Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology
Simple Summary
Abstract
1. Introduction
2. Aptamers
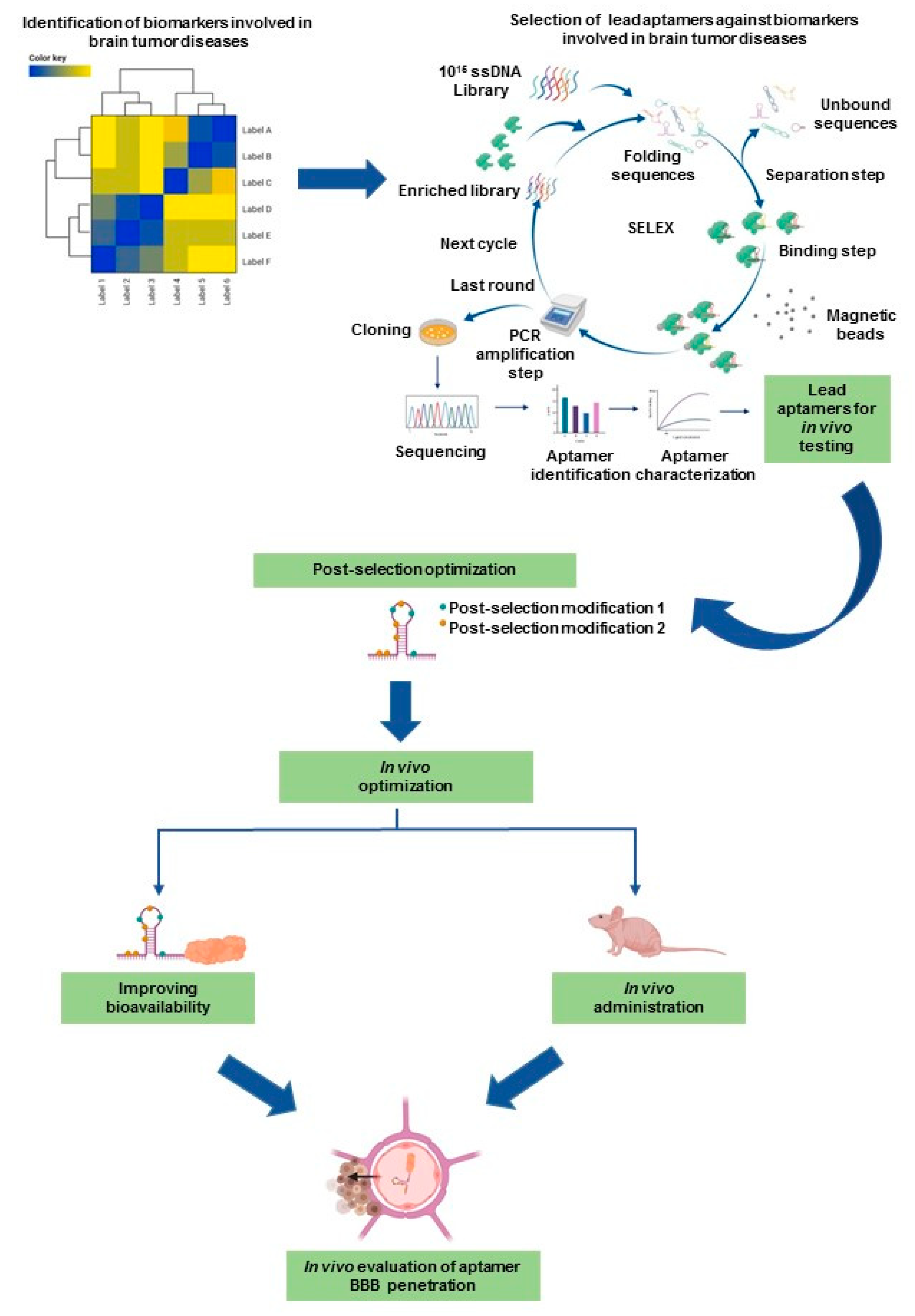
2.1. Latest Advanced SELEX Methods
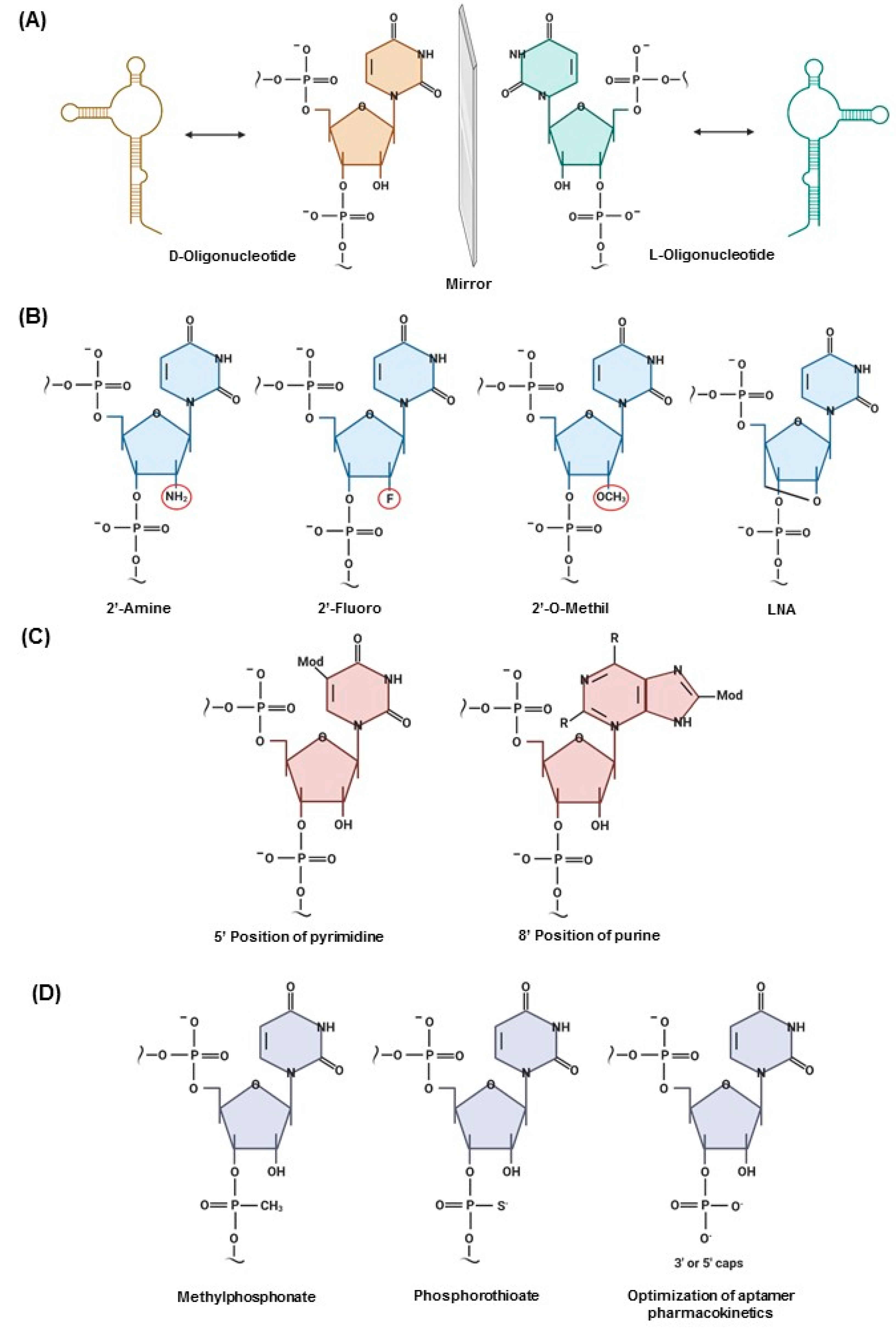
2.2. Structure and Target Association/Binding of Aptamers
2.3. Post-SELEX Modifications That Impact the Clinical Translation of Aptamers
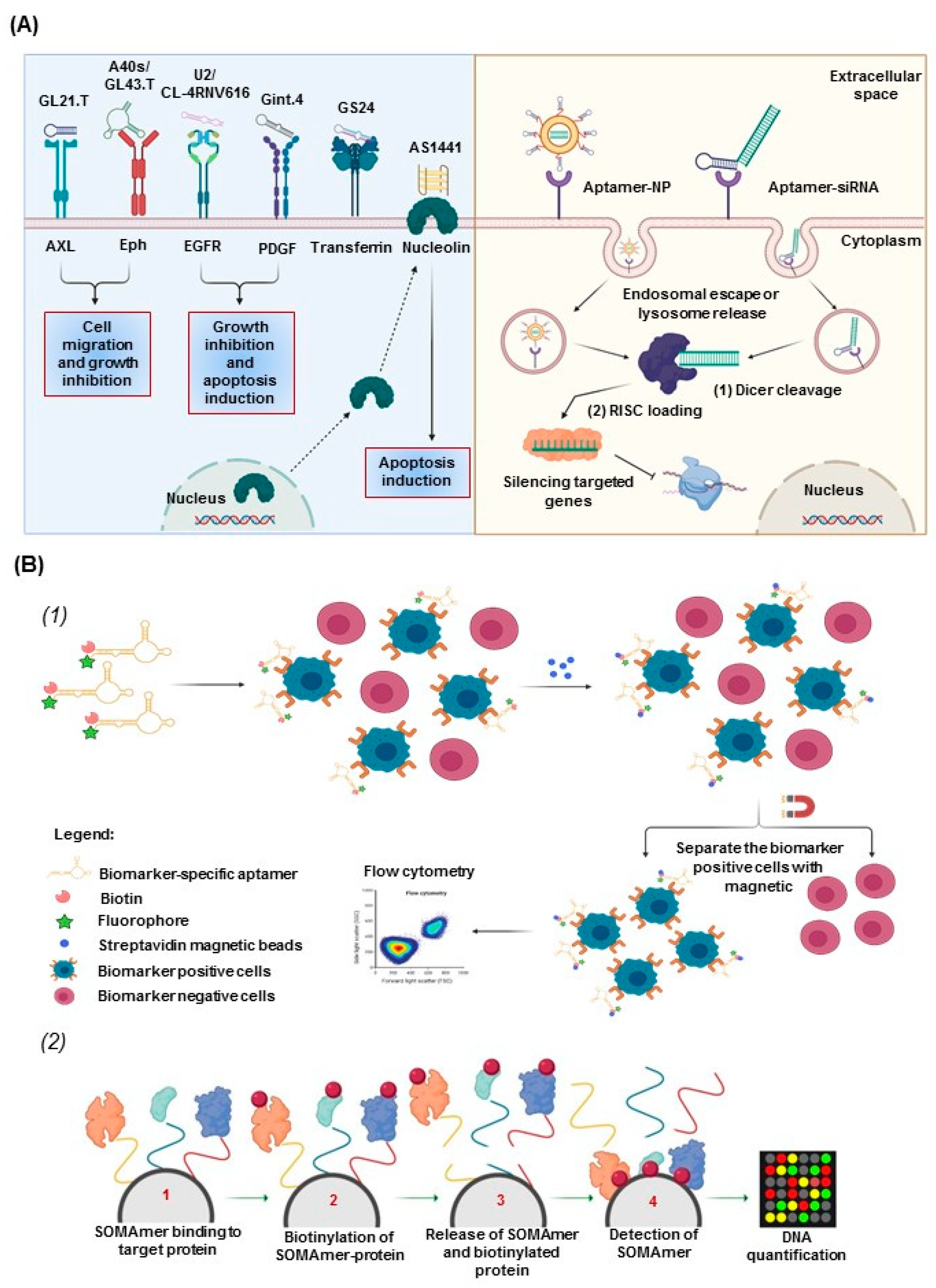
2.4. Aptamers for Therapeutic and Diagnostic Applications
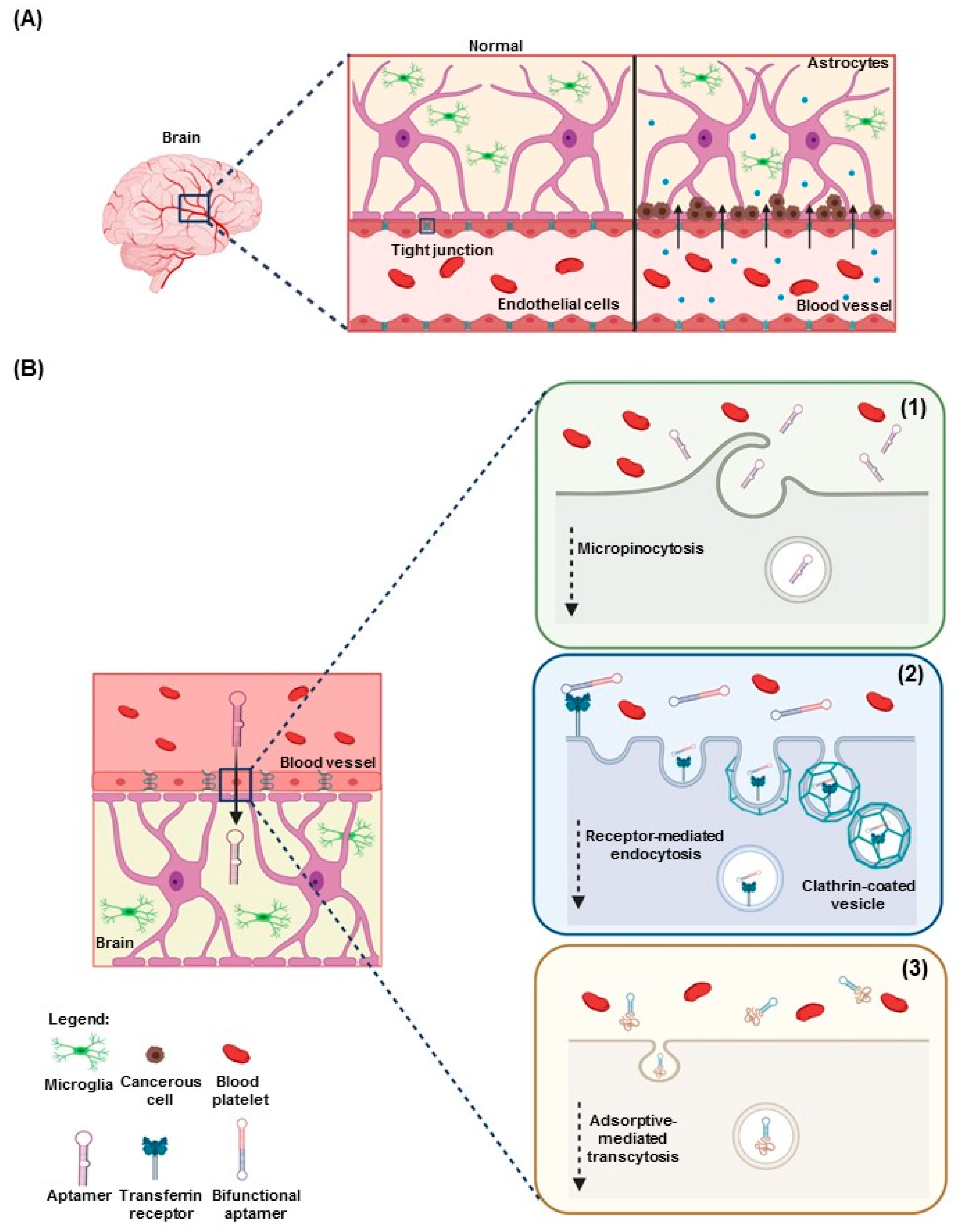
3. Aptamers in Neuro-Oncology
3.1. Aptamers as Therapeutics in Neuro-Oncology
3.2. Aptamers for Diagnostics and Imaging in Neuro-Oncology
4. Potential Role of Aptamers in Pediatric Neuro-Oncology
4.1. Pediatric Brain Tumors and Current Treatments
4.2. Potential Aptamer Targets in Pediatric Brain Tumors
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEG | Polyethylene Glycol |
| SELEX | Systemic Evolution of Ligands by Exponential enrichment |
| KD | Dissociation Constant |
| SOMAmer | Slow off-rate modified aptamer |
| WHO | World Health Organization |
| BBB | Blood–brain barrier |
| tFNA | Tetrahedral framework nucleic acid |
| GTG | Gint4.TtFNA-GMT8 |
| TMZ | Temozolomide |
| PLGA-b-PEG | Poly(lactic-co-glycolic)-block-Poly Ethylene Glycol |
| GBM | Glioblastoma |
| siRNA | Small interfering RNA |
| PDGFβ | Platelet-derived growth factor β |
| OPN | Osteopontin |
| EGFR | Epidermal growth factor receptor |
| EDB | Extra-domain B |
| VEGF | Vascular endothelial growth factor |
| HH | Hedgehog |
| PTCH1 | Patched1 |
| SMO | Smoothened |
| IDH1 | Isocitrate dehydrogenase |
| SOX2 | SRY (sex determining region Y)-box 2 |
References
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Kovacevic, K.D.; Gilbert, J.C.; Jilma, B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv. Drug Deliv. Rev. 2018, 134, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Sullenger, B.A.; Gallardo, H.F.; Ungers, G.E.; Gilboa, E. Analysis of trans-acting response decoy RNA-mediated inhibition of human immunodeficiency virus type 1 transactivation. J. Virol. 1991, 65, 6811–6816. [Google Scholar] [CrossRef]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 440. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Gold, L. Oligonucleotides as research, diagnostic, and therapeutic agents. J. Biol. Chem. 1995, 270, 13581–13584. [Google Scholar] [CrossRef]
- Xiang, D.; Zheng, C.; Zhou, S.F.; Qiao, S.; Tran, P.H.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Araujo, O.L.; Trindade, K.M.; Trompieri, N.M.; Fontenele, J.B.; Felix, F.H. Analysis of survival and prognostic factors of pediatric patients with brain tumor. J. Pediatr. 2011, 87, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, A.Y.; Frost, I.M.; Mastrodimos, M.B.; Plant, A.S.; Wang, A.C.; Moore, T.B.; Prins, R.M.; Weiss, P.S.; Jonas, S.J. Precision Medicine in Pediatric Neurooncology: A Review. ACS Chem. Neurosci. 2018, 9, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ciancio, D.; Vargas, M.R.; Thiel, W.H.; Bruno, M.A.; Giangrande, P.H.; Mestre, M.B. Aptamers as Diagnostic Tools in Cancer. Pharmaceuticals 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Rossi, J.J. Targeted Molecular Imaging Using Aptamers in Cancer. Pharmaceuticals 2018, 11, 71. [Google Scholar] [CrossRef]
- Wolter, O.; Mayer, G. Aptamers as Valuable Molecular Tools in Neurosciences. J. Neurosci. 2017, 37, 2517–2523. [Google Scholar] [CrossRef]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef]
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef]
- Yuce, M.; Ullah, N.; Budak, H. Trends in aptamer selection methods and applications. Analyst 2015, 140, 5379–5399. [Google Scholar] [CrossRef]
