Simple Summary
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) is an emerging treatment modality for patients with peritoneal cancer with good safety profile and promising early response rates. The aim of this study was to analyze survival and surrogates for oncological response after PIPAC ± systemic chemotherapy for appendiceal tumours. Median overall survival of this cohort was 30 months from time of diagnosis and 22 months from PIPAC1 (per protocol) comparing favorably with 20.4 months of OS reported for patients with palliative chemotherapy alone. However, without prospective comparative data, the role of PIPAC for appendicular cancer with peritoneal metastases remains unclear.
Abstract
Background The aim of this study was to analyse survival and surrogates for oncological response after PIPAC for appendiceal tumours. Methods This retrospective cohort study included consecutive patients with appendiceal peritoneal metastases (PM) treated in experienced PIPAC centers. Primary outcome measure was overall survival (OS) from the date of diagnosis of PM and from the start of PIPAC. Predefined secondary outcome included radiological response (RECIST criteria), repeat laparoscopy and peritoneal cancer index (PCI), histological response assessed by the Peritoneal regression grading system (PRGS) and clinical response. Results Final analysis included 77 consecutive patients (208 PIPAC procedures) from 15 centres. Median OS was 30 months (23.00–46.00) from time of diagnosis and 19 months (13.00–28.00) from start of PIPAC. 35/77 patients (45%) had ≥3 procedures (pp: per protocol). Objective response at PIPAC3 was as follows: RECIST: complete response 4 (11.4%), 11 (31.4%) partial/stable; mean PRGS at PIPAC3: 1.8 ± 0.9. Median PCI: 21 (IQR 18–27) vs. 22 (IQR 17–28) at baseline (p = 0.59); 21 (60%) and 18 (51%) patients were symptomatic at baseline and PIPAC3, respectively (p = 0.873). Median OS in the pp cohort was 22.00 months (19.00–NA) from 1st PIPAC. Conclusion Patients with PM of appendiceal origin had objective treatment response after PIPAC and encouraging survival curves call for further prospective evaluation.
1. Introduction
Peritoneal metastases (PM) from appendiceal tumours are a distinct entity of peritoneal disease with important differences in terms of prognosis and treatments compared to colorectal tumours [1,2]. The estimated incidence of cancers and tumors (neoplasms) of the appendix is 0.15–0.9 per 100,000 people [3]. The literature on appendiceal neoplasms consists due to its rarity mostly of retrospective studies with limited sample size and risk for selection bias. Metastatic appendiceal cancer has a bad prognosis with 5-year OS between 18% to 19% under 5-FU-based palliative chemotherapy with or without combination of capecitabine and oxaliplatin [1,4,5].
Complete cytoreductive surgery (CRS) ± Hyperthermic Intraperitoneal Chemotherapy (HIPEC) is the preferred treatment option for resectable patients offering favourable survival in selected patients [6,7]. Systemic chemotherapy can be added in analogy with colorectal adenocarcinoma although the molecular profile of appendiceal adenocarcinoma is different from colorectal with implications for response to systemic treatment [8,9]. Therapeutic options are limited for patients unfit for major surgery, or those with relapse refractory to systemic treatment options. Retrospective reports of OS after palliative treatment alone with systemic chemotherapy (in majority of cases with capecitabine or fluorouracil) showed a median OS up to 20.4 months [10,11]. After CRS-HIPEC for mucinous appendiceal primaries, 5-year OS for the low- and high-grade mucinous cohorts was 62.5% and 37.7%, respectively. A 5-year survival for the high-grade group who had a complete cytoreduction was of 45% for patients with PCI > 20 and 66% for patients with PCI < 20. High-grade non mucinous appendiceal primaries including adenocarcinoma, goblet cell, and carcinoid tumors derive significantly less benefit from a CRS-HIPEC procedure, with a 3-year survival of approximately 15% [1,5,12,13].
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) was suggested as an alternative in the palliative situation combining minimal-invasive approach, enhanced pharmacokinetic properties and repeated administration of IP chemotherapy [14,15]. The existing evidence suggests a favourable safety profile and promising reponse rates but these results come mainly from single-center experiences on different tumour entities [16,17,18].
The aim of this multicenter study was to study survival and various surrogates for treatment response after PIPAC specifically for PM of appendiceal origin.
2. Materials and Methods
This is a multicenter retrospective cohort study on consecutive PIPAC patients treated for PM of appendiceal origin. All PIPAC centres having performed more than 60 procedures in total by November 2018 were contacted for participation and no center was deliberately excluded [19]. Exclusion criteria were other tumor entities, patient refusal, and patients treated outside current indications. Low-grade appendiceal mucinous neoplasms (LAMN) and High-grade appendiceal mucinous neoplasms (HAMN) were not included into this analysis [16]. The study was conducted according to the declaration of Helsinki (IRB approval: #ICM-ART-2020/05).
2.1. Pressurized IntraPeritoneal Aerosol Chemotherapy
Surgical technique, safety and treatment protocols underlie little variation between centers according to recent investigations [18,19] due to a standardized training curriculum [20]. Main features of PIPAC treatment including technical aspects have been summarized recently [21] and include a two-trocar technique (balloon trocars), a standardized safety procotol (advanced ventilation system, zero flow, remote application), and constant pressure conditions of 12 mmHg [22,23]. The diagnostic phase includes documentation of disease extent (Peritoneal cancer index: PCI), aspiration of ascites (volume, cytology) and 3–4 biopsies from different areas of the abdomen for grading of histological response (outlines below). The empirical drug regimen used for most patients was oxaliplatin at 92 mg/m2.
