Simple Summary
Malignant melanoma is the most aggressive skin cancer; its incidence is constantly growing in the white population. In the advanced stage of the disease, after metastatic dissemination, patients have a poor prognosis. Nanomedicine represents a new frontier in cancer treatment; in this field, synthetic and natural nanoparticles (NPs) may represent an important therapeutic and diagnostic opportunity. This review provides an overview of current knowledge in this area: several kinds of NPs, like PLGA, chitosan, liposome and gold-NPs, are used to increase the specificity of drug delivery, allowing a dose reduction and, consequently, a lower toxic effect. Particular attention is given to exosomes (EXOs), an example of natural NPs, important both in conveying molecules with a therapeutic function and in the diagnostic field.
Abstract
Advanced melanoma is still a major challenge in oncology. In the early stages, melanoma can be treated successfully with surgery and the survival rate is high, nevertheless the survival rate drops drastically after metastasis dissemination. The identification of parameters predictive of the prognosis to support clinical decisions and of new efficacious therapies are important to ensure patients the best possible prognosis. Recent progress in nanotechnology allowed the development of nanoparticles able to protect drugs from degradation and to deliver the drug to the tumor. Modification of the nanoparticle surface by specific molecules improves retention and accumulation in the target tissue. In this review, we describe the potential role of nanoparticles in advanced melanoma treatment and discuss the current efforts of designing polymeric nanoparticles for controlled drug release at the site upon injection. In addition, we highlight the advances as well as the challenges of exosome-based nanocarriers as drug vehicles. We place special focus on the advantages of these natural nanocarriers in delivering various cargoes in advanced melanoma treatment. We also describe the current advances in knowledge of melanoma-related exosomes, including their biogenesis, molecular contents and biological functions, focusing our attention on their utilization for early diagnosis and prognosis in melanoma disease.
1. Introduction
Advanced cutaneous melanoma is a highly aggressive and drug-resistant cancer [1,2]. The main problem associated with treating melanoma is its low response rate to conventional therapy. Nevertheless, the rapidly developed fields of melanoma research are introducing new potential therapeutic approaches, focusing on developing new efficient drugs and delivery systems. Before 2011, metastatic melanoma was considered incurable, leading to death within 18 months of diagnosis [3,4]. The therapies used were based on the administration of chemotherapeutic agents such as dacarbazine and temozolomide [5]. In order to circumvent the intrinsic resistance against chemotherapy, researchers explored the possibility of potentiating the immune response by using immune stimulators, such as interleukin 2 (IL-2), interferon alpha (IFN-α), ipilimumab and thymosin alpha 1 [6,7]. Afterwards, as result of genome sequencing and identification of the main mutation, a series of therapeutic agents have been discovered; about half of all melanomas have mutations in the BRAF (v-raf murine sarcoma viral oncogene homolog B1) gene [8,9]. These mutations constitutively activate the MAPK (mitogen-activated protein kinase) pathway, resulting in uncontrolled cell proliferation, tumor development and growth [1,10]. Several tyrosine kinase inhibitors (TKIs) have been developed that block the MAPK pathway and inhibit cell growth and survival. The use of TKIs has shown a significant improvement of patient overall survival and the combinations therapy with BRAF/MEK inhibitors is now accepted as the standard care for BRAF-muted advanced melanoma. Unfortunately, in most treated patients, responses to these inhibitory molecules are transient, and, in a few months, a therapy-resistant phenotype occurs. Recently, the inhibition of the programmed cell death 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) axis, a suppressor of T-cell response, turned out to be an effective therapeutic strategy with a low toxicity profile and contributed to better clinical outcomes compared to chemotherapy and also to other immune mediated approaches with cytotoxic T lymphocyte-associated 4 (CTLA-4) inhibition [11,12,13]. However, substantial heterogeneity exists in metastatic melanoma response to treatments; therefore, the development of multimodality strategies is the major challenge to fight this deadly disease. Several types of nanoparticles (NPs) and nanovesicles have been explored for their employment as drug carriers in melanoma therapy and their functionalization with specific molecules, such as antibodies, can generate different smart nanodrugs for application in melanoma therapy (Figure 1). Herein, we will discuss the new frontiers in the treatment of patients with unresectable or metastatic melanoma, paying special attention to new anticancer molecules and their delivery through natural or synthetic nanoparticles.
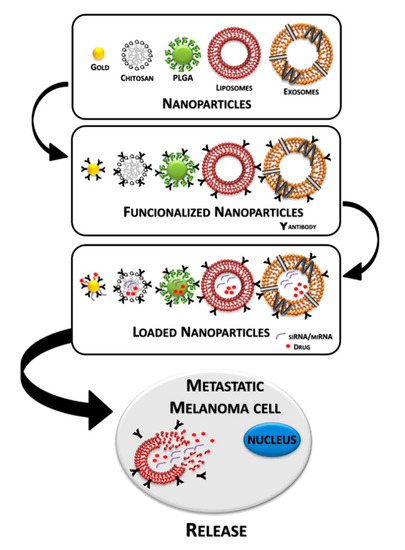
Figure 1.
Step-by-step representation of a nanoparticles-based delivery system for melanoma therapy.
2. Nanoparticles and Drug Delivery
Nanomaterials are delivery vehicles used to protect the intended drug against degradation and enhance its stability. Using a nanotechnology treatment for several types of cancer enhances targeted delivery to cancer cells and uptake, as well as reducing cytotoxic side effects on normal tissues [14].
Chemotherapeutic agents generally exhibit a short plasma half-life due to rapid clearance and fast biodegradation; these elements, together with the low specificity, make it necessary to use high doses, resulting in severe toxic effects. In addition, since cancer diagnosis is often late, cancer cells have already invaded other parts of the body [15].
Nanotechnologies are based on the use of different polymeric systems whose modifiable physical and chemical systems provide tools to improve different aspects of the therapy. Some examples are the release of the drug in a tissue-specific way, the decrease in side effects, the protection from drug degradation, the possibility to reduce the frequency of administrations and the toxicity of the substance itself [16]. Depending on their specific structural characteristics, nanoparticles can easily penetrate tissues and cells, circumscribe the biological effect of the drug on a specific cell type and modify the pharmacokinetic properties, thus allowing a prolonged drug release over time. The Food and Drug Administration (FDA) approved only a few nanodrugs for use and clinical trials, as they have been shown to either target and directly kill tumor cells or improve, overall, targeted chemotherapy drug delivery. The formulation of these nanoparticles increases the drug concentration into the tumor by 100–400% [17].
2.1. PLGA
The FDA and the European Medicine Agency (EMA) approved PLGA nanoparticles (PLGA-NPs) in 2011 as a system for conveying nucleic acids, drugs and other molecules for cancer and other diseases. PLGA is a biocompatible and biodegradable polymer (poly-lactic acid and poly-glycolic acid) whose degradation produces non-toxic substances, such as water and carbon dioxide [18]. The PLGA-NPs’ cellular uptake depends on both the size of the NP and the composition of the plasma membrane of the cells themselves [19]. The different physical characteristics of PLGA-NPs, such as their size and morphology, can be determined by varying the parameters of the synthesis method used. Nanoparticles have a large surface area that can be functionalized with different agents to make the vehicle target-specific [20].
