.A.; Riccardi, D.; Bercu, B.B.; Cantor, A.; Minton, S.; Balducci, L.; Jacobsen, P.B. Fatigue, weight gain, lethargy and amenorrhea in breast cancer patients on chemotherapy: Is subclinical hypothyroidism the culprit? Breast Cancer Res. Treat. 2004, 83, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Bhurani, D.; Agarwal, N.B. Alteration of Thyroid Function in Indian HER 2-Negative Breast Cancer Patients Undergoing Chemotherapy. Asian Pac. J. Cancer Prev. 2015, 16, 7701–7705. [Google Scholar] [CrossRef][Green Version]
- Mortezaee, K.; Ahmadi, A.; Haghi-Aminjan, H.; Khanlarkhani, N.; Salehi, E.; Shabani Nashtaei, M.; Farhood, B.; Najafi, M.; Sahebkar, A. Thyroid function following breast cancer chemotherapy: A systematic review. J. Cell Biochem. 2019, 120, 12101–12107. [Google Scholar] [CrossRef]
- Rahu, K.; Hakulinen, T.; Smailyte, G.; Stengrevics, A.; Auvinen, A.; Inskip, P.D.; Boice, J.D.; Rahu, M. Site-specific cancer risk in the Baltic cohort of Chernobyl cleanup workers, 1986–2007. Eur. J. Cancer 2013, 49, 2926–2933. [Google Scholar] [CrossRef]
- Ivanov, V.K.; Tsyb, A.F.; Petrov, A.V.; Maksioutov, M.A.; Shilyaeva, T.P.; Kochergina, E.V. Thyroid cancer incidence among liquidators of the Chernobyl accident. Absence of dependence of radiation risks on external radiation dose. Radiat. Environ. Biophys. 2002, 41, 195–198. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.P.; van Eggermond, A.M.; Janus, C.P.M.; Krol, A.D.G.; van der Maazen, R.W.M.; Roesink, J.; Raemaekers, J.M.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Wijnen, M.; van den Heuvel-Eibrink, M.M.; Medici, M.; Peeters, R.P.; van der Lely, A.J.; Neggers, S.J.C.M.M. Risk factors for subsequent endocrine-related cancer in childhood cancer survivors. Endocr. Relat. Cancer 2016, 23, R299–R321. [Google Scholar] [CrossRef]
- Stovall, M.; Weathers, R.; Kasper, C.; Smith, S.A.; Travis, L.; Ron, E.; Kleinerman, R. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat. Res. 2006, 166, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Grantzau, T.; Mellemkjær, L.; Overgaard, J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother. Oncol. 2013, 106, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Akın, M.; Ergen, A.; Unal, A.; Bese, N. Irradiation doses on thyroid gland during the postoperative irradiation for breast cancer. J. Cancer Res. Ther. 2014, 10, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.M.; Ying, J.; Poppe, M.M.; Suneja, G.; Gaffney, D.K. Risk of secondary malignancies after radiation therapy for breast cancer: Comprehensive results. Breast 2017, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chang, J.S.; Byun, H.K.; Son, N.-H.; Hong, C.-S.; Hong, N.; Park Ms, Y.-I.; Kim, J.; Kim, J.S.; Kim, Y.B. Risk of Hypothyroidism in Women After Radiation Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 462–472. [Google Scholar] [CrossRef]
- Darvish, L.; Ghorbani, M.; Teshnizi, S.H.; Roozbeh, N.; Seif, F.; Bayatiani, M.R.; Knaup, C.; Amraee, A. Evaluation of thyroid gland as an organ at risk after breast cancer radiotherapy: A systematic review and meta-analysis. Clin. Transl. Oncol. 2018, 20, 1430–1438. [Google Scholar] [CrossRef]
- Reinertsen, K.V.; Cvancarova, M.; Wist, E.; Bjøro, T.; Dahl, A.A.; Danielsen, T.; Fosså, S.D. Thyroid function in women after multimodal treatment for breast cancer stage II/III: Comparison with controls from a population sample. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 764–770. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Hendrick, R.E. Mammography and the Risk of Thyroid Cancer. Am. J. Roentgenol. 2012, 198, 705–707. [Google Scholar] [CrossRef]
- Yuan, M.-K.; Chang, S.-C.; Hsu, L.-C.; Pan, P.-J.; Huang, C.-C.; Leu, H.-B. Mammography and the Risk of Thyroid and Hematological Cancers: A Nationwide Population-based Study. Breast J. 2014, 20, 496–501. [Google Scholar] [CrossRef]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Zheng, G.; Yu, H.; Hemminki, A.; Försti, A.; Sundquist, K.; Hemminki, K. Familial associations of female breast cancer with other cancers. Int. J. Cancer 2017, 141, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: {158350}: {01/10/2019}. Available online: https://omim.org/ (accessed on 13 September 2022).
