A Phase I Dose-Escalation and Dose-Expansion Study of FCN-437c, a Novel CDK4/6 Inhibitor, in Patients with Advanced Solid Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Study Endpoints
2.3. Study Assessments
2.4. Statistical Analyses
3. Results
3.1. Patient Demographics and Baseline Characteristics
3.2. Dose-Limiting Toxicities
3.3. Safety and Tolerability
3.4. Other Safety Parameters
3.5. Pharmacokinetics
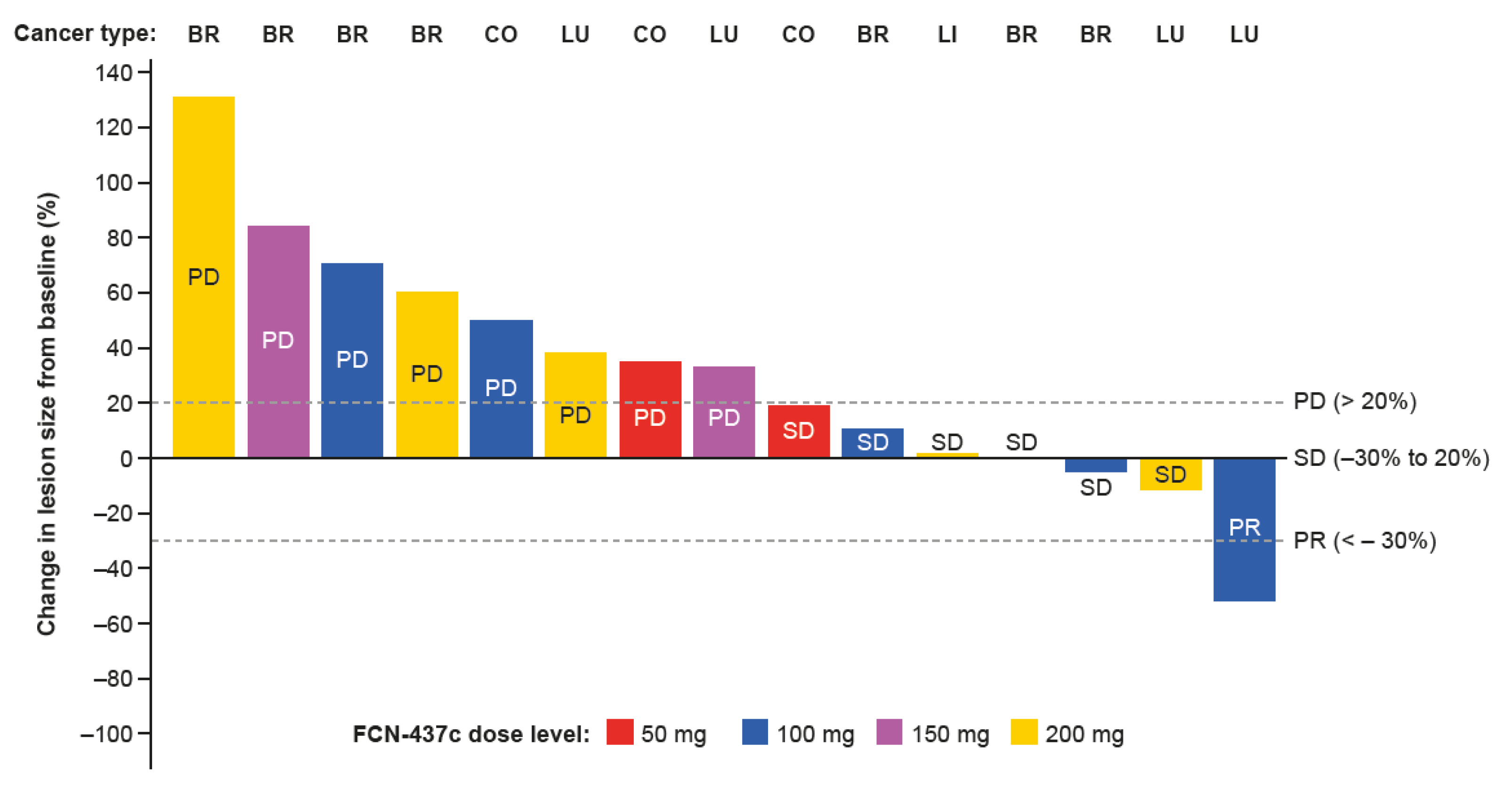
3.6. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Q.; Guo, X.; Wang, M.; Li, Y.; Sun, X.; Li, Q. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J. Hematol. Oncol. 2020, 13, 41. [Google Scholar] [CrossRef]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ettl, T.; Schulz, D.; Bauer, R.J. The renaissance of cyclin dependent kinase inhibitors. Cancers 2022, 14, 293. [Google Scholar] [CrossRef]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front. Oncol. 2018, 8, 608. [Google Scholar] [CrossRef]
- Pfizer Inc. IBRANCE US Prescribing Information; Pfizer Inc.: New York, NY, USA, 2019. [Google Scholar]
- Novartis Pharmaceuticals Corp. KISQALI US Prescribing Information; Novartis Pharmaceuticals Corp.: Cambridge, MA, USA, 2022. [Google Scholar]
- Eli Lilly Co. VERZENIO US Prescribing, Information; Eli Lilly, Co.: Indianapolis, IN, USA, 2021. [Google Scholar]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: A randomized clinical trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO International Consensus Guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer, Version 3.2022; National Comprehensive Cancer Network: Plymouth Meeting, PN, USA, 2022. [Google Scholar]
- Zhang, J.; Xu, D.; Zhou, Y.; Zhu, Z.; Yang, X. Mechanisms and implications of CDK4/6 inhibitors for the treatment of NSCLC. Front. Oncol. 2021, 11, 676041. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Ke, Y.; Liao, C.-G.; Zhao, Z.-Q.; Li, X.-M.; Lin, R.-J.; Yang, L.; Zhang, H.-L.; Kong, L.-M. Combining a CDK4/6 inhibitor with pemetrexed inhibits cell proliferation and metastasis in human lung adenocarcinoma. Front. Oncol. 2022, 12, 880153. [Google Scholar] [CrossRef]