In the last period, several publications highlighted the efficiency of PLGA-NPs in delivering anticancer agents; in a recent work, Arruda and his co-workers showed that the encapsulation of a peptide with antitumoral activity, P20 (CSSRTMHH), within the PLGA-NPs conjugated to the C peptide with a homing function (CVNHPAFACGYGHTMYYHHYQHHL), strongly increases their functionality. The therapeutic effect of these NPs was evaluated in a syngeneic model of metastasis and was shown to produce the same inhibitory effect of free P20 at fivefold higher concentration, reducing by 28% the number of lung nodules [21].
In another recent study, trastuzumab has been used as a recognizing unit attached to NPs for Her-2 positive breast cancer therapy. Targeted nanoparticles were prepared by combining PLGA/ polyethylenimine (PEI)/lipid nanoparticles (PPLNs) with trastuzumab through the electrostatic adsorption method to deliver the antitumor drug docetaxel. The results showed that trastuzumab-PPLNs had a cell-targeting effect and could effectively inhibit the proliferation of cancer cells. This work demonstrates that trastuzumab-PPLNs are a promising treatment for breast cancer and can be considered a proof of concept for anticancer therapies, including melanoma [22].
Following a similar approach, Kou et al. selected paclitaxel as anti-metastatic and anti-angiogenic model drug to produce paclitaxel-loaded PLGA-NPs (Ptx-NPs), coated with a polylysine cationic SM5-1 single-chain antibody (Ptx-NP-S) which binds to a membrane protein specifically expressed in melanoma, hepatocellular carcinoma (HCC) and breast cancer cells. The authors demonstrated that Ptx-NP-S not only showed very high affinity to SM5-1 but also was internalized by the target cells. They proved a significantly enhanced cytotoxicity of Ptx-NP-S when compared to non-targeted paclitaxel-loaded PLGA-NPs, suggesting SM5-1 single-chain antibody-polylys as a good candidate to synthesize cancer-targeted PLGA nanoparticles [23].
Another important field of application of PLGA-NPs is as peptide transporters in vaccines. Vaccines could be promising tools for treating cancers, including melanoma [24]; however, several studies obtained unsatisfying results, possibly because of the low stability of peptides and delivery approaches. Thus, the appropriate and efficient peptide carrier systems still continue to be one of the major obstacles. In order to overcome this difficulty, Li and coworkers recently used PLGA-NPs as a new method to deliver tumor-associated antigen (TAA) peptides to dendritic cells (DC) [25].
2.2. Chitosan
Chitosan is a bio-poly-amino-saccharide cationic polymer obtained from chitin deacetylation moieties formed by the repetition of two units: N-acetyl-D-glucosamine and D- glucosamine [26]. The molecular weight and the extent of deacetylation of the chitosan influence its properties; this is employed specially to prepare micro and nanoparticles capable of conveying nucleic acids or medicines. The chitosan backbone consists of multiple free amino and hydroxyl groups that can be functionalized. Versatile approaches have been reported for the construction of chitosan-based nanomaterials, such as nanogels, NPs, micelles, liposomes, nanofibers and nanospheres [27]. The polymer’s success is due to its properties such as: biocompatibility and biodegradability, FDA approval in clinical use, the possibility of modifying its surface and to complex it in nanoparticles conveyed against cells and/or target tissues. Chitosan exhibits no or minimal toxicity, which made it widely regarded as a safe material in drug delivery [28]. Various cargos, hydrophilic and also hydrophobic, can be incorporated into chitosan-NPs. Their release is influenced by solubility, diffusion, and size of the nanoparticles [29]. Many publications show innovative ways for antitumor drug delivery based on environmental response and targeting principles [30,31].
A recent study evaluated the effect of doxorubicin encapsulated into chitosan/alginate nanoparticles to inhibit viability and tumor growth of melanoma cells in a mouse melanoma model. The experiments revealed that the encapsulation of doxorubicin could be considered advantageous because it increases intracellular accumulation and produces a long-lasting cytotoxic effect [32]. The cationic nature of chitosan makes it suitable to complex anionic nucleic acids, protecting them from degradation by nucleases in serum [33].
One of the possible applications of this property is the transport of two specific categories of non-coding RNAs called microRNA (miRs) and short interfering RNA (siRNAs) [34,35]. MiRs regulate the expression of messenger RNAs (mRNAs) at a post-transcriptional level. They are implicated in various aspects of tumor progression, including proliferation, apoptosis, and epithelial–mesenchymal transition (EMT), and they can promote or suppress tumorigenesis and metastatization, thus acting either as onco-suppressors or oncogenes [14,36,37,38].
The possibility of protecting these molecules from enzymatic degradation using chitosan-NPs represents a very important therapeutic option. SiRNAs have broad potential as therapeutic agents because they are able to silence any gene in a reversible way. To achieve the clinical potential of siRNAs, delivery materials are required to transport them in the cells of target tissues.
Rostami and coworkers, in a recent study, developed the alginate-conjugated trimethyl chitosan (ATMC) nanoparticles loaded with specific siRNAs. In particular, in the cancer cells the activation of S1P/sphingosine-1-phosphate receptor 1 (S1PR1) and IL-6/glycoprotein 130 (GP130) genes induces STAT3 phosphorylation, which in turn stimulates their expression.
On this basis, the authors investigated the potential anticancer effect by silencing the S1PR1 and GP130 genes, through ATMC-NPs containing specific siRNAs; the results obtained in different cancer cell lines, including melanoma B16F10, although in need of further study, have been encouraging [35].
2.3. Liposomes
Liposomes are small lipid vesicles (50 to 100 nm) generated from cholesterol, which reduces their permeability and increases their in vivo and in vitro stability [39]. Liposomes, categorized by the composition and mechanism of intracellular delivery, fall under five types: conventional liposomes (CL), pH-sensitive liposomes, cationic liposomes, immunoliposomes, and long-circulating liposomes (LCL). Liposomes with modified surfaces have also been developed using several molecules, such as glycolipids or sialic acid [40]. To prolong their circulation time, liposome surfaces have been coated with a hydrophilic polymer, such as polyethylene glycol (PEG), thus increasing repulsive forces between liposomes and plasma components [41]. Liposomes seem to be an almost ideal drug-carrier system due to their morphology being similar to that of cellular membranes and for their ability to incorporate various substances.
Recently, Gao et al. demonstrated that liposome-PEI encapsulation improved the uptake of n-Butylidenephthalide (BP) through enhancement of cell endocytosis, indicating that the BP/lipo PEG PEI complex (LPPC) has great potential in melanoma treatment. In particular, because BP is metabolized very rapidly, its antitumor role, already demonstrated in various types of cancer, including melanoma, would be limited. To avoid this problem, the researchers developed the complex BP/LPPC, improving both the uptake of this drug by the cells and its stability over time [42].
