Systemic Therapy Is Associated with Improved Oncologic Outcomes in Resectable Stage II/III Intrahepatic Cholangiocarcinoma: An Examination of the National Cancer Database over the Past Decade
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Cohort
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Profile
3.2. Use of Chemotherapy
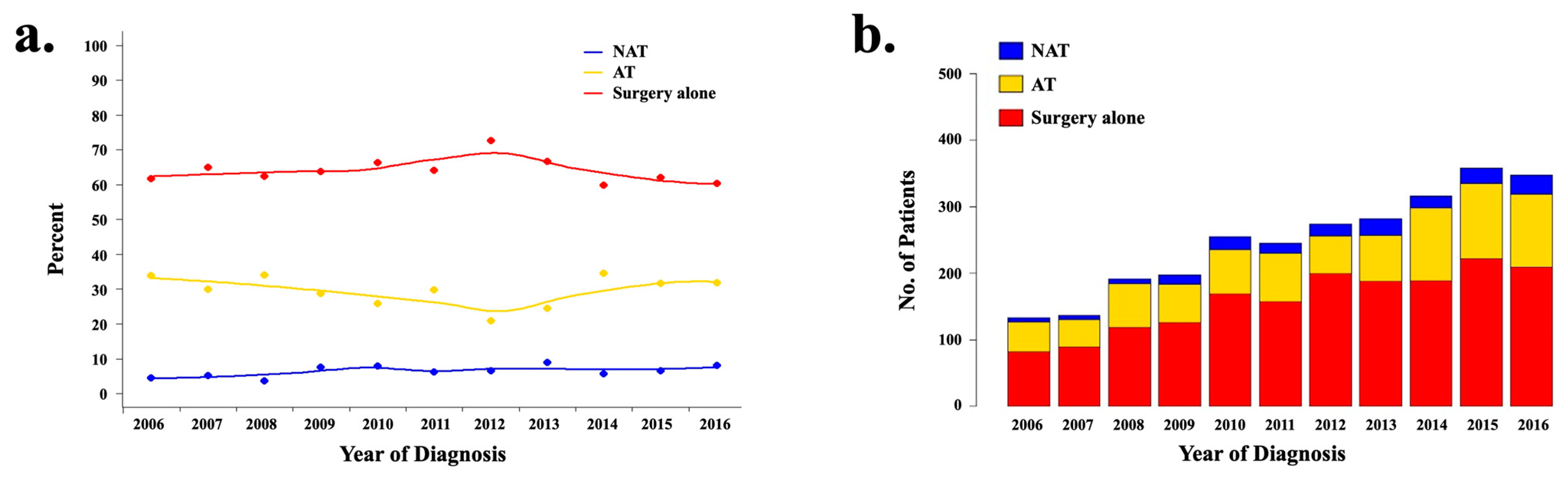
3.3. Trends over Time and Predictors of NAT Utilization
3.4. Trends over Time and Predictors of AT Utilization
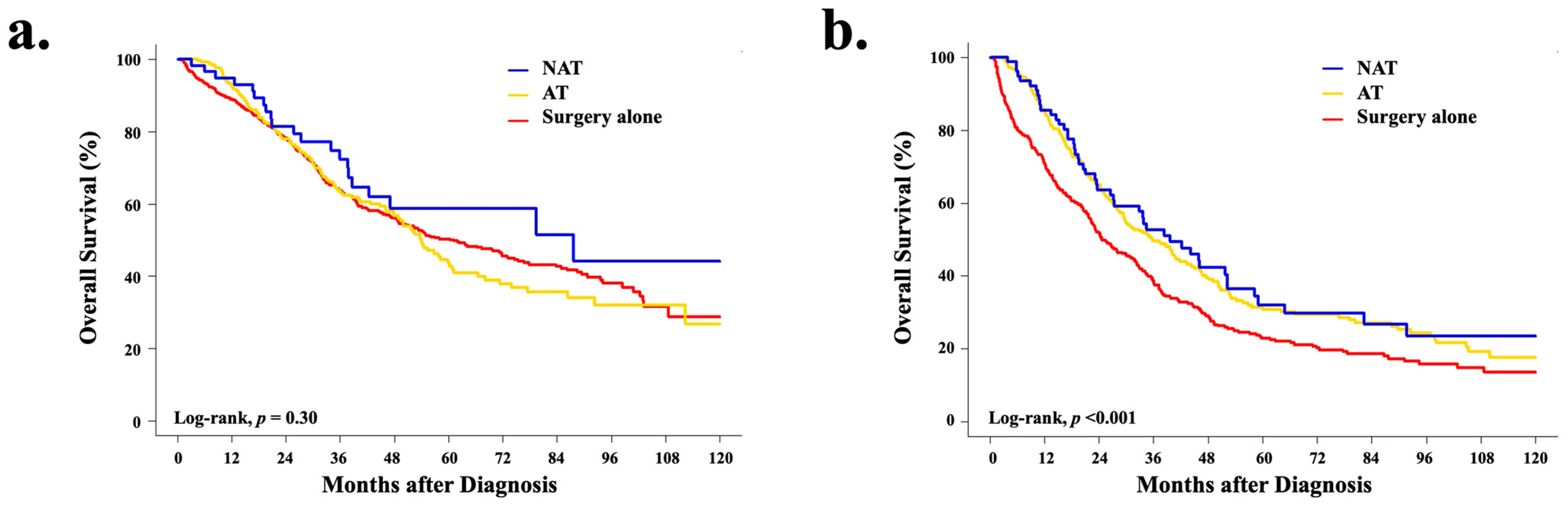
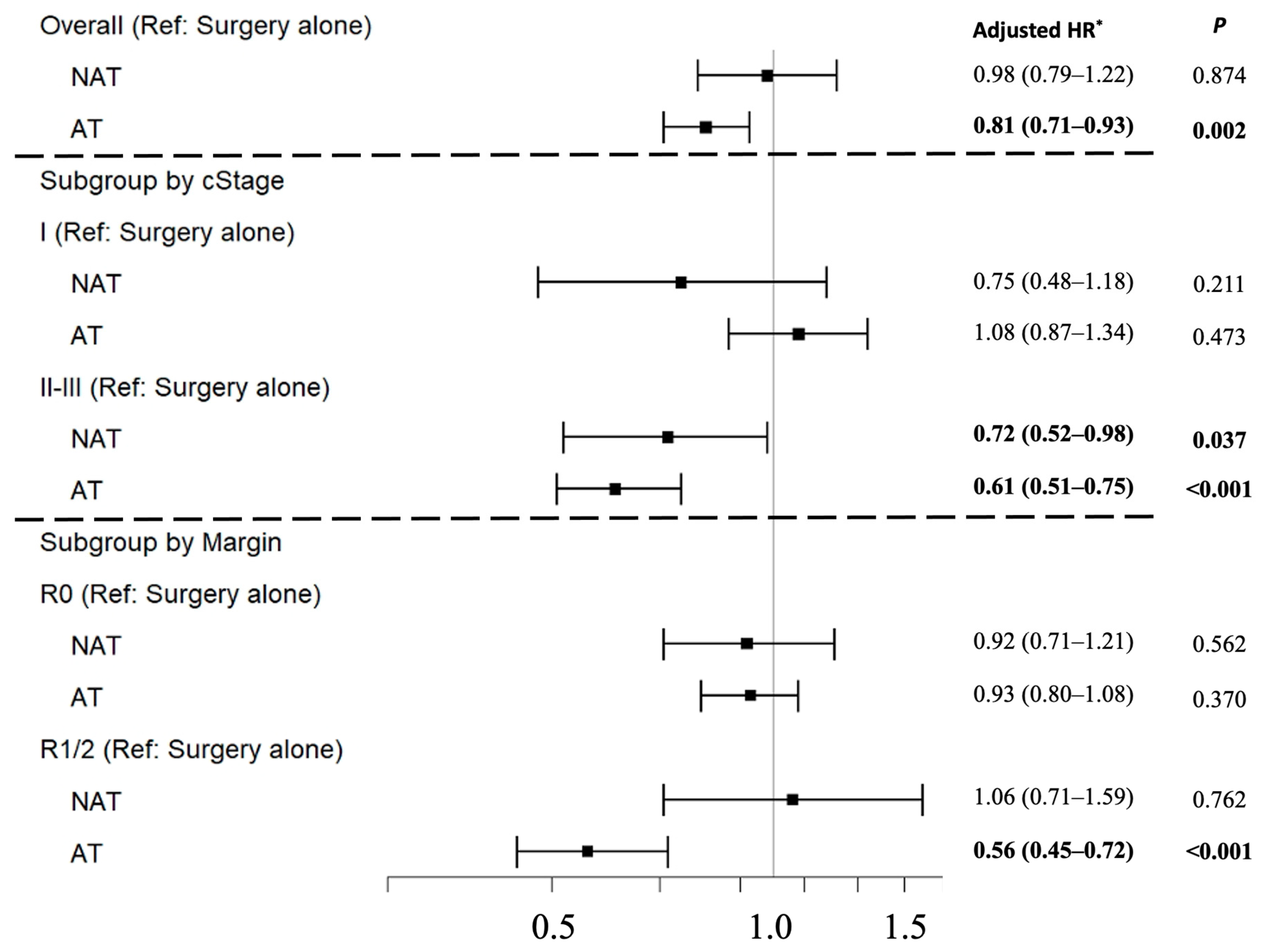
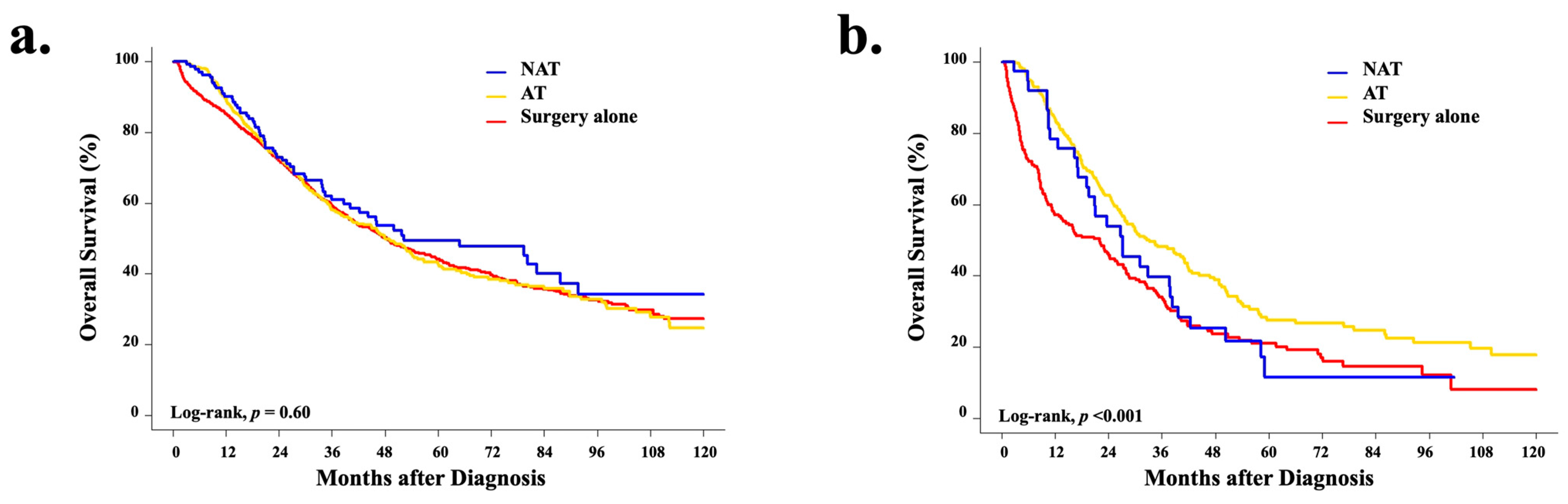
3.5. Overall Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirstein, M.M.; Vogel, A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc. Med. 2016, 32, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477. [Google