- McCartney, A.; Migliaccio, I.; Bonechi, M.; Biagioni, C.; Romagnoli, D.; De Luca, F.; Galardi, F.; Risi, E.; De Santo, I.; Benelli, M.; et al. Mechanisms of resistance to CDK4/6 inhibitors: Potential implications and biomarkers for clinical practice. Front. Oncol. 2019, 9, 666. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; DeMichele, A.; Clark, A.S.; Zelnak, A.; Yardley, D.A.; Karuturi, M.; Sanft, T.; Blau, S.; Hart, L.; et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin. Cancer Res. 2021, 27, 4177–4185. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, E.; Sánchez-Rodríguez, I.; Balderrama-Ibarra, R.; Fuentes-Lara, J.; Rios-Martínez, A.; Vázquez Aldana Arroyo, I.; Bayardo-López, L.; Hernández Chávez, A.; Puebla-Mora Ana, G.; Nader-Roa, L.; et al. Diagnosis and management of brain metastases: An updated review from a radiation oncology perspective. J. Cancer Metastasis Treat. 2019, 5, 54. [Google Scholar] [CrossRef]
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front. Oncol. 2021, 11, 693104. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Searle, K.; Jerzak, K.J. Central nervous system-specific efficacy of CDK4/6 inhibitors in randomized controlled trials for metastatic breast cancer. Oncotarget 2019, 10, 6317–6322. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, X.; Li, T.; Zhang, H.; Tan, H.; Wang, X.; Jiang, L.; Liu, Y.; Sun, J.; Linghu, L.; et al. Abstract 4425: FCN-437: A novel, potent and selective oral inhibitor of CDK4/6 for the treatment of solid tumors. Cancer Res. 2019, 79, 4425. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, X.; Hui, A.; Wu, Z.; Tian, L.; Xu, C.; Yang, Y.; Zhang, W.; Hu, X. Phase 1a study of the CDK4/6 inhibitor, FCN-437c, in Chinese patients with HR + /HER2- advanced breast cancer. Investig. New Drugs 2021, 39, 1549–1558. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.G.; Gwyther, S.; Mooney, M.M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Reuben, A. Hy’s law. Hepatology 2004, 39, 574–578. [Google Scholar] [CrossRef]
- National Cancer Institute (USA). Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0; National Cancer Institute (USA): Bethesda, MD, USA, 2017. [Google Scholar]
- Malorni, L.; Curigliano, G.; Minisini, A.M.; Cinieri, S.; Tondini, C.A.; D’Hollander, K.; Arpino, G.; Bernardo, A.; Martignetti, A.; Criscitiello, C.; et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann. Oncol. 2018, 29, 1748–1754. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Wander, S.A.; Han, H.S.; Zangardi, M.L.; Niemierko, A.; Mariotti, V.; Kim, L.S.L.; Xi, J.; Pandey, A.; Dunne, S.; Nasrazadani, A.; et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: A multicenter experience. J. Natl. Compr. Canc. Netw. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- dos Anjos, C.H.; Razavi, P.; Herbert, J.; Colon, J.; Gill, K.; Modi, S.; Bromberg, J.; Dang, C.T.; Liu, D.; Norton, L.; et al. A large retrospective analysis of CDK 4/6 inhibitor retreatment in ER+ metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, 1053. [Google Scholar] [CrossRef]
- Infante, J.R.; Shapiro, G.; Witteveen, P.; Gerecitano, J.F.; Ribrag, V.; Chugh, R.; Issa, I.; Chakraborty, A.; Matano, A.; Zhao, X.; et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J. Clin. Oncol. 2014, 32, 2528. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Shapiro, G.I.; Tolaney, S.M. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care 2016, 11, 167–173. [Google Scholar] [CrossRef]
- Rugo, H.S.; Huober, J.; García-Sáenz, J.A.; Masuda, N.; Sohn, J.H.; Andre, V.A.M.; Barriga, S.; Cox, J.; Goetz, M. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Safety analysis of MONARCH 2 and MONARCH 3. Oncologist 2021, 26, e53–e65. [Google Scholar] [CrossRef] [PubMed]
- West, M.T.; Smith, C.E.; Kaempf, A.; Kohs, T.C.L.; Amirsoltani, R.; Ribkoff, J.; Choung, J.L.; Palumbo, A.; Mitri, Z.; Shatzel, J.J. CDK 4/6 inhibitors are associated with a high incidence of thrombotic events in women with breast cancer in real-world practice. Eur. J. Haematol. 2021, 106, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, L.; Montero, A.J.; Jia, X.; Khorana, A.A. Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J. Thromb. Haemost. 2020, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Mazieres, J.; Barlesi, F.; Dragnev, K.H.; Koczywas, M.; Göskel, T.; Cortot, A.B.; Girard, N.; Wesseler, C.; Bischoff, H.; et al. A randomized phase III Study of abemaciclib versus erlotinib in patients with stage IV non-small cell lung cancer with a detectable KRAS mutation who failed prior platinum-based therapy: JUNIPER. Front. Oncol. 2020, 10, 578756. [Google Scholar] [CrossRef]
- Ahon Pharmaceutical, Co. Ltd. Safety, Tolerability, Pharmacokinetics and Antitumor Activity of FCN-437c. Available online: https://clinicaltrials.gov/ct2/show/NCT04488107 (accessed on 17 March 2022).
- Ahon Pharmaceutical, Co. Ltd. Study of Efficacy, Safety, and Pharmacokinetics of FCN-437c in Combination with Fulvestrant or Letrozole+Goserelin. Available online: https://clinicaltrials.gov/ct2/show/NCT05004142 (accessed on 17 March 2022).
