Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines
Abstract
1. Introduction
2. Results
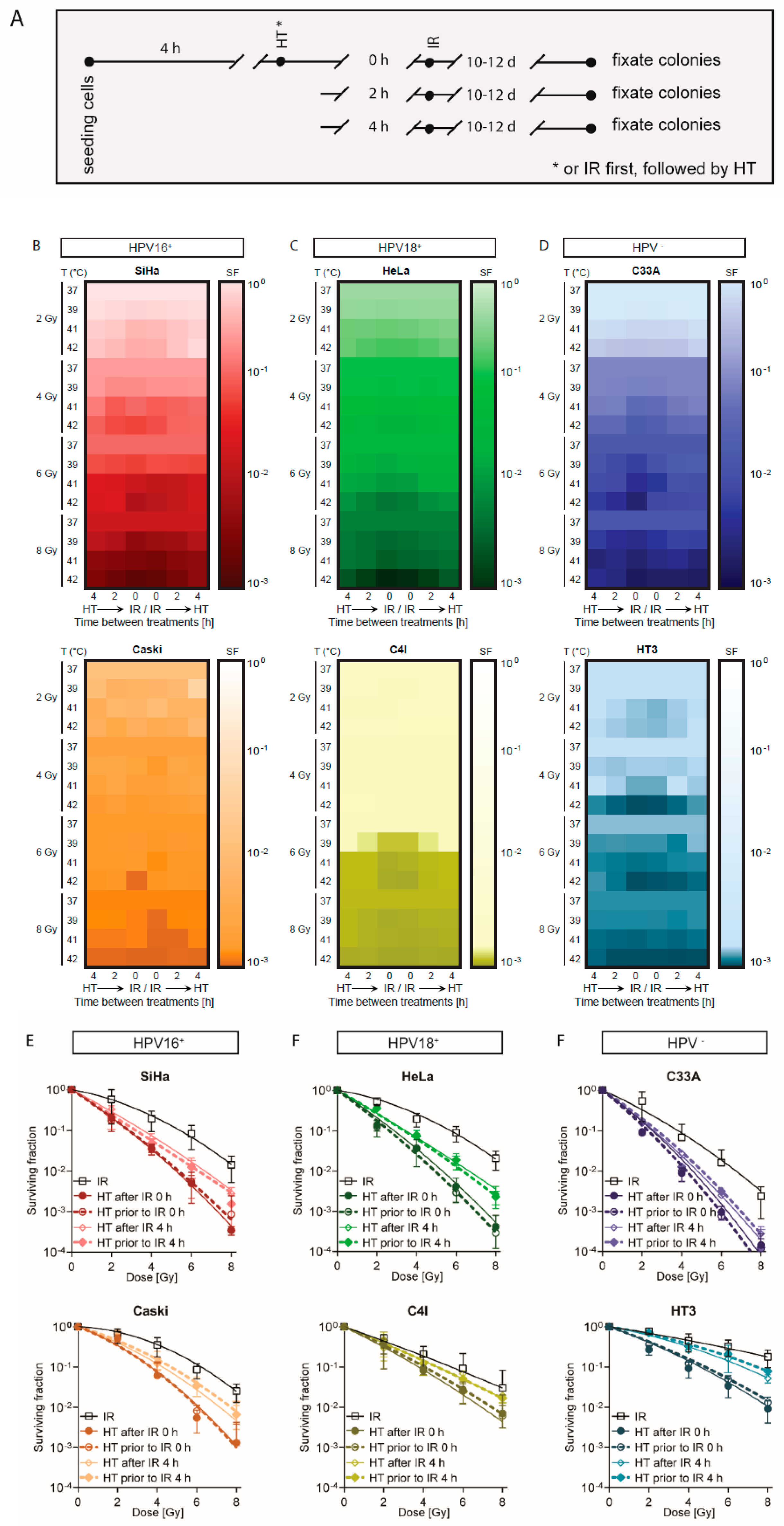
2.1. A Short Time Interval between Ionizing Radiation and Hyperthermia Results in a Lower Cell Survival
2.2. A Higher G2 Arrest after a Short Time Interval between Hyperthermia and Ionizing Radiation
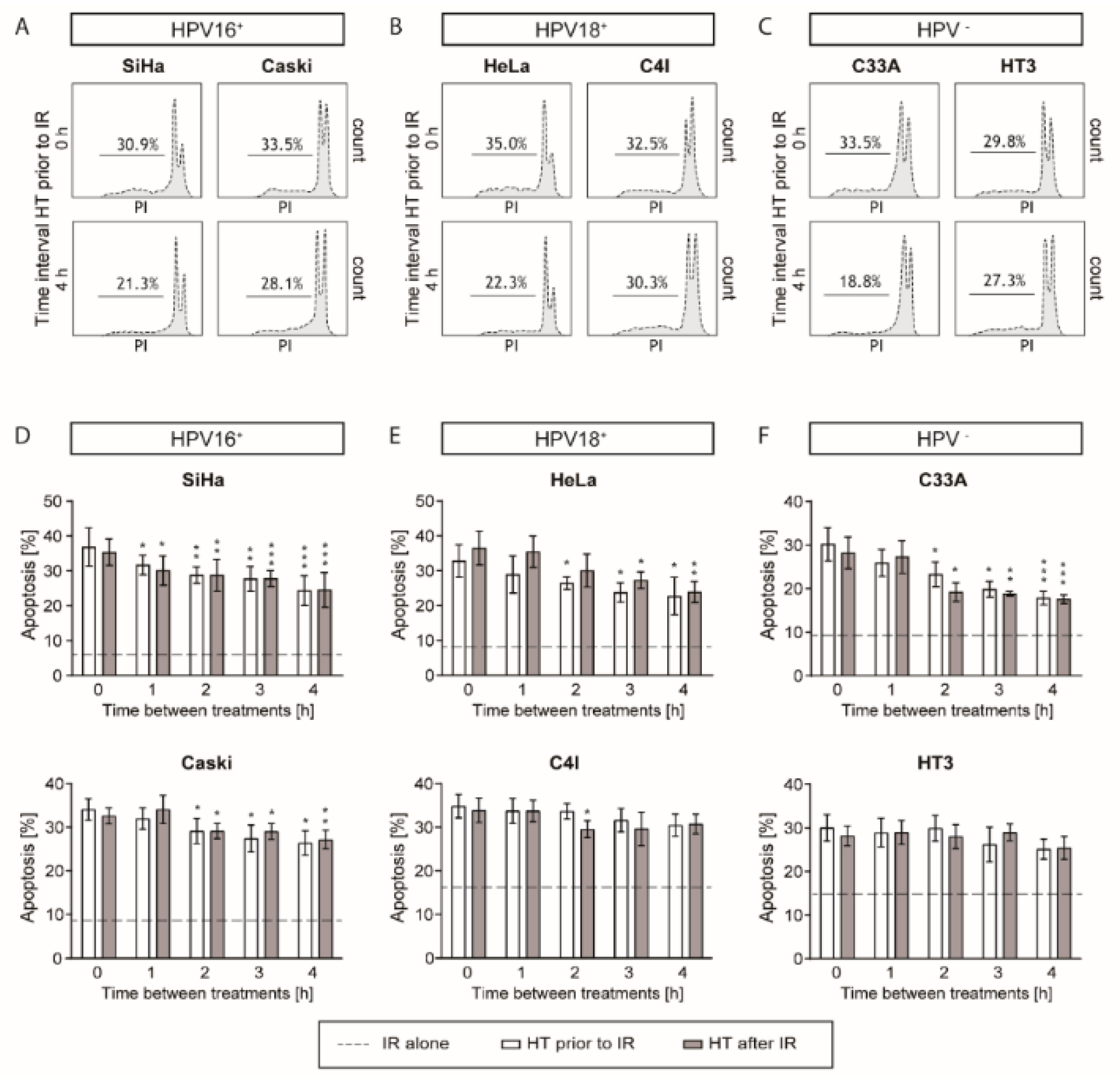
2.3. Apoptotic Levels are the Highest after a Short Time Interval between Ionizing Radiation and Hyperthermia
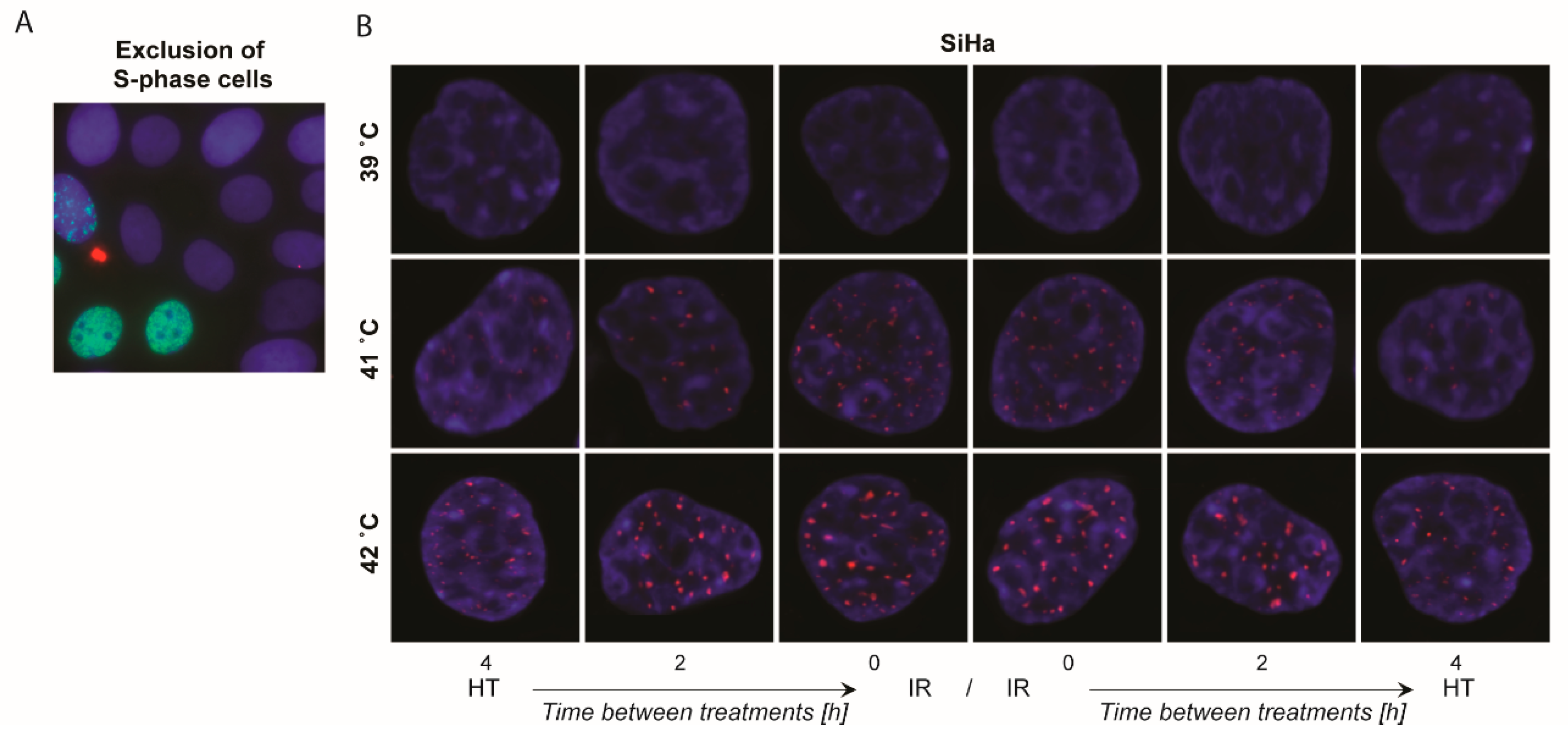
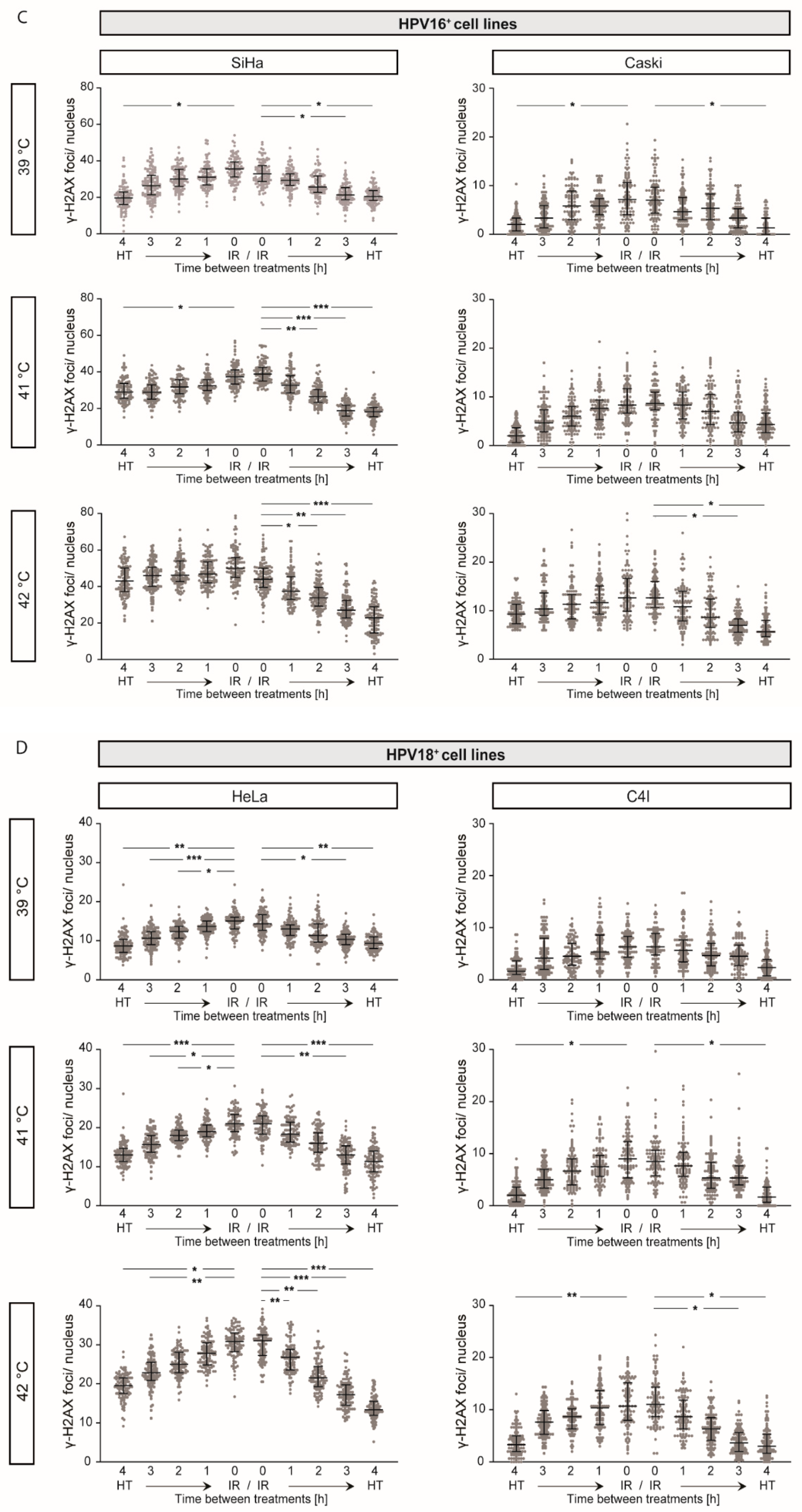
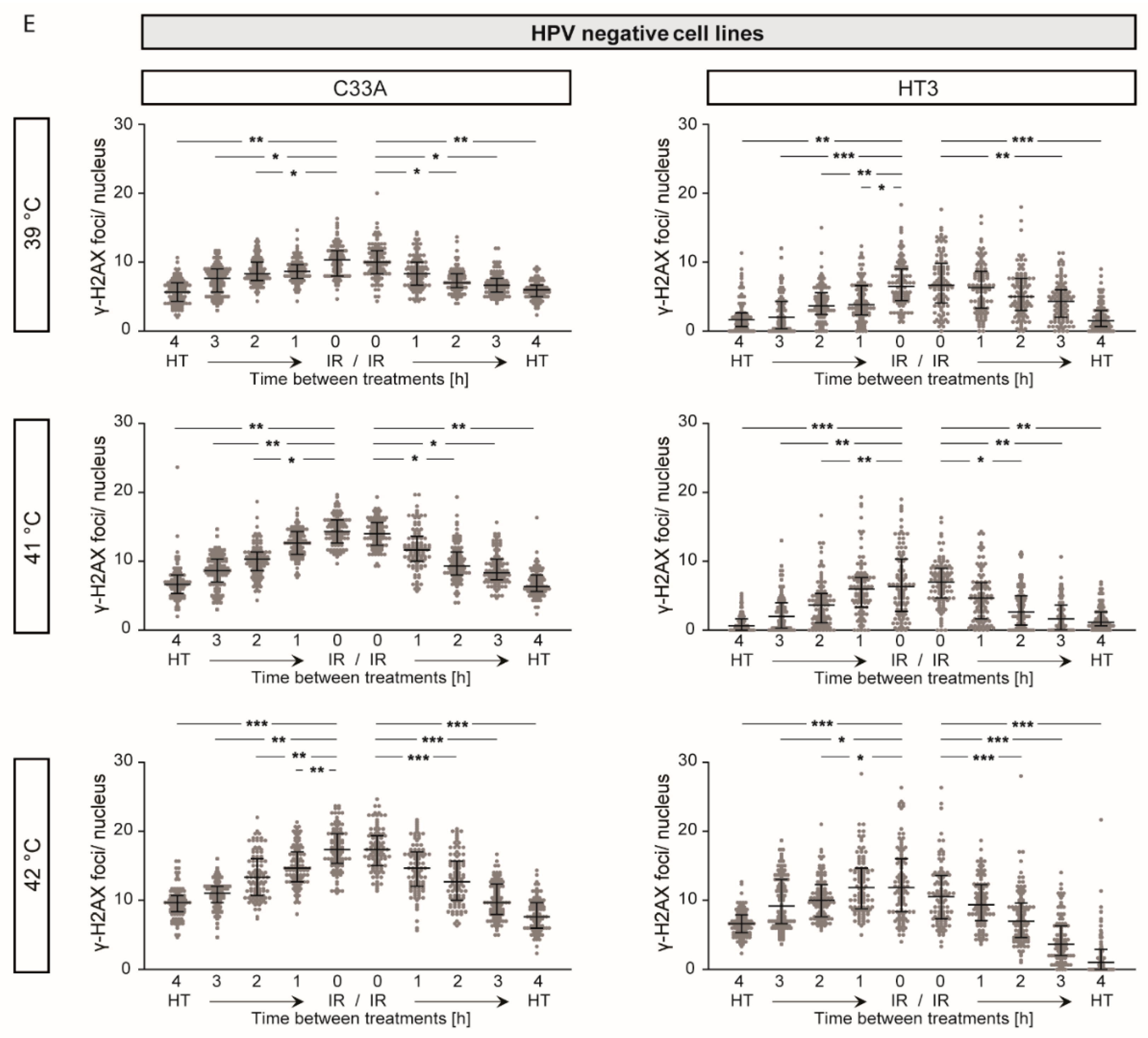
2.4. γ-H2AX Foci Levels Are Increased at Higher Temperatures and at Shorter Time Intervals between Ionizing Radiation and Hyperthermia
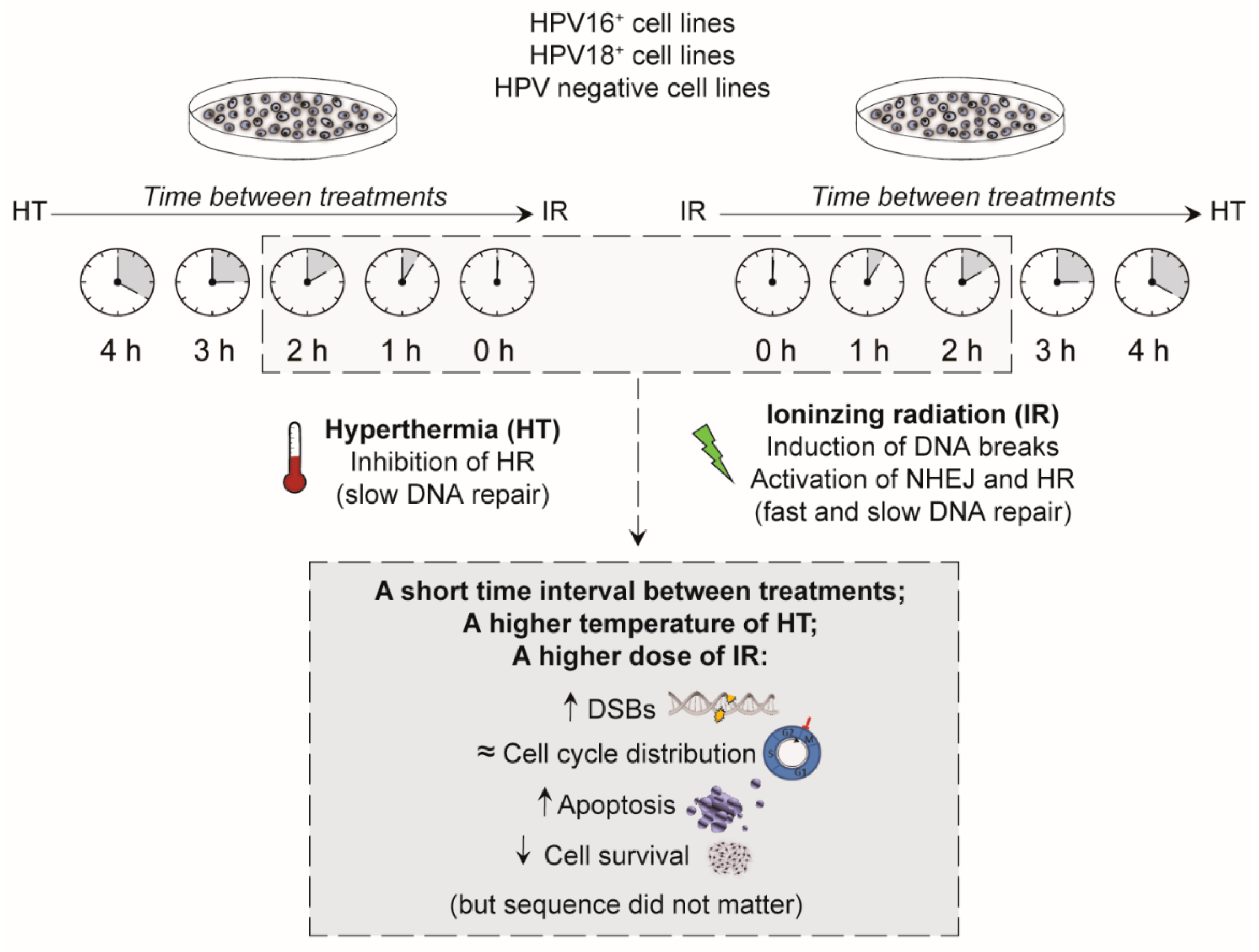
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Cell Treatments
4.3. Cell Survival Assay
4.4. Detection of DNA DSBs via γ-H2AX Staining
4.5. Cell Cycle Analysis
4.6. Apoptosis Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Šarenac, T.; Momir, M. Cervical cancer, different treatments and importance of bile acids as therapeutic agents in this disease. Front. Pharmacol. 