2.2. Outcomes Measures
Primary endpoint was OS from first PIPAC and from diagnosis of PM. Predefined subgroup analysis was performed for patients having received at least 3 PIPAC treatments (per protocol; pp). Secondary outcome measures were all available potential surrogates for treatment response: symptoms, quality of life (QoL), repeated documentation of PCI [24], radiological response according to Response Evaluation Criteria in Solid Tumours (RECIST) [25] and histological response by use of the peritoneal regression grading score (PRGS) [26,27]. Symptoms were accounted as dichotomous variables including abdominal pain, distension, nausea, and altered intestinal transit (including obstruction) [16,18]. QoL was assessed by use of the validated EORTC QLQ-C30 survey analysing overall QoL as well as its components and main symptoms [28]. Repeated imaging was performed before, during (mostly after 2nd PIPAC) and after treatment, mainly by computed tomography or by magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT if indicated. Treatment response was assessed by use of RECIST criteria [25]. Assessment of histological response and cytology were performed during repeated PIPAC procedures. Aspiration of ascites or peritoneal washing to be sent for cytology and conversion of positive (presence of malignant cells) to negative cytology was counted as treatment response. In addition, 3–4 representative biopsies of PM were performed during PIPAC procedures and analysed according to PRGS. PRGS was strongly propagated and encouraged after its proposal in 2016 [26] and validated recently [27]. PRGS evaluates the histological response of treatment on PM by evaluating the number of tumor cells, fibrosis, acellular mucin pools and necrosis. As described by Solass et al., the PRGS score is defined as follows: 1 corresponds to a complete regression with absence of tumor cells; 2 to major regression features with only a few residual tumor cells; 3 to minor regression with predominance of residual tumor cells and only few regressive features; and 4 corresponds to an absence of response to therapy and where the tumor cells are not accompanied by any regressive features. A PRGS was assessed for each biopsy taken during each PIPAC procedure. The mean PRGS (out of a minimum of 4 biopsies) is calculated according to current recommendations in order to illustrate overall histological response [26,27].
2.3. Statistics
For the descriptive analysis, Student’s t-test for continuous data, Kruskal-Wallis test for non-continuous data and a chi-squared test for categorical data was performed. Descriptive statistics are expressed as mean ± SD, median (IQR) or n (%). Repeated measures t-test was performed for comparing means before and after treatment. Overall survival from the time of diagnosis and from first PIPAC were calculated by use of the Kaplan–Meier method. Variables for survival outcomes were fitted to univariate Cox models, and multivariate Cox models were then created using forward selection strategy. The assumption of proportional hazard was tested. The statistical significance level was considered as <0.05. For all the statistical analysis Statistical software RStudio (Version 1.4.1106) was used. Percentages were calculated based on the availability of information and not to the total number of patients per group.
3. Results
A total of 77 patients from 15 centres having 208 PIPAC procedures were included in the analysis. 35 patients had ≥3 PIPAC treatments per protocol (pp) as detailed in the patient flow chart (Figure 1). Patients’ and tumour characteristics overall and for the pp cohort are displayed in Table 1. PIPAC was applied as monotherapy in 50 (65%) patients, while it was combined with systemic therapy in 27 (35%) patients.
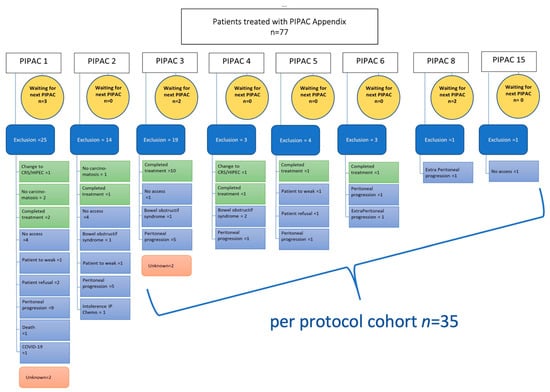
Figure 1.
Flow chart of all patients undergoing PIPAC procedures. Causes of PIPAC treatment interruption are described precisely. PIPAC = Pressurized IntraPeritoneal Aerosol Chemotherapy; CRS = Cyto Reductive Surgery, HIPEC = Hyperthermic intraperitoneal chemotherapy; IP = intraperitoneal chemotherapy.

Table 1.
Baseline characteristics of patients undergoing Pressurized IntraPeritoneal Aerosol Chemotherapy for appendiceal peritoneal metastases.
Median follow-up from diagnosis and from start of PIPAC treatment was 20.6 (IQR 13.5–32.8) months and 9.8 (IQR 3.9–21.1) months, respectively. Figure 2A,B depict OS of patients with PM of appendiceal origin computed from the date of diagnosis and from PIPAC1, respectively.
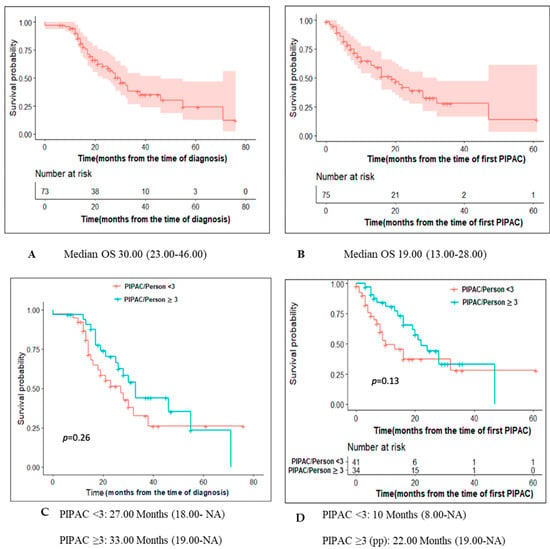
Figure 2.
