Evaluation of Cognitive Function in Trials Testing New-Generation Hormonal Therapy in Patients with Prostate Cancer: A Systematic Review
Simple Summary
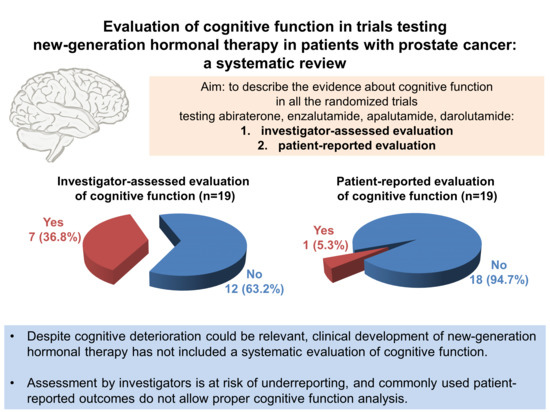
Abstract
1. Introduction
2. Investigator-Assessed Cognitive Impairment
3. PROs Commonly Used in Clinical Trials of Prostate Cancer
4. Available Validated Instruments for Evaluation of Cognitive Function
5. Different Modalities of Data Analysis and Presentation of PROs: Opportunities and Limitations
6. Methods: Literature Search
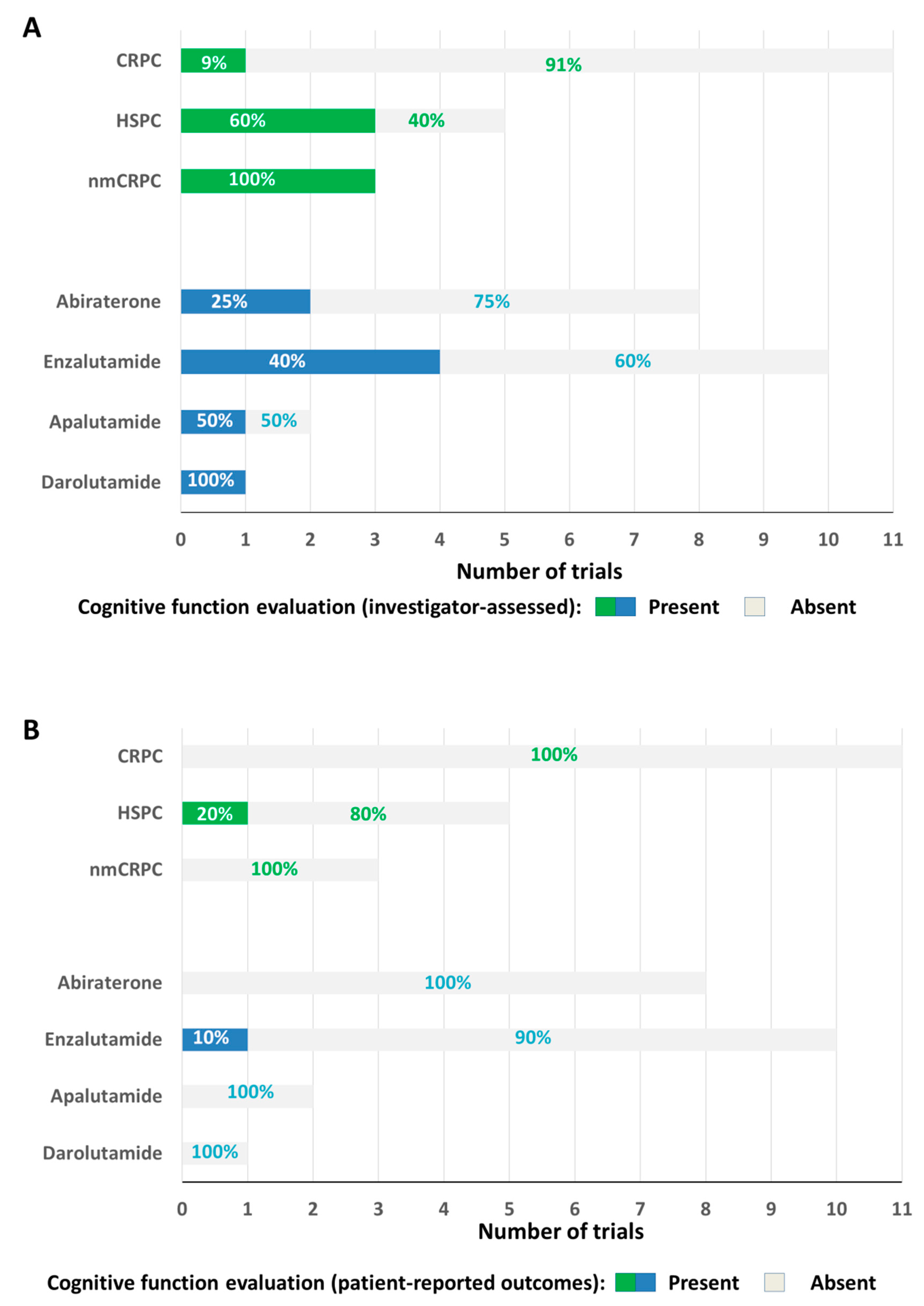
7. Trials Testing Abiraterone, Enzalutamide, Apalutamide, Darolutamide: Available Data on Cognitive Function
8. Trials Testing Abiraterone
9. Trials Testing Enzalutamide
10. Trials Testing Both Abiraterone and Enzalutamide
11. Trials Testing Apalutamide
12. Trials Testing Darolutamide
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Bandelow, S.; Hogervorst, E. Testosterone levels and cognition in elderly men: A review. Maturitas 2011, 69, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Wefel, J.S.; Morgans, A.K. A review of prostate cancer treatment impact on the CNS and cognitive function. Prostate Cancer Prostatic Dis. 2020, 23, 207–219. [Google Scholar] [CrossRef]
- McGinty, H.L.; Phillips, K.M.; Jim, H.S.; Cessna, J.M.; Asvat, Y.; Cases, M.G.; Small, B.J.; Jacobsen, P.B. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: A systematic review and meta-analysis. Support Care Cancer 2014, 22, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Amidi, A. Cognitive impairment following hormone therapy: Current opinion of research in breast and prostate cancer patients. Curr. Opin. Support. Palliat. Care 2017, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.D.; Jim, H.S.; Booth-Jones, M.; Small, B.J.; Sutton, S.K.; Lin, H.Y.; Park, J.Y.; Spiess, P.E.; Fishman, M.N.; Jacobsen, P.B. Course and Predictors of Cognitive Function in Patients with Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison. J. Clin. Oncol. 2015, 33, 2021–2027. [Google Scholar] [CrossRef]
- Da Costa, R.; Passos, G.F.; Quintão, N.L.M.; Fernandes, E.S.; Maia, J.R.L.C.B.; Campos, M.M.; Calixto, J.B. Taxane-induced neurotoxicity: Pathophysiology and therapeutic perspectives. Br. J. Pharmacol. 2020, 177, 3127–3146. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. LATITUDE Investigators. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; National Institute of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf (accessed on 13 July 2020).