2019, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordonez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperthermia 2015, 31, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Klingbiel, D.; Gomez, S.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperthermia 2016, 32, 809–821. [Google Scholar] [CrossRef]
- Lutgens, L.; van der Zee, J.; Pijls-Johannesma, M.; De Haas-Kock, D.F.; Buijsen, J.; Mastrigt, G.A.; Lammering, G.; De Ruysscher, D.K.; Lambin, P. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst. Rev. 2010, 3, CD006377. [Google Scholar] [CrossRef]
- Datta, N.R.; Stutz, E.; Gomez, S.; Bodis, S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 411–437. [Google Scholar] [CrossRef]
- Lutgens, L.C.; Koper, P.C.; Jobsen, J.J.; van der Steen-Banasik, E.M.; Creutzberg, C.L.; van den Berg, H.A.; Ottevanger, P.B.; van Rhoon, G.C.; van Doorn, H.C.; Houben, R.; et al. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: Results of the randomized RADCHOC trial. Radiother. Oncol. 2016, 120, 378–382. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef]
- Moon, E.J.; Sonveaux, P.; Porporato, P.E.; Danhier, P.; Gallez, B.; Batinic-Haberle, I.; Nien, Y.C.; Schroeder, T.; Dewhirst, M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 2010, 107, 20477–20482. [Google Scholar] [CrossRef]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Dewhirst. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer cell 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Tamulevicius, P.; Streffer, C. Bioluminescence imaging of metabolites in a human tumour xenograft after treatment with hyperthermia and/or the radiosensitizer pimonidazole. Int. J. Hyperthermia 1997, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Streffer, C. Metabolic changes during and after hyperthermia. Int. J. Hyperthermia 1985, 1, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Crezee, J.; van Leeuwen, C.M.; Oei, A.L.; van Heerden, L.E.; Bel, A.; Stalpers, L.J.; Ghadjar, P.; Franken, N.A.; Kok, H.P. Biological modelling of the radiation dose escalation effect of regional hyperthermia in cervical cancer. Radiat. Oncol. 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperthermia 2016, 32, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, H.; Griffin, R.J. Improvement of tumor oxygenation by mild hyperthermia. Radiat. Res. 2001, 155, 515–528. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R.; Ong, E.T.; Gross, J.F.; Dewhirst, M.W. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995, 34, 313–316. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lee, C.T.; Ashcraft, K.A. The future of biology in driving the field of hyperthermia. Int. J. Hyperthermia 2016, 32, 4–13. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Dikomey, E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int. J. Radiat. Biol. 2001, 77, 399–408. [Google Scholar] [CrossRef]
- Fleck, O.; Nielsen, O. DNA repair. J. Cell Sci. 2004, 117, 515–517. [Google Scholar] [CrossRef]
- Iliakis, G.; Wu, W.; Wang, M. DNA double strand break repair inhibition as a cause of heat radiosensitization: Re-evaluation considering backup pathways of NHEJ. Int. J. Hyperthermia 2008, 24, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumour and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Wachsberger, P.R.; Burd, R.; Bhala, A.; Bobyock, S.B.; Wahl, M.L.; Owen, C.S.; Rifat, S.B.; Leeper, D.B. Quercetin sensitizes cells in a tumour-like low pH environment to hyperthermia. Int. J. Hyperthermia 2003, 19, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Nygaard, T.G.; Burlett, M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res. 1979, 39, 966–972. [Google Scholar]