Scholar] [CrossRef]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced Intrahepatic Cholangiocarcinoma: Post Hoc Analysis of the ABC-01, -02, and -03 Clinical Trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Survival Rates for Bile Duct Cancer. Available online: https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html (accessed on 7 July 2022).
- Rizzo, A.; Brandi, G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: Reflections on a standard of care. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 483–485. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; van Vugt, J.L.; Ijzermans, J.N.; Koerkamp, B.G. Intrahepatic cholangiocarcinoma: Current perspectives. OncoTargets Ther. 2017, 10, 1131–1142. [Google Scholar] [CrossRef]
- Goere, D.; Wagholikar, G.D.; Pessaux, P.; Carrère, N.; Sibert, A.; Vilgrain, V.; Belghiti, J. Utility of staging laparoscopy in subsets of biliary cancers: Laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg. Endosc. 2006, 20, 721–725. [Google Scholar] [CrossRef]
- Franken, L.C.; Coelen, R.J.S.; Roos, E.; Verheij, J.; Phoa, S.S.; Besselink, M.G.; Busch, O.R.C.; van Gulik, T.M. Staging Laparoscopy in Patients with Intrahepatic Cholangiocarcinoma: Is It Still Useful? Visc. Med. 2020, 36, 501–505. [Google Scholar] [CrossRef]
- Utuama, O.; Ms, J.B.P.; Dagne, G.; Sanchez-Anguiano, A.; Alman, A.; Kumar, A.; Denbo, J.; Kim, R.; Fleming, J.B.; Anaya, D.A. Neoadjuvant Chemotherapy for Intrahepatic Cholangiocarcinoma: A Propensity Score Survival Analysis Supporting Use in Patients with High-Risk Disease. Ann. Surg. Oncol. 2021, 28, 1939–1949. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines: Hepatobiliary Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 10 January 2020).