- Volk, D.E.; Lokesh, G.L.R. Development of Phosphorothioate DNA and DNA Thioaptamers. Biomedicines 2017, 5, 41. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Paul, A.; Wilhelm, N.; Ziemer, G.; Wendel, H.P. Upgrading SELEX technology by using lambda exonuclease digestion for single-stranded DNA generation. Molecules 2009, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berezovski, M.V.; Musheev, M.U.; Drabovich, A.P.; Jitkova, J.V.; Krylov, S.N. Non-SELEX: Selection of aptamers without intermediate amplification of candidate oligonucleotides. Nat. Protoc. 2006, 1, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, E.; Foley, J.H.; Conway, E.M.; Haynes, C. Hi-Fi SELEX: A High-Fidelity Digital-PCR Based Therapeutic Aptamer Discovery Platform. Biotechnol. Bioeng. 2015, 112, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of Cell-SELEX Technology and Its Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.; Ding, H.; Huang, Y.; Cao, X.; Yang, G.; Li, J.; Xie, Z.; Meng, Y.; Li, X.; et al. Identification of an aptamer targeting hnRNP A1 by tissue slide-based SELEX. J. Pathol. 2009, 218, 327–336. [Google Scholar] [CrossRef]
- Mi, J.; Liu, Y.; Rabbani, Z.N.; Yang, Z.; Urban, J.H.; Sullenger, B.A.; Clary, B.M. In vivo selection of tumor-targeting RNA motifs. Nat. Chem. Biol. 2010, 6, 22–24. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Volk, D.E.; Lokesh, G.L.; Elizondo-Riojas, M.A.; Li, L.; Nick, A.M.; Sood, A.K.; Rosenblatt, K.P.; Gorenstein, D.G. Morph-X-Select: Morphology-based tissue aptamer selection for ovarian cancer biomarker discovery. Biotechniques 2016, 61, 249–259. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e67. [Google Scholar] [CrossRef]
- Djordjevic, M. SELEX experiments: New prospects, applications and data analysis in inferring regulatory pathways. Biomol. Eng. 2007, 24, 179–189. [Google Scholar] [CrossRef]
- Tomilin, F.N.; Moryachkov, R.; Shchugoreva, I.; Zabluda, V.N.; Peters, G.; Platunov, M.; Spiridonova, V.; Melnichuk, A.; Atrokhova, A.; Zamay, S.S.; et al. Four steps for revealing and adjusting the 3D structure of aptamers in solution by small-angle X-ray scattering and computer simulation. Anal. Bioanal. Chem. 2019, 411, 6723–6732. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Adams, M.C.; Naik, R.R.; Milam, V.T. Analyzing Secondary Structure Patterns in DNA Aptamers Identified via CompELS. Molecules 2019, 24, 1572. [Google Scholar] [CrossRef] [PubMed]
- Tapp, M.J.N.; Slocik, J.M.; Dennis, P.B.; Naik, R.R.; Milam, V.T. Competition-Enhanced Ligand Selection to Identify DNA Aptamers. ACS Comb. Sci. 2018, 20, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.B.; Fraser, L.A.; Lang, S.; Shiu, S.C.C.; Tanner, J.A. Aptamer Bioinformatics. Int. J. Mol. Sci. 2017, 18, 2516. [Google Scholar] [CrossRef]
- Eaton, B.E.; Gold, L.; Hicke, B.J.; Janjic, N.; Jucker, F.M.; Sebesta, D.P.; Tarasow, T.M.; Willis, M.C.; Zichi, D.A. Post-SELEX combinatorial optimization of aptamers. Bioorg. Med. Chem. 1997, 5, 1087–1096. [Google Scholar] [CrossRef]
- Shigdar, S.; Macdonald, J.; O’Connor, M.; Wang, T.; Xiang, D.; Al Shamaileh, H.; Qiao, L.; Wei, M.; Zhou, S.F.; Zhu, Y.; et al. Aptamers as theranostic agents: Modifications, serum stability and functionalisation. Sensors 2013, 13, 13624–13637. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Eulberg, D.; Klussmann, S. Spiegelmers: Biostable Aptamers. ChemBioChem 2003, 4, 979–983. [Google Scholar] [CrossRef]
- Kong, H.Y.; Byun, J. Nucleic Acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomol. Ther. 2013, 21, 423–434. [Google Scholar] [CrossRef]
- Wang, R.E.; Wu, H.; Niu, Y.; Cai, J. Improving the stability of aptamers by chemical modification. Curr. Med. Chem. 2011, 18, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakatani, M.; Narukawa, K.; Obika, S. Antisense drug discovery and development. Future Med. Chem. 2011, 3, 339–365. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, C.; Strehlitz, B. Aptamer-modified nanoparticles and their use in cancer diagnostics and treatment. Swiss Med. Wkly. 2014, 144, w13908. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef] [PubMed]