Yin et al. prepared R8-dGR peptide modified paclitaxel (PTX) and hydroxychloroquine (HCQ) co-loaded liposomes (PTX/HCQ-R8-dGR-Lip) to enhance delivery through recognition of integrin αvβ3 receptors and neuropilin-1 receptors on B16F10 melanoma cells. The results showed that R8-dGR modified liposomes (R8-dGR-Lip) enhanced tumor-targeting delivery in vitro and in vivo [43].
Health authorities have approved different liposomal formulations of small molecule therapeutics; their ability to deliver macromolecules, such as nucleic acid-based therapeutics plasmid DNA (pDNA) and siRNA, to disease sites is under investigation. In a recent study, lipid nanocapsules were used to combine two anti-melanoma strategies, gene therapy by using Bcl-2 siRNA and ferrocifens as novel anticancer agents. The results showed a 50% reduction in tumor volume in tumor-bearing animals compared to the control group [44].
Sadhu et al. utilized liposomes to deliver glutathione disulfide to melanoma cells. These liposomes slowed down tumor proliferation by 85–90% in melanoma-bearing mice and significantly improved the survival rate [45]. Interestingly, lipid constituents of liposomal vesicles can be tailored to achieve particular immunogenic responses. Manipulation of surface charge density in cationic liposomes can regulate immune response [46].
Fu and coworkers developed paclitaxel (PTX)-loaded liposomes functionalized with trans activator of transcription (TAT), the most frequently used cell-penetrating peptide, and cleavable PEG. Under physiological conditions, the TAT peptide is shielded by a PEG layer and so liposomes exhibit a good stability in the bloodstream. When liposomes arrive at the tumor site, exogenous reducing agents could remove PEG, causing TAT exposure and facilitating cell internalization. In this study, the authors showed a tumor inhibition of around 70% in B16F1-bearing mice [47].
Lastly, the liposomes may also be used as an adjuvant to boost the protective or therapeutic immune response [48].
2.4. Gold Nanoparticles
Gold nanoparticles (AuNPs) are a type of nanoparticles produced in various sizes and shapes (i.e., gold nanospheres, nanorods, nanocages, nanoshells, and nanostars). They are dependent on the synthetic methods adopted for their preparation [49]. These nanoparticles can be prepared in a broad range of core sizes (1 to 150 nm), which makes it easier to control their dispersion. The presence of a negative charge on the surface of gold nanoparticles makes them easily modifiable.
Literature reports that various types of gold nanoparticles are effective tools in interfering with human cancer cell biology, including melanoma [50]. Indeed, their application in therapeutic development and in cancer diagnosis could provide a potential step forward in cancer theranostics [51]. Thanks to their physical, chemical and biological features, AuNPs can be used in imaging technology, such as computed tomography, ultrasound and magnetic resonance and a few of them have been approved by the FDA in medicine [52].
Kim et al. demonstrated the use of AuNPs as a contrast agent for the quantitative molecular combining of high-resolution photoacoustic tomography (PAT) of melanomas in vivo. To avoid the limitations of conventional diagnostic techniques, the authors exploited the enormous optical absorbing gold nanocages (AuNCs), obtaining a 300% increase in the contrast when AuNCs were bioconjugated with [Nle4,d-Phe7]-α-melanocyte-stimulating hormone compared to the control nanocages [53].
Through the addition of various biomolecules such as drugs, target ligands and genes, the nanoparticles could function in an enhanced way. To overcome in vivo delivery barriers, Au nanocomplexes are normally modified with functional moieties such as stabilizing materials, target ligands, and bio responsive linkers [54,55].
Conjugation with the anti-nucleosome monoclonal antibody (mAb 2C5) of PEGylated liposomes improves cancer cell-specific delivery targeting the matrix metalloprotease 2 in the tumor microenvironment [56]. Many studies showed that the use of magnetic fields or altered temperatures and light improve the delivery of gold particles including anticancer agents [57].
2.5. Other Nano-Agents
Other nanoparticles have a potential use as anti-melanoma agents (Table 1) [58,59,60,61,62,63,64,65].

Table 1.
Several types of nanoparticles used in melanoma treatment.
2.6. Specific Drug Delivery
The ideal drug carrier would have the ability to deliver the drug to the cell/tissue/organ in a specific way to make the cancer treatment more efficient. A potentially powerful technology is represented by targeted nanoparticles. The most used approach is to conjugate to nanoparticles antibodies or antibody-derivatives which must be able to overcome the physiological barriers and conditions that limit access to the target, and to bind target cells through specific antibody–receptor interactions [66].
Primary tumors as well as metastatic tumors overexpress some antigens on their surfaces. Particularly, for melanoma treatment, the literature shows the specificity of many antigens.
Secreted protein acidic and rich in cysteine (SPARC) was deepened in the tumorigenicity and progression of melanoma, resulting as a nonstructural matricellular protein [67]. The conjugation of nanoparticles with a peptide able to specifically recognizing the SPARC protein, has made it possible to obtain an important tool that exhibited high tumor affinity with a very low binding in negative cell lines. In in vivo targeting experiments, an increase of SPARC-targeted nanoparticles was observed in tumor tissue as compared with the control group. Moreover, a very important result was the detection of both primary tumors and metastatic growth obtained by using the fluorescent version of these nanoparticles [68].
Melanoma antigen genes (MAGE)-A proteins are a melanoma-specific-antigen family [69]. Saeed and coworkers in a recent work described that the single-chain variable fragment (scFv) antibodies directed against MAGE-A1 peptide, presented by human leukocyte antigen A1 (HLA-A1), in short M1/A1, have been coupled to liposomes to achieve specific melanoma targeting. Through experiments by flow cytometry and confocal microscopy, the authors demonstrated the bind between M1/A1-positive and B-cells as well as with melanoma cells and the internalization by these cells [70].
The chondroitin sulfate proteoglycan 4 (CSPG4) is a highly specific marker of the nevomelanocyte lineage and has been utilized to target melanoma. In a recent work, Falvo and coworkers produced nanoparticles conjugated to a monoclonal antibody specific for CSPG4 encapsulating cisplatin molecules. Flow cytometry showed a specific binding to CSPG4 (+) melanoma cell lines. Selectivity towards melanoma was also confirmed in xenograft models: targeted nanoparticles significantly inhibited growth of melanoma xenografts whereas modestly affected breast carcinoma xenografts [71].
These studies highlight the importance of the potential active targeting to improve the outcome of a nanoparticle-based therapy.
2.7. Natural Nanoparticles
All cell types of the body release extracellular Vesicles (EVs), which are present in all body fluids. They are defined by their mechanism of cellular production and include exosomes (EXOs), microvesicles (MVs) and apoptotic bodies [72]. The diameter of the EXOs ranges from 30 to 150 nm; they are secreted by most cell types into the extracellular microenvironment under normal and pathological conditions and can be found in blood, sweat, saliva, urine, amniotic fluids, breast milk, cerebrospinal fluids and malignant ascites [73,74,75,76]. EXOs have a membrane consisting of a lipid bilayer and contain various biomolecules, including proteins, lipids, RNA and DNA, suggesting their involvement in the regulation of various biological functions through different molecular mechanisms [77,78,79].