- Miura, J.T.; Johnston, F.M.; Tsai, S.; George, B.; Thomas, J.; Eastwood, D.; Banerjee, A.; Christians, K.K.; Turaga, K.K.; Pawlik, T.M.; et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Campagnaro, T.; Pachera, S.; Valdegamberi, A.; Nicoli, P.; Cappellani, A.; Malfermoni, G.; Iacono, C. Intrahepatic Cholangiocarcinoma: Prognostic Factors after Surgical Resection. World J. Surg. 2009, 33, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Marques, H.; Pulitano, C.; Marsh, J.W.; Alexandrescu, S.; Bauer, T.W.; Gamblin, T.C.; Sotiropoulos, G.C.; Paul, A.; Barroso, E.; et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 2014, 149, 432–438. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Sur, M.D.; In, H.; Sharpe, S.M.; Baker, M.S.; Weichselbaum, R.R.; Talamonti, M.S.; Posner, M.C. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 2209–2217. [Google Scholar] [CrossRef]
- Malka, D.; Edeline, J. Adjuvant capecitabine in biliary tract cancer: A standard option? Lancet Oncol. 2019, 20, 606–608. [Google Scholar] [CrossRef]
- Aquina, C.T.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. National Trends in the Use of Neoadjuvant Therapy before Cancer Surgery in the US from 2004 to 2016. JAMA Netw. Open 2021, 4, e211031. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7948056/ (accessed on 2 April 2021).
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Le, V.H.; O’Connor, V.V.; Li, D.; Melstrom, L.G.; Fong, Y.; DiFronzo, A.L. Outcomes of neoadjuvant therapy for cholangiocarcinoma: A review of existing evidence assessing treatment response and R0 resection rate. J. Surg. Oncol. 2021, 123, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical Resection after Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer: A Retrospective Single-center Study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef]
- Maithel, S.K.; Gamblin, T.C.; Kamel, I.; Corona-Villalobos, C.P.; Thomas, M.; Pawlik, T.M. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013, 119, 3929–3942. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.M.; Wirth, K.; Marmor, S.; Lou, E.; Chang, K.; Hui, J.Y.; Tuttle, T.; Jensen, E.; Denbo, J.W. Completion of Adjuvant Chemotherapy after Upfront Surgical Resection for Pancreatic Cancer Is Uncommon Yet Associated with Improved Survival. Ann. Surg. Oncol. 2019, 26, 4108–4116. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Cameron, J.L.; Makary, M.; Soares, K.; Ahuja, N.; Rezaee, N.; Herman, J.; Zheng, L.; Laheru, D.; et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017, 3, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.W.; Pan, W.; Hong, H.J.; Chen, J.Z.; Chen, Y.L. Modified staging classification for intrahepatic cholangiocarcinoma based on the sixth and seventh editions of the AJCC/UICC TNM staging systems. Medicine 2017, 96, e7891. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Dafni, U. Landmark analysis at the 25-year landmark point. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 363–371. [Google Scholar] [CrossRef]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma: Chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef]