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Nucleic Acid Ligands With Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol. Ther. Nucleic Acids 2014, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Yuan, W.F.; Ai, W.B.; Ai, Y.W.; Wang, J.J.; Chu, L.Y.; Zhang, Y.Q.; Wu, J.F. An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 207–218. [Google Scholar] [CrossRef]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer Based Targeted Drug Delivery Systems: Current Potential and Challenges. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Zhu, G.; Zheng, J.; Song, E.; Donovan, M.; Zhang, K.; Liu, C.; Tan, W. Self-assembled, aptamer-tethered DNA nanotrains for targeted transport of molecular drugs in cancer theranostics. Proc. Natl. Acad. Sci. USA 2013, 110, 7998–8003. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Catuogno, S.; Romano, S.; de Nigris, F.; de Franciscis, V. STAT3 Gene Silencing by Aptamer-siRNA Chimera as Selective Therapeutic for Glioblastoma. Mol. Ther. Nucleic Acids 2018, 10, 398–411. [Google Scholar] [CrossRef]
- Esposito, C.L.; Cerchia, L.; Catuogno, S.; De Vita, G.; Dassie, J.P.; Santamaria, G.; Swiderski, P.; Condorelli, G.; Giangrande, P.H.; de Franciscis, V. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol. Ther. 2014, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Catuogno, S.; de Franciscis, V. Aptamer-mediated selective delivery of short RNA therapeutics in cancer cells. J. RNAi Gene Silenc. 2014, 10, 500–506. [Google Scholar]
- Li, X.; Zhao, Q.; Qiu, L. Smart ligand: Aptamer-mediated targeted delivery of chemotherapeutic drugs and siRNA for cancer therapy. J. Control Release 2013, 171, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Katilius, E.; Ostroff, R.M.; Kim, Y.; Seok, M.; Lee, S.; Jang, S.; Kim, W.S.; Choi, C.M. Development of a Protein Biomarker Panel to Detect Non-Small-Cell Lung Cancer in Korea. Clin. Lung Cancer 2017, 18, e99–e107. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, P.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2018, 8, 4016–4032. [Google Scholar] [CrossRef]
- Muller, J.; Friedrich, M.; Becher, T.; Braunstein, J.; Kupper, T.; Berdel, P.; Gravius, S.; Rohrbach, F.; Oldenburg, J.; Mayer, G.; et al. Monitoring of plasma levels of activated protein C using a clinically applicable oligonucleotide-based enzyme capture assay. J. Thromb. Haemost. 2012, 10, 390–398. [Google Scholar] [CrossRef]
- Candia, J.; Cheung, F.; Kotliarov, Y.; Fantoni, G.; Sellers, B.; Griesman, T.; Huang, J.; Stuccio, S.; Zingone, A.; Ryan, B.M.; et al. Assessment of Variability in the SOMAscan Assay. Sci. Rep. 2017, 7, 14248. [Google Scholar] [CrossRef]
- Delgado-Lopez, P.D.; Saiz-Lopez, P.; Gargini, R.; Sola-Vendrell, E.; Tejada, S. A comprehensive overview on the molecular biology of human glioma: What the clinician needs to know. Clin. Transl. Oncol. 2020. [Google Scholar] [CrossRef]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Bovenberg, M.S.; Degeling, M.H.; Tannous, B.A. Advances in stem cell therapy against gliomas. Trends Mol. Med. 2013, 19, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control Release 2018, 281, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Drean, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M.; et al. Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J. Neurooncol. 2019, 144, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Fortin, D. Blood-brain barrier disruption in the treatment of brain tumors. Methods Mol. Biol. 2011, 686, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Maurizi, P.; Mastrangelo, S.; Attina, G.; Capozza, M.A.; Ruggiero, A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers 2019, 11, 824. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2017, 20, 184–191. [Google Scholar] [CrossRef]
- Nuzzo, S.; Brancato, V.; Affinito, A.; Salvatore, M.; Cavaliere, C.; Condorelli, G. The Role of RNA and DNA Aptamers in Glioblastoma Diagnosis and Therapy: A Systematic Review of the Literature. Cancers 2020, 12, 2173. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Ibba, M.L.; Ricci-Vitiani, L.; Pallini, R.; Condorelli, G.; Catuogno, S.; de Franciscis, V. Combined Targeting of Glioblastoma Stem-Like Cells by Neutralizing RNA-Bio-Drugs for STAT3. Cancers 2020, 12, 1434. [Google Scholar] [CrossRef]