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; De Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Marandino, L.; De Luca, E.; Zichi, C.; Lombardi, P.; Reale, M.L.; Pignataro, D.; Di Stefano, R.F.; Ghisoni, E.; Mariniello, A.; Trevisi, E.; et al. Quality-of-Life Assessment and Reporting in Prostate Cancer: Systematic Review of Phase 3 Trials Testing Anticancer Drugs Published Between 2012 and 2018. Clin. Genitourin. Cancer 2019, 17, 332–347.e2. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, G.; Bottomley, A.; Fosså, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Gunlusoy, B.; Ceylan, Y.; Koskderelioglu, A.; Gedizlioglu, M.; Degirmenci, T.; Ortan, P.; Kozacioglu, Z. Cognitive Effects of Androgen Deprivation Therapy in Men with Advanced Prostate Cancer. Urology 2017, 103, 167–172. [Google Scholar] [CrossRef]
- Lange, M.; Laviec, H.; Castel, H.; Heutte, N.; Leconte, A.; Léger, I.; Giffard, B.; Capel, A.; Dubois, M.; Clarisse, B.; et al. Impact of new generation hormone-therapy on cognitive function in elderly patients treated for a metastatic prostate cancer: Cog-Pro trial protocol. BMC Cancer 2017, 17, 549. [Google Scholar] [CrossRef]
- Wagner, L.I.; Sabatino, T.; Cella, D.; Sweet, J.; Havlin, K.; Gitelman, D.; Beaumont, J. Cognitive function during cancer treatment: The FACT-Cog study. J. Robert H. Lurie Compr. Cancer Center Northwest Univ. 2005, 10, 10–15. [Google Scholar]
- Costa, D.S.J.; Loh, V.; Birney, D.P.; Dhillon, H.M.; Fardell, J.E.; Gessler, D.; Vardy, J.L. The Structure of the FACT-Cog v3 in Cancer Patients, Students, and Older Adults. J. Pain Symptom Manag. 2018, 55, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Dhillon, H.M.; Bray, V.J.; Vardy, J.L. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog). J. Patient Rep. Outcomes 2018, 2, 48. [Google Scholar] [CrossRef]
- Fallowfield, L.J. Quality of life assessment using patient-reported outcome (PRO) measures: Still a Cinderella outcome? Ann. Oncol. 2018, 29, 2286–2287. [Google Scholar] [CrossRef]
- Charton, E.; Cuer, B.; Cottone, F.; Efficace, F.; Touraine, C.; Hamidou, Z.; Fiteni, F.; Bonnetain, F.; Woronoff-Lemsi, M.C.; Bascoul-Mollevi, C.; et al. Time to deterioration in cancer randomized clinical trials for patient-reported outcomes data: A systematic review. Qual. Life Res. 2020, 29, 867–878. [Google Scholar] [CrossRef]
- Marandino, L.; La Salvia, A.; Sonetto, C.; De Luca, E.; Pignataro, D.; Zichi, C.; Di Stefano, R.F.; Ghisoni, E.; Lombardi, P.; Mariniello, A.; et al. Deficiencies in health-related quality-of-life assessment and reporting: A systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann. Oncol. 2018, 29, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- De Wit, R.; De Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. CARD Investigators. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, Q.; Sun, Z.; Li, C.; Du, C.; Chen, Z.; Shan, Y.; Huang, Y.; Jin, J.; Ye, Z.Q.; et al. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: A randomized, double-blind, placebo-controlled phase 3 bridging study. Int. J. Urol. 2016, 23, 404–411. [Google Scholar] [CrossRef]