- Ngeow, J.; Stanuch, K.; Mester, J.L.; Barnholtz-Sloan, J.S.; Eng, C. Second malignant neoplasms in patients with Cowden syndrome with underlying germline PTEN mutations. J. Clin. Oncol. 2014, 32, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Bennett, K.L.; Mester, J.; Eng, C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010, 304, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.-B.; Gunnarsdóttir, K.Á.; Højris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef]
- Engeland, A.; Tretli, S.; Akslen, L.A.; Bjørge, T. Body size and thyroid cancer in two million Norwegian men and women. Br. J. Cancer 2006, 95, 366–370. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Berrington de González, A. Body fat distribution, weight change during adulthood, and thyroid cancer risk in the NIH-AARP Diet and Health Study. Int. J. Cancer 2012, 130, 1411–1419. [Google Scholar] [CrossRef]
- He, Q.; Sun, H.; Li, F.; Liang, N. Obesity and risk of differentiated thyroid cancer: A large-scale case-control study. Clin. Endocrinol. 2019, 91, 869–878. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, K.E.; Park, Y.J.; Kim, S.-J.; Kwon, H.; Park, D.J.; Cho, B.; Choi, H.-C.; Kang, D.; Park, S.K. Annual Average Changes in Adult Obesity as a Risk Factor for Papillary Thyroid Cancer: A Large-Scale Case-Control Study. Medicine 2016, 95, e2893. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Lise, M.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Guillas, G.; Overvad, K.; Tjønneland, A.; Halkjær, J.; Lukanova, A.; Kaaks, R.; et al. Body size and risk of differentiated thyroid carcinomas: Findings from the EPIC study. Int. J. Cancer 2012, 131, E1004–E1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, N.K.; Choi, J.H.; Sohn, S.Y.; Kim, S.W.; Jin, S.-M.; Jang, H.W.; Suh, S.; Min, Y.-K.; Chung, J.H.; et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin. Endocrinol. 2013, 78, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.M.; Bookwalter, D.B.; O’Brien, K.M.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann. Oncol. 2021, 32, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Farvid, M.S.; Kang, J.H.; Holmes, M.D.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Diabetes Risk Reduction Diet and Survival after Breast Cancer Diagnosis. Cancer Res. 2021, 81, 4155–4162. [Google Scholar] [CrossRef]
- Fussey, J.M.; Beaumont, R.N.; Wood, A.R.; Vaidya, B.; Smith, J.; Tyrrell, J. Does Obesity Cause Thyroid Cancer? A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2020, 105, e2398–e2407. [Google Scholar] [CrossRef]
- Langballe, R.; Olsen, J.H.; Andersson, M.; Mellemkjær, L. Risk for second primary non-breast cancer in pre- and postmenopausal women with breast cancer not treated with chemotherapy, radiotherapy or endocrine therapy. Eur. J. Cancer 2011, 47, 946–952. [Google Scholar] [CrossRef]
- Jin, Y.J.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Choi, H.G. Association between Thyroid Cancer and Breast Cancer: Two Longitudinal Follow-Up Studies Using a National Health Screening Cohort. J. Pers. Med. 2022, 12, 133. [Google Scholar] [CrossRef]
- Cieszyńska, M.; Kluźniak, W.; Wokołorczyk, D.; Cybulski, C.; Huzarski, T.; Gronwald, J.; Falco, M.; Dębniak, T.; Jakubowska, A.; Derkacz, R.; et al. Risk of Second Primary Thyroid Cancer in Women with Breast Cancer. Cancers 2022, 14, 957. [Google Scholar] [CrossRef]
- Bright, C.J.; Reulen, R.C.; Winter, D.L.; Stark, D.P.; McCabe, M.G.; Edgar, A.B.; Frobisher, C.; Hawkins, M.M. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): A population-based, cohort study. Lancet. Oncol. 2019, 20, 531–545. [Google Scholar] [CrossRef]