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.Y.; Ling, X.; Caruso, H.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2019, 129, 137–149. [Google Scholar] [CrossRef]
- Shi, S.; Fu, W.; Lin, S.; Tian, T.; Li, S.; Shao, X.; Zhang, Y.; Zhang, T.; Tang, Z.; Zhou, Y.; et al. Targeted and effective glioblastoma therapy via aptamer-modified tetrahedral framework nucleic acid-paclitaxel nanoconjugates that can pass the blood brain barrier. Nanomedicine 2019, 21, 102061. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Wu, X.; Armstrong, B.; Habib, N.; Rossi, J.J. An RNA Aptamer Targeting the Receptor Tyrosine Kinase PDGFRalpha Induces Anti-tumor Effects through STAT3 and p53 in Glioblastoma. Mol. Ther. Nucleic Acids 2019, 14, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Philippovich, S.; Mao, J.; Veedu, R.N. Efficient Epidermal Growth Factor Receptor Targeting Oligonucleotide as a Potential Molecule for Targeted Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4700. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; You, C.; Ma, L.; Li, H.; Ju, Y.; Guo, X.; Shi, S.; Zhang, T.; Zhou, R.; Lin, Y. Enhanced Efficacy of Temozolomide Loaded by a Tetrahedral Framework DNA Nanoparticle in the Therapy for Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 39525–39533. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Zhao, H.; Qiao, G.; Liu, M.; Zhang, C.; Cui, D.; Ma, L. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018, 25, 1718–1727. [Google Scholar] [CrossRef]
- Larcher, L.M.; Wang, T.; Veedu, R.N. Development of Novel antimiRzymes for Targeted Inhibition of miR-21 Expression in Solid Cancer Cells. Molecules 2019, 24, 2489. [Google Scholar] [CrossRef]
- Saw, P.E.; Zhang, A.; Nie, Y.; Zhang, L.; Xu, Y.; Xu, X. Tumor-Associated Fibronectin Targeted Liposomal Nanoplatform for Cyclophilin A siRNA Delivery and Targeted Malignant Glioblastoma Therapy. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Z.; Jin, K.; Pang, Q.; Jiang, T.; Lu, H.; Liu, X.; Pang, Z.; Yu, L.; Jiang, X. Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (l-gamma-glutamylglutamine)-paclitaxel nanoconjugates. J. Colloid Interface Sci. 2017, 490, 783–796. [Google Scholar] [CrossRef]
- Monaco, I.; Camorani, S.; Colecchia, D.; Locatelli, E.; Calandro, P.; Oudin, A.; Niclou, S.; Arra, C.; Chiariello, M.; Cerchia, L.; et al. Aptamer Functionalization of Nanosystems for Glioblastoma Targeting through the Blood-Brain Barrier. J. Med. Chem. 2017, 60, 4510–4516. [Google Scholar] [CrossRef]
- Amero, P.; Esposito, C.L.; Rienzo, A.; Moscato, F.; Catuogno, S.; de Franciscis, V. Identification of an Interfering Ligand Aptamer for EphB2/3 Receptors. Nucleic Acid Ther. 2016, 26, 102–110. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Kumar, S.A.; Rienzo, A.; Lawrence, C.L.; Pallini, R.; Shaw, L.; Alder, J.E.; Ricci-Vitiani, L.; Catuogno, S.; et al. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J. Control Release 2016, 238, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Qi, X.; Liu, X.; Zhou, Y.; Woolf, E.C.; Valeri, A.; Al-Atrache, Z.; Belykh, E.; Feuerstein, B.G.; Preul, M.; et al. Provision of rapid and specific ex vivo diagnosis of central nervous system lymphoma from rodent xenograft biopsies by a fluorescent aptamer. J. Neurosurg. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Vilardo, C.; Nuzzo, S.; Ricci-Vitiani, L.; De Luca, G.; Pallini, R.; Kichkailo, A.S.; et al. The Discovery of RNA Aptamers that Selectively Bind Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2019, 18, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Fechter, P.; Cruz Da Silva, E.; Mercier, M.C.; Noulet, F.; Etienne-Seloum, N.; Guenot, D.; Lehmann, M.; Vauchelles, R.; Martin, S.; Lelong-Rebel, I.; et al. RNA Aptamers Targeting Integrin alpha5beta1 as Probes for Cyto- and Histofluorescence in Glioblastoma. Mol. Ther. Nucleic Acids 2019, 17, 63–77. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Wang, H.; Wu, L.; Zhang, H.; Song, Y.; Zhu, Z.; Kang, D.; Yang, C. DNA aptamers from whole-cell SELEX as new diagnostic agents against glioblastoma multiforme cells. Analyst 2018, 143, 2267–2275. [Google Scholar] [CrossRef]