- Ye, D.; Huang, Y.; Zhou, F.; Xie, K.; Matveev, V.; Li, C.; Alexeev, B.; Tian, Y.; Qiu, M.; Li, H.; et al. A phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in Chemotherapy-naïve patients with mCRPC in China, Malaysia, Thailand and Russia. Asian J. Urol. 2017, 4, 75–85. [Google Scholar] [CrossRef]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. STAMPEDE Investigators. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Khalaf, D.J.; Sunderland, K.; Eigl, B.J.; Kollmannsberger, C.K.; Ivanov, N.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Gleave, M.E.; et al. Health-related Quality of Life for Abiraterone Plus Prednisone Versus Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer: Results from a Phase II Randomized Trial. Eur. Urol. 2019, 75, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Harland, S.; Staffurth, J.; Molina, A.; Hao, Y.; Gagnon, D.D.; Sternberg, C.N.; Cella, D.; Fizazi, K.; Logothetis, C.J.; Kheoh, T.; et al. COU-AA-301 Investigators. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur. J. Cancer 2013, 49, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Autio, K.; Ryan, C.J.; Mulders, P.; Shore, N.; Kheoh, T.; Fizazi, K.; Logothetis, C.J.; Rathkopf, D.; Smith, M.R.; et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013, 14, 1193–1199. [Google Scholar] [CrossRef]
- Rush, H.L.; Cook, A.D.; Brawley, C.D.; Murphy, L.; Macnair, A.; Millman, R.; Attard, G.; Clarke, N.; Morgans, A.K.; Chowdhury, S.; et al. STAMPEDE Investigators. Comparative quality of life in patients randomized contemporaneously to docetaxel or abiraterone in the STAMPEDE trial. J. Clin. Oncol. 2020, 38 (Suppl. 6), 14. [Google Scholar] [CrossRef]
- Chi, K.N.; Protheroe, A.; Rodríguez-Antolín, A.; Facchini, G.; Suttman, H.; Matsubara, N.; Ye, Z.; Keam, B.; Damião, R.; Li, T.; et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Poulsen, M.H.; Lagneau, E.; Ploussard, G.; Birtle, A.; Dourthe, L.M.; Beal-Ardisson, D.; Pintus, E.; Trepiakas, R.; Antoni, L.; et al. Impact of abiraterone acetate plus prednisone or enzalutamide on fatigue and cognition in patients with metastatic castration-resistant prostate cancer: Initial results from the observational AQUARiUS study. ESMO Open 2018, 3, e000397. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Poulsen, M.H.; Lagneau, E.; Ploussard, G.; Birtle, A.; Dourthe, L.M.; Beal-Ardisson, D.; Pintus, E.; Trepiakas, R.; Lefresne, F.; et al. AQUARiUS Investigators. Impact of Abiraterone Acetate plus Prednisone or Enzalutamide on Patient-reported Outcomes in Patients with Metastatic Castration-resistant Prostate Cancer: Final 12-mo Analysis from the Observational AQUARiUS Study. Eur. Urol. 2020, 77, 380–387. [Google Scholar] [CrossRef]
- Shore, N.D.; Saltzstein, D.; Sieber, P.; Mehlhaff, B.; Gervasi, L.; Phillips, J.; Wong, Y.-N.; Pei, H.; McGowan, T. Results of a Real-world Study of Enzalutamide and Abiraterone Acetate with Prednisone Tolerability (REAAcT). Clin. Genitourin. Cancer 2019, 17, 457–463.e6. [Google Scholar] [CrossRef]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- Shore, N.D.; Chowdhury, S.; Villers, A.; Klotz, L.; Siemens, D.R.; Phung, D.; van Os, S.; Hasabou, N.; Wang, F.; Bhattacharya, S.; et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): A randomised, double-blind, phase 2 study. Lancet Oncol. 2016, 17, 153–163. [Google Scholar] [CrossRef]
- Penson, D.F.; Armstrong, A.J.; Concepcion, R.; Agarwal, N.; Olsson, C.; Karsh, L.; Dunshee, C.; Wang, F.; Wu, K.; Krivoshik, A.; et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J. Clin. Oncol. 2016, 34, 2098–2106. [Google Scholar] [CrossRef]
- Iguchi, T.; Tamada, S.; Kato, M.; Yasuda, S.; Yamasaki, T.; Nakatani, T. Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: Study protocol for a multicenter randomized phase II trial (the OCUU-CRPC study). BMC Cancer 2019, 19, 339. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. PROSPER Investigators. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Cella, D.; Ivanescu, C.; Holmstrom, S.; Bui, C.N.; Spalding, J.; Fizazi, K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: Additional analyses from the AFFIRM randomized clinical trial. Ann. Oncol. 2015, 26, 179–185. [Google Scholar] [CrossRef]
