Simple Summary
Head and neck squamous cell carcinoma (HNSCC) incidence has been escalating in the last two decades, particularly in Western Europe and North America. Human papillomavirus (HPV) has been identified as the main culprit for this rise with significant implications for the treatment and outcomes of these patients. The purpose of this retrospective study was to investigate HPV’s impact on HNSCC patient outcomes in the Irish population, which has never been performed before. HPV positivity of HNSCC appears to be associated with improved survival in patients and highlights the importance of surgery, perhaps with less severe chemo-radiation therapy, in HPV-related HNSCC treatment.
Abstract
Human papillomavirus (HPV) infection has been identified as a significant etiological agent in the development of head and neck squamous cell carcinoma (HNSCC). HPV’s involvement has alluded to better survival and prognosis in patients and suggests that different treatment strategies may be appropriate for them. Only some data on the epidemiology of HPV infection in the oropharyngeal, oral cavity, and laryngeal SCC exists in Europe. Thus, this study was carried out to investigate HPV’s impact on HNSCC patient outcomes in the Irish population, one of the largest studies of its kind using consistent HPV testing techniques. A total of 861 primary oropharyngeal, oral cavity, and laryngeal SCC (OPSCC, OSCC, LSCC) cases diagnosed between 1994 and 2013, identified through the National Cancer Registry of Ireland (NCRI), were obtained from hospitals across Ireland and tested for HPV DNA using Multiplex PCR Luminex technology based in and sanctioned by the International Agency for Research on Cancer (IARC). Both overall and cancer-specific survival were significantly improved amongst all HPV-positive patients together, though HPV status was only a significant predictor of survival in the oropharynx. Amongst HPV-positive patients in the oropharynx, surgery alone was associated with prolonged survival, alluding to the potential for de-escalation of treatment in HPV-related OPSCC in particular. Cumulatively, these findings highlight the need for continued investigation into treatment pathways for HPV-related OPSCC, the relevance of introducing boys into national HPV vaccination programs, and the relevance of the nona-valent Gardasil-9 vaccine to HNSCC prevention.
1. Introduction
Prognosis and survival for head and neck squamous cell carcinoma (HNSCC) are generally poor. Approximately half of all patients with HNSCC have advanced-stage disease at the time of diagnosis, with an expected 5-year survival rate between 10% to 40% [1]. This is mostly attributed to the fact that diagnosis of HNSCC is frequently delayed because symptoms for which patients will seek medical attention such as pain, dysphagia, and shortness of breath occur late in the stage of the disease [2].
HPV-positive HNSCC, and more specifically oropharyngeal squamous cell carcinoma (OPSCC), has a unique relationship to diagnosis, prognosis, and treatment. These tumors generally present with a more advanced clinical stage, with a higher nodal category [3,4], despite lower tumor extent [4,5], and have different tendencies for extracapsular spread and perineural invasion [6]. Tonsil squamous cell carcinoma (SCC) in general is known to present with early lymph node metastases [7] and it may simply be that the anatomy of the site itself facilitates the early spread and depth of invasion [8].
Despite more advanced presentation, improved survival, which is consistently higher than 30% [9], is evident in HPV-related OPSCC [8,10,11,12,13,14], irrespective of treatment modality [5,15,16,17,18,19,20,21]. The improved prognosis and response to treatment holds true for all indicators of HPV-positivity including seropositivity, mRNA, oncoprotein expression, and viral load and copy number [22]. It also remains salient in the case of HPV-positive OPSCC biomarkers, including p16, p53, EGFR, and Bcl-xL [22,23].
For most patients with high-risk, resected HNSCC, the standard treatment constitutes adjuvant radiation therapy with high doses of cisplatin. This course of treatment appears to work well for HPV-positive tumors. Adjuvant chemoradiation therapy with one dose of weekly cisplatin had 3-year overall survival rates of 86% and 91% and 3-year recurrence free survival of 82% and 84% in one study, suggesting that cisplatin is a good treatment for HPV-positive OPSCC to preserve survival and minimize toxicity [24,25].
Given this positive response to therapies [5,26,27,28,29], de-escalation of therapy might be appropriate for these HPV-positive HNSCCs. This is particularly important given the long-term consequences and associated morbidities amongst those patients who do survive. Though patients express gratitude for the success of their treatments, many suffer from difficulty swallowing, breathing, and speaking, chronic pain, osteoradionecrosis, hypertension, pneumonia, dysphagia, weight loss, malnutrition, dental issues, and third-degree burns. These are acute hindrances to the quality of the rest of their lives.
Despite extensive reports in the literature, most studies regarding the differential prognosis and treatment modalities of HPV-related and HPV-unrelated HNSCC analyze fewer than 300 cases [13,14,30]. When pooled for meta-analysis [21], the definition of what constitutes an HPV-positive case is heavily dependent upon the HPV indicator chosen and the technology used, which varies by study. Furthermore, there is an evident gap in the literature regarding the Irish population’s HPV-related and HPV-unrelated HNSCC survival. This gap provides a unique opportunity to study historical samples from a period before the widespread assessment of HPV status determined patient management, allowing for a prognostic comparison without management as a confounder.
In the context of the worldwide meta-analysis being conducted by the International Agency for Research on Cancer (IARC) on the subject (HPV-AHEAD, http://hpv-ahead.iarc.fr, accessed on 1 July 2022), we collected over 1115 FFPE HNSCC samples from six different hospital sites across Ireland, aiming to determine the relationships between HR HPV status, treatment scheme, and survival. We also standardized the definition of an HPV-positive case, using DNA alone detected by an extremely sensitive Multiplex PCR Luminex technology. In this article, we describe the first results of comprehensive survival analysis of HPV DNA-positive and negative OPSCC, oral cavity SCC (OSCC), and laryngeal SCC (LSCC) diagnosed in Ireland between 1994 and 2013. These patient samples were not routinely analyzed for HPV in this time period.
2. Materials and Methods
2.1. Specimen Collection
Through the National Cancer Registry of Ireland (NCRI) database, an initial incident population of 5767 OPSCC, OSCC, and LSCC was identified. ICD10 codes used to define these sites were in line with the most up-to-date World Health Organization (WHO) classifications [31]. Strict inclusion criteria were applied to this initial database including that cases should be: (i) archival primary tumors (ii) diagnosed between 1994 and 2013 (iii) purely and histologically confirmed squamous cell carcinoma (iv) plentiful enough to conduct all necessary analyses. A total of 1115 specimens fulfilling these criteria based on review of associated pathology reports were retrieved. All cases were re-cut, H+E stained, and re-reviewed by pathologists to confirm there was still tumor remaining in specimens selected for HPV analysis. Following this, 861 cases remained for molecular testing. Ethical approval for the study was obtained from 11 different research ethics committees across Ireland representing 14 different hospitals, 6 of which were ultimately included and sourced for specimens. The study’s use of only archival material ensured no contact with patients, and cases were anonymized by the NCRI using a random study number. Consent was obtained from patients still alive through the NCRI, and a waiver on consent was obtained from the different RECs at that time for use of anonymized material from deceased patients. The blocks were used in the manner detailed below to generate the coming results.