- Hasan, M.R.; Hassan, N.; Khan, R.; Kim, Y.T.; Iqbal, S.M. Classification of cancer cells using computational analysis of dynamic morphology. Comput. Methods Programs Biomed. 2018, 156, 105–112. [Google Scholar] [CrossRef]
- Tang, J.; Huang, N.; Zhang, X.; Zhou, T.; Tan, Y.; Pi, J.; Pi, L.; Cheng, S.; Zheng, H.; Cheng, Y. Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. Int. J. Nanomed. 2017, 12, 3899–3911. [Google Scholar] [CrossRef]
- Georges, J.F.; Liu, X.; Eschbacher, J.; Nichols, J.; Mooney, M.A.; Joy, A.; Spetzler, R.F.; Feuerstein, B.G.; Preul, M.C.; Anderson, T.; et al. Use of a conformational switching aptamer for rapid and specific ex vivo identification of central nervous system lymphoma in a xenograft model. PLoS ONE 2015, 10, e0123607. [Google Scholar] [CrossRef]
- Gu, M.J.; Li, K.F.; Zhang, L.X.; Wang, H.; Liu, L.S.; Zheng, Z.Z.; Han, N.Y.; Yang, Z.J.; Fan, T.Y. In vitro study of novel gadolinium-loaded liposomes guided by GBI-10 aptamer for promising tumor targeting and tumor diagnosis by magnetic resonance imaging. Int. J. Nanomed. 2015, 10, 5187–5204. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Hasan, M.R.; Khan, U.J.; Allen, P.B.; Kim, Y.T.; Ellington, A.D.; Iqbal, S.M. One-step tumor detection from dynamic morphology tracking on aptamer-grafted surfaces. Technology 2015, 3, 194–200. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Henri, J.; Goodman, L.; Xiang, D.; Duan, W.; Shigdar, S. Development of a Bifunctional Aptamer Targeting the Transferrin Receptor and Epithelial Cell Adhesion Molecule (EpCAM) for the Treatment of Brain Cancer Metastases. ACS Chem. Neurosci. 2017, 8, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Perlas, C.; Oller-Salvia, B.; Sánchez-Navarro, M.; Teixidó, M.; Giralt, E. Branched BBB-shuttle peptides: Chemoselective modification of proteins to enhance blood-brain barrier transport. Chem. Sci. 2018, 9, 8409–8415. [Google Scholar]
- Ferda, J.; Ferdova, E.; Hes, O.; Mracek, J.; Kreuzberg, B.; Baxa, J. PET/MRI: Multiparametric imaging of brain tumors. Eur. J. Radiol. 2017, 94, A14–A25. [Google Scholar] [CrossRef]
- Delac, M.; Motaln, H.; Ulrich, H.; Lah, T.T. Aptamer for imaging and therapeutic targeting of brain tumor glioblastoma. Cytometry A 2015, 87, 806–816. [Google Scholar] [CrossRef]
- Hays, E.M.; Duan, W.; Shigdar, S. Aptamers and Glioblastoma: Their Potential Use for Imaging and Therapeutic Applications. Int. J. Mol. Sci. 2017, 18, 2576. [Google Scholar] [CrossRef]
- Khalid, U.; Vi, C.; Henri, J.; Macdonald, J.; Eu, P.; Mandarano, G.; Shigdar, S. Radiolabelled Aptamers for Theranostic Treatment of Cancer. Pharmaceuticals 2018, 12, 2. [Google Scholar] [CrossRef]
- Dang, M.; Phillips, P.C. Pediatric Brain Tumors. Continuum 2017, 23, 1727–1757. [Google Scholar] [CrossRef]
- Northcott, P.A.; Korshunov, A.; Witt, H.; Hielscher, T.; Eberhart, C.G.; Mack, S.; Bouffet, E.; Clifford, S.C.; Hawkins, C.E.; French, P.; et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 2011, 29, 1408–1414. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Cho, Y.J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Garzia, L.; Kijima, N.; Morrissy, A.S.; De Antonellis, P.; Guerreiro-Stucklin, A.; Holgado, B.L.; Wu, X.; Wang, X.; Parsons, M.; Zayne, K.; et al. A Hematogenous Route for Medulloblastoma Leptomeningeal Metastases. Cell 2018, 173, 1549. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.D.; Jamshidi, A.; Shah, S.; Martin, S.; Wolters, P.L.; Argersinger, D.P.; Warren, K.E.; Lonser, R.R. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2018, 23, 333–342. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.Y.; Jones, D.T.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.A.; Tonjes, M.; et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Lindroth, A.M.; Plass, C. Recurrent H3.3 alterations in childhood tumors. Nat. Genet. 2013, 45, 1413–1414. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef]