- Fruscione, M.; Pickens, R.C.; Baker, E.H.; Martinie, J.B.; Iannitti, D.A.; Hwang, J.J.; Vrochides, D. Conversion therapy for intrahepatic cholangiocarcinoma and tumor downsizing to increase resection rates: A systematic review. Curr. Probl. Cancer 2021, 45, 100614. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Song, J.; Wang, F.; Zhu, G.; Chen, B. Combination of TACE and Lenvatinib as a promising option for downstaging to surgery of initially unresectable intrahepatic cholangiocarcinoma. Investig. New Drugs 2022, 40, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.; Giovinazzo, F.; Roberts, K.J.; Punia, P.; Sutcliffe, R.P.; Marudanayagam, R.; Chatzizacharias, N.; Isaac, J.; Mirza, D.F.; Muiesan, P.; et al. The role of down staging treatment in the management of locally advanced intrahepatic cholangiocarcinoma: Review of literature and pooled analysis. Ann. Hepato-Biliary-Pancreatic Surg. 2020, 24, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Akateh, C.; Ejaz, A.M.; Pawlik, T.M.; Cloyd, J.M. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J. Hepatol. 2020, 12, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.C.; Massarweh, N.N.; Tzeng, C.-W.D.; Chiang, Y.-J.; Chun, Y.S.; Aloia, T.A.; Javle, M.; Vauthey, J.-N.; Cao, H.S.T. Time to Rethink Upfront Surgery for Resectable Intrahepatic Cholangiocarcinoma? Implications from the Neoadjuvant Experience. Ann. Surg. Oncol. 2021, 28, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Wolfe, A.R.; Prabhakar, D.; Yildiz, V.O.; Cloyd, J.M.; Dillhoff, M.; Abushahin, L.; Diaz, D.A.; Miller, E.D.; Chen, W.; Frankel, W.L.; et al. Neoadjuvant-modified FOLFIRINOX vs. nab-paclitaxel plus gemcitabine for borderline resectable or locally advanced pancreatic cancer patients who achieved surgical resection. Cancer Med. 2020, 9, 4711–4723. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Spring: Berlin, Germany, 2017. [Google Scholar]
- Bridgewater, J.; Fletcher, P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J. Clin. Oncol. 2022, 40, 2048–2057. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Wang, H.; Overman, M.; Zhao, J.; Denbo, J.; Prakash, L.; Kim, M.P.; Shroff, R.; Javle, M.; Varadhachary, G.R.; et al. Influence of Preoperative Therapy on Short- and Long-Term Outcomes of Patients with Adenocarcinoma of the Ampulla of Vater. Ann. Surg. Oncol. 2017, 24, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Prakash, L.; Vauthey, J.-N.; Aloia, T.A.; Chun, Y.S.; Tzeng, C.-W.; Kim, M.P.; Lee, J.E.; Katz, M.H. The role of preoperative therapy prior to pancreatoduodenectomy for distal cholangiocarcinoma. Am. J. Surg. 2018, 218, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Maithel, S.K. Gemcitabine, Cisplatin, and Nab-Paclitaxel before Surgery in Patients with High-Risk Liver Bile Duct Cancer. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03579771 (accessed on 8 July 2022).

| Treatment Strategy | ||||
|---|---|---|---|---|
| Characteristic, N (%) | Surgery Alone | NAT | AT | p Value |
| Year of diagnosis | 0.011 | |||
| 2006–2008 | 290 (16.6) | 20 (11.0) | 151 (18.8) | |
| 2009–2012 | 651 (37.2) | 68 (37.4) | 253 (31.4) | |