- Huang, N.S.; Chen, X.X.; Wei, W.J.; Mo, M.; Chen, J.Y.; Ma, B.; Yang, S.W.; Xu, W.B.; Wu, J.; Ji, Q.H.; et al. Association between breast cancer and thyroid cancer: A study based on 13 978 patients with breast cancer. Cancer Med. 2018, 7, 6393–6400. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Veronesi, P.; Santomauro, G.I.; Maisonneuve, P.; Morigi, C.; Peruzzotti, G.; Intra, M.; Sacchini, V.; Galimberti, V. Multiple primary non-breast tumors in breast cancer survivors. J. Cancer Res. Clin. Oncol. 2018, 144, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.G.; Lipshitz, I.; Keinan-Boker, L. Second Primary Cancers After Primary Breast Cancer Diagnosis in Israeli Women, 1992 to 2006. J. Glob. Oncol. 2017, 3, 135–142. [Google Scholar] [CrossRef]
- Jung, H.K.; Park, S.; Kim, N.W.; Lee, J.E.; Kim, Z.; Han, S.W.; Hur, S.M.; Kim, S.Y.; Lim, C.W.; Lee, M.H.; et al. Development of second primary cancer in Korean breast cancer survivors. Ann. Surg. Treat Res. 2017, 93, 287–292. [Google Scholar] [CrossRef]
- Hung, M.H.; Liu, C.J.; Teng, C.J.; Hu, Y.W.; Yeh, C.M.; Chen, S.C.; Chien, S.H.; Hung, Y.P.; Shen, C.C.; Chen, T.J.; et al. Risk of Second Non-Breast Primary Cancer in Male and Female Breast Cancer Patients: A Population-Based Cohort Study. PLoS ONE 2016, 11, e0148597. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Pollán, M.; Payer, T.; Molina, E.; Dávila-Arias, C.; Sánchez, M.-J. Risk of second primary cancer among women with breast cancer: A population-based study in Granada (Spain). Gynecol. Oncol. 2013, 130, 340–345. [Google Scholar] [CrossRef]
- Lee, K.-D.; Chen, S.-C.; Chan, C.H.; Lu, C.-H.; Chen, C.-C.; Lin, J.-T.; Chen, M.-F.; Huang, S.-H.; Yeh, C.-M.; Chen, M.-C. Increased risk for second primary malignancies in women with breast cancer diagnosed at young age: A population-based study in Taiwan. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2647–2655. [Google Scholar] [CrossRef][Green Version]
- Kirova, Y.M.; De Rycke, Y.; Gambotti, L.; Pierga, J.Y.; Asselain, B.; Fourquet, A. Second malignancies after breast cancer: The impact of different treatment modalities. Br. J. Cancer 2008, 98, 870–874. [Google Scholar] [CrossRef]
- Raymond, J.S.; Hogue, C.J.R. Multiple primary tumours in women following breast cancer, 1973-2000. Br. J. Cancer 2006, 94, 1745–1750. [Google Scholar] [CrossRef]
- Sadetzki, S.; Calderon-Margalit, R.; Peretz, C.; Novikov, I.; Barchana, M.; Papa, M.Z. Second primary breast and thyroid cancers (Israel). Cancer Causes Control 2003, 14, 367–375. [Google Scholar] [CrossRef]
- Levi, F.; Te, V.C.; Randimbison, L.; La Vecchia, C. Cancer risk in women with previous breast cancer. Ann. Oncol. 2003, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tsukuma, H.; Koyama, H.; Kinoshita, Y.; Kinoshita, N.; Oshima, A. Second primary cancers following breast cancer in the Japanese female population. Jpn. J. Cancer Res. 2001, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Walker, R.; Groome, P.G.; Shelley, W.; Mackillop, W.J. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer 2001, 92, 1411–1418. [Google Scholar] [CrossRef]
- Rubino, C.; de Vathaire, F.; Diallo, I.; Shamsaldin, A.; Lê, M.G. Increased risk of second cancers following breast cancer: Role of the initial treatment. Breast Cancer Res. Treat 2000, 61, 183–195. [Google Scholar] [CrossRef]
- Volk, N.; Pompe-Kirn, V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control 1997, 8, 764–770. [Google Scholar] [CrossRef]
- Doherty, M.A.; Rodger, A.; Langlands, A.O.; Kerr, G.R. Multiple primary tumours in patients treated with radiotherapy for breast cancer. Radiother. Oncol. 1993, 26, 125–131. [Google Scholar] [CrossRef]
- Murakami, R.; Hiyama, T.; Hanai, A.; Fujimoto, I. Second primary cancers following female breast cancer in Osaka, Japan--a population-based cohort study. Jpn. J. Clin. Oncol. 1987, 17, 293–302. [Google Scholar]