Survival of patients undergoing Pressurized IntraPeritoneal Aerosol Chemotherapy for peritoneal metastases of appendiceal origin. OS for the entire cohort from time of diagnosis (A) and first PIPAC (B). By protocol analysis for OS (C) from diagnosis and OS (D) from first PIPAC.
For the pp cohort, OS from diagnosis and start of PIPAC treatment were 33 months (19.00–NA) and 22 months (19.00–NA) respectively (Figure 2C,D). Figure 3 depict OS of patients computed from PIPAC1 stratified by PCI < 11, 11–20, >20.
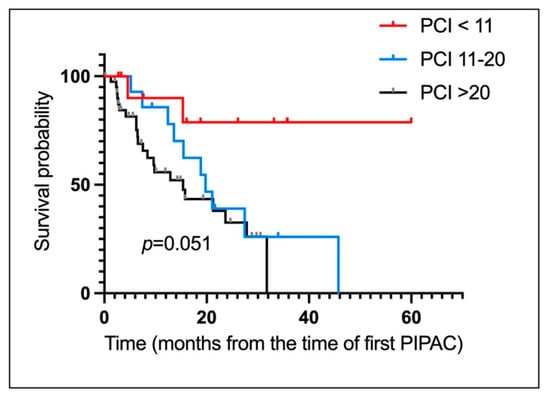
Figure 3.
Survival of patients undergoing Pressurized IntraPeritoneal Aerosol Chemotherapy for peritoneal metastases of appendiceal origin. OS for the entire cohort from time first PIPAC stratified by PCI. (PCI < 11, 11–20, >20). PCI < 11: NA (5.00-NA). PCI 11–20: 20.00 Months (14.00–29.00). PCI: >20: 15.00 Months (9.00–22.00).
Variables indicating treatment response are displayed for both cohorts in Table 2. No significant difference was observed in pp cohort for parameters such as cytology, ΔPCI and presence of any symptoms when compared to the values at base line.

Table 2.
Treatment response of patients undergoing Pressurized IntraPeritoneal Aerosol Chemotherapy for peritoneal metastases of appendiceal origin.
In univariate analysis, total number of cycles of chemotherapy, HR 0.75 (95% CI 0.6–0.93), CEA pre PIPAC, HR1 (95% CI 1–1), Albumin pre –PIPAC, HR 0.8 (95% CI 0.66–0.96), ascites at first PIPAC, HR 1 (95% CI 1–1) and patients who showed radiological response after 3rd PIPAC, HR 3.7(95% CI 1–14) were significant predictors for overall survival (Table S1). However, none of these factors was retained after multivariate analysis (Table S2).
4. Discussion
This international cohort of patients with appendiceal cancer peritoneal metastases showed encouraging survival results and objective response after repeated PIPAC treatment.
The estimated incidence of tumors of the appendix is 0.15–0.9 per 100,000 people [29]. Therefore, most of the existing reports in [16] the literature are small retrospective studies with high risk for potential selection bias. Systemic chemotherapy and CRS ± HIPEC are part of current management protocols [12,30], but the sequence of recommend treatments is inconsistent [6].
The histopathological classification of appendiceal tumors evolved during the last two decades [31]. The most recent classifications return to a three-scale grading system for all appendiceal neoplasms where G1 is a low-grade appendiceal neoplasms that features a particularly favourable prognostic. However, given the moment of the data collection for this study that preceded the WHO 2019 classification, our grading refers to the mucinous adenocarcinoma alone [32]. The patients with adenocarcinoma have significantly worse prognostic compared to the other entities [2].
This multicentric study includes patients from diverse geographic areas of the world with inherent differences with regards to the socio-economic situation and healthcare infrastructure. Common features of the participating centers are special expertise in peritoneal surface malignancies and similar indications and treatment protocols, reserving PIPAC for patients with unresectable disease, mostly beyond the first line.
Currently there is no standard for systemic chemotherapy regimen for surgically unresectable patients diagnosed with metastatic appendiceal cancer. Many chemotherapy regimens have been extrapolated from the metastatic colorectal population and are largely 5-fluorouracil based [32]. However, there are important molecular differences described between the two entities that explain the lower rates of response and survival in the appendiceal cancer population [2]. The latter also has higher rates of signet ring cells (SRC) subtype that are associated with ominous prognosis in any primary [33]. In spite of the absence of specific chemotherapy protocols, it has been shown that patients with mucinous adenocarcinoma (MAC), non-mucinous adenocarcinoma (NMAC) and SRC all benefit from adjuvant chemotherapy, while there is little data about the results of induction treatment [34,35].
In this series, 80% of the patients received oxaliplatin-based treatment in their 1st chemotherapy line with irinotecan-based treatment in the 2nd line. In a retrospective analysis by Shapiro et al. systemic chemotherapy prolonged disease control by 7.6 months and OS up to 20.4 months in patients who are deemed suboptimal candidates for CRS +/− HIPEC [10,11,12,30,31,36]. In comparison to this, the median OS in our study was 20.9 months (13.7–31.4) from diagnosis and 9.9 months (4.5–20.8) from the time of the first PIPAC. For the pp cohort, the median OS was 22 months (19.00-NA) from first PIPAC. The current results seem encouraging, and they suggest that the use of PIPAC in this setting can further be explored as stand-alone treatment or in combination with systemic chemotherapy (bi-directional). The latter poses obvious methodological challenge, namely to attribute potential benefits of the combined treatment to either modality, as it was the case also in the present study. Further evidence arising from large registry data is expected in the future.