2.2. Preparation of the Tissue Sections
FFPE tissue sections were cut in the Trinity College Dublin CERVIVA Molecular Pathology Research Laboratory based at the Coombe Women and Infants’ University Hospital (CWIUH), Dublin, Ireland in accordance with the HPV-AHEAD sectioning protocol [32] and using a Leica® RM2135 (Leica Biosystems, Wetzlar, Germany) [33] instrument. Briefly, for DNA analysis, five sections were cut in order from S1 to S5. S1 and S5 were cut for H+E slides (Leica® Bond Plus charged slides (Leica Biosystems, Wetzlar, Germany) [34]) at 5 μm to confirm the presence of appropriate tumor tissue for all sections used for molecular testing. S2, S3, and S4 were cut for HPV DNA analysis at 10 μm and placed in a 1.5 mL DNase/RNase-free 1.5 mL Micro tube (Sarstedt, Wexford, Ireland) [35]. To minimize the risk of cross-contamination during sectioning, the microtome was cleaned extensively between each FFPE block using ethanol 70% and DNA ZAPTM (ThermoFisherTM Scientific, Waltham, MA, USA) [36]. A new blade was used for each block and after 10 tumor tissue blocks were cut, sections were generated from an empty paraffin block and a known HPV DNA-positive block comprised of SiHa or HeLa cells. These were all included in the DNA analyses.
2.3. Histological Analysis
Two H+E slides were generated for every block, resulting in 2230 slides from the original 1115 FFPE blocks retrieved. These were all analyzed by a Pathology Review Board (PRB) comprised of 6 pathologists based in St. James’ University Hospital, Dublin, Ireland, and the CWIUH, Dublin, Ireland. A subset of 20% of cases was reviewed a second time by another member of the Pathology Review Board to confirm diagnosis, with little to no divergence in assessment. Only cases with relevant tumor tissue in both associated H+Es were brought forward for molecular testing, resulting in 861 cases ultimately used in the study.
2.4. HPV DNA Genotyping
At the Trinity College Dublin CERVIVA Molecular Pathology Laboratory at the CWIUH, HPV DNA was extracted from pooled sections S2 to S4 using a 250 μL of digestion buffer (10 mM Tris/HCl pH 7.4, proteinase K 0.5mg/mL, and Tween 20 0.4%) and a 2 h incubation at 56 °C. Water samples were also included to signal contamination at any stage of DNA preparation.
HPV DNA was detected by a type-specific multiplex genotyping assay developed previously [37] and based in IARC, Lyon, France. This method combined Multiplex PCR and bead-based Luminex Technology (Luminex Corp, Austin, TX, USA) [38,39]. The Multiplex PCR uses HPV type-specific primers targeting the E7 region of 21 genotypes. A total of 19 of these are high-risk (HR) or possible HR (pHR) and include HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68a, 68b, 70, 73, and 82. The remaining 2 are low-risk types HPV6 and 11. Detection limits of the PCR range from 10 to 1000 copies per reaction. To control for quality of the template DNA, two primers for amplification of the beta-globin were included. The slight modification of the protocol described previously [32,40] for the amplification of shorter (~100 bp) fragments for ten HPV genotypes (16, 18, 31, 33, 35, 52, 56, 66, 6, 11) and 117 bp for β-globin were applied.
Post PCR, 10 μL of each reaction mixture was analyzed by Multiplex HPV genotyping using Luminex xMAP® technology (Luminex Corp, Austin, TX, USA) [38,39] as described previously [41,42]. The aforementioned empty paraffin blocks, known positive blocks, and water samples were analyzed alongside all specimens blindly, with no evidence of contamination at any stage.
2.5. Statistical Analysis
Independent variables, survival, and treatment data were provided for each patient case by the NCRI including all those in Table 1, Table 2 and Table 3. The NCRI provided anonymized study numbers to the researcher that linked all HPV analyses the researcher performed to the associated characteristics in the national database. These variables were then used to compare and contrast survival, prognosis, and treatment administered between HPV-related and HPV-unrelated groups.

Table 1.
Independent variables made available by the NCRI for the population of the study and notes on any adjustments made for the purposes of the analysis.

Table 2.
Variables regarding patient treatment provided by the NCRI. These variables were used individually and in combination with one another for the analysis.

Table 3.
Variables regarding patient survival provided by the NCRI.
Statistics were generated using IBM SPSS Statistics Version 25 (IBM, Chicago, IL, USA), XLSTAT 2019.1.3, and Microsoft Excel Version 16.25. Overall and cancer-specific survival for all oropharyngeal, oral cavity, and laryngeal cancer, and within each sub-site, based on HR HPV status was assessed by Kaplan–Meier curves and log-rank test. Additional Cox proportional hazard statistics were generated to confirm Kaplan–Meier results. The relationship between treatment and HPV status for all cases and for each sub-site was evaluated using chi-square statistics and Fisher’s exact tests in cases where expected counts fell below 5. The cohort of patients assessed for treatment was limited to those who received treatment of any kind within 12 months of diagnosis as these were patients of interest. Predictors of overall and cancer-specific survival were evaluated individually by univariable Cox proportional hazard models. For variables from which more than 10% of data were missing, “missing” was included as a category of its own as is convention in the literature to account for or detect any bias responsible for significance. For those variables with between 0% to 10% missing data, cases with missing data were excluded for univariable and multivariable analyses. Those variables significant in univariable analysis were brought forward for multivariable analysis. All significant variables by univariable models were included in the initial multivariable model. The least significant predictor was then taken out, and the model was run again. The least significant predictor was again taken out, and the model was run again. This continued until all variables remaining in the model proved significantly predictive of survival and risk of death, or until taking another variable out rendered the model as a whole insignificant.
3. Results
3.1. Summary of the Study Population
To contextualize the study population, its basic demographic characteristics are presented in Table 4 below.

Table 4.
Summary of the study population.
A basic summary of the number of HPV positive and negative cases by sub-site is provided below in Table 5.

Table 5.
HPV DNA prevalence for oropharyngeal, oral cavity, and laryngeal cancer diagnosed in Ireland between 1994 and 2013.
3.2. Survival by HPV DNA Status
Figure 1 and Figure 2 show the result of the Kaplan–Meier analysis of overall and cancer-specific survival for the population stratified by HPV status, respectively. There was significantly worse survival for the HPV-negative group than for the HPV-positive group (Log-rank: Chi-square = 12.593, 1 d.f., p < 0.0001). Cox proportional hazard model for HPV status and overall survival confirmed the increased risk of death for HPV-negative patients (HR = 0.372, 1 d.f., p < 0.0001). Figure 2 mirrors findings in Figure 1, showing better survival for HPV-positive cases than HPV-negative cases (Log-rank: Chi-square = 4.582, d.f. = 1, p = 0.032). Cox proportional hazard model seconded the significantly increased risk of cancer-specific death in the HPV-negative group (HR = 0.257, SE = 0.122, p = 0.035).