- Teppo, L.; Pukkala, E.; Saxén, E. Multiple cancer--an epidemiologic exercise in Finland. J. Natl. Cancer Inst. 1985, 75, 207–217. [Google Scholar]
- Harvey, E.B.; Brinton, L.A. Second cancer following cancer of the breast in Connecticut, 1935-82. Natl. Cancer Inst. Monogr. 1985, 68, 99–112. [Google Scholar]
- Schenker, J.G.; Levinsky, R.; Ohel, G. Multiple primary malignant neoplasms in breast cancer patients in Israel. Cancer 1984, 54, 145–150. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lee, K.-D.; Chen, P.-T.; Chen, C.-C.; Kuan, F.-C.; Huang, C.-E.; Chen, M.-F.; Chen, M.-C. Second primary malignancies following thyroid cancer: A population-based study in Taiwan. Eur. J. Endocrinol. 2013, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Consorti, F.; Di Tanna, G.; Milazzo, F.; Antonaci, A. Nulliparity enhances the risk of second primary malignancy of the breast in a cohort of women treated for thyroid cancer. World J. Surg. Oncol. 2011, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Verkooijen, R.B.; Smit, J.W.; Romijn, J.A.; Stokkel, M.P. The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. Eur. J. Endocrinol. 2006, 155, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Berthe, E.; Henry-Amar, M.; Michels, J.-J.; Rame, J.-P.; Berthet, P.; Babin, E.; Icard, P.; Samama, G.; Galateau-Sallé, F.; Mahoudeau, J.; et al. Risk of second primary cancer following differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Rubino, C.; de Vathaire, F.; Dottorini, M.E.; Hall, P.; Schvartz, C.; Couette, J.E.; Dondon, M.G.; Abbas, M.T.; Langlois, C.; Schlumberger, M. Second primary malignancies in thyroid cancer patients. Br. J. Cancer 2003, 89, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Adjadj, E.; Rubino, C.; Shamsaldim, A.; Lê, M.G.; Schlumberger, M.; de Vathaire, F. The risk of multiple primary breast and thyroid carcinomas. Cancer 2003, 98, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Jiang, Y. Second primary neoplasms after 19281 endocrine gland tumours: Aetiological links? Eur. J. Cancer 2001, 37, 1886–1894. [Google Scholar] [CrossRef]
- Dottorini, M.E.; Lomuscio, G.; Mazzucchelli, L.; Vignati, A.; Colombo, L. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J. Nucl. Med. 1995, 36, 21–27. [Google Scholar]
- Glanzmann, C. Subsequent malignancies in patients treated with 131-iodine for thyroid cancer. Strahlenther Onkol. 1992, 168, 337–343. [Google Scholar]
- Hall, P.; Holm, L.E.; Lundell, G.; Bjelkengren, G.; Larsson, L.G.; Lindberg, S.; Tennvall, J.; Wicklund, H.; Boice, J.D. Cancer risks in thyroid cancer patients. Br. J. Cancer 1991, 64, 159–163. [Google Scholar] [CrossRef]
- Johns, M.E.; Shikhani, A.H.; Kashima, H.K.; Matanoski, G.M. Multiple primary neoplasms in patients with salivary gland or thyroid gland tumors. Laryngoscope 1986, 96, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.J.; Smith, T. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br. J. Radiol. 1986, 59, 45–51. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Liu, H.; Zheng, B.; Sun, S.; Chen, C. Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions. Cancers 2022, 14, 5117. https://doi.org/10.3390/cancers14205117
Lu M, Liu H, Zheng B, Sun S, Chen C. Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions. Cancers. 2022; 14(20):5117. https://doi.org/10.3390/cancers14205117
Chicago/Turabian StyleLu, Man, Hanqing Liu, Bilian Zheng, Shengrong Sun, and Chuang Chen. 2022. "Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions" Cancers 14, no. 20: 5117. https://doi.org/10.3390/cancers14205117
APA StyleLu, M., Liu, H., Zheng, B., Sun, S., & Chen, C. (2022). Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions. Cancers, 14(20), 5117. https://doi.org/10.3390/cancers14205117