IP chemotherapy allows a higher drug concentration intraperitoneally compared to systemic chemotherapy, resulting In better response in terms of peritoneal metastasis, along with less systemic toxicity [37]. The pp cohort set of patients in this study showed an improved survival benefit when compared to patients who received less than 3 cycles of PIPAC: median OS from time of first PIPAC of 22 months (19.00-NA) vs 10 months (8.00-NA) (p-value = 0.13). However, the results did not reach statistical significance (potential type II error). The survival benefit was even higher in patients who prior received two lines of chemotherapy as it gained statistical significance (28 months versus 8 months, p-value = 0.02).
The potential role of neoadjuvant intraperitoneal chemotherapy was already emphasized in other appendiceal entities (pseudomyxoma) [38]. The probable effectiveness of other types of intraperitoneal administrations in the context of peritoneal disease of appendiceal origin adds to the rationale of PIPAC, in spite of the clinical setting of the present study that is limited to appendiceal adenocarcinoma [12,33].
In the current study we mostly used oxaliplatin as a PIPAC drug. This attitude is still considered up-to-date by the recent consensus on drug regimens [39]. On the other side, the recent literature questions the role of oxaliplatin as an IP drug after oxaliplatin-based neoadjuvant treatment due to potential resistance. The current data concerning the potential resistance are scarce and have low-quality, and PIPAC can potentially overcome some of the limitations of HIPEC through repeated administration of low-dose, long duration IP treatment [40]. Furthermore, in the particular setting of resectable appendiceal adenocarcinoma, a randomized control trial showed similar survival results with HIPEC with oxaliplatin and mitomycine C but HIPEC with oxaliplatin was associated with a better quality of life [30,41]. All these aspects indirectly support the further use of PIPAC-Ox in the metastatic appendiceal adenocarcinoma patients while pursuing efforts to identify new IP drugs.
Although on multivariate regression analysis we could not identify any significant variables that can predict survival in the pp cohort, factors that showed promising trends were radiological response at PIPAC 3, tumour markers CA 19-9 and CA 125. PRGS was expected to confirm its prognostic nature for PM similar to histologic response in other metastatic sites [42]. In this cohort, it failed to show a significant signal which suggest that a more complex use of the score may be required in order to enhance its value. Small sample size and missing data inherent to the retrospective and multicentric nature of the study are probable causes for the lack of more conclusive results.
The main limitations are the retrospective study design, the small and heterogeneous cohort of patients and the limited follow-up time. However, the patient demographics, disease presentation, and treatments are consistent with the literature and can hence be considered as representative. Despite the limitations, the current data is the best available evidence on this topic and it can guide the management of the disease in the adapted clinical context. However further prospective studies are needed in order to further elucidate the role of PIPAC for appendiceal PM.
5. Conclusions
In conclusion, PIPAC appears to be a promising treatment option for patients with PM of appendiceal origin. PIPAC can hence be discussed for patients in this situation with no standard treatment option available. Large-scale registry data and prospective comparative data are needed to confirm oncological efficacy before use of PIPAC can be validated for this indication and potential others.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14204998/s1, Table S1: Univariate analysis; Table S2: Multivariate analysis.
Author Contributions
S.S., J.A., O.S., M.A., H.T.F., R.G.R., W.W. and M.H. meet all the criteria for the definition of authorship and contributed substantially to the manuscript. S.S., W.W. and M.H. conceived and designed the study. J.A., M.A., R.G.R. and H.T.F. managed the data and analyzed the data. All authors have read and agreed to the published version of the manuscript. M.H. is the corresponding author.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Informed written consent was obtained for all participants and the study was approved by the Ethics Committee of canton de Vaud (CER-VD), Lausanne, Switzerland: #2019-00747.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
A preliminary analysis of the data was presented as oral presentation at the ISSPP meeting 2021 in Rome. The full and final analysis has not been presented yet and was not submitted elsewhere. The list of PIPAC study group members are as follows: Brigand Cécile, Strasbourg University Hospital- Hautepierre Hospital Strasbourg, University Hospital—Hautepierre Hospital, Department of general and digestive surgery; Delhorme Jean-Baptiste, Strasbourg University Hospital-Hautepierre Hospital Strasbourg, University Hospital—Hautepierre Hospital, Department of general and digestive surgery; Romain Benoit, Strasbourg University Hospital—Hautepierre Hospital Strasbourg University Hospital—Hautepierre Hospital, Department of general and digestive surgery; Charleux-Muller Diane, Strasbourg University Hospital—Hautepierre Hospital Strasbourg University Hospital—Hautepierre Hospital, Department of General and Digestive