- Loriot, Y.; Miller, K.; Sternberg, C.N.; Fizazi, K.; De Bono, J.S.; Chowdhury, S.; Higano, C.S.; Noonberg, S.; Holmstrom, S.; Mansbach, H.; et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol. 2015, 16, 509–521. [Google Scholar] [CrossRef]
- Heidenreich, A.; Chowdhury, S.; Klotz, L.; Siemens, D.R.; Villers, A.; Ivanescu, C.; Holmstrom, S.; Baron, B.; Wang, F.; Lin, P.; et al. Impact of Enzalutamide Compared with Bicalutamide on Quality of Life in Men with Metastatic Castration-resistant Prostate Cancer: Additional Analyses from the TERRAIN Randomised Clinical Trial. Eur. Urol. 2017, 71, 534–542. [Google Scholar] [CrossRef]
- Tombal, B.; Saad, F.; Penson, D.; Hussain, M.; Sternberg, C.N.; Morlock, R.; Ramaswamy, K.; Ivanescu, C.; Attard, G. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 556–569. [Google Scholar] [CrossRef]
- Stenzl, A.; Dunshee, C.; De Giorgi, U.; Alekseev, B.; Iguchi, T.; Szmulewitz, R.Z.; Flaig, T.W.; Tombal, B.; Morlock, R.; Ivanescu, C.; et al. Effect of Enzalutamide plus Androgen Deprivation Therapy on Health-related Quality of Life in Patients with Metastatic Hormone-sensitive Prostate Cancer: An Analysis of the ARCHES Randomised, Placebo-controlled, Phase 3 Study. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Stockler, M.R.; Martin, A.J.; Dhillon, H.; Davis, I.D.; Chi, K.N.; Chowdhury, S.; Horvath, L.G.; Lawrence, N.J.; Marx, G.M.; Mc Caffrey, J.; et al. Health-Related Quality of Life in a Randomized Phase 3 Trial of Enzalutamide with Standard First Line Therapy for Metastatic Hormone-Sensitive Prostate Cancer: ENZAMET, ANZUP-led, International, Co-Operative Group Trial. Ann. Oncol. 2019, 30 (Suppl. 5), v851–v934. [Google Scholar] [CrossRef]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cai, L.; et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012, 72, 1494–1503. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Antonarakis, E.S.; Shore, N.D.; Tutrone, R.F.; Alumkal, J.J.; Ryan, C.J.; Saleh, M.; Hauke, R.J.; Bandekar, R.; Maneval, E.C.; et al. Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone. Clin. Cancer Res. 2017, 23, 3544–3551. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. SPARTAN Investigators. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. TITAN Investigators. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Cella, D.; Basch, E.; Hadaschik, B.A.; Mainwaring, P.N.; Oudard, S.; Graff, J.N.; McQuarrie, K.; Li, S.; Hudgens, S.; et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1404–1416. [Google Scholar] [CrossRef]
- Agarwal, N.; McQuarrie, K.; Bjartell, A.; Chowdhury, S.; Pereira de Santana Gomes, A.J.; Chung, B.H.; Özgüroğlu, M.; Juárez Soto, Á.; Merseburger, A.S.; Uemura, H.; et al. TITAN investigators. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): A randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019, 20, 1518–1530. [Google Scholar] [CrossRef]
- Fizazi, K.; Massard, C.; Bono, P.; Jones, R.; Kataja, V.; James, N.; Garcia, J.A.; Protheroe, A.; Tammela, T.L.; Elliott, T.; et al. ARADES study group. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): An open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014, 15, 975–985. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. ARAMIS Investigators. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
| Study | Setting | Comparison | Investigator-Assessed Evaluation of Cognitive Function | PRO Tools Adopted in the Study | PRO-Based Evaluation of Cognitive Function |
|---|---|---|---|---|---|