When EXOs are taken up by other cells, their cargo (mRNAs, miRs and protein) is transferred and influences the phenotype of recipient cells [80,81]. EXOs mediate cell-to-cell communication and are involved in different physiological and pathological processes, also playing an important role in cancer [82]. Like other tumors, melanoma cells modify their microenvironment to enable growth and metastasis via direct cellular interactions or the indirect release of soluble factors and/or EXOs.
Similarly to synthetic nanoparticles, EXOs can be considered drug delivery systems and possess important advantages such as long-term circulatory capability, good biocompatibility, minimal toxicity and intrinsic ability to target specific cells [83]. Moreover, one of the most useful properties of EXOs is their ability to cross the cytoplasmic membrane [84] and the blood/brain barrier [85].
Altogether, these characteristics make EXOs a very interesting drug delivery system [86]. Recently, several studies have focused on specific modification of EXOs surface to make them a proper candidate for cellular uptake and target specificity [87]. In particular, it has been proved that modification of EXO–tetraspanin complexes strongly influences target cell selection both in vitro and in vivo [88], improving the targeting of tissues and cell types of interest. Other modified transmembrane proteins are the platelet-derived growth factor receptors [89]. In 2018, Kim et al. engineered macrophage-derived EXOs loaded with the anticancer drug paclitaxel to improve their circulation time in the blood and allow them to target pulmonary metastases. By using this modified EXOs, the drug can selectively deliver to target cancer cells and also can increase the survival rate in an in vivo lung cancer model [90].
In the last decade, several studies have focused on immune system stimulation for the treatment of different cancers. Dendritic cell-derived EXOs (dexosome) have been successfully engineered to target helper T cells to stimulate cytotoxic T cell proliferation, influence T cell differentiation and create an anti-tumor environment [91]. Dexosomes have entered clinical trials for colorectal cancer [92], non-small cell lung cancer [93] and metastatic melanoma [94]. Particularly in melanoma cancer, EXOs are studied as therapeutic cell-free vaccines, e.g., dendritic cell-derived EXOs loaded with MAGE3 antibodies were administered subcutaneously or intradermally to stimulate the immune response of melanoma stage IIIb/IV patients obtaining positive clinical effects [95].
Moreover, Zhu et al. analyzed the fundamental role of EXOs derived from natural killer cells to exert cytotoxic effects on melanoma cells, suggesting this could be a potential immunotherapeutic strategy for cancer [96]. More recently, Luigini L. et al. performed a homemade test for the analysis of natural killer exosomes derived from plasma of melanoma patients and healthy donors. Their results showed that these vesicles could be used to improve cancer treatment in combination with NK cells or immune checkpoint-based therapies [97].
Among the new therapeutic approaches, very interesting results were reviewed by Chillà A. et al. In their paper, they refer to the therapeutic use of some particular cells used as natural nanoparticles [98]. For example, the possibility to target an experimental melanoma with red blood cells (RBCs) and platelet membrane-coated gold nanoparticles containing curcumin was described. In this study, the natural cell membranes have been used as biointerfaces that interact with the host environment. Platelet membrane coating has been used to target cancer cells while RBC membrane coating has been used to provide clearance by macrophages [99]. This cell-mediated delivery would provide controlled dosing, no systemic toxicity, and high tolerance by the patient and could be possibly evaluated as a therapeutic strategy in melanoma [98].
2.8. Natural Nanoparticles for Metastatic Melanoma Diagnosis
Early diagnosis and effective therapy are undoubtedly the primary issues for prolonging the survival of cancer patients. At present, the solid biopsy used for diagnosis is mostly the basis for treatment of cancer. Unfortunately, this method has considerable limitations as it is invasive and sometimes not feasible. Accumulating evidence over the past few decades has highlighted the potential use of circulating molecular biomarkers as a promising platform for the noninvasive diagnosis and definition of prognosis. As previously reported, the cargos incorporated in EXOs reflect the contents and the pathological status or signaling alterations of the original cells. Moreover, the cargos are protected from degradation and are isolable from patients’ body fluids. Thus, analyses of circulating EXOs and their contents in blood could represent a promising strategy in cancer diagnosis advancement and monitor patients’ therapeutic response.
For instance, PD-L1 expression in tumor tissues is widely used in selecting patients who will benefit from treatment with immunotherapy. Recently, evaluating the role of exosomal PD-L1 expressed in melanoma patients treated with anti-PD-1/PD-L1 antibodies, Danesi R. and his group showed that exosomal PD-L1 expression increased in subjects with disease progression and decreased in patients responding to treatment, while no significant changes were observed in patients with disease stabilization [100]. In the same year, Chen G. et al. [101] showed that Interferon-γ (IFN-γ) up-regulates PD-L1-containing EXOs secretion and changes during the course of anti-PD-1 therapy. In accordance, a very recent prospective study to evaluate exosomal-PD-L1 expression from 100 melanoma patients treated with immune checkpoint inhibitors and BRAF/MEK inhibitors, showed that tumor EXOs carrying PD-L1 had immunosuppressive properties since they were as efficient as the melanoma cell at inhibiting T-cell activation. This study provided a rationale for monitoring the EXO-PD-L1 level as a potential predictor of treatment response in melanoma patients [102].
Functional nucleic acids contained in EXO (miRs, long non-coding RNAs, circulating RNAs, mRNAs, and DNA) are chemically stable and resistant to degradation by RNAases. Numerous previous studies have shown that exosomal miRs could be potential diagnostic and prognostic biomarkers for melanoma [103]. In particular, González and his group, in one of the first publications that identified circulating exosomal miRs in patients with melanoma, reported that the EXOs from patients with melanoma contain a level of miR-125b that is significantly lower compared to that contained in EXOs from disease-free melanoma patients or healthy controls [104]. Consistently, miR-125b appears to function as a tumor suppressor in melanoma, by direct regulation of c-Jun and MLK3 protein expressions [105,106] and inducing cell senescence [107]. Recently, Huber et al. have described seven miRs, including miR-125b-5p, that were released in extracellular vesicles and associated with myeloid-derived suppressor cells (MDSCs) and resistance to treatment with immune checkpoint inhibitors in melanoma patients [108]. Moreover, it was reported that the regulation of the miR-125b-5p/EIF5A2 axis in melanoma, through the long intergenic non-protein coding RNA 520 (LICN00520), promotes the proliferation, invasion and migration of melanoma cells. The authors suggest that long non-coding RNA (lncRNA) expression level may be possibly evaluated in blood circulation as a therapeutic biomarker [109].
Another report by Tengda et al. reported that the analysis of a panel of five exosomal miRs, in particular miR-532-5p and miR-106b, was able to distinguish patients with or without metastases, patients with stage I–II from patients with III–IV of disease and patients who received pembrolizumab treatment from those not treated [110].