- Miklja, Z.; Pasternak, A.; Stallard, S.; Nicolaides, T.; Kline-Nunnally, C.; Cole, B.; Beroukhim, R.; Bandopadhayay, P.; Chi, S.; Ramkissoon, S.H.; et al. Molecular profiling and targeted therapy in pediatric gliomas: Review and consensus recommendations. Neuro-Oncology 2019. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017, 133, 5–12. [Google Scholar] [CrossRef]
- Vladoiu, M.C.; El-Hamamy, I.; Donovan, L.K.; Farooq, H.; Holgado, B.L.; Sundaravadanam, Y.; Ramaswamy, V.; Hendrikse, L.D.; Kumar, S.; Mack, S.C.; et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 2019, 572, 67–73. [Google Scholar] [CrossRef]
- Jones, R.M.; Pattwell, S.S. Future considerations for pediatric cancer survivorship: Translational perspectives from developmental neuroscience. Dev. Cogn. Neurosci. 2019, 38, 100657. [Google Scholar] [CrossRef] [PubMed]
- Koschmann, C.; Calinescu, A.A.; Nunez, F.J.; Mackay, A.; Fazal-Salom, J.; Thomas, D.; Mendez, F.; Kamran, N.; Dzaman, M.; Mulpuri, L.; et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci. Transl. Med. 2016, 8, 328ra328. [Google Scholar] [CrossRef]
- Jones, C.; Baker, S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Koschmann, C.; Zamler, D.; MacKay, A.; Robinson, D.; Wu, Y.M.; Doherty, R.; Marini, B.; Tran, D.; Garton, H.; Muraszko, K.; et al. Characterizing and targeting PDGFRA alterations in pediatric high-grade glioma. Oncotarget 2016, 7, 65696–65706. [Google Scholar] [CrossRef] [PubMed]
- Selt, F.; Deiss, A.; Korshunov, A.; Capper, D.; Witt, H.; van Tilburg, C.M.; Jones, D.T.; Witt, R.; Sahm, F.; Reuss, D.; et al. Pediatric Targeted Therapy: Clinical Feasibility of Personalized Diagnostics in Children with Relapsed and Progressive Tumors. Brain Pathol. 2016, 26, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, M.; Hwang, E.I. Molecularly Targeted Agents in the Therapy of Pediatric Brain Tumors. Paediatr. Drugs 2020, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nageswara Rao, A.A.; Scafidi, J.; Wells, E.M.; Packer, R.J. Biologically targeted therapeutics in pediatric brain tumors. Pediatr. Neurol. 2012, 46, 203–211. [Google Scholar] [CrossRef]
- Ke, W. Molecularly Targeted Therapy for Pediatric Brain Tumors. J. Neuro-Oncol. 2005, 75, 335–343. [Google Scholar] [CrossRef]
- Sadahiro, H.; Kang, K.D.; Gibson, J.T.; Minata, M.; Yu, H.; Shi, J.; Chhipa, R.; Chen, Z.; Lu, S.; Simoni, Y.; et al. Activation of the Receptor Tyrosine Kinase AXL Regulates the Immune Microenvironment in Glioblastoma. Cancer Res. 2018, 78, 3002–3013. [Google Scholar] [CrossRef]
- Meel, M.H.; de Gooijer, M.C.; Metselaar, D.S.; Sewing, A.C.P.; Zwaan, K.; Waranecki, P.; Breur, M.; Buil, L.C.M.; Lagerweij, T.; Wedekind, L.E.; et al. Combined therapy of AXL and HDAC inhibition reverses mesenchymal transition in diffuse intrinsic pontine glioma. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Huang, S.Y.; Yang, J.Y. Targeting the Hedgehog Pathway in Pediatric Medulloblastoma. Cancers 2015, 7, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.J. Hedgehog Pathway in Pediatric Cancers: They’re Not Just for Brain Tumors Anymore. Am. Soc. Clin. Oncol. Educ. Book 2012, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Brandt, W.D.; Schreck, K.C.; Bar, E.E.; Taylor, I.; Marchionni, L.; Raabe, E.; Eberhart, C.G.; Rodriguez, F.J. Notch signaling activation in pediatric low-grade astrocytoma. J. Neuropathol. Exp. Neurol. 2015, 74, 121–131. [Google Scholar] [CrossRef] [PubMed]
- de la Rocha, A.M.; Sampron, N.; Alonso, M.M.; Matheu, A. Role of SOX family of transcription factors in central nervous system tumors. Am. J. Cancer Res. 2014, 4, 312–324. [Google Scholar] [PubMed]
- Pollack, I.F.; Hamilton, R.L.; Sobol, R.W.; Nikiforova, M.N.; Lyons-Weiler, M.A.; LaFramboise, W.A.; Burger, P.C.; Brat, D.J.; Rosenblum, M.K.; Holmes, E.J.; et al. IDH1 mutations are common in malignant gliomas arising in adolescents: A report from the Children’s Oncology Group. Childs Nerv. Syst. 2011, 27, 87–94. [Google Scholar] [CrossRef]