- Koutcher, J.A.; Barnett, D.; Kornblith, A.B.; Cowburn, D.; Brady, T.J.; Gerweck, L.E. Relationship of changes in pH and energy status to hypoxic cell fraction and hyperthermia sensitivity. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1429–1435. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Oei, A.L.; Ten Cate, R.; Franken, N.A.P.; Bel, A.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P. Measurement and analysis of the impact of time-interval, temperature and radiation dose on tumour cell survival and its application in thermoradiotherapy plan evaluation. Int. J. Hyperthermia 2018, 34, 30–38. [Google Scholar] [CrossRef]
- Giunta, S.; Belotserkovskaya, R.; Jackson, S.P. DNA damage signaling in response to double-strand breaks during mitosis. J. Cell Biol. 2010, 190, 197–207. [Google Scholar] [CrossRef]
- Sinclair, W.; Morton, R. X-ray and ultraviolet sensitivity of synchronized Chinese hamster cells at various stages of the cell cycle. Biophys. J. 1965, 5, 1–25. [Google Scholar] [CrossRef]
- Oei, A.L.; Ahire, V.R.; van Leeuwen, C.M.; Ten Cate, R.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P. Enhancing radiosensitisation of BRCA2-proficient and BRCA2-deficient cell lines with hyperthermia and PARP1-i. Int. J. Hyperthermia 2018, 34, 39–48. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Crezee, J.