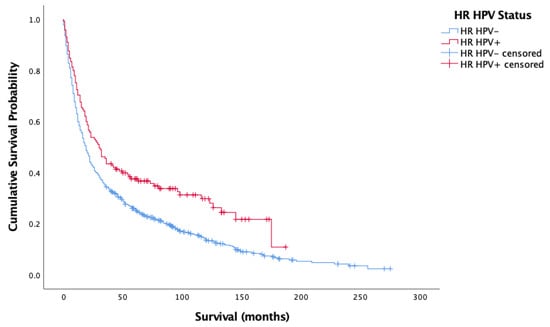
Figure 1.
Kaplan–Meier analysis of overall survival in months based on HR HPV status for oropharyngeal, oral cavity, and laryngeal cancer (n = 861).
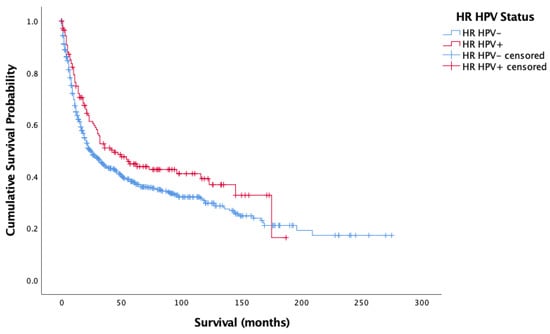
Figure 2.
Kaplan–Meier analysis of disease-specific (cancer-specific) survival in months based on HR HPV status for oropharyngeal, oral cavity, and laryngeal cancer (n = 861).
Figure 3 and Figure 4 showcase the Kaplan–Meier analysis of overall and cancer-specific survival stratified by HPV status for the oropharyngeal sub-site alone, respectively. Much like for all cases, there was significantly worse prognosis for HPV-negative cases (Overall-Log-rank: Chi-square = 17.017, 1 d.f., p < 0.0001; Cancer-specific-Log-rank: Chi-square 11.902, 1 d.f., p = 0.001). Cox proportional hazard model confirmed this finding (HR = 0.659, SE = 0.165, p < 0.0001; HR = 00.620, SE = 0.185, p = 0.001).
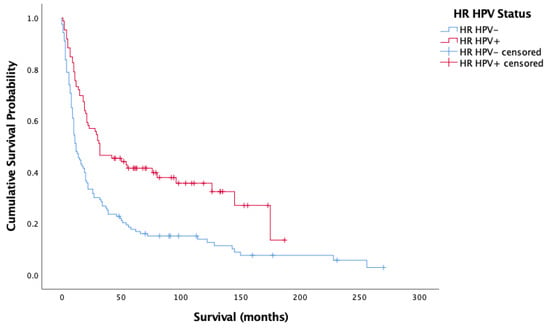
Figure 3.
Kaplan–Meier analysis of overall survival in months based on HR HPV status for oropharyngeal cancer (n = 209).
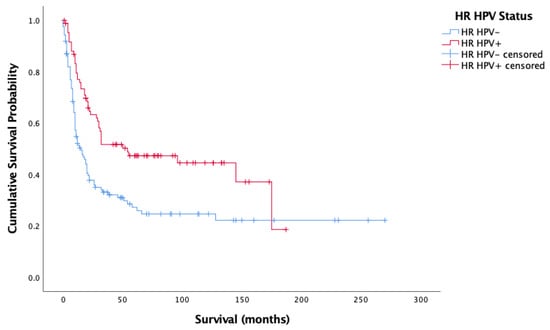
Figure 4.
Kaplan–Meier analysis of disease-specific (cancer-specific) survival in months based on HR HPV status for oropharyngeal cancer (n = 209).
No significant differences in survival by HPV status were noted for LSCC or OSCC sub-sites alone.
3.3. The Relationship between Treatment Modality and HPV Status
There was significant relationship between HPV status and treatment modality administered (Chi-square = 49.732, 4 d.f., p < 0.0001). HPV-positive patients were almost twice as likely to be treated more aggressively with all three treatment modalities (surgery/radiotherapy/chemotherapy) than HPV-negative cases. More HPV-negative patients were treated with surgery or radiotherapy alone and almost three times as many HPV-positive patients were treated chemically with radiotherapy/chemotherapy than HPV-negative patients. These patterns were mimicked within the population of oropharyngeal patients alone (chi-square = 14.401, 4 d.f., p = 0.006). No associations were seen within oral cavity cases alone (chi-square = 7.837, 4 d.f., p = 0.098) but were within the laryngeal case population alone (Fisher’s exact = 12.423, p = 0.007). These differences emanated from the disproportionate treatment of HPV-negative cases with radiotherapy alone, and the more frequent treatment of HPV-positive patients with all three modalities.
3.4. The Relationship between HPV Status, Treatment Modality, and Survival
Given the significant relationship between HPV status and treatment modality, and HPV status and survival only for OPSCC, the relationship between all three variables for OPSCC alone is presented. For HPV-positive cases in the oropharynx, there was a significant difference in overall survival by treatment type (Log-rank = 10.481, 4 d.f., p = 0.033) (n = 80) (Figure 5). Cox proportional hazard model reflected this significance for all treatments (HR = 1.166, 0.579, −0.055, 0.096, SE = 0.460, 0.443, 0.803, 0.559, p = 0.049, 0.011, 0.192, 0.946, 0.864). Surgery alone and all three treatment modalities maximized overall survival for HPV-positive OPSCC patients. Treatment with radiotherapy alone was associated with decreased survival. For cancer-specific survival amongst HPV-positive oropharyngeal cases, there was a significant difference by treatment type (Log-rank = 11.398, 4 d.f., p = 0.022). Figure 6 showcases this difference. Indeed, this significance was reflected by Cox proportional hazard model, with surgery having the lowest risk of death, followed by surgery/radiotherapy and surgery/radiotherapy/chemotherapy, followed by radiotherapy/chemotherapy, with radiotherapy having the worst risk of death (HR = 1.186, 0.490, −0.567, −0.137, SE = 0.496, 0.484, 1.082, 0.649, p = 0.039, 0.017, 0.312, 0.600, 0.833).
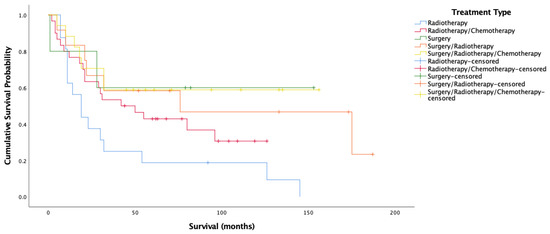
Figure 5.
Kaplan–Meier analysis for overall survival amongst HPV-positive oropharyngeal cancer stratified by treatment type (n = 80).
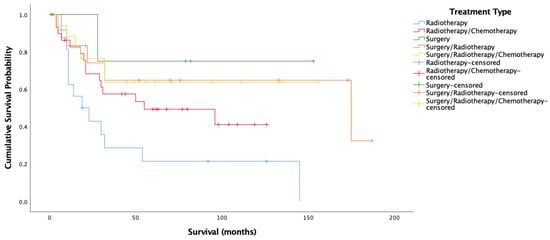
Figure 6.