Surgery; Bertin Jean- Baptiste, Strasbourg University Hospital—Hautepierre Hospital Strasbourg University Hospital—Hautepierre Hospital, Department of General and Digestive Surgery; Rohr Serge, Strasbourg University Hospital—Hautepierre Hospital Strasbourg University Hospital—Hautepierre Hospital, Department of General and Digestive Surgery; Jäger Tarkan, Department of Surgery, Paracelsus Medical University Salzburg, 5020 Salzburg, Austria; Neureiter Daniel, Institute of Pathology, Paracelsus Medical University Salzburg, 5020 Salzburg, Austria; Schredl Philipp, Department of Surgery, Paracelsus Medical University Salzburg, 5020 Salzburg, Austria; Weiss Lukas, Department of Internal Medicine III with Haematology, Medical Oncology, Haemostaseology, Infectiology and Rheumatology, Oncologic Center, Salzburg Cancer Research Institute-Laboratory for Immunological and Molecular Cancer Research (SCRI-LIMCR), Paracelsus Medical University, 5020 Salzburg, Austria and Cancer Cluster Salzburg, 5020 Salzburg, Austria; Klieser Eckhard, Institute of Pathology, Paracelsus Medical University Salzburg, 5020 Salzburg, Austria; Emmanuel Klaus, Department of Surgery, Paracelsus Medical University Salzburg, 5020 Salzburg, Austria; Sgarbura Olivia, Institut du Cancer Montpellier (1) Department of Surgical Oncology, Institut du Cancer Montpellier, Montpellier France; (2) IRCM, Institut de Recherche en Cancérologie de Montpellier, INSERM U1194, Université de Montpellier, Institut régional du Cancer de Montpellier, Montpellier, F-34298, France; Bouillin Alix, Institut du Cancer Montpellier, Department of Surgical Oncology, Institut du Cancer Montpellier, Montpellier, France; Khellaf Lakhdar, Institut du Cancer Montpellier, Department of Pathology, Institut du Cancer Montpellier; Nougaret Stéphanie, Institut du Cancer Montpellier, Department of Radiology, Institut du Cancer Montpellier; Samalin Emmanuelle, Institut du Cancer Montpellier, Department of Medical Oncology, Institut du Cancer Montpellier; Mazard Thibault, Institut du Cancer Montpellier, Department of Medical Oncology, Institut du Cancer Montpellier; Glehen Olivier, Hôpital Lyon Sud, Department of Surgical Oncology, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France, Hôpital Lyon Sud, Department of Surgical Oncology, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France; Kepenekian Vahan, Hôpital Lyon Sud, Department of Surgical Oncology, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France; Isabelle Bonnefoy, Hôpital Lyon Sud, Department of Surgical Oncology, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France; Parisot Marlene, Hôpital Lyon, Sud Department of Surgical Oncology, Hôpital Lyon Sud, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France; Villeneuve Laurent, Hôpital Lyon Sud Service de Recherche et d’Epidémiologie Cliniques, Pôle de Santé Publique, Hospices Civils de Lyon, Lyon, France; EA 3738 CICLY, Université de Lyon, Lyon, France; Hübner Martin, CHUV Lausanne, Department of Visceral Surgery, Lausanne University Hospital CHUV, University of Lausanne (UNIL), Lausanne, Switzerland; Demartines Nicolas, CHUV Lausanne Department of Visceral Surgery, Lausanne University Hospital CHUV, University of Lausanne (UNIL), Lausanne, Switzerland; Teixeira-Farinha Hugo, CHUV Lausanne, Department of Visceral Surgery, Lausanne University Hospital CHUV, University of Lausanne (UNIL), Lausanne, Switzerland; Clerc Daniel, CHUV Lausanne Department of Visceral Surgery, Lausanne University Hospital CHUV, University of Lausanne (UNIL), Lausanne, Switzerland; Dromain Clarisse, CHUV Lausanne, Department of Medical Oncology, Institut du Cancer Montpellier; Sempoux Christine; CHUV Lausanne, Department of Medical Oncology, Institut du Cancer Montpellier; Robella Manuela, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy Unit of Surgical Oncology, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy; Vaira Marco, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy Unit of Surgical Oncology, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy; De Simone Michele, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy Unit of Surgical Oncology, Candiolo Cancer Institute, FPO—IRCCS, Candiolo, Italy; Di Giorgio Andrea, Fondazione Policlinico Universitario A. Gemelli IRCCS Surgical Unit of Peritoneum and Retroperitoneum, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Ferracci Federica, Fondazione Policlinico Universitario A. Gemelli IRCCS Surgical Unit of Peritoneum and Retroperitoneum, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Rotolo Stefano, Fondazione Policlinico Universitario A. Gemelli IRCCS, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Palermo, Italy; Schena Carlo Alberto, Fondazione Policlinico Universitario A. Gemelli IRCCS General Surgery, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Inzani Frediano, Fondazione Policlinico Universitario A. Gemelli IRCCS Surgical Unit of Peritoneum and Retroperitoneum, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Bagalà Cinzia, Fondazione Policlinico Universitario A. Gemelli IRCCS Division of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy; Babucke Maximilian, department of general surgery Krankenhaus Barmherzige Brüder Regensburg, Prüfeninger Str. 86, 93049 Regensburg, Germany; Piso Pompiliu, department of general surgery Krankenhaus Barmherzige Brüder Regensburg, Prüfeninger Str. 86, 93049 Regensburg, Germany; Somashekhar s p, Manipal Comprehensive Cancer Center, Bengaluru, Department of Surgical