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A.; Bel, A.; Kok, H.P. The effect of time interval between radiotherapy and hyperthermia on planned equivalent radiation dose. Int. J. Hyperthermia 2018, 34, 901–909. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.M.; Oei, A.L.; Chin, K.; Crezee, J.; Bel, A.; Westermann, A.M.; Buist, M.R.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat. Oncol. 2017, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; van Leeuwen, C.M.; ten Cate, R.; Rodermond, H.M.; Buist, M.R.; Stalpers, L.J.; Crezee, J.; Kok, H.P.; Medema, J.P.; Franken, N.A. Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res. 2015, 75, 5120–5129. [Google Scholar] [CrossRef]
- Li, G.C.; Kal, H.B. Effect of hyperthermia on the radiation response of two mammalian cell lines. Eur. J. Cancer 1977, 13, 65–69. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Vujaskovic, Z.; Jones, E.; Thrall, D. Re-setting the biologic rationale for thermal therapy. Int. J. Hyperthermia 2005, 21, 779–790. [Google Scholar] [CrossRef]
- Patel, J.K.; Vasques, R.; Ganapol, B.D. Towards a Multiphysics Model for Tumor Response to Combined-Hyperthermia-Radiotherapy Treatment. In Proceedings of the 2019 International Conference on Mathematics and Computational Methods Applied to Nuclear Science and Engineering, M and C 2019, Portland, OR, USA, 25–29 August 2019; pp. 1002–1011. [Google Scholar]