Kaplan–Meier analysis for cancer-specific survival amongst HPV-positive oropharyngeal cancer stratified by treatment type (n = 80).
For HPV-negative oropharyngeal cases, there was no difference in overall or cancer-specific survival by treatment type, something also reflected by the Cox proportional hazard model.
3.5. Modeling Predictors of Survival
Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11 summarize the variables significantly predicting overall and cancer-specific survival, respectively, for OPSCC, OSCC, and LSCC populations.

Table 6.
Patient and tumor characteristics significantly predicting overall survival amongst oropharyngeal cancer patients by multivariable Cox proportional hazard model (n = 189).

Table 7.
Patient and tumor characteristics significantly predicting disease-specific (cancer-specific) survival amongst oropharyngeal cancer patients by multivariable Cox proportional hazard model (n = 209).

Table 8.
Patient and tumor characteristics significantly predicting overall survival amongst oral cavity cancer patients by multivariable Cox proportional hazard model (n = 282).

Table 9.
Patient and tumor characteristics significantly predicting disease-specific (cancer-specific) survival amongst oral cavity cancer patients by multivariable Cox proportional hazard model. The initial model included all those variables significant by univariable.

Table 10.
Patient and tumor characteristics significantly predicting overall survival amongst laryngeal cancer patients by multivariable Cox proportional hazard model (n = 306).

Table 11.
Patient and tumor characteristics significantly predicting disease-specific (cancer-specific) survival amongst laryngeal cancer patients by multivariable Cox proportional hazard model. The initial model included all significant variables by univariable.
4. Discussion
The key finding of the present analysis was that both overall and cancer-specific survival were significantly improved for HPV-positive cases in all oropharyngeal, oral cavity, and laryngeal SCC grouped together. This relationship emanated, however, from the OPSCC population alone. This is reflective of most studies in the literature [14,30,43]. This is likely given that HPV-related patients, being younger, are less likely to have had significant exposure to tobacco, marijuana, alcohol, diabetes, chronic obstructive pulmonary disease, anxiety disorders, and major depression. The most at-risk populations are thus those with the best immune ability to combat HPV-related disease. Furthermore, the current results support the notion that the viral origins of HPV-positive tumors, accompanied by their expression of viral oncoproteins and related HPV-positive tumor antigens at sites of huge immune and lymphatic activity likely attracts a more aggressive and specific immune response that improves both overall and cancer-specific survival [44,45]. Younger patients are also more likely to better survive harsh treatments and their potential side effects, which is particularly important in the case of HPV-positive tumors in this population given that they were more likely to be treated harshly than HPV-negative patients.
Figure 5 and Figure 6 show very clearly that survival amongst HPV-positive OPSCC patients was longest amongst those treated with surgery alone. Figure 6 shows over 70% cancer-specific survival rates after 10 years for patients treated with surgery alone, with those treated by all three modalities and surgery/radiotherapy following closely behind.
These results highlight the importance of surgical intervention for HPV-positive OPSCC, with treatment approaches not involving surgery seeing very poor survival. The findings are extremely promising in terms of the potential for de-escalation of treatment for these patients. This is indicative of the chance that HPV-related OPSCC presents for drastically improving the quality of life for patients by avoiding the administration of extremely harsh treatments and the long-term side effects that accompany them including difficulties swallowing, breathing, and speaking, chronic pain, osteoradionecrosis, hypertension, pneumonia, dysphagia, weight loss, malnutrition, dental issues, and third-degree burns [46,47] along with the increased risk of heart disease and failure [48,49], risk of another (non-recurrence) primary tumor at another site [50,51,52,53], and complications due to immunosuppression.
The present data thus supports the notion that robotic trans-oral resection (TOR) alone yields extremely good results for HPV-related patients [54,55,56] regardless of stage and posits that this kind of non-chemical curative approach gives patients better functional outcomes [57,58,59] may be the way forward. Other studies are in agreement that TOR without adjuvant therapy is often an adequate treatment for HPV-related OPSCC, with anywhere between 48% to 74% of patients not requiring chemotherapy after TORs [54,55,60]. This said, it is understandable that patients may feel more comfortable being treated with more than just surgery, with studies showing that nearly 70% of patients are not willing to risk a 5% or less drop in survival likelihood to switch from chemoradiation to radiation alone after surgery [61]. In the present population, this 5% drop in survival is not evident amongst HPV-positive OPSCC patients, with surgery alone seeing better survival than surgery/radiotherapy, and the margin between surgery/radiotherapy and all three treatments being minimal (Figure 6). This is something that may give patients more incentive to opt for less harsh schemes. Nonetheless, many trials currently underway are based on the suggestion that surgery with de-escalated radiotherapy yields maximal survival with decreased morbidity and associated side effects [62,63,64], a scheme that might satisfy survival outcomes, minimize side effects, and ensure patient peace of mind simultaneously.
Despite positive indications of de-escalation potential in HPV-positive OPSCC, the present analysis indicates that HPV-positive HNSCC and OPSCC were more likely to be treated harshly than their HPV-negative counterparts. The population that might have benefited most from less severe treatment schemes was thus the population being treated most severely. The present data and the literature explain that this irony is due to the later stage at which HPV-related OPSCC is diagnosed [9,43,65,66]. Specifically, they disproportionately present at Stage IV due to the late N stage according to the 5th edition AJCC guidelines relevant to this population between 1994 and 2013 [67]. The current analysis posits therefore that the new 2017 8th edition AJCC guidelines [68] updated for the oropharyngeal sub-site alone, reflecting the role of HR HPV, are very highly relevant. This is especially true since neither the N stage nor the TNM stage were significant predictors of overall survival in OPSCC. TNM stage was barely significant in predicting survival in OPSCC at the cancer-specific level (Table 6), and the N stage remained insignificant. This implies that the nodal and cumulative staging of the older staging systems were not accurate assessors of the aggressivity of these tumors, likely due to the unique features of HPV-related tumors in this region. Those HPV-related cases diagnosed as Stage IV before 2017 will now be downgraded to at least Stage III if not even Stage I due to adjustments in the N stage relating to nodal metastasis. It is very likely that the consequent down-grading of the stage in OPSCC will act as a de-escalation mechanism of its own, implicating less severe treatment requirements from the moment the cancer is diagnosed. On the basis of pending results of current trials [64], the clinical context may need to adapt.
To note is that it is increasingly recognized that HPV-negative oropharyngeal cancer represents a treatment-resistant entity and a distinct therapeutic challenge. Comparison among treatment modalities for oropharyngeal cancer has been limited, and very few studies have evaluated differences based on the HPV subtype. Our findings that HPV-negative cases were treated less aggressively than HPV-positive tumors may in fact just be reflective of clinical practices at that time, or indeed may be related to the patient demographic where smoking and alcohol use may play a role in treatment responses. Further prospective studies into outcomes for HPV-negative tumors are warranted.