- Garrido-Castro, A.C.; Goel, S. CDK4/6 inhibition in breast cancer: Mechanisms of response and treatment failure. Curr. Breast Cancer Rep. 2017, 9, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic review of molecular biomarkers predictive of resistance to CDK4/6 inhibition in metastatic breast cancer. JCO Precis. Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef]
- Migliaccio, I.; Leo, A.; Galardi, F.; Guarducci, C.; Fusco, G.M.; Benelli, M.; Di Leo, A.; Biganzoli, L.; Malorni, L. Circulating biomarkers of CDK4/6 inhibitors response in hormone receptor positive and HER2 negative breast cancer. Cancers 2021, 13, 2640. [Google Scholar] [CrossRef] [PubMed]
- Puyol, M.; Martin, A.; Dubus, P.; Mulero, F.; Pizcueta, P.; Khan, G.; Guerra, C.; Santamaria, D.; Barbacid, M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010, 18, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; You, J.; Wu, Q.; Meng, W.; He, Q.; Yang, B.; Zhu, C.; Cao, J. Cyclin-dependent kinases-based synthetic lethality: Evidence, concept, and strategy. Acta Pharm. Sin. B 2021, 11, 2738–2748. [Google Scholar] [CrossRef]
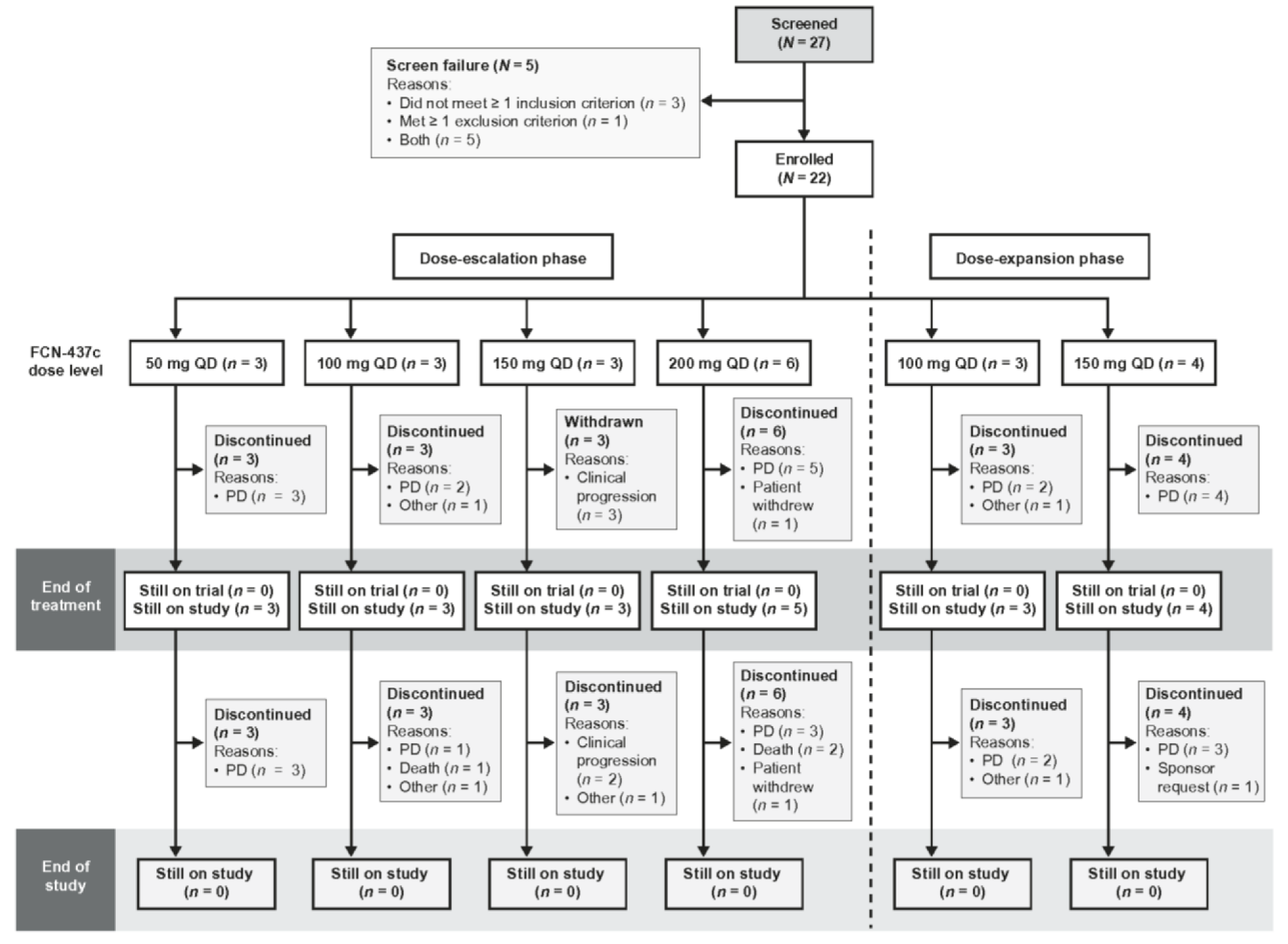

| Characteristic n (%), Unless Otherwise Stated | FCN-437c Dose-Escalation Phase N = 15 | FCN-437c Dose-Expansion Phase N = 7 | Total N = 22 | ||||
|---|---|---|---|---|---|---|---|
| 50 mg n = 3 | 100 mg n = 3 | 150 mg n = 3 | 200 mg n = 6 | 100 mg n = 3 | 150 mg n = 4 | ||
| Age, years | |||||||
| Median (range) | 64.0 (52–71) | 70.0 (69–77) | 64.0 (51–64) | 62.5 (45–88) | 56.0 (39–67) | 59.0 (54–77) | 64.0 (39–88) |
| <65 years | 2 (66.7) | 0 | 3 (100) | 3 (50.0) | 2 (66.7) | 3 (75.0) | 13 (59.1) |
| ≥65 years | 1 33.37) | 3 (100) | 0 | 3 (50.0) | 1 (33.3) | 1 (25.0) | 9 (40.9) |
| Sex | |||||||
| Female | 1 (33.3) | 1 (33.3) | 2 (66.7) | 5 (83.3) | 3 (100) | 2 (50.0) | 14 (63.6) |
| Male | 2 (66.7) | 2 (66.7) | 1 (33.3) | 1 (16.7) | 0 | 2 (50.0) | 8 (36.4) |
| Ethnicity | |||||||
| Hispanic or Latino | 1 (33.3) | 0 | 2 (66.7) | 1 (16.7) | 1 (33.3) | 1 (25.0) | 6 (27.3) |
| Not Hispanic or Latino | 2 (66.7) | 3 (100) | 1 (33.3) | 4 (66.7) | 1 (33.3) | 3 (75.0) | 14 (63.6) |
| Not reported | 0 | 0 | 0 | 1 (16.7) | 1 (33.3) | 0 | 2 (9.1) |
| Primary tumor site | |||||||
| Breast | 1 (3.33) | 0 | 1 (3.33) | 2 (3.33) | 3 (100) | 2 (50.0) | 9 (40.9) |