Most studies have shown the potential role of tumor-derived EVs, mainly EXOs, as biomarkers to predict cutaneous malignant melanoma (CMM) outcome and resistance to MAPKis [110,111,112]. A recent work published by Svedman has led to the identification of the EV-derived miRs, let-7g-5p and miR-497-5p, that can be used as putative good predictive biomarkers after MAPKi treatment in metastatic CMM patients [113]. As reported by Lunavat et al., the expression of miR-211–5p is up-regulated upon vemurafenib and dabrafenib treatment in EXOs and melanoma cells. Interestingly, the authors suggested that stable expression of miR-211–5p reduces sensitivity to BRAF inhibition by regulating cellular proliferation. The authors indicate this miR as a promising prognostic biomarker in melanoma [112].
MiR-21, one of the most frequently up-regulated miRs in solid tumors, controls important tumor suppressor genes as well as genes involved in carcinogenesis, such as phosphatase and tensin homolog (PTEN), programmed cell death protein 4 (PDCD4), phosphoinositide 3-kinase (PI3K), Sprouty and reversion inducing cysteine rich protein with Kazal motifs (RECK) [114,115,116,117,118]. As a result, miR-21 has been proposed as a plausible diagnostic and prognostic biomarker, as well as a therapeutic target for several types of cancer [119]. Moreover, the use of exosomal miR-21 as a potential biomarker has been proposed. Specifically, a systematic review provided evidence that is consistent with this hypothesis although the limited number of each cancer type analyzed should be considered. In addition, the author suggested that a combination of miR panels or cancer antigens may be a good strategy to have better diagnostic results or prognostic predictions in most circumstances [120].
MiR-21 plays a pivotal role in melanomagenesis and is released in EXOs produced from melanoma cells [121]. In an interesting work, Pfeffer et al. performed a miR profiling on RNA prepared from plasma-derived EXOs from specific patient cohorts [122]. As stated in the cited review [120], they found that a panel of five miRs (miR-17, miR-19a, miR-21, miR-126, and miR-149) was expressed at higher levels in plasma-derived EXOs from patients with metastatic melanoma. In addition, these miRs were found to be over-expressed in patients with metastatic sporadic melanoma compared to familial melanoma patients or healthy controls. This panel is proposed as a predictive biomarker to monitor remission as well as relapse following therapeutic intervention [122]. Of interest, miR-21 and miR-146a up-regulations were found in vitreal EXOs of patients with uveal melanoma (UM) as a consequence of dysregulation arising from tumor cells. Data shown in that paper also suggest the possibility of detecting these miRs in VH (vitreous humor) and that serum of UM patients could be considered a potential circulating marker [123]. In addition, the plasma level of miR-21 was positively correlated with tumor burden in metastatic melanoma patients [124] and its relative expression was positively correlated with the tumor nodes and metastasis (TNM) stage of melanoma [125]. All these studies confirmed that the expression of miR-21 in melanoma patients can be used as a potential serum biomarker for melanoma metastasis diagnosis and disease assessment.
Several proteins involved in melanoma progression and metastasis have been identified in EXOs and could be considered as possible prognostic biomarkers [126].
A large increase in total exosomal protein derived from malignant melanoma occurs during disease progression. Specifically, each melanoma cell line appears to exhibit an individual type of heat shock protein (HSP)70 expression, likely reflecting a selection during tumor progression and therapy [127]. Very interesting results were obtained from Peinado and coworkers who identified a melanoma-specific exosomal signature in blood from patients with stage IV melanoma that included expression of tyrosinase-related protein-2 (TYRP-2), very late antigen-4 (VLA-4), HSP70, HSP90 and mesenchymal epithelial transition (MET) oncoprotein [128]. More recently, Alegre et al. evaluated the presence of the melanoma biomarkers melanoma inhibitory activity (MIA) protein, S100B and tyrosinase-related protein 2 (TYRP2) in EXOs derived from serum obtained from stage IV melanoma patients, melanoma-free patients and healthy controls. They observed that MIA and S100B can be detected in EXOs and their quantification presents diagnostic and prognostic utility in melanoma patients [129].
Recently, lncRNAs have emerged as an important regulatory factor in tumor growth and transformation [130]. In particular, Cantile et al. have analyzed the fundamental role played by HOTAIR in the malignant transformation and progression of melanoma cells. Moreover, they have identified this lncRNA in the blood and have suggested its potential role as circulating marker [131]. Numerous reports have shown that exosomal lncHOTAIR could be a sensitive liquid biomarker of different types of cancers, such as glioblastoma multiform, breast cancer and non-small-cell lung cancer (NSCLC) [132,133,134]. However, the potential use of EXOs for HOTAIR detection in melanoma cells has not yet been explored.
In a very recent and interesting study, the authors describe a comprehensive proteomic analysis of 426 human samples from tissue explants (TEs), plasma and other body fluids. They reported that the “tiny packages of materials released by tumors”, called EVPs (extracellular vesicles and non-vesicular particles <50 nm), may serve as biomarkers for detecting a number of different types of cancers (including melanoma) in its early stages. In particular, the analysis of some specific proteins (e.g., versican, tenascin C, and thrombospondin 2 can distinguish tumors from normal tissues with 90% sensitivity and 94% specificity. Moreover, they have defined a panel of tumor-type-specific EVP proteins in TEs and plasma, which can classify tumors of unknown primary origin [135].
3. Conclusions
Advanced melanoma is a rapid metastasizing and drug-resistant cancer [1,2]. Despite the availability of new therapies discovered in recent years, metastatic melanoma remains a highly lethal disease. Recent advances in nanotechnologies opened up new perspectives for treating melanoma, potentially effective in overcoming the limitations of conventional approaches: toxicity to normal tissues, development of drug resistance and rapid blood clearance. Nanotechnologies are based on the use of different polymers whose specific characteristics are adapted to different aspects of therapies. Both the Food and Drug Administration (FDA) and European Medicines Agency (EMA) recently approved several types of nanoparticles as a system for conveying nucleic acids, drugs and other molecules for cancer and other disease treatment, including melanoma [17,18,28]. More optimized nanocarriers for the delivery of drugs in a specific way that is able to make the melanoma treatment more efficient is represented by targeted nanoparticles. Melanoma-specific antibodies are conjugated to nanoparticles, thus overcoming the physiological barriers and conditions to reach the target and to recognize and actively bind to target cells through specific antibody–receptor interactions [66].
The discovery of EXOs as natural carriers of RNA, DNA, proteins and lipids have highlighted the potential use of these vesicles as circulating molecular biomarkers for both diagnostic and therapeutic purposes. Due to their easy handling and their ability to transfer molecules between cells, EXOs may be a very performant delivery system [86]. Moreover, naturally secreted EXOs loaded with therapeutic molecules may optimize efficacy of treatment while also reducing off-target delivery. Specifically, molecules may be included in EXOs that are difficult to deliver intracellularly without the use of a carrier (ncRNA, recombinant proteins, small-molecule drugs). Particularly in melanoma, EXOs are also studied as a therapeutic cell-free vaccine [95]. The purification of pure populations of EXOs from EXO-secreting cell lines, unlike those released from autologous primary cells, have immunogenic and oncogenic potential. However, the modification of the complex structure of naturally secreted EXOs may limit their functional activity and their pharmaceutical acceptability. As a potential alternative, some authors have proposed the development of EXO-mimetics manufactured using selected components of EXOs that can be incorporated into synthetic nanoparticles to enhance their stability, immunogenicity, targeting, and uptake [136]. However, the main and useful components of exosomes to therapeutic delivery have not been identified yet.