Oncology; Ashwin KR, DNB, (Surgical Oncology) Manipal Comprehensive Cancer Center, Bengaluru, Department of Surgical Oncology; Rohit Kumar DNB, ( Surgical Oncology) Manipal Comprehensive Cancer Center, Bengaluru, Department of Surgical Oncology; Priya Kapoor, MS, DNB, (General Surgery) Manipal Comprehensive Cancer Center, Bengaluru, Department of Surgical Oncology; Susmita Rakshit, MBBS, (Pathology) Manipal Comprehensive Cancer Center, Bengaluru, Department of Pathology; Amit Rauthan, DM, (Medical Oncology) Manipal Comprehensive Cancer Center, Bengaluru, Department of Medical Oncology; Bhatt Aditi, MS, MCh., Zydus Hospital, Ahmedabad, Department of Surgical Oncology; Shaikh Sakina, BH, MS, MBA Zydus Hospital, Ahmedabad Department of surgical Oncology; Parikh Loma, Zydus Hospital, Ahmedabad, Department of Pathology; Sheth Sandeep, Zydus Hospital, Ahmedabad, Department of Pathology; Panchal Amee, Zydus Hospital, Ahmedabad, Department of Radiodiagnosis and Imaging; Thakkar Shweta, Zydus Hospital, Ahmedabad, Department of Radiodiagnosis and Imaging; Pocard Marc, INSERM, U1275 CAP Paris-Tech 1. Université de Paris, INSERM, U1275 CAP Paris-Tech, F-75010 Paris, France. 2. Hepato-Biliary-pancreatic Gastrointestinal Surgery and Liver Transplantation, Pitié Salpêtrière Hospital, AP-HP, F-75013 Paris, France; Ezzano Anne-Cécile, Hôpital Bégin Department of visceral surgery, BEGIN Military Hospital, 69, avenue de Paris, 94160 St Mandé, France; Aime Adeline, Hôpital Bégin Department of visceral surgery, BEGIN Military Hospital, 69, avenue de Paris, 94160 St Mandé, France; Eveno Clarisse, CHU Lille Department of Digestive and Oncological Surgery Claude Huriez University Hospital, 59000 Lille, France; UMR-S1277-CANTHER laboratory, “Cancer Heterogeneity Plasticity and Resistance to Therapies”, Lille, France; Noiret Barbara, CHU Lille Department of Digestive and Oncological Surgery Claude Huriez University Hospital, 59000 Lille, France; Khomiakov Vladimir, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology, Moscow, Russia; Ryabov Andrey, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia; Utkina Anna, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology, Moscow, Russia P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia; Aksenov Sergey, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology, Moscow, Russia P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology, Moscow, Russia; Bolotina Larisa, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology, Moscow, Russia; Kaprin Andrey, P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia; P.A. Hertsen Moscow Research Oncological Institute—Branch of the National Medical Research Centre of Radiology., Moscow, Russia; Willaert Wouter, Ghent University, Department of Human Structure and Repair, Laboratory of Experimental Surgery; Cosyns Sarah, Ghent University, Department of Human Structure and Repair, Laboratory of Experimental Surgery; Akhayad Soumaya, BSN, Ghent University Hospital, Department of Gastro-intestinal Surgery; Ceelen Wim, Ghent University, Department of Human Structure and Repair, Laboratory of Experimental Surgery; Gockel Ines, University Hospital Leipzig “Department of Visceral, Transplant, Thoracic and Vascular Surgery”; Jansen-Winkeln Boris, University Hospital Leipzig, Department of Visceral, Transplant, Thoracic and Vascular Surgery and Department of General, Visceral and Oncological Surgery, St. Georg Hospital, Leipzig, Germany”; Thieme René, University Hospital Leipzig, Department of Visceral, Transplant, Thoracic and Vascular Surgery”; Schierle Katrin, University Hospital Leipzig Institute of Pathology; Moulla Yusef, University Hospital Leipzig, Department of Visceral, Transplant, Thoracic and Vascular Surgery; Mehdorn Matthias, University Hospital Leipzig, Department of Visceral, Transplant, Thoracic and Vascular Surgery; Abba Julio, Centre Hospitalier Universitaire Grenoble Alpes Service de chirurgie Digestive; Trilling Bertrand, Centre Hospitalier Universitaire Grenoble Alpes Service de chirurgie Digestive; Tidadini Fatah, Centre Hospitalier Universitaire Grenoble Alpes Service de chirurgie Digestive; Bonne Aline, Centre Hospitalier Universitaire Grenoble Alpes Service de chirurgie Digestive; Arvieux Catherine, Centre Hospitalier Universitaire Grenoble Alpes Service de chirurgie Digestive; Orry David, Centre Georges François Leclerc; Basso Valeria, Centre Georges François Leclerc; Ghiringhelli François, Centre Georges François Leclerc; Escayola Cecilia, HOSPITAL EL PILAR QUIRON SALUD Quenet- Torrent Institute; Torrent Juan José, HOSPITAL EL PILAR QUIRON SALUD, Quenet-Torrent Institute; Alyami Mohammad, Department of General Surgery and Surgical Oncology, King Khalid Hospital, Najran, Saudi Arabia; Alqannas Mashhour, Department of General Surgery and Surgical Oncology, King Khalid Hospital, Najran, Saudi Arabia; Cortés Guiral Delia, Department of General Surgery and Surgical Oncology, King Khalid Hospital, Najran, Saudi Arabia; Alammari samer, Department of General Surgery and Surgical Oncology, King Khalid Hospital, Najran, Saudi Arabia; Bashanfer Galal, Department of Pathology King Khalid Hospital, Najran, Saudi Arabia; Alshukami Anwar, Department of Radiology King Khalid Hospital, Najran, Saudi Arabia; Reymond Marc A, Department of General and Transplant Surgery, University of Tuebingen; Solass Wiebke, Department of Pathology, University Hospital Tuebingen; Nadiradze Giorgi, Department of General and Transplant Surgery, University of Tuebingen.