- van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperthermia 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Crezee, H.; van Leeuwen, C.M.; Oei, A.L.; Stalpers, L.J.; Bel, A.; Franken, N.A.; Kok, H.P. Thermoradiotherapy planning: Integration in routine clinical practice. Int. J. Hyperthermia 2016, 32, 41–49. [Google Scholar] [CrossRef]
- Bergs, J.W.; Oei, A.L.; Ten Cate, R.; Rodermond, H.M.; Stalpers, L.J.; Barendsen, G.W.; Franken, N.A. Dynamics of chromosomal aberrations, induction of apoptosis, BRCA2 degradation and sensitization to radiation by hyperthermia. Int. J. Mol. Med. 2016, 38, 243–250. [Google Scholar] [CrossRef]
- van Bree, C.; Franken, N.A.; Snel, F.A.; Haveman, J.; Bakker, P.J. Wild-type p53-function is not required for hyperthermia-enhanced cytotoxicity of cisplatin. Int. J. of Hyperthermia 2001, 17, 337–346. [Google Scholar] [CrossRef]
- van Bree, C.; Franken, N.A.; Rodermond, H.M.; Stalpers, L.J.; Haveman, J. Repair of potentially lethal damage does not depend on functional TP53 in human glioblastoma cells. Radiat. Res. 2004, 161, 511–516. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, X.; ten Cate, R.; van Leeuwen, C.M.; Rodermond, H.M.; de Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P.; et al. Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines. Cancers 2020, 12, 582. https://doi.org/10.3390/cancers12030582
Mei X, ten Cate R, van Leeuwen CM, Rodermond HM, de Leeuw L, Dimitrakopoulou D, Stalpers LJA, Crezee J, Kok HP, Franken NAP, et al. Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines. Cancers. 2020; 12(3):582. https://doi.org/10.3390/cancers12030582
Chicago/Turabian StyleMei, Xionge, Rosemarie ten Cate, Caspar M. van Leeuwen, Hans M. Rodermond, Lidewij de Leeuw, Dionysia Dimitrakopoulou, Lukas J. A. Stalpers, Johannes Crezee, H. Petra Kok, Nicolaas A. P. Franken, and et al. 2020. "Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines" Cancers 12, no. 3: 582. https://doi.org/10.3390/cancers12030582
APA StyleMei, X., ten Cate, R., van Leeuwen, C. M., Rodermond, H. M., de Leeuw, L., Dimitrakopoulou, D., Stalpers, L. J. A., Crezee, J., Kok, H. P., Franken, N. A. P., & Oei, A. L. (2020). Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines. Cancers, 12(3), 582. https://doi.org/10.3390/cancers12030582