- Mascelli, S.; Raso, A.; Biassoni, R.; Severino, M.; Sak, K.; Joost, K.; Milanaccio, C.; Barra, S.; Grillo-Ruggieri, F.; Vanni, I.; et al. Analysis of NADP+-dependent isocitrate dehydrogenase-1/2 gene mutations in pediatric brain tumors: Report of a secondary anaplastic astrocytoma carrying the IDH1 mutation. J. Neurooncol. 2012, 109, 477–484. [Google Scholar] [CrossRef]
- Liu, X.; McEachron, T.A.; Schwartzentruber, J.; Wu, G. Histone H3 mutations in pediatric brain tumors. Cold Spring Harb. Perspect. Biol. 2014, 6, a018689. [Google Scholar] [CrossRef]
- Sikkema, A.H.; den Dunnen, W.F.; Hulleman, E.; van Vuurden, D.G.; Garcia-Manero, G.; Yang, H.; Scherpen, F.J.; Kampen, K.R.; Hoving, E.W.; Kamps, W.A.; et al. EphB2 activity plays a pivotal role in pediatric medulloblastoma cell adhesion and invasion. Neuro-Oncology 2012, 14, 1125–1135. [Google Scholar] [CrossRef]
- Chen, P.; Rossi, N.; Priddy, S.; Pierson, C.R.; Studebaker, A.W.; Johnson, R.A. EphB2 activation is required for ependymoma development as well as inhibits differentiation and promotes proliferation of the transformed cell. Sci. Rep. 2015, 5, 9248. [Google Scholar] [CrossRef]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.E.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
| Aptamer Name | Year | Molecular Target | Aptamer Role | SELEX Method | KD (nmol/L) | Composition | Therapeutic Application | References |
|---|---|---|---|---|---|---|---|---|
| Gint4.T | 2020 | PDGFRβ | Antagonist | Cell-based SELEX | 9.6 | 2′-Fluoro RNA; conjugated with STAT3 siRNA | Reduce GBM tumor growth and relapse | [69] |
| GL21.T | 2020 | AXL | Antagonist | Cell-based SELEX | 13 | 2′-Fluoro RNA; conjugated with miR-10b | Reduce GBM tumor growth and relapse | [69] |
| 4-1BB–OPN | 2019 | CD8+/OPN | Agonist/antagonist | SELEX primer/in vitro SELEX | 40/18 | 2′-Fluoro RNA/RNA bispecific aptamer | CD8+ T cell activation/block M0 and M2 macrophage migration | [70] |
| Gint4.T/GMT8 | 2019 | PDGFRβ/U87 cell line | Antagonist/unknown | Cell-based SELEX/whole-cell SELEX | 9.6/unknown | 2′-Fluoro RNA/DNA nanocarrier | Cross BBB and deliver paclitaxel | [71] |
| PDR3 | 2019 | PDGFRα | Antagonist | Protein-based SELEX | 0.25 | RNA | Reduce GBM tumor growth | [72] |
| CL-4RNV616 | 2019 | EGFR | Antagonist | Cell-based SELEX | 18.24 | 2′-O-methyl RNA and DNA | Reduce cell proliferation | [73] |
| AS1411/GS24 | 2019 | Nucleolin/transferrin receptor | G-rich DNA/DNA nanocarrier | Cross BBB and deliver TMZ, reduce drug resistance | [74] | |||
| A15 | 2018 | CD133 | In vivo SELEX | RNA/dual-targeting ligand/nanoparticles | Cross BBB/deliver siRNA | [75] | ||
| TfR Aptamer | 2018 | Transferrin Receptor | 365 | Conjugated with RNV541 DNAzyme/antimiRzymes chimera | Suppress miR-21 expression in U87MG malignant glioblastoma | [76] | ||
| Gint4.T | 2018 | PDGFRβ | Antagonist | Cell-based SELEX | 9.6 | 2′-Fluoro RNA; conjugated with STAT3 siRNA | Inhibit cancer cell survival and migration. | [50] |
| Aptamer-like peptide | 2018 | EDB-fibronectin | 16 | APTEDB-PEG2000-DSPE | Liposome-based nanoparticle platform for systemic siRNA delivery | [77] | ||
| AS1411 | 2017 | Nucleolin | Poly (L-c-glutamylglutamine)–paclitaxel nanoconjugates | Increased median survival time of GBM tumor-bearing mice | [78] | |||
| Gint4.T | 2017 | PDGFRβ | Antagonist | Cell-based SELEX | 9.6 | Conjugated with BODIPY@PNPs | Cross BBB and deliver drugs | [79] |
| GL43.T | 2016 | EphB3/2 | Antagonist | Cell-based SELEX | 433.5 | 2′-Fluoro RNA | Inhibit cell migration | [80] |
| GL21.T/Gint4.T | 2016 | AXL/PDGFRβ | Antagonist | Cell-based SELEX | 13/9.6 | Conjugated with miR-137 and antimiR-10b | Target glioma stem-like cells | [81] |
| Aptamer Name | Year | Molecular Target | KD (nmol/L) | SELEX Method | Composition | Diagnostic or Imaging Application | References |
|---|---|---|---|---|---|---|---|
| TD05 | 2020 | CD20+ B cells | 256 | Cell-based SELEX | Conjugated with Alexa-488 | Diagnostic (intraoperative tumor-specific) Imaging (ex vivo fluorescence microscopy) | [82] |
| A40s | 2019 | EphA2 | 41.92 | Cell-based SELEX | 2’-Fluoro RNA conjugated with siRNA or miRNA | Imaging (confocal fluorescence microscopy) | [83] |