Although the high number of publications in this field suggests the high diagnostic and prognostic potential of EVs in melanoma, our knowledge remains limited. Several factors hinder the real use of EVs or EXOs as biomarkers. For example, the differences in the methods used by researchers to isolate EXOs from patients’ blood introduce heterogeneity in the composition of the different preparations, thus precluding the identification of the active components. Furthermore, different populations of vesicles are found in the plasma of patients and the isolation of those released by melanoma tumor cells would be of great scientific importance. In this regard, a recent publication by Ferrone et al. suggests the use of an antibody directed against CSPG4 antigen to separate exosomes released by melanoma from those secreted by normal cells [137].
In conclusion, natural or synthetic nanoparticles are promising tools to design new therapeutic, diagnostic and prognostic approaches to cancer and, in particular, to melanoma. Studies are needed to fill the gap between the large amount of evidence available in the preclinical setting and the limited evidence obtained in the real clinical situation.
Funding
This work was supported by the following grants: MAECI, Progetti di Grande Rilevanza, Domanda di contributo; L. 401/90 (PGR01049) to Nadia Felli (N.F.); Regione Lazio “Progetto di Gruppo di Ricerca finanziato ai sensi della LR Lazio 13/08” (Protocollo 15263) to Mauro Biffoni (M.B.) for an M.B.A. fellowship.
Acknowledgments
We thank Mauro Biffoni (M.B.) for critical reading of the manuscript and Deborah Paradiso for English-language editing and proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular pathogenesis and therapeutic management. Mol. Cell. Pharmacol. 2014, 6, 228. [Google Scholar] [CrossRef]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105. [Google Scholar] [CrossRef]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 23, 488–496. [Google Scholar] [PubMed]
- Di Franco, S.; Turdo, A.; Todaro, M.; Stassi, G. Role of Type I and II Interferons in Colorectal Cancer and Melanoma. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, R.; Zhang, X.D.; Chen, C. Applications of nanotechnology for melanoma treatment, diagnosis, and theranostics. Int. J. Nanomed. 2013, 8, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Puzanov, I.; Kim, K.B.; Ribas, A.; McArthur, G.A.; Sosman, J.A.; O’Dwyer, P.J.; Lee, R.J.; Grippo, J.F.; Nolop, K.; et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Viros, A.; Sanchez-Laorden, B.; Pedersen, M.; Furney, S.J.; Rae, J.; Hogan, K.; Ejiama, S.; Girotti, M.R.; Cook, M.; Dhomen, N.; et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nat. Cell Biol. 2014, 511, 478–482. [Google Scholar] [CrossRef]
- Dankort, D.; Curley, D.P.; Cartlidge, R.A.; Nelson, B.; Karnezis, A.N.; Damsky, W.E., Jr.; You, M.J.; Depinho, R.A.; McMahon, M.; Bosenberg, M. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009, 41, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.M.; Soares, P.; Populo, H. Melanoma treatment in review. Immuno Targets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014, 3, 111–122. [Google Scholar] [CrossRef]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122. [Google Scholar] [CrossRef]
- Park, K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef]
- Arruda, D.C.; De Oliveira, T.D.; Cursino, P.H.F.; Maia, V.S.C.; Berzaghi, R.; Travassos, L.R.; Tada, D.B. Inhibition of melanoma metastasis by dual-peptide PLGA NPS. Pept. Sci. 2017, 108, e23029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-Coated Nanoparticles Loaded With Docetaxel for Breast Cancer Therapy. Dose-Response 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Kou, G.; Gao, J.; Wang, H.; Chen, H.; Li, B.; Zhang, D.; Wang, S.; Hou, S.; Qian, W.; Dai, J.; et al. Preparation and Characterization of Paclitaxel-loaded PLGA nanoparticles coated with cationic SM5-1 single-chain antibody. J. Biochem. Mol. Biol. 2007, 40, 731–739. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; López-Barcenas, A.; Sánchez-Blanco, E.; Sánchez-Blanco, B.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Arenas, R. Advances in Immunotherapy for Melanoma: A Comprehensive Review. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, W. Intracellular Delivery of Tumor Antigenic Peptides in Biodegradablepolymer Adjuvant for Enhancing Cancer Immunotherapy. Curr. Med. Chem. 2014, 21, 2357–2366. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-S. Nanomaterials as Delivery Vehicles and Components of New Strategies to Combat Bacterial Infections: Advantages and Limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Vahed, S.Z.; Fathi, N.; Samiei, M.; Dizaj, S.M.; Sharifi, S. Targeted cancer drug delivery with aptamer-functionalized polymeric nanoparticles. J. Drug Target. 2018, 27, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Paolino, D.; Paolino, D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579. [Google Scholar] [CrossRef]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; Petkov, P.S.; Vayssilov, G.N.; Garrido, M.J. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.E.; Aminabhavi, T. Chitosan as a carrier for targeted delivery of small interfering RNA. Int. J. Pharm. 2010, 399, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cryan, S.-A.; McKiernan, P.J.; Cunningham, O.; Greene, C.M. Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int. J. Nanomed. 2013, 8, 3907–3915. [Google Scholar] [CrossRef] [PubMed]
- Rostami, N.; Nikkhoo, A.; Khazaei-Poul, Y.; Farhadi, S.; Haeri, M.S.; Ardebili, S.M.; Vanda, N.A.; Atyabi, F.; Namdar, A.; Baghaei, M.; et al. Coinhibition of S1PR1 and GP130 by siRNA-loaded alginate-conjugated trimethyl chitosan nanoparticles robustly blocks development of cancer cells. J. Cell. Physiol. 2020, 235, 9702–9717. [Google Scholar] [CrossRef] [PubMed]
- Felli, N.; Errico, M.C.; Pedini, F.; Petrini, M.; Puglisi, R.; Bellenghi, M.; Boe, A.; Felicetti, F.; Mattia, G.; De Feo, A.; et al. AP2α controls the dynamic balance between miR-126&126* and miR-221&222 during melanoma progression. Oncogene 2015, 35, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.P.; Saghafinia, S.; Hanahan, D. A set of microRNAs coordinately controls tumorigenesis, invasion, and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 24184–24195. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Gao, H.-W.; Chang, K.-F.; Huang, X.-F.; Lin, Y.-L.; Weng, J.-C.; Liao, K.-W.; Tsai, N.-M. Antitumor Effect of n-Butylidenephthalide Encapsulated on B16/F10 Melanoma Cells In Vitro with a Polycationic Liposome Containing PEI and Polyethylene Glycol Complex. Molecules 2018, 23, 3224. [Google Scholar] [CrossRef]