Conflicts of Interest
Martin Hübner: ENCARE: Consultant fee (institution); Nestlé: Research funding. Capnomed: Sponsoring of scientific meetings; MSD, Fresenius: Speaker honorary (institution); ERAS society: Board member, chair education; ISSPP: Board member, chair education. Other authors declare no conflict of interest.
Abbreviations
| PRGS | Peritoneal regression grading system |
| PIPAC | Pressurized IntraPeritoneal Aerosol Chemotherapy |
| PM | Peritoneal Metastasis |
| RECIST | Response Evaluation Criteria in Solid Tumors, |
| OS | overall survival |
| PCI | Peritoneal Cancer Index. |
References
- Votanopoulos, K.I.; Shen, P.; Skardal, A.; Levine, E.A. Peritoneal Metastases from Appendiceal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Xiu, J.; Johnston, C.; Goldberg, R.M.; Philip, P.A.; Seeber, A.; Naseem, M.; Lo, J.H.; Arai, H.; Battaglin, F.; et al. Molecular Profiling of Appendiceal Adenocarcinoma and Comparison with Right-sided and Left-sided Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.L.; Assi, R.; Shamseddine, A.; Alese, O.B.; Staley, C., 3rd; Memis, B.; Adsay, V.; Bekaii-Saab, T.; El-Rayes, B.F. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist 2017, 22, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.H.; Holt, N.; Langer, S.W.; Hasselby, J.P.; Gronbaek, H.; Hillingso, J.; Mahmoud, M.; Ladekarl, M.; Iversen, L.H.; Kjaer, A.; et al. Goblet cell carcinoids: Characteristics of a Danish cohort of 83 patients. PLoS ONE 2015, 10, e0117627. [Google Scholar] [CrossRef]
- Palmer, K.; Weerasuriya, S.; Chandrakumaran, K.; Rous, B.; White, B.E.; Paisey, S.; Srirajaskanthan, R.; Ramage, J.K. Goblet Cell Adenocarcinoma of the Appendix: A Systematic Review and Incidence and Survival of 1225 Cases From an English Cancer Registry. Front. Oncol. 2022, 12, 915028. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Raghav, K.; Shen, J.P.; Jacome, A.A.; Guerra, J.L.; Scally, C.P.; Taggart, M.W.; Foo, W.C.; Matamoros, A.; Shaw, K.R.; Fournier, K.; et al. Integrated clinico-molecular profiling of appendiceal adenocarcinoma reveals a unique grade-driven entity distinct from colorectal cancer. Br. J. Cancer 2020, 123, 1262–1270. [Google Scholar] [CrossRef]
- Lu, P.; Fields, A.C.; Meyerhardt, J.A.; Davids, J.S.; Shabat, G.; Bleday, R.; Goldberg, J.E.; Nash, G.M.; Melnitchouk, N. Systemic chemotherapy and survival in patients with metastatic low-grade appendiceal mucinous adenocarcinoma. J. Surg. Oncol. 2019, 120, 446–451. [Google Scholar] [CrossRef]
- Tejani, M.A.; ter Veer, A.; Milne, D.; Ottesen, R.; Bekaii-Saab, T.; Benson, A.B., 3rd; Schrag, D.; Shibata, S.; Skibber, J.; Weiser, M.; et al. Systemic therapy for advanced appendiceal adenocarcinoma: An analysis from the NCCN Oncology Outcomes Database for colorectal cancer. J. Natl. Compr. Cancer Netw. 2014, 12, 1123–1130. [Google Scholar] [CrossRef]
- Lieu, C.H.; Lambert, L.A.; Wolff, R.A.; Eng, C.; Zhang, N.; Wen, S.; Rafeeq, S.; Taggart, M.; Fournier, K.; Royal, R.; et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann. Oncol. 2012, 23, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- El Halabi, H.; Gushchin, V.; Francis, J.; Athas, N.; Macdonald, R.; Nieroda, C.; Studeman, K.; Sardi, A. The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann. Surg. Oncol. 2012, 19, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Solass, W.; Kerb, R.; Murdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Grass, F.; Vuagniaux, A.; Teixeira-Farinha, H.; Lehmann, K.; Demartines, N.; Hubner, M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br. J. Surg. 2017, 104, 669–678. [Google Scholar] [CrossRef]
- Alyami, M.; Hubner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Lurvink, R.J.; Rovers, K.P.; Nienhuijs, S.W.; Creemers, G.J.; Burger, J.W.A.; de Hingh, I.H.J. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC-OX) in patients with colorectal peritoneal metastases-a systematic review. J. Gastrointest. Oncol. 2021, 12, S242–S258. [Google Scholar] [CrossRef]
- Nowacki, M.; Alyami, M.; Villeneuve, L.; Mercier, F.; Hubner, M.; Willaert, W.; Ceelen, W.; Reymond, M.; Pezet, D.; Arvieux, C.; et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur. J. Surg. Oncol. 2018, 44, 991–996. [Google Scholar] [CrossRef]
- Sgarbura, O.; Villeneuve, L.; Alyami, M.; Bakrin, N.; Torrent, J.J.; Eveno, C.; Hubner, M.; ISSPP PIPAC Study Group. Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): Still standardized or on the verge of diversification? Eur. J. Surg. Oncol. 2021, 47, 149–156. [Google Scholar] [CrossRef]
- Alyami, M.; Gagniere, J.; Sgarbura, O.; Cabelguenne, D.; Villeneuve, L.; Pezet, D.; Quenet, F.; Glehen, O.; Bakrin, N.; Passot, G. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2017, 43, 2178–2183. [Google Scholar] [CrossRef]