| H02 | 2019 | Integrinα5β1 | 72–277.8 | Protein SELEX | 2’-Fluoro RNA conjugated with Cy5, Alexa-564 | Imaging (confocal fluorescence microscopy) | [84] |
| WYZ-41a WYZ-50a | 2018 | A172 cells | 75.27–168.56 | Cell-based SELEX | DNA conjugated with Cy5, FITC | Imaging (confocal fluorescence microscopy) | [85] |
| A15 | 2018 | CD133 | In vivo SELEX | RNA/dual-targeting ligand/nanoparticles | Imaging (in vivo bioluminescency) | [75] | |
| Anti-EGFR | 2018 | EGFRvIII | 2′-Fluoro RNA | Dynamic morphology | [86] | ||
| QD-A32 Apt, | 2017 | EGFRvIII | DNA conjugated with streptavidin-PEG-CdSe/ZnS QDs | Imaging (in vivo imaging) | [87] | ||
| Quenched-TD05 | 2015 | CD20+ B cells | 256 | Cell-based SELEX | FRET-based switchable aptamer | Imaging (confocal fluorescence microscopy) Diagnostic (intraoperative diagnoses) | [88] |
| GBI-10 | 2015 | Tenascin-C | 150 | In vitro SELEX | Gadolinium-loaded liposomes | Diagnostic (magnetic resonance imaging) | [89] |
| Anti-EGFR | 2015 | EGFRvIII | 2′-Fluoro RNA | Dynamic morphology | [90] |
| Aptamer Name | Molecular Target | Composition | Administration Route | Therapeutic Applications | Clinical Trials | Status | Clinical Trials.gov Identifier |
|---|---|---|---|---|---|---|---|
| AS1411 (AGRO001) | Nucleolin | 26-mer G-rich DNA | Intravenous | Glioblastomas, acute myeloid leukemia, metastatic renal cell carcinoma | Phase II, phase 1, and phase II | Completed, completed, and unknown | NTC01034410, NTC00881244, and NTC00740441 |
| Anti-VEGF PEGylated aptamer (EYE001) | VEGF | Intravitreal injection | Retinal tumors in patients with Von Hippel-Lindau syndrome | Phase I | Completed | NCT00056199 | |
| NOX-A12 | Angiogenic chemokine (C-X-C motif) ligand 12 (CXCL12, also known as SDF-1α) | 45-mer L-RNA with 3′-PEG (Spiegelmer) | Intravenous | Multiple myeloma, non-Hodgkin lymphoma, leukemia, metastatic colorectal cancer | Phase II, phase II, and phase I | Completed, completed, and unknown | NTC01521533, NTC01486797, and NTC03168139 |
| Aptamer sensors | Discover urinary biomarker(s) | Electro-phage and colorimetric aptamer sensors for clinical staging and monitoring by FRET system | Urine test | Bladder cancer | Recruiting patients | NCT02957370 | |
| 68Ga-Sgc8 | PTK7 | 41-mer DNA bi-functional aptamer; diagnostic performance and evaluation efficacy of a novel PTK7 positron emission tomography radiotracer 68Ga-SGC8 | Intravenous | Colorectal cancer | Early-phase I | NTC03385148 | |
| Aptamers to target tumor cells In the laboratory | Unknown | Tailored neoadjuvant epirubicin and cyclophosphamide and nanoparticle albumin bound paclitaxel for newly diagnosed breast cancer | Tumor tissue in vitro | Breast cancer | Phase II | NCT01830244 | |
| X-aptamers library | Unknown | Novel proteomic biomarkers for HCC patients treated with Lipiodol TACE using beads-based X-aptamer library, then validate and create a biomarker panel that can be used to predict the outcome of patients with hepatocellular carcinoma after treatment with Lipiodol TACE | Blood test | Hepatocellular carcinoma | July 2020 | Not yet recruiting | NCT04459468 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amero, P.; Khatua, S.; Rodriguez-Aguayo, C.; Lopez-Berestein, G. Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology. Cancers 2020, 12, 2889. https://doi.org/10.3390/cancers12102889
Amero P, Khatua S, Rodriguez-Aguayo C, Lopez-Berestein G. Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology. Cancers. 2020; 12(10):2889. https://doi.org/10.3390/cancers12102889
Chicago/Turabian StyleAmero, Paola, Soumen Khatua, Cristian Rodriguez-Aguayo, and Gabriel Lopez-Berestein. 2020. "Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology" Cancers 12, no. 10: 2889. https://doi.org/10.3390/cancers12102889
APA StyleAmero, P., Khatua, S., Rodriguez-Aguayo, C., & Lopez-Berestein, G. (2020). Aptamers: Novel Therapeutics and Potential Role in Neuro-Oncology. Cancers, 12(10), 2889. https://doi.org/10.3390/cancers12102889