| 2013–2016 | 808 (46.2) | 94 (51.6) | 401 (49.8) | |
| Age, years, mean (SD) | 66.1 (10.7) | 60.4 (10.9) | 61.9 (10.9) | <0.001 |
| Age, years, range | <0.001 | |||
| 18–55 | 236 (13.5) | 56 (30.8) | 192 (23.9) | |
| 56–69 | 797 (45.6) | 93 (51.1) | 403 (50.1) | |
| 70+ | 716 (40.9) | 33 (18.1) | 210 (26.1) | |
| Gender | 0.001 | |||
| Male | 817 (46.7) | 63 (34.6) | 334 (41.5) | |
| Female | 932 (53.3) | 119 (65.4) | 471 (58.5) | |
| Race | 0.165 | |||
| White | 1373 (78.5) | 155 (85.2) | 617 (76.6) | |
| Black | 116 (6.6) | 8 (4.4) | 48 (6.0) | |
| Hispanic | 88 (5.0) | 9 (4.9) | 46 (5.7) | |
| Asian Pacific Islander | 88 (5.0) | 4 (2.2) | 56 (7.0) | |
| Other/Missing | 84 (4.8) | 6 (3.3) | 38 (4.7) | |
| Insurance Status | <0.001 | |||
| Uninsured | 35 (2.0) | 1 (0.5) | 9 (1.1) | |
| Private | 616 (35.2) | 78 (42.9) | 386 (48.0) | |
| Public/Government | 1048 (59.9) | 97 (53.3) | 395 (49.1) | |
| Missing | 50 (2.9) | 6 (3.3) | 15 (1.9) | |
| Charlson comorbidity score | <0.001 | |||
| 0–1 | 1525 (87.2) | 171 (94.0) | 735 (91.3) | |
| ≥2 | 224 (12.8) | 11 (6.0) | 70 (8.7) | |
| Facility Type | <0.001 | |||
| Community Cancer Program, Comprehensive Community Cancer Program, or Integrated Network Cancer Program | 585 (33.4) | 45 (24.7) | 311 (38.6) | |
| Academic/Research Program | 1137 (65.1) | 129 (70.9) | 465 (57.8) | |
| Other/Unknown | 27 (1.5) | 8 (4.4) | 29 (3.6) | |
| Tumor size, cm | <0.001 | |||
| <2 | 127 (7.3) | 4 (2.2) | 39 (4.8) | |
| 2–5 | 766 43.8) | 38 (20.9) | 297 (36.9) | |
| >5 | 767 (43.9) | 116 (63.7) | 400 (49.7) | |
| Missing | 89 (5.1) | 24 (13.2) | 69 (8.6) | |
| Tumor grade | <0.001 | |||
| Well-differentiated | 216 (12.3) | 16 (8.8) | 79 (9.8) | |
| Moderately differentiated | 896 (51.2) | 78 (42.9) | 375 (46.6) | |
| Poorly or undifferentiated | 415 (23.7) | 48 (26.4) | 241 (29.9) | |
| Missing | 222 (12.7) | 40 (22.0) | 110 (13.7) | |
| TNM Clinical T | <0.001 | |||
| T1 | 781 (44.7) | 57 (31.3) | 251 (31.2) | |
| T2 | 276 (15.8) | 57 (31.3) | 178 (22.1) | |
| T3 | 84 (4.8) | 15 (8.2) | 84 (10.4) | |
| T4 | 13 (0.7) | 3 (1.6) | 5 (0.6) | |
| Tx | 595 (34.0) | 50 (27.5) | 287 (35.7) | |
| TNM Clinical N | 0.186 | |||
| N0 | 1353 (77.4) | 139 (76.4) | 606 (75.3) | |
| N1 | 29 (1.7) | 6 (3.3) | 24 (3.0) | |
| Nx | 367 (21.0) | 37 (20.3) | 175 (21.7) | |
| Surgery type | <0.001 | |||
| Wedge, segmentectomy, or sectionectomy | 899 (51.4) | 82 (45.1) | 372 (46.2) | |
| Hemi-hepatectomy | 607 (34.7) | 56 (30.8) | 272 (33.8) | |
| Extended hepatectomy | 217 (12.4) | 36 (19.8) | 130 (16.1) | |
| Surgery NOS | 26 (1.5) | 8 (4.4) | 31 (3.9) | |
| Resection Margin | <0.001 | |||
| Negative | 1446 (82.7) | 132 (72.5) | 504 (62.6) | |
| Positive | 212 (12.1) | 37 (20.3) | 242 (30.1) | |
| Missing | 91 (5.2) | 13 (7.1) | 59 (7.3) | |
| Regional nodes positive | <0.001 | |||
| 0 | 743 (42.5) | 80 (44.0) | 386 (48.0) | |
| ≥1 | 75 (4.3) | 9 (4.9) | 63 (7.8) | |
| No LN examined | 919 (52.5) | 89 (48.9) | 342 (42.5) | |
| Unknown | 12 (0.7) | 4 (2.2) | 14 (1.7) | |
| Number of lymph nodes examined | <0.001 | |||
| 0 | 919 (52.5) | 89 (48.9) | 342 (42.5) | |
| 1–5 | 627 (35.8) | 72 (39.6) | 334 (41.5) | |