| COU-AA-301 [8,28,35] | Postdocetaxel CRPC | Randomized trial (abiraterone + prednisone + ADT vs. placebo + prednisone + ADT) | Not available | FACT-P BPI-SF BFI | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| COU-AA-302 [9,29,36] | Chemotherapy-naïve CRPC | Randomized trial (abiraterone + prednisone + ADT vs. placebo + prednisone + ADT) | Not available | FACT-P BPI-SF | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| NCT01695135 [30] | Postdocetaxel CRPC | Randomized trial (abiraterone + prednisone + ADT vs. placebo + prednisone + ADT) | Not available | FACT-P BPI-SF BFI-SF | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| NCT01591122 [31] | CRPC | Randomized trial (abiraterone + prednisone + ADT vs. placebo + prednisone + ADT) | Not available | FACT-P BPI-SF | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| STAMPEDE [32,37] | HSPC | Randomized trial (abiraterone + prednisolone + ADT vs. ADT alone) | Cognitive disturbance:
| EORTC C30 EORTC P25 | Not available (EORTC C30 includes evaluation of cognitive functioning but results are not reported in the publication) |
| LATITUDE [10,33,38] | HSPC | Randomized trial (abiraterone + prednisone + ADT vs. placebo + prednisone +ADT) | Not available | FACT-P BPI-SF BFI EQ-5D-5L | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| Study | Setting | Comparison | Investigator-Assessed Evaluation of Cognitive Function | PRO Tools Adopted in the Study | PRO-Based Evaluation of Cognitive Function |
|---|---|---|---|---|---|
| CARD [27] | CRPC | Randomized trial (Cabazitaxel vs. abiraterone + prednisone or enzalutamide; drug in the arm randomized to hormonal treatment was at Investigator’s discretion) | Not available | FACT-P BPI-SF EQ-5D-5L | Not available (the adopted PRO tools do not include evaluation of cognitive function) |
| NCT02125357 [34] | CRPC | Randomized trial (Abiraterone + prednisone vs. Enzalutamide) | Montreal Cognitive Assessment (screening test): Proportion of patients with a MoCA score < 26 similar between arms at week 12 (47% abiraterone vs. 54% enzalutamide), 95% CI for difference: −8.6% to 23.8%, p = 0.4; Distribution of score change from baseline was similar between arms (p = 0.11). | FACT-P PHQ-9 | Not available (the adopted PRO tools do not include evaluation of cognitive function) |
| AQUARiUS [39,40] | CRPC | Abiraterone + prednisone vs. Enzalutamide (observational, non randomized study) | Not available | FACT-Cog EORTC QLQ- C30 BFI-SF BPI-SF | Scales:
|
| REAAcT [41] | CRPC | Abiraterone + prednisone vs. Enzalutamide (observational, non randomized study) | Cogstate tests: mean changes from baseline were similar for both arms and showed no meaningful change over the first 2 months of treatment; clinically meaningful cognitive decline in 1/46 pts (2.2%) with abiraterone and 4/46 pts (8.7%) with enzalutamide. | FACT-Cog FACIT-Fatigue EORTC QLQ-C30 |
|
| Study | Setting | Comparison | Investigator-Assessed Evaluation of Cognitive Function | PRO Tools Adopted in the Study | PRO-Based Evaluation of Cognitive Function |
|---|---|---|---|---|---|
| AFFIRM [43,53] | Postchemotherapy CRPC | Randomized trial (enzalutamide + ADT vs. placebo + ADT) | Not available | FACT-P BPI-SF EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| PREVAIL [44,45,54] | Chemotherapy-naïve CRPC | Randomized trial (enzalutamide + ADT vs. placebo + ADT) | Not available | FACT-P BPI-SF EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| TERRAIN [46,55] | Chemotherapy-naïve CRPC | Randomized trial (enzalutamide + placebo + ADT vs. placebo + bicalutamide + ADT) | Not available | FACT-P BPI-SF EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| STRIVE [47] | Chemotherapy-naïve CRPC | Randomized trial (enzalutamide + ADT vs. bicalutamide + ADT) | Not available | FACT-P | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| OCUU-CRPC [48] | CRPC after ADT + bicalutamide | Randomized trial (enzalutamide + ADT vs. flutamide + ADT) | Not available | FACT-P | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| PROSPER [49,50,56] | Nonmetastatic CRPC | Randomized trial (enzalutamide + ADT vs. placebo + ADT) | Cognitive and memory impairment (disturbance in attention, cognitive disorders, amnesia, Alzheimer’s disease, dementia, senile dementia, mental impairment and vascular dementia):
| FACT-P EORTC QLQ PR25 BPI-SF EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| ARCHES [51,57] | HSPC | Randomized trial (enzalutamide + ADT vs. placebo + ADT) | Cognitive/memory impairment:
| BPI-SF FACT-P EORTC QLQ PR25 EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| ENZAMET [52,58] | HSPC | Randomized trial (enzalutamide + ADT +/– docetaxel vs. standard nonsteroidal antiandrogen therapy + ADT +/– docetaxel | Cognitive disturbance:
| EORTC QLQ C30 EORTC PR25 EQ5D | Scale: Cognitive functioning (EORTC QLQ C30) Deterioration-free survival at 3 years:
|
| Study | Setting | Comparison | Investigator-Assessed Evaluation of Cognitive Function | PRO Tools Adopted in the Study | PRO-Based Evaluation of Cognitive Function |
|---|---|---|---|---|---|
| SPARTAN [61,63] | Nonmetastatic CRPC | Randomized trial (apalutamide + ADT vs. placebo + ADT) | Mental impairment disorders (disturbance in attention, memory impairment, cognitive disorder and amnesia):
| FACT-P EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| TITAN [62,64] | HSPC | Randomized trial (apalutamide + ADT vs. placebo + ADT) | Not available | BPI-SF BFI FACT-P EQ5D | Not available (the adopted PRO tools do not include an evaluation of cognitive function) |
| Study | Setting | Comparison | Investigator-Assessed Evaluation of Cognitive Function | PRO Tools Adopted in the Study | PRO-Based Evaluation of Cognitive Function |
|---|---|---|---|---|---|
| ARAMIS [66] | Nonmetastatic CRPC | Randomized trial (darolutamide + ADT vs. placebo + ADT) |
| FACT-P EORTC QLQ PR25 EQ5D | Not available (FACT-P and EORTC PR-25 do not include an evaluation of cognitive function) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marandino, L.; Vignani, F.; Buttigliero, C.; Gamba, T.; Necchi, A.; Tucci, M.; Di Maio, M. Evaluation of Cognitive Function in Trials Testing New-Generation Hormonal Therapy in Patients with Prostate Cancer: A Systematic Review. Cancers 2020, 12, 2568. https://doi.org/10.3390/cancers12092568
Marandino L, Vignani F, Buttigliero C, Gamba T, Necchi A, Tucci M, Di Maio M. Evaluation of Cognitive Function in Trials Testing New-Generation Hormonal Therapy in Patients with Prostate Cancer: A Systematic Review. Cancers. 2020; 12(9):2568. https://doi.org/10.3390/cancers12092568
Chicago/Turabian StyleMarandino, Laura, Francesca Vignani, Consuelo Buttigliero, Teresa Gamba, Andrea Necchi, Marcello Tucci, and Massimo Di Maio. 2020. "Evaluation of Cognitive Function in Trials Testing New-Generation Hormonal Therapy in Patients with Prostate Cancer: A Systematic Review" Cancers 12, no. 9: 2568. https://doi.org/10.3390/cancers12092568
APA StyleMarandino, L., Vignani, F., Buttigliero, C., Gamba, T., Necchi, A., Tucci, M., & Di Maio, M. (2020). Evaluation of Cognitive Function in Trials Testing New-Generation Hormonal Therapy in Patients with Prostate Cancer: A Systematic Review. Cancers, 12(9), 2568. https://doi.org/10.3390/cancers12092568