Two caveats to this study’s support of de-escalation should be noted. First, there were smaller sample sizes available when sub-dividing all 861 cases into their sub-site, HPV status, and treatment groups. Targeted sampling of OPSCC alone is needed for further confirmation of these promising findings. Second, the analysis also emphasizes that in terms of potential de-escalation, it would be unethical to make treatment decisions for these patients, or their negative counterparts, based solely on HPV status. For oropharyngeal, oral cavity, and laryngeal SCC, multivariable predictors of the overall risk of death did include HPV negativity, but HPV status was not confounded by other patient characteristics including older age and current smoker status (Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11). For OPSCC, HPV positivity was predictive of decreased risk of death at the overall and cancer-specific levels (Table 6 and Table 7). However, HPV was not confounded by age or social deprivation. It also did not predict the risk of death for any survival in LSCC and OPSCC (Table 8, Table 9, Table 10 and Table 11).
The findings indicate that the oropharynx is the sub-site in which HPV-related tumors occur and that it is therefore the region for which any HPV-related treatment alterations should be made. They also highlight that though HPV-related tumors are already significantly associated with younger aged patients [43,64,65] and never-/ex-smokers [69,70], it would be extremely prudent to select patients who might benefit from de-escalation based on not only HPV-positive status but also on other survival-maximizing characteristics at both the cancer-specific and overall levels. Table 12 summarizes those patient characteristics that the present multivariable analysis indicates as being stereotypically HPV-driven cases and might benefit from de-escalated treatment.

Table 12.
Patient characteristics indicative of stereotypically HPV-driven oropharyngeal, oral cavity, and laryngeal SCC that may be the basis for the precise selection of patients for whom treatment de-escalation is possible.
This collection of patient characteristics has recently been recognized in the literature as the only group of oropharyngeal, oral cavity, and laryngeal SCC patients for which de-escalation of treatment is acceptable. In fact, several of the ongoing trials regarding de-escalation only include patients meeting these criteria to assure no jeopardizing of patient safety [62,63,64,71], but also to target the group that will likely benefit most from less severe treatment. This said, very recent publications from the largest de-escalation trials for these patients prove a cautionary tale. DE-ESCALATE and RTOG 1016 trials have thus far shown that omitting cisplatin or substituting it shows a detrimental impact on survival in HPV-positive OPSCC [72]. This said, several non-randomized phase II cohort studies attempting lower radiotherapy doses have shown promising results despite lacking control arms [62,73,74]. The details of the definition of “de-escalation” are thus still being clinically determined and much like these newly published studies suggest, no amendments to current treatment regimes for HPV-positive patients should be implemented in the clinic until this time [72].
With respect to Table 6 and Table 7, it should also be noted that there is still a need to distinguish clinically significant HR HPV infections from transient ones. While in this analysis HR HPV DNA was used to determine HPV-related status, many trials only use p16 as a representative biomarker of an active HPV infection [64]. Neither of these alone is entirely satisfactory in the clinical context given the potential for transient HR HPV infections, and the expression of p16 regardless of HPV status. In fact, HPV DNA may be misleading even if other patient characteristics are suggestive of a classically HPV-related case. In the clinic, these kinds of risks resulting in the potential under-treatment of patients cannot be taken. Further specification of “HPV positivity” as a necessary characteristic for de-escalation will likely make treatment decisions and thus survival determinations even more accurate. Pairing p16 with HR HPV DNA [32,75], or simply using HPV mRNA [76], represents mechanisms to refine this process in the clinic, though the present HR HPV DNA data are a resounding start.
Thus, this study population is further proof of the suggestion that HPV-positive oropharyngeal, oral cavity, and laryngeal SCC, and more specifically, OPSCC alone, is a better-surviving cancer than its HPV-negative counterparts. Furthermore, though more than simply surgery is and will continue to be necessary to treat some late-stage patients, the benefit of treating all HNSCC patients indiscriminately with all three modalities is questionable. The data also indicate that other indicators of clinically relevant HPV infections including patient characteristics will play a significant role in determining treatment options for oropharyngeal, oral cavity, and laryngeal SCC in addition to, and not instead of sensitive HR HPV detection.
Importantly, the diverse treatment, survival, and HPV characteristics observed in the present data converge and point to the crucial nature of prevention and early detection in oropharyngeal, oral cavity, and laryngeal SCC if survival, overall and cancer-specific, is to be maximized. All HNSCC are overwhelmingly behaviorally driven cancers, whether by exposure to HPV and/or to smoking (and likely alcohol).
For OPSCC specifically, data are still emerging on the impact of the quadra- and nona-valent Gardasil vaccines on HR HPV prevalence in the oral cavity [77], though preliminary data from cervical trials testing oral rinses show that HPV16/HPV18 prevalence is lower in vaccinated groups compared to control groups, with an estimated efficacy of 93.3% for HPV16/18 [78]. Predictive modeling studies also suggest that with a 50% vaccination uptake and 50% vaccine efficacy, the vaccination of young boys for the prevention of HPV-related OPSCC would be cost-effective [77,79]. The need for more data is evident, but the systemic nature of vaccines logically suggests that the administration of the vaccine in early adolescence should be as effective in preventing HNSCC as it is in the cervical context. The FDA has recently approved the indication of Gardasil-9 to include the prevention of oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58, and while in Europe, Gardasil-9 is yet licensed this indicates that many governments including Ireland have now expanded the public HPV vaccination scheme to include boys is, therefore, encouraging as an HNSCC prevention strategy.
Lastly, the present analysis underlines the urgent need for effective and systematic HNC screening tools. Early detection of those SCC that do go on to develop despite preventative measures is tantamount to prolonging overall survival, no matter how promising or poor cancer-specific survival is and regardless of HPV status. For HPV-unrelated HNSCC in this analysis, especially in the larynx, diagnosis at a later TNM stage was the only predictor of cancer-specific survival after adjustment for other variables (Table 11). Efforts are currently being made to investigate the best ways to sample tissue from the oral site, but it is made difficult by the region’s confined nature and the dense, complex network of MALT tissues that line it [80,81]. Mobile microscopy with a simple brush biopsy has shown to be an effective screening mechanism for oral cavity cancer, even in low-resource areas [82], but such a sampling method is not ideal for the deep, hidden, crypts of the oropharynx. The role that HPV might play in this screening is also uncertain, though monitoring systems such as those established in the cervical case [83,84] are a promising way of catching HR HPV patients who, perhaps even after vaccination, go on to develop lesions.
5. Conclusions
In all, pairing early detection with preventative mechanisms and curative approaches suitable to the tumor and patient characteristics will render oropharyngeal, oral cavity, and laryngeal SCC an imminently manageable and rare disease. These public health and clinical measures will ultimately mean huge cost savings, and more importantly, the difference between life and death for potential and current oropharyngeal, oral cavity, and laryngeal SCC patients.