| ≥6 | 176 (10.1) | 17 (9.3) | 102 (12.7) | |
| Unknown | 27 (1.5) | 4 (2.2) | 27 (3.4) | |
| Chemotherapy regimen | <0.001 | |||
| None | 1749 (100) | 0 (0) | 0 (0) | |
| Single-agent | 0 (0) | 34 (18.7) | 404 (50.2) | |
| Multi-agent | 0 (0) | 134 (73.6) | 345 (42.9) | |
| Missing | 0 (0) | 14 (7.7) | 56 (7.0) | |
| Radiation | <0.001 | |||
| No | 1689 (96.6) | 144 (79.1) | 495 (61.5) | |
| Yes | 60 (3.4) | 38 (20.9) | 310 (38.5) | |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Year of diagnosis | ||||
| 2006–2008 | ||||
| 2009–2012 | 1.66 (0.99–2.77) | 0.052 | 2.09 (1.19–3.68) | 0.011 |
| 2013–2016 | 1.71 (1.05–2.81) | 0.033 | 2.41 (1.37–4.24) | 0.002 |
| Age, years, range | ||||
| 18–55 | ||||
| 56–69 | 0.59 (0.42–0.84) | 0.003 | 0.55 (0.36–0.83) | 0.004 |
| 70+ | 0.27 (0.17–0.43) | <0.001 | 0.20 (0.12–0.34) | <0.001 |
| Gender | ||||
| Male | ||||
| Female | 1.55 (1.13–2.12) | 0.007 | 1.46 (1.05–2.04) | 0.025 |
| Race | ||||
| White | ||||
| Black | 0.63 (0.30–1.30) | 0.208 | 0.52 (0.24–1.13) | 0.097 |
| Hispanic | 0.86 (0.43–1.73) | 0.676 | 0.71 (0.34–1.51) | 0.379 |
| Asian Pacific Islander | 0.36 (0.13–0.98) | 0.045 | 0.32 (0.12–0.92) | 0.033 |
| Other/Missing | 0.63 (0.27–1.46) | 0.281 | 0.51 (0.21–1.26) | 0.143 |
| Insurance Status | ||||
| Uninsured | 0.29 (0.04–2.15) | 0.227 | 0.24 (0.03–1.90) | 0.177 |
| Private | ||||
| Public/Government | 0.86 (0.63–1.18) | 0.352 | 1.63 (1.12–2.38) | 0.012 |
| Charlson comorbidity score | ||||
| 0–1 | ||||
| ≥2 | 0.49 (0.27–0.92) | 0.026 | 0.56 (0.29–1.06) | 0.076 |
| Facility Type | ||||
| Community Cancer Program, Comprehensive Community Cancer Program, or Integrated Network Cancer Program | ||||
| Academic/Research Program | 1.6 (1.13–2.27) | 0.008 | 1.84 (1.26–2.69) | 0.002 |
| Tumor size, cm | ||||
| <2 | ||||
| 2–5 | 1.48 (0.52–4.21) | 0.459 | 1.35 (0.46–3.92) | 0.584 |
| >5 | 4.13 (1.5–11.32) | 0.006 | 3.8 (1.34–10.72) | 0.012 |
| Clinical stage | ||||
| I | ||||
| II | 2.08 (1.41–3.06) | <0.001 | 1.84 (1.22–2.78) | 0.004 |
| IIIA | 1.48 (0.80–2.71) | 0.209 | 1.38 (0.72–2.63) | 0.329 |
| IIIB | 2.74 (1.24–6.04) | 0.013 | 3.28 (1.30–8.24) | 0.012 |
| Radiation | ||||
| No | ||||
| Yes | 1.56 (1.07–2.26) | 0.020 | 1.28 (0.85–1.93) | 0.245 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Year of diagnosis | ||||
| 2006–2008 | ||||
| 2009–2012 | 0.75 (0.58–0.95) | 0.019 | 1.15 (0.84–1.57) | 0.395 |
| 2013–2016 | 0.95 (0.76–1.20) | 0.683 | 1.63 (1.20–2.25) | 0.002 |
| Age, years, range | ||||
| 18–55 | ||||
| 56–69 | 0.62 (0.50–0.78) | <0.001 | 0.69 (0.51–0.92) | 0.012 |
| 70+ | 0.36 (0.28–0.46) | <0.001 | 0.39 (0.28–0.55) | <0.001 |
| Gender | ||||
| Male | ||||
| Female | 1.24 (1.04–1.46) | 0.014 | 1.43 (1.16–1.75) | 0.001 |
| Race | ||||
| White | ||||
| Black | 0.92 (0.65–1.31) | 0.644 | 0.74 (0.47–1.16) | 0.193 |
| Hispanic | 1.16 (0.80–1.68) | 0.422 | 0.98 (0.61–1.58) | 0.945 |
| Asian Pacific Islander | 1.42 (1.00–2.01) | 0.050 | 1.47 (0.96–2.23) | 0.074 |
| Other/Missing | 1.01 (0.68–1.49) | 0.974 | 1.00 (0.62–1.60) | 0.996 |
| Insurance Status | ||||
| Uninsured | 0.41 (0.20–0.86) | 0.019 | 0.44 (0.19–1.03) | 0.059 |
| Private | ||||