| Uterine a | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (4.5) |
| Colorectal | 2 (66.7) | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 | 4 (18.2) |
| Liver and bile duct | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (4.5) |
| Pancreatic b | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (4.5) |
| Lung | 0 | 2 (66.7) | 0 | 2 (3.33) | 0 | 2 (50.0) | 6 (27.3) |
| Tumor stage | |||||||
| III | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (4.5) |
| IV | 3 (100) | 3 (100) | 3 (100) | 5 (83.3) | 3 (100) | 4 (100) | 21 (95.5) |
| TEAEs, n (%) | FCN-437c Dose-Escalation Phase (N = 15) | FCN-437c Dose-Expansion Phase (N = 7) | Total (N = 22) | ||||
|---|---|---|---|---|---|---|---|
| 50 mg (n = 3) | 100 mg (n = 3) | 150 mg (n = 3) | 200 mg (n = 6) | 100 mg (n = 3) | 150 mg (n = 4) | ||
| Any TEAE | 3 (100) | 3 (100) | 3 (100) | 6 (100) | 3 (100) | 4 (100) | 22 (100) |
| Related to FCN-437c a | 3 (100) | 3 (100) | 3 (100) | 6 (100) | 1 (33.3) | 4 (100) | 20 (90.9) |
| Grade ≥ 3 | 2 (66.7) | 2 (66.7) | 3 (100) | 4 (66.7) | 1 (33.3) | 4 (100) | 16 (72.7) |
| SAEs | 0 | 1 (33.3) | 0 | 1 (16.7) | 0 | 0 | 2 (9.1) |
| Leading to dose interruption | 1 (33.3) | 1 (33.3) | 3 (100) | 4 (66.7) | 0 | 2 (50.0) | 11 (50.0) |
| Leading to dose modification b | 1 (33.3) | 0 | 1 (33.3) | 1 (16.7) | 0 | 1 (25.0) | 4 (18.2) |
| TEAEs occurring in ≥10% of the total population | |||||||
| Neutrophil count decreased | 1 (33.3) | 1 (33.3) | 0 | 3 (50.0) | 0 | 3 (75.0) | 8 (36.4) |
| WBC decreased | 0 | 1 (33.3) | 2 (66.7) | 2 (33.3) | 0 | 2 (50.0) | 7 (31.8) |
| Fatigue | 1 (33.3) | 0 | 0 | 4 (66.7) | 0 | 1 (25.0) | 6 (27.3) |
| Lymphocyte decreased | 0 | 0 | 0 | 2 (33.3) | 1 (33.3) | 2 (50.0) | 5 (22.7) |
| Nausea | 0 | 1 (33.3) | 1 (33.3) | 2 (33.3) | 0 | 1 (25.0) | 5 (22.7) |
| Diarrhea | 0 | 1 (33.3) | 0 | 2 (33.3) | 0 | 1 (25.0) | 4 (18.2) |
| Dyspnea | 1 (33.3) | 0 | 0 | 1 (16.7) | 1 (33.3) | 1 (25.0) | 4 (18.2) |
| Platelet count decreased | 0 | 0 | 1 (33.3) | 1 (16.7) | 0 | 2 (50.0) | 4 (18.2) |
| Anemia | 1 (33.3) | 0 | 0 | 2 (33.3) | 0 | 0 | 3 (13.6) |
| Cough | 1 (33.3) | 0 | 1 (33.3) | 0 | 0 | 1 (25.0) | 3 (13.6) |
| Dehydration | 0 | 0 | 0 | 2 (33.3) | 0 | 1 (25.0) | 3 (13.6) |
| Fall | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 | 1 (25.0) | 3 (13.6) |
| Upper respiratory tract infection | 1 (33.3) | 0 | 2 (66.7) | 0 | 0 | 0 | 3 (13.6) |