- Yin, S.; Xia, C.; Wang, Y.; Wan, D.; Rao, J.; Tang, X.; Wei, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Dual receptor recognizing liposomes containing paclitaxel and hydroxychloroquine for primary and metastatic melanoma treatment via autophagy-dependent and independent pathways. J. Control. Release 2018, 288, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Resnier, P.; Galopin, N.; Sibiril, Y.; Clavreul, A.; Cayon, J.; Briganti, A.; Legras, P.; Vessières, A.; Montier, T.; Jaouen, G.; et al. Efficient ferrocifen anticancer drug and Bcl-2 gene therapy using lipid nanocapsules on human melanoma xenograft in mouse. Pharmacol. Res. 2017, 126, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.S.; Wang, S.; Dachineni, R.; Averineni, R.K.; Yang, Y.; Yin, H.; Bhat, G.J.; Guan, X. In Vitro and In Vivo Tumor Growth Inhibition by Glutathione Disulfide Liposomes. Cancer Growth Metastasis 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shi, K.; Hu, G.; Yang, Y.; Kuang, Q.; Lu, L.; Zhang, L.; Chen, W.; Dong, M.; Chen, Y.; et al. Tumor-Targeted Paclitaxel Delivery and Enhanced Penetration Using TAT-Decorated Liposomes Comprising Redox-Responsive Poly(Ethylene Glycol). J. Pharm. Sci. 2015, 104, 1160–1173. [Google Scholar] [CrossRef]
- De Serrano, L.O.; Burkhart, D.J. Liposomal vaccine formulations as prophylactic agents: Design considerations for modern vaccines. J. Nanobiotechnol. 2017, 15, 83. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In Vivo Molecular Photoacoustic Tomography of Melanomas Targeted by Bioconjugated Gold Nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of Gold Nanoparticle-based Drug Delivery System in Cancer Therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Guo, J.; Rahme, K.; He, Y.; Li, L.-L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef]
- Zhu, L.; Kate, P.; Torchilin, V.P. Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. ACS Nano 2012, 6, 3491–3498. [Google Scholar] [CrossRef]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Li, Z.; Xie, X.; Gao, X.; Xu, X. Inducing Controlled Release and Increased Tumor-Targeted Delivery of Chlorambucil via Albumin/Liposome Hybrid Nanoparticles. AAPS PharmSciTech 2017, 18, 2977–2986. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Hassanzadeh, F.; Javanmard, S.H.; Rostami, M. Self-assembly micelles with lipid core of cholesterol for docetaxel delivery to B16F10 melanoma and HepG2 cells. J. Liposome Res. 2014, 25, 157–165. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomology 2019, 9, 330. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kazi, J.; Debnath, M.C. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: Evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother. 2018, 97, 1373–1385. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Esfeh, F.A.; Kaviani, M.; Ashtari, K.; Kheirbakhsh, R.; Salimi, A.; Pourahmad, J. Single-walled carbon nanotube, multi-walled carbon nanotube and Fe2O3 nanoparticles induced mitochondria mediated apoptosis in melanoma cells. Cutan. Ocul. Toxicol. 2017, 37, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hung, C.-F.; Chen, B.-H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Chemoprevention of skin cancer using low HLB surfactant nanoemulsion of 5-fluorouracil: A preliminary study. Drug Deliv. 2013, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wu, T.; Zhao, Y.; Hu, X.; Bao, Y.; Guo, Y.; Song, Q.; Li, G.; Tan, S.; Zhang, Z. Lipid-enveloped zinc phosphate hybrid nanoparticles for codelivery of H-2Kb and H-2Db-restricted antigenic peptides and monophosphoryl lipid A to induce antitumor immunity against melanoma. J. Control. Release 2016, 228, 26–37. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Wong, G.S.; Rustgi, A.K. Matricellular proteins: Priming the tumour microenvironment for cancer development and metastasis. Br. J. Cancer 2013, 108, 755–761. [Google Scholar] [CrossRef]
- Thomas, S.; Waterman, P.; Chen, S.; Marinelli, B.; Seaman, M.; Rodig, S.; Ross, R.W.; Josephson, L.; Weissleder, R.; Kelly, K.A. Development of Secreted Protein and Acidic and Rich in Cysteine (SPARC) Targeted Nanoparticles for the Prognostic Molecular Imaging of Metastatic Prostate Cancer. J. Nanomed. Nanotechnol. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Weon, J.L.; Potts, P.R. The MAGE protein family and cancer. Curr. Opin. Cell Biol. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Saeed, M.; Van Brakel, M.; Zalba, S.; Schooten, E.; Rens, J.A.P.; Debets, R.; Koning, G.A. Targeting melanoma with immunoliposomes coupled to anti-MAGE A1 TCR-like single-chain antibody. Int. J. Nanomed. 2016, 955–975. [Google Scholar] [CrossRef]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody–drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, M.I.; Brisson, A.R.; Van Herwijnen, M.J.C.; Tan, S.; Van De Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.M.; Nolte-’t Hoen, E.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Balaj, L.; Liau, L.M.; Samuels, M.L.; Kotsopoulos, S.K.; Maguire, C.A.; Loguidice, L.; Soto, H.; Garrett, M.; Zhu, L.D.; et al. BEAMing and Droplet Digital PCR Analysis of Mutant IDH1 mRNA in Glioma Patient Serum and Cerebrospinal Fluid Extracellular Vesicles. Mol. Ther. Nucleic Acids 2013, 2, e109. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-X.; Liu, Z.-F. Proteomic Profiling of Sweat Exosome Suggests its Involvement in Skin Immunity. J. Investig. Dermatol. 2018, 138, 89–97. [Google Scholar] [CrossRef]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T.W. Detection of Tumor Cell-Specific mRNA and Protein in Exosome-Like Microvesicles from Blood and Saliva. PLoS ONE 2014, 9, e110641. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Keeerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2018, 47, D516–D519. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef]
- Ogawa, Y.; Taketomi, Y.; Murakami, M.; Tsujimoto, M.; Yanoshita, R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol. Pharm. Bull. 2013, 36, 66–75. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, R.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf–Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome–like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Zöller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2008, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Stickney, Z.; Losacco, J.; McDevitt, S.; Zhang, Z.; Lu, B. Development of exosome surface display technology in living human cells. Biochem. Biophys. Res. Commun. 2016, 472, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In Vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Hao, S.; Liu, Y.; Yuan, J.; Zhang, X.; He, T.; Wu, X.; Wei, Y.; Sun, D.; Xiang, J. Novel Exosome-Targeted CD4+ T Cell Vaccine Counteracting CD4+25+ Regulatory T Cell-Mediated Immune Suppression and Stimulating Efficient Central Memory CD8+ CTL Responses. J. Immunol. 2007, 179, 2731–2740. [Google Scholar] [CrossRef]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.C.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Boorn, J.G.V.D.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Chillà, A.; Margheri, F.; Biagioni, A.; Del Rosso, T.; Fibbi, G.; Del Rosso, M.; Laurenzana, A. Cell-Mediated Release of Nanoparticles as a Preferential Option for Future Treatment of Melanoma. Cancers 2020, 12, 1771. [Google Scholar] [CrossRef]