| Public/Government | 0.60 (0.51–0.71) | <0.001 | 0.85 (0.67–1.08) | 0.189 |
| Charlson comorbidity score | ||||
| 0–1 | ||||
| ≥2 | 0.65 (0.49–0.86) | 0.003 | 0.76 (0.54–1.06) | 0.107 |
| Facility Type | ||||
| Community Cancer Program, Comprehensive Community Cancer Program, or Integrated Network Cancer Program | ||||
| Academic/Research Program | 0.77 (0.65–0.92) | 0.003 | 0.69 (0.55–0.86) | 0.001 |
| Tumor size, cm | ||||
| <2 | ||||
| 2–5 | 1.26 (0.86–1.85) | 0.233 | 1.53 (0.96–2.44) | 0.077 |
| >5 | 1.70 (1.16–2.48) | 0.006 | 1.93 (1.21–3.07) | 0.006 |
| Tumor grade | ||||
| Well-differentiated | ||||
| Moderately differentiated | 1.14 (0.86–1.52) | 0.353 | 0.81 (0.57–1.15) | 0.238 |
| Poorly or undifferentiated | 1.59 (1.17–2.15) | 0.003 | 1.11 (0.76–1.61) | 0.598 |
| Pathologic stage | ||||
| I | ||||
| II | 2.3 (1.82–2.91) | <0.001 | 2.50 (1.87–3.34) | <0.001 |
| IIIA | 3.21 (2.39–4.33) | <0.001 | 3.36 (2.32–4.84) | <0.001 |
| IIIB | 3.51 (2.33–5.28) | <0.001 | 3.33 (1.94–5.72) | <0.001 |
| Surgery type | ||||
| Wedge, segmentectomy, or sectionectomy | ||||
| Hemi-hepatectomy | 1.08 (0.90–1.31) | 0.404 | 1.08 (0.86–1.35) | 0.531 |
| Extended hepatectomy | 1.45 (1.13–1.86) | 0.004 | 1.06 (0.78–1.45) | 0.710 |
| Surgery NOS | 2.88 (1.69–4.92) | <0.001 | 1.90 (0.98–3.69) | 0.058 |
| Resection Margin | ||||
| Negative | ||||
| Positive | 3.27 (2.65–4.04) | <0.001 | 1.83 (1.41–2.39) | <0.001 |
| Regional lymph nodes examined | ||||
| No | ||||
| Yes | 1.46 (1.23–1.73) | <0.001 | 1.12 (0.88–1.42) | 0.351 |
| Radiation | ||||
| No | ||||
| Yes | 17.63 (13.14–23.66) | <0.001 | 16.81 (12.15–23.26) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcus, R.; Christopher, W.; Keller, J.; Nassoiy, S.; Chang, S.-C.; Goldfarb, M.; Wolf, R.; Jutric, Z. Systemic Therapy Is Associated with Improved Oncologic Outcomes in Resectable Stage II/III Intrahepatic Cholangiocarcinoma: An Examination of the National Cancer Database over the Past Decade. Cancers 2022, 14, 4320. https://doi.org/10.3390/cancers14174320
Marcus R, Christopher W, Keller J, Nassoiy S, Chang S-C, Goldfarb M, Wolf R, Jutric Z. Systemic Therapy Is Associated with Improved Oncologic Outcomes in Resectable Stage II/III Intrahepatic Cholangiocarcinoma: An Examination of the National Cancer Database over the Past Decade. Cancers. 2022; 14(17):4320. https://doi.org/10.3390/cancers14174320
Chicago/Turabian StyleMarcus, Rebecca, Wade Christopher, Jennifer Keller, Sean Nassoiy, Shu-Ching Chang, Melanie Goldfarb, Ronald Wolf, and Zeljka Jutric. 2022. "Systemic Therapy Is Associated with Improved Oncologic Outcomes in Resectable Stage II/III Intrahepatic Cholangiocarcinoma: An Examination of the National Cancer Database over the Past Decade" Cancers 14, no. 17: 4320. https://doi.org/10.3390/cancers14174320
APA StyleMarcus, R., Christopher, W., Keller, J., Nassoiy, S., Chang, S.-C., Goldfarb, M., Wolf, R., & Jutric, Z. (2022). Systemic Therapy Is Associated with Improved Oncologic Outcomes in Resectable Stage II/III Intrahepatic Cholangiocarcinoma: An Examination of the National Cancer Database over the Past Decade. Cancers, 14(17), 4320. https://doi.org/10.3390/cancers14174320






