Abstract
The survival rates of patients with metastatic colorectal cancer (mCRC) have improved in recent years. We analysed the survival of mCRC patients followed at a single institution over the last 17 years. We retrospectively collected data from 899 mCRC patients treated from 2001 to 2016. Patients were divided into two groups based on the year of diagnosis: Cohort A (2001–2006) and Cohort B (2007–2014). A total of 788 patients were analysed. The median survival of the whole population was 32.0 months with a significant difference between Cohort A and B (29.2 vs. 33.5 months; p = 0.041). Surgical procedures significantly increased in Cohort B, however, no significant changes in survival were observed in patients undergoing surgery (58.9 months Cohort A vs. 58.2 months Cohort B, p = 0.822). Similarly, we did not demonstrate survival improvement in patients treated with systemic therapy alone (18.9 months Cohort A vs. 20.7 months Cohort B; p = 0.948). At the multivariate analysis, right-sided primary and synchronous metastatic tumour were found to be independent unfavorable prognostic factors. Improvements of mCRC patient survival might relate to integrated approach, with more patients undergoing extra-hepatic surgery. The medical approach seems to have had a more favourable impact on subgroups characterized by a worse prognosis.
1. Introduction
Colorectal cancer (CRC) represents the second most commonly diagnosed cancer in Europe, with 215,000 deaths in 2012 [1,2].
Over the last two decades, the outcome of patients with metastatic CRC (mCRC) has significantly improved, reaching a median overall survival (mOS) of around 30 months, more than double that 20 years ago [3,4].
This advancement may result from the contribution of a more aggressive systemic approach—including three-drug chemotherapy (CT) schemes and CT with targeted therapy combinations—together with a substantial change in surgical indication [5,6,7].
In our study, we investigated the possible influence of tumour characteristics as well as the changes in treatment practice through the years, on the evolution of survival of mCRC patients.
2. Results
2.1. Demographics
A total of 1702 patients with CRC were treated at our institution from 1999 to 2016.
Patients with localized CRC who received adjuvant CT only and those with mCRC who had never been treated with chemotherapy were excluded from the analysis.
Overall, 899 patients were available for the analysis, 788 of whom displayed a sufficient completeness of treatment and outcome data to be included in the survival analysis.
The median age at diagnosis was 62.58 years.
All main disease and treatment characteristics are summarized in Table 1.

Table 1.
Population characteristics.
2.2. Treatment
Considering the whole population, patients who underwent surgery significantly increased (42% in Cohort A vs. 58% in Cohort B; p < 0.009). A total of 203 patients only received hepatic surgery (93 in Cohort A vs. 110 in Cohort B). The details of surgery in the two cohorts are listed in Table 2 and Table 3.

Table 2.
Liver surgery vs. surgery of other sites.

Table 3.
Surgery sites and distribution.
Eleven patients in Cohort B received hypertermic intraperitoneal chemotherapy (HIPEC) and/or pressurised intraperitoneal aerosol chemotherapy (PIPAC) procedures.
In the first line setting, two-drug CT containing oxaliplatin was the main choice in both cohorts (65.1% in Cohort A and 66.3% in Cohort B). Conversely, the use of irinotecan-based doublets was significantly higher in