- Kim, M.W.; Lee, G.; Niidome, T.; Komohara, Y.; Lee, R.; Park, Y.I. Platelet-Like Gold Nanostars for Cancer Therapy: The Ability to Treat Cancer and Evade Immune Reactions. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nat. Cell Biol. 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.-P.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, D.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying mRNA, MicroRNA and Protein Profiles of Melanoma Exosomes. PLoS ONE 2012, 7, e46874. [Google Scholar] [CrossRef] [PubMed]
- Alegre, E.; Sanmamed, M.F.; Rodriguez, C.; Carranza, O.; Martin-Algarra, S.; González, A. Study of Circulating MicroRNA-125b Levels in Serum Exosomes in Advanced Melanoma. Arch. Pathol. Lab. Med. 2014, 138, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, M.; Kuphal, S.; Meister, G.; Vardimon, L.; Bosserhoff, A.-K. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene 2012, 32, 2984–2991. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, L.; Xiong, Y.; Qin, W.; Zhang, Y.; Qian, Y.; Jiang, H.; Liu, W. MLK3 promotes melanoma proliferation and invasion and is a target of microRNA-125b. Clin. Exp. Dermatol. 2014, 39, 376–384. [Google Scholar] [CrossRef]
- Nyholm, A.M.; Lerche, C.M.; Manfé, V.; Biskup, E.; Johansen, P.; Morling, N.; Thomsen, B.M.; Glud, M.; Gniadecki, R. miR-125b induces cellular senescence in malignant melanoma. BMC Dermatol. 2014, 14, 8. [Google Scholar] [CrossRef]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J. Clin. Investig. 2018, 128, 5505–5516. [Google Scholar] [CrossRef]
- Luan, W.; Ding, Y.; Yuan, H.; Ma, S.; Ruan, H.; Wang, J.; Lu, F.; Bu, X. Long non-coding RNA LINC00520 promotes the proliferation and metastasis of malignant melanoma by inducing the miR-125b-5p/EIF5A2 axis. J. Exp. Clin. Cancer Res. 2020, 39, 96. [Google Scholar] [CrossRef]
- Tengda, L.; Shuping, L.; Mingli, G.; Jie, G.; Yun, L.; Weiwei, Z.; Anmei, D. Serum exosomal microRNAs as potent circulating biomarkers for melanoma. Melanoma Res. 2018, 28, 295–303. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Lunavat, T.R.; Echeng, L.; Einarsdottir, B.O.; Bagge, R.O.; Muralidharan, S.V.; Sharples, R.A.; Lässer, C.; Gho, Y.S.; Hill, A.F.; Nilsson, J.A.; et al. BRAFV600 inhibition alters the microRNA cargo in the vesicular secretome of malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2017, 114, E5930–E5939. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Lohcharoenkal, W.; Bottai, M.; Brage, S.E.; Sonkoly, E.; Hansson, J.; Pivarcsi, A.; Eriksson, H. Extracellular microvesicle microRNAs as predictive biomarkers for targeted therapy in metastastic cutaneous malignant melanoma. PLoS ONE 2018, 13, e0206942. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Henson, R.; Wehbe–Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2007, 27, 2128–2136. [Google Scholar] [CrossRef]
- Xiong, B.; Cheng, Y.; Ma, L.; Zhang, C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int. J. Oncol. 2012, 42, 219–228. [Google Scholar] [CrossRef]
- Sayed, D.; Rane, S.; Lypowy, J.; He, M.; Chen, I.-Y.; Vashistha, H.; Yan, L.; Malhotra, A.; Vatner, D.; Abdellatif, M. MicroRNA-21 Targets Sprouty2 and Promotes Cellular Outgrowths. Mol. Biol. Cell 2008, 19, 3272–3282. [Google Scholar] [CrossRef]
- Leite, K.R.; Reis, S.T.; Viana, N.I.; Morais, D.R.; Moura, C.M.; Silva, I.A.; Pontes, J.; Katz, B.; Srougi, M. Controlling RECK miR21 Promotes Tumor Cell Invasion and Is Related to Biochemical Recurrence in Prostate Cancer. J. Cancer 2015, 6, 292–301. [Google Scholar] [CrossRef]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef]
- Shi, J. Considering Exosomal miR-21 as a Biomarker for Cancer. J. Clin. Med. 2016, 5, 42. [Google Scholar] [CrossRef]
- Melnik, B.C. MiR-21: An environmental driver of malignant melanoma? J. Transl. Med. 2015, 13, 202. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Grossmann, K.F.; Cassidy, P.B.; Yang, C.H.; Fan, M.; Kopelovich, L.; Leachman, S.A.; Pfeffer, L.M. Detection of Exosomal miRNAs in the Plasma of Melanoma Patients. J. Clin. Med. 2015, 4, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, G.; Potter, L.; Shendge, P.; Osborne, J.; Nicholson, S.; Yii, N.; Varma, S.; Aslam, M.I.; Elshaw, S.; Papadogeorgakis, E.; et al. Plasma MicroRNA-21 Is Associated with Tumor Burden in Cutaneous Melanoma. J. Investig. Dermatol. 2013, 133, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Guan, J.; Yuan, Z.-C.; Lin, X.; Wu, Z.-J.; Liu, B.; He, J.-L. Expression and predictive value of miR-489 and miR-21 in melanoma metastasis. World J. Clin. Cases 2019, 7, 2930–2941. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Clement, E.; Ducoux-Petit, M.; Denat, L.; Soldan, V.; Dauvillier, S.; Balor, S.; Burlet-Schiltz, O.; LaRue, L.; Muller, C.; et al. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment. Cell Melanoma Res. 2015, 28, 464–475. [Google Scholar] [CrossRef]
- Dressel, R.; Johnson, J.P.; Günther, E. Heterogeneous patterns of constitutive and heat shock induced expression of HLA-linked HSP70–1 and HSP70–2 heat shock genes in human melanoma cell lines. Melanoma Res. 1998, 8, 482–492. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Alegre, E.; Zubiri, L.; Pérez-Gracia, J.L.; María, G.-C.; Soria, L.; Martín-Algarra, S.; González, A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin. Chim. Acta 2016, 454, 28–32. [Google Scholar] [CrossRef]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef]
- Cantile, M.; Scognamiglio, G.; Marra, L.; Aquino, G.; Botti, C.; Falcone, M.R.; Malzone, M.G.; Liguori, G.; Di Bonito, M.; Franco, R.; et al. HOTAIR role in melanoma progression and its identification in the blood of patients with advanced disease. J. Cell. Physiol. 2017, 232, 3422–3432. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Liu, L.-C.; Hung, Y.; Chen, C.-J.; Lin, Y.-Z.; Wu, W.-R.; Wang, S.-C. Long non-coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer. Breast 2019, 46, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yin, Z.; Yang, L.; Fan, J.; Xu, J.; Jin, Y.; Yu, J.; Zhang, D.; Yang, G. Smoking Induced Extracellular Vesicles Release and Their Distinct Properties in Non-Small Cell Lung Cancer. J. Cancer 2019, 10, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, S.; Whiteside, T.L. Targeting CSPG4 for isolation of melanoma cell-derived exosomes from body fluids. HNO 2020, 68, 100–105. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).