| Dose-Escalation Phase (N = 15) | Dose-Expansion Phase (N = 7) | Total (N = 22) | |||||
|---|---|---|---|---|---|---|---|
| 50 mg (n = 3) | 100 mg (n = 3) | 150 mg (n = 3) | 200 mg (n = 6) | 100 mg (n = 3) | 150 mg (n = 4) | ||
| Best overall response, n (%) | 3 (100) | 3 (100) | 3 (100) | 6 (100) | 1 (33.3) | 4 (100) | 20 (90.9) |
| PR (confirmed) | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 1 (4.5) |
| SD | 1 (33.3) | 0 | 1 (33.3) | 2 (33.3) | 1 (33.3) | 0 | 5 (22.7) |
| PD | 2 (66.7) | 1 (33.3) | 0 | 3 (50.0) | 2 (66.7) | 2 (50.0) | 10 (45.5) |
| Not evaluable | 0 | 1 (33.3) | 2 (66.7) | 1 (16.7) | 0 | 2 (50.0) | 6 (27.3) |
| ORR, a % (95% CI) | 0 | 33.3 (0.8–90.6) | 0 | 0 | 0 | 0 | 4.5 (0.1–22.8) |
| DCR b at 12 weeks, n | 1 | 1 | 1 | 2 | 1 | 0 | 6 |
| % (95% CI) | 33.3 (0.8–90.6) | 33.3 (0.8–90.6) | 33.3 (0.8–90.6) | 33.3 (4.3–77.7) | 33.3 (0.8–90.6) | 0 | 27.3 (10.7–50.2) |
| DoR, median (95% CI) | NR | NR | NR | NR | NR | NR | NR |
| Duration of SD (days), median (95% C) | 57 (49–NR) | NR (112–NR) | NR (NR–NR) | 69 (56–NR) | 56 (54–NR) | 56 (51–NR) | 112 (56–NR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnaik, A.; Hamilton, E.; Xing, Y.; Rasco, D.W.; Smith, L.; Lee, Y.-L.; Fang, S.; Wei, J.; Hui, A.-M. A Phase I Dose-Escalation and Dose-Expansion Study of FCN-437c, a Novel CDK4/6 Inhibitor, in Patients with Advanced Solid Tumors. Cancers 2022, 14, 4996. https://doi.org/10.3390/cancers14204996
Patnaik A, Hamilton E, Xing Y, Rasco DW, Smith L, Lee Y-L, Fang S, Wei J, Hui A-M. A Phase I Dose-Escalation and Dose-Expansion Study of FCN-437c, a Novel CDK4/6 Inhibitor, in Patients with Advanced Solid Tumors. Cancers. 2022; 14(20):4996. https://doi.org/10.3390/cancers14204996
Chicago/Turabian StylePatnaik, Amita, Erika Hamilton, Yan Xing, Drew W. Rasco, Lon Smith, Ya-Li Lee, Steven Fang, Jiao Wei, and Ai-Min Hui. 2022. "A Phase I Dose-Escalation and Dose-Expansion Study of FCN-437c, a Novel CDK4/6 Inhibitor, in Patients with Advanced Solid Tumors" Cancers 14, no. 20: 4996. https://doi.org/10.3390/cancers14204996
APA StylePatnaik, A., Hamilton, E., Xing, Y., Rasco, D. W., Smith, L., Lee, Y.-L., Fang, S., Wei, J., & Hui, A.-M. (2022). A Phase I Dose-Escalation and Dose-Expansion Study of FCN-437c, a Novel CDK4/6 Inhibitor, in Patients with Advanced Solid Tumors. Cancers, 14(20), 4996. https://doi.org/10.3390/cancers14204996