- Hubner, M.; Grass, F.; Teixeira-Farinha, H.; Pache, B.; Mathevet, P.; Demartines, N. Pressurized IntraPeritoneal Aerosol Chemotherapy—Practical aspects. Eur. J. Surg. Oncol. 2017, 43, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Hubner, M.; Alyami, M.; Villeneuve, L.; Cortes-Guiral, D.; Nowacki, M.; So, J.; Sgarbura, O.; Abba, J.; Afifi, A.; Mortensen, M.B.; et al. Consensus guidelines for pressurized intraperitoneal aerosol chemotherapy: Technical aspects and treatment protocols. Eur. J. Surg. Oncol. 2022, 48, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Girardot-Miglierina, A.; Clerc, D.; Alyami, M.; Villeneuve, L.; Sgarbura, O.; Reymond, M.-A.; Hübner, M.; on behalf of the ISSPP PIPAC Study Group. Consensus statement on safety measures for pressurized intraperitoneal aerosol chemotherapy. Pleura Peritoneum 2021, 6, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Solass, W.; Sempoux, C.; Carr, N.J.; Bibeau, F.; Neureiter, D.; Jager, T.; Di Caterino, T.; Brunel, C.; Klieser, E.; Fristrup, C.W.; et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 2019, 74, 1014–1024. [Google Scholar] [CrossRef]
- Solass, W.; Sempoux, C.; Detlefsen, S.; Carr, N.J.; Bibeau, F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: Proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016, 1, 99–107. [Google Scholar] [CrossRef]
- Teixeira Farinha, H.; Grass, F.; Kefleyesus, A.; Achtari, C.; Romain, B.; Montemurro, M.; Demartines, N.; Hubner, M. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 4596176. [Google Scholar] [CrossRef]
- Marmor, S.; Portschy, P.R.; Tuttle, T.M.; Virnig, B.A. The rise in appendiceal cancer incidence: 2000–2009. J. Gastrointest. Surg. 2015, 19, 743–750. [Google Scholar] [CrossRef]
- Levine, E.A.; Votanopoulos, K.I.; Shen, P.; Russell, G.; Fenstermaker, J.; Mansfield, P.; Bartlett, D.; Stewart, J.H. A Multicenter Randomized Trial to Evaluate Hematologic Toxicities after Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin or Mitomycin in Patients with Appendiceal Tumors. J. Am. Coll. Surg. 2018, 226, 434–443. [Google Scholar] [CrossRef]
- Hissong, E.; Yantiss, R.K. The Frontiers of Appendiceal Controversies: Mucinous Neoplasms and Pseudomyxoma Peritonei. Am. J. Surg. Pathol. 2022, 46, e27–e42. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.J. Management of Appendix Cancer. Clin. Colon Rectal. Surg. 2015, 28, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Le, V.H.; Tee, M.C.; Lin, M.; Sedinkin, J.; Raman, S.; Frankova, D. Signet ring cell carcinoma of the gastrointestinal tract: National trends on treatment effects and prognostic outcomes. Cancer Treat. Res. Commun. 2021, 29, 100475. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.A.; Compton, C.C.; Hanna, N.N.; Kosinski, L.A.; Washington, M.K.; Kakar, S.; Weiser, M.R.; Overman, M.J. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: Analysis of the National Cancer Data Base. Cancer 2016, 122, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Qiao, W.; Fournier, K.; Morris, J.; Mansfield, P.; Eng, C.; Royal, R.E.; Wolff, R.A.; Raghav, K.; Mann, G.N.; et al. Retrospective study of nonmucinous appendiceal adenocarcinomas: Role of systemic chemotherapy and cytoreductive surgery. BMC Cancer 2017, 17, 331. [Google Scholar] [CrossRef]
- Shapiro, J.F.; Chase, J.L.; Wolff, R.A.; Lambert, L.A.; Mansfield, P.F.; Overman, M.J.; Ohinata, A.; Liu, J.; Wang, X.; Eng, C. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: A single-institution experience. Cancer 2010, 116, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Guiral, D.; Hubner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Noguchi, K.; Motoi, S.; Ichinose, M.; et al. Neoadjuvant Intraperitoneal Chemotherapy in Patients with Pseudomyxoma Peritonei—A Novel Treatment Approach. Cancers 2020, 12, 2212. [Google Scholar] [CrossRef]
- Sgarbura, O.; Eveno, C.; Alyami, M.; Bakrin, N.; Guiral, D.C.; Ceelen, W.; Delgadillo, X.; Dellinger, T.; Di Giorgio, A.; Kefleyesus, A.; et al. Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Taibi, A.; Hubner, M.; Eveno, C.; Dumont, F.; Glehen, O.; Sgarbura, O. ASO Author Reflections: Is There Still a Role for Intraperitoneal Oxaliplatin for Colorectal Peritoneal Metastases? Ann. Surg. Oncol. 2022, 29, 5252–5253. [Google Scholar] [CrossRef]
- Moaven, O.; Perry, K.C.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. ASO Author Reflections: Patient-Reported Outcomes of Mucinous Appendiceal Cancer Improve with Oxaliplatin HIPEC. Ann. Surg. Oncol. 2020, 27, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Rubbia-Brandt, L.; Giostra, E.; Brezault, C.; Roth, A.D.; Andres, A.; Audard, V.; Sartoretti, P.; Dousset, B.; Majno, P.E.; Soubrane, O.; et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2007, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).