Author Contributions
Conceptualization, E.O. (Eamon O’Murchu), M.T. (Mary Toner), C.O., E.O. (Eamon O’Murchu), S.O., L.S., C.M.M. and J.J.O.; data curation, I.S.O., E.O. (Eamon O’Murchu) and R.W.; formal analysis, I.S.O., E.O., M.T. (Mary Toner), R.O. (Esther O’Regan), D.M. and M.N.; funding acquisition, E.O. (Esther O’Regan), E.O. (Eamon O’Murchu), S.O., L.S., C.M.M. and J.J.O.; investigation, I.S.O. and P.T.; methodology, I.S.O., E.O. (Eamon O’Murchu), L.S. and C.M.M.; project administration, I.S.O., E.O. (Eamon O’Murchu) and C.M.M.; resources, I.S.O., E.O. (Esther O’Regan), M.T. (Mary Toner), E.K., P.F., J.B.-O., N.K., H.K., S.K., K.F., T.G., M.T. (Massimo Tommasino), C.M.M. and J.J.O.; supervision, C.M.M. and J.J.O.; validation, P.T., H.K. and R.W.; visualization, I.S.O.; writing—original draft, I.S.O.; writing—review and editing, I.S.O., E.O. (Esther O’Regan), C.O., P.T., L.S., T.G., M.T. (Massimo Tommasino), J.J.O. and C.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Research Board (Ireland), grant number CARG/2012/29, and the primary author (ISO) PhD studentship was funded by the Coombe Women and Infants University Hospital (Ireland), grant number: 205566.
Institutional Review Board Statement
The study was ethically approved by 11 Hospital Research Ethics Committees in 2015, including Beaumont University Hospital (approved 2 July 2015), Cork University Hospital (approved 29 May 2015), Cork Dental Hospital (approved 29 May 2015), Dublin Dental Hospital (approved 29 May 2015), Kerry General Hospital (29 May 2015), Mater Misericordiae University Hospital (approved 9 December 2015), Midlands Regional Hospital (approved 15 September 2015), Sligo General Hospital (24 August 2015), South Infirmary Victoria University Hospital (approved 29 May 2015), St. James’ University Hospital (26 May 2015), St. Vincent’s University Hospital (approved 25 February 2016), Royal Eye and Ear Hospital (approved 8 July 2016), University Hospital Limerick (approved 14 May 2015), and Waterford Regional Hospital (approved 23 June 2015).
Informed Consent Statement
The study was conducted in compliance with the HSE National Consent Policy of May 2013. Informed consent was obtained from all live subjects whose FFPE tissue was involved in the study. For those who were no longer alive, consent was needed and obtained from the managing pathologist in the relevant hospital. Written informed consent was obtained from the live patient(s) to publish research yielded from their specimens.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Thank you to all those named as authors, administrative staff in all participating hospitals who facilitated so much of the process, and managers of all the hospital warehouses that the authors sifted through to make this study possible.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible of the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
References
- Goldberg, H.I.; Lockwood, S.A.; Crossett, L.S.; Wyatt, S.W. Trends and differentials in mortality from cancers of the oral cavity and pharynx in the United States, 1973–1987. Cancer 1994, 74, 565–572. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8033034 (accessed on 7 February 2017). [CrossRef]
- Thomas, G.R.; Nadiminti, H.; Regalado, J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int. J. Exp. Pathol. 2005, 86, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Hafkamp, H.C.; Manni, J.; Haesevoets, A.; Voogd, A.; Schepers, M.; Bot, F.; Hopman, A.; Ramaekers, F.; Speel, E.-J.M. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer 2008, 122, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Krane, J.F. Role of cytology in the diagnosis and management of HPV-associated head and neck carcinoma. Acta Cytol. 2013, 57, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. JNCI J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Forastiere, A.A.; Rocco, J.W. Implications of the oropharyngeal cancer epidemic. J. Clin. Oncol. 2011, 29, 4222–4223. [Google Scholar] [CrossRef]
- Thompson, L.D.; Heffner, D.K. The clinical importance of cystic squamous cell carcinomas in the neck: A study of 136 cases. Cancer 1998, 82, 944–956. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9486586 (accessed on 7 February 2017). [CrossRef]
- Joo, Y.-H.; Jung, C.-K.; Sun, D.-I.; Park, J.-O.; Cho, K.-J.; Kim, M.-S. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck 2011, 34, 10–14. [Google Scholar] [CrossRef]
- Psyrri, A.; Sasaki, C.; Vassilakopoulou, M.; Dimitriadis, G.; Rampias, T. Future directions in research, treatment and prevention of HPV-related squamous cell carcinoma of the head and neck. Head Neck Pathol. 2012, 6 (Suppl. S1), 121–128. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Su, C.-Y.; Fang, F.-M.; Huang, H.-Y.; Chuang, H.-C.; Chen, C.-M. Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol. 2008, 44, 174–179. [Google Scholar] [CrossRef]
- Fischer, C.A.; Kampmann, M.; Zlobec, I.; Green, E.; Tornillo, L.; Lugli, A.; Wolfensberger, M.; Terracciano, L.M. p16 expression in oropharyngeal cancer: Its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann. Oncol. 2010, 21, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kimple, R.J.; Harari, P.M. The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy. Ann. Transl. Med. 2015, 3 (Suppl. S1), S14. [Google Scholar] [CrossRef]
- Nichols, A.C.; Dhaliwal, S.S.; Palma, D.A.; Basmaji, J.; Chapeskie, C.; Dowthwaite, S.; Franklin, J.H.; Fung, K.; Kwan, K.; Wehrli, B.; et al. Does HPV type affect outcome in oropharyngeal cancer? J. Otolaryngol. Head Neck Surg. 2013, 42, 9. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Gillison, M.L. HPV and prognosis for patients with oropharynx cancer. Eur. J. Cancer 2009, 45, 383–385. [Google Scholar] [CrossRef]
- Lassen, P.; Eriksen, J.G.; Krogdahl, A.; Therkildsen, M.H.; Ulhøi, B.P.; Overgaard, M.; Specht, L.; Andersen, E.; Johansen, J.; Neck Cancer Group (DAHANCA); et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: Evaluation of the randomised DAHANCA 6 & 7 trial. Radiother. Oncol. 2011, 100, 49–55. [Google Scholar] [CrossRef]
- Posner, M.R.; Lorch, J.H.; Goloubeva, O.; Tan, M.; Schumaker, L.M.; Sarlis, N.J.; Haddad, R.I.; Cullen, K.J. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann. Oncol. 2011, 22, 1071–1077. [Google Scholar] [CrossRef]
- Rischin, D.; Young, R.J.; Fisher, R.; Fox, S.B.; Le, Q.-T.; Peters, L.J.; Solomon, B.; Choi, J.; O’Sullivan, B.; Kenny, L.M.; et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III Trial. J. Clin. Oncol. 2010, 28, 4142–4148. [Google Scholar] [CrossRef]
- Shaw, R.; Robinson, M. The increasing clinical relevance of human papillomavirus type 16 (HPV-16) infection in oropharyngeal cancer. Br. J. Oral Maxillofac. Surg. 2011, 49, 423–429. [Google Scholar] [CrossRef]
- Albers, A.E.; Qian, X.; Kaufmann, A.M.; Coordes, A. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci. Rep. 2017, 7, 16715. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Cordell, K.G.; Lee, J.S.; Worden, F.P.; Prince, M.E.; Tran, H.H.; Wolf, G.T.; Urba, S.G.; Chepeha, D.B.; Teknos, T.N.; et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 2008, 26, 3128–3137. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Anderson, K.S.; Cheng, J.N.; Chowell, D.; Li, G.; Posner, M.; Sturgis, E.M. HPV serum antibodies as predictors of survival and disease progression in patients with HPV-positive squamous cell carcinoma of the oropharynx. Clin. Cancer Res. 2015, 21, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; Szyfter, K.; Milecki, P.; Składowski, K.; Ramlau, R. The rationale for HPV-related oropharyngeal cancer de-escalation treatment strategies. Contemp. Oncol. 2015, 4, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Lazim, A.F.; Walsh, F.J.; Foote, R.L.; Moore, E.J.; Okuno, S.H.; Olsen, K.D.; Kasperbauer, J.L.; Price, D.L.; Garces, Y.I.; et al. Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol. 2014, 50, 311–318. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. JNCI J. Natl. Cancer Inst. 2000, 92, 709–720. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10793107 (accessed on 7 February 2017). [CrossRef]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef]
- Rieckmann, T.; Tribius, S.; Grob, T.J.; Meyer, F.; Busch, C.-J.; Petersen, C.; Dikomey, E.; Kriegs, M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. 2013, 107, 242–246. [Google Scholar] [CrossRef]
- Vu, H.L.; Sikora, A.G.; Fu, S.; Kao, J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett. 2010, 288, 149–155. [Google Scholar] [CrossRef]
- Woods, R. Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma—Prevalence and Incidence in a Defined Population and Analysis of the Expression of Specific Cellular Biomarkers. Ph.D. Thesis, Trinity College Dublin, Dublin, Ireland, 2016. [Google Scholar]
- World Health Organization. ICD-10 Code Descriptions for All Disease Types; World Health Organization: Geneva, Switzerland, 2016; Available online: https://icd.who.int/browse10/2016/en (accessed on 27 May 2019).
- Gheit, T.; Anantharaman, D.; Holzinger, D.; Alemany, L.; Tous, S.; Lucas, E.; Prabhu, P.R.; Pawlita, M.; Ridder, R.; Rehm, S.; et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in central India. Int. J. Cancer 2017, 141, 143–151. [Google Scholar] [CrossRef]
- Leica Biosystems. 2019. Available online: https://www.leicabiosystems.com/ (accessed on 28 May 2019).
- Leica Biosystems. Leica Microsystems BOND Plus Slides. 2019. Available online: https://www.leicabiosystems.com/ihc-ish-fish/bond-ancillary-ihc-ish-reagents/consumables/products/leica-microsystems-plus-slides/ (accessed on 27 May 2019).
- Life Technologies. Life Technologies: ThermoFisher Scientific. 2019. Available online: https://www.thermofisher.com/ie/en/home/brands/life-technologies.html (accessed on 27 May 2019).
- ThermoFisher Scientific. DNAZap PCR DNA Degradation Solutions. 2019. Available online: https://www.thermofisher.com/order/catalog/product/AM9890?SID=srch-srp-AM9890 (accessed on 28 May 2019).
- Gheit, T.; Landi, S.; Gemignani, F.; Snijders, P.J.F.; Vaccarella, S.; Franceschi, S.; Canzian, F.; Tommasino, M. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J. Clin. Microbiol. 2006, 44, 2025–2031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- xMAP® Technology for Research & Applied Markets. 2017. Available online: https://www.luminexcorp.com/research/our-technology/xmap-technology/ (accessed on 22 August 2017).
- Affymetrix Panomics. Luminex Assays: How it Works. 2014. Available online: http://cdn.panomics.com/products/luninex-assays/technical-overview/how-it-works (accessed on 15 November 2017).
- Mena, M.; Lloveras, B.; Tous, S.; Bogers, J.; Maffini, F.; Gangane, N.; Kumar, R.V.; Somanathan, T.; Lucas, D.; H.-A. Study Group; et al. Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: The example from the HPV-AHEAD study. PLoS ONE 2017, 12, e0184520. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Bravo, I.G.; Snijders, P.J.F.; Gissmann, L.; Pawlita, M.; Waterboer, T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006, 44, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk Human Papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef]
- Woods, E.M.O.R.S.; Regan, S.K.; Kennedy, S.; Martin, C.; Leary, C.T.; Timon, C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J. Clin. Cases 2014, 2, 172–193. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2013, 110, 501–509. [Google Scholar] [CrossRef]
- Jung, A.C.; Guihard, S.; Krugell, S.; Ledrappier, S.; Brochot, A.; Dalstein, V.; Job, S.; de Reynies, A.; Noël, G.; Wasylyk, B.; et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int. J. Cancer 2012, 132, E26–E36. [Google Scholar] [CrossRef]
- Cancer.Net. Side Effects of Radiation Therapy. ASCO. 2019. Available online: https://www.cancer.net/navigating-cancer-care/how-cancer-treated/radiation-therapy/side-effects-radiation-therapy (accessed on 4 July 2019).
- Tolentino, E.; Centurion, B.S.; Ferreira, L.H.C.; De Souza, A.P.; Damante, J.H.; Rubira-Bullen, I.R. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [CrossRef]
- Lyon, A.R. Heart failure resulting from cancer treatment: Still serious but an opportunity for prevention. Heart 2018, 105, 6–8. [Google Scholar] [CrossRef]
- Aleman, B.M.P.; Moser, E.C.; Nuver, J.; Suter, T.M.; Maraldo, M.V.; Specht, L.; Vrieling, C.; Darby, S.C. Cardiovascular disease after cancer therapy. Eur. J. Cancer Suppl. 2014, 12, 18–28. [Google Scholar] [CrossRef]
- Atienza, J.A.S.; Dasanu, C.A. Incidence of second primary malignancies in patients with treated head and neck cancer: A comprehensive review of literature. Curr. Med. Res. Opin. 2012, 28, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-C.; Scelo, G.; Tonita, J.M.; Tamaro, S.; Jonasson, J.G.; Kliewer, E.V.; Hemminki, K.; Weiderpass, E.; Pukkala, E.; Tracey, E.; et al. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int. J. Cancer 2008, 123, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Sermaxhaj, B.; Bojaxhiu, B.; Shelan, M.; Giger, R.; Rauch, D.; Aebersold, D.M. Incidence of second primary cancers after radiotherapy combined with platinum and/or cetuximab in head and neck cancer patients. Strahlenther. Onkol. 2018, 195, 468–474. [Google Scholar] [CrossRef]
- Wong, S.J.; Heron, D.E.; Stenson, K.; Ling, D.C.; Vargo, J.A. Locoregional recurrent or second primary head and neck cancer: Management strategies and challenges. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e284–e292. [Google Scholar] [CrossRef] [PubMed]
- White, H.N.; Moore, E.J.; Rosenthal, E.L.; Carroll, W.R.; Olsen, K.D.; Desmond, R.A.; Magnuson, J.S. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1248–1252. [Google Scholar] [CrossRef][Green Version]
- Iseli, T.; Kulbersh, B.D.; Iseli, C.E.; Carroll, W.R.; Rosenthal, E.L.; Magnuson, J.S. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol. Neck Surg. 2009, 141, 166–171. [Google Scholar] [CrossRef]
- Genden, E.M. The role for surgical management of HPV-related oropharyngeal carcinoma. Head Neck Pathol. 2012, 6, 98–103. [Google Scholar] [CrossRef]
- Golusiński, W. Functional organ preservation surgery in head and neck cancer: Transoral robotic surgery and beyond. Front. Oncol. 2019, 9, 293. [Google Scholar] [CrossRef]
- Mahmoud, O.; Sung, K.; Civantos, F.J.; Thomas, G.R.; Samuels, M.A. Transoral robotic surgery for oropharyngeal squamous cell carcinoma in the era of human papillomavirus. Head Neck 2017, 40, 710–721. [Google Scholar] [CrossRef]
- Weinstein, G.S.; Quon, H.; Newman, H.J.; Chalian, J.A.; Malloy, K.; Lin, A.; Desai, A.; Livolsi, V.A.; Montone, K.T.; Cohen, K.R.; et al. Transoral robotic surgery alone for oropharyngeal cancer. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 628–634. [Google Scholar] [CrossRef]
- Genden, E.M.; Kotz, T.; Ms, C.C.L.T.; Smith, C.; Sikora, A.G.; Teng, M.S.; Packer, S.H.; Lawson, W.L.; Kao, J. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope 2011, 121, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Brotherston, D.C.; Poon, I.; Le, T.; Leung, M.; Kiss, A.; Ringash, J.; Balogh, J.; Lee, J.; Wright, J.R. Patient preferences for oropharyngeal cancer treatment de-escalation. Head Neck 2012, 35, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Felix, C.; Wang, P.-C.; Hsu, S.; Basehart, V.; Garst, J.; Beron, P.; Wong, D.; Rosove, M.H.; Rao, S.; et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017, 18, 803–811. [Google Scholar] [CrossRef]
- Villaflor, V.; Melotek, J.; Karrison, T.; Brisson, R.; Blair, E.; Portugal, L.; De Souza, J.; Ginat, D.; Stenson, K.; Langerman, A.; et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann. Oncol. 2016, 27, 908–913. [Google Scholar] [CrossRef]
- Mirghani, H.; Blanchard, P. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin. Transl. Radiat. Oncol. 2017, 8, 4–11. [Google Scholar] [CrossRef]
- Smith, E.M.; Ritchie, J.M.; Summersgill, K.F.; Klussmann, J.P.; Lee, J.H.; Wang, D.; Haugen, T.H.; Turek, L.P. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int. J. Cancer 2003, 108, 766–772. [Google Scholar] [CrossRef]
- Husain, N.; Neyaz, A. Human papillomavirus associated head and neck squamous cell carcinoma: Controversies and new concepts. J. Oral Biol. Craniofacial Res. 2017, 7, 198–205. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer and American Cancer Society. Head and neck sites. In AJCC Cancer Staging Manual, 5th ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1997; pp. 24–59. Available online: https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC5thEdCancerStagingManual.pdf (accessed on 14 April 2019).
- American Joint Committee on Cancer and American Cancer Society. AJCC Cancer Staging Manual, 8th ed.; Springer Publishing: New York, NY, USA, 2016; Available online: https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx (accessed on 14 April 2019).
- Boscolo-Rizzo, P.; Del Mistro, A.; Bussu, F.; Lupato, V.; Baboci, L.; Almadori, G.; Da Mosto, M.; Paludetti, G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 77–87. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23853396 (accessed on 3 February 2017).
- D’Souza, G.; McNeel, T.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef]
- Brisson, R.J.; Kochanny, S.; Foster, C.C.; Vokes, E.E.; Haraf, D.J.; Seiwert, T.Y. De-escalation in HPV-negative locally advanced head and neck squamous cell cancer (LA-HNSCC) in patients after induction chemotherapy: A retrospective case series. J. Clin. Oncol. 2018, 36 (Suppl. S15), e18090. [Google Scholar] [CrossRef]
- Mehanna, H.; Rischin, D.; Wong, S.J.; Gregoire, V.; Ferris, R.; Waldron, J.; Le, Q.-T.; Forster, M.; Gillison, M.; Laskar, S.; et al. De-escalation after De-ESCALATE and RTOG 1016: A head and neck cancer InterGroup framework for future de-escalation studies. J. Clin. Oncol. 2020, 38, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN cancer research group. J. Clin. Oncol. 2017, 35, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.; Foster, C.; Blair, E.; Karrison, T.; Agrawal, N.; Melotek, J.; Portugal, L.; Brisson, R.; Dekker, A.; Kochanny, S.; et al. OPTIMA: A phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Psyrri, A.; Mesía, R.; Peyrade, F.; Beier, F.; de Blas, B.; Celik, I.; Licitra, L. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: Retrospective analysis of the phase III EXTREME trial. Ann. Oncol. 2014, 25, 801–807. [Google Scholar] [CrossRef]
- Jung, A.C.; Briolat, J.; Millon, R.; de Reyniès, A.; Rickman, D.; Thomas, E.; Abecassis, J.; Clavel, C.; Wasylyk, B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int. J. Cancer 2009, 126, 1882–1894. [Google Scholar] [CrossRef]
- Guo, T.; Eisele, D.W.; Fakhry, C. The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer 2016, 122, 2313–2323. [Google Scholar] [CrossRef]
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; CVT Vaccine Group; et al. Reduced prevalence of oral Human Papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in costa Rica. PLoS ONE 2013, 8, e68329. [Google Scholar] [CrossRef]
- Graham, D.M.; Isaranuwatchai, W.; Habbous, S.; de Oliveira, C.; Liu, G.; Siu, L.L.; Ma, J.S.H. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer 2015, 121, 1785–1792. [Google Scholar] [CrossRef]
- Fakhry, C.; Rosenthal, B.T.; Clark, D.P.; Gillison, M.L. Associations between oral HPV16 infection and cytopathology: Evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev. Res. 2011, 4, 1378–1384. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Chaturvedi, A.K. HPV-associated oropharyngeal cancers—Are they preventable? Cancer Prev. Res. 2011, 4, 1346–1349. [Google Scholar] [CrossRef]
- Skandarajah, A.; Sunny, S.P.; Gurpur, P.; Reber, C.D.; D’Ambrosio, M.V.; Raghavan, N.; James, B.L.; Ramanjinappa, R.D.; Suresh, A.; Kandasarma, U.; et al. Mobile microscopy as a screening tool for oral cancer in India: A pilot study. PLoS ONE 2017, 12, e0188440. [Google Scholar] [CrossRef] [PubMed]
- Health Service Executive. Cervical Check. 2019. Available online: https://www.hse.ie/eng/cervicalcheck/ (accessed on 19 May 2019).
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2015, 76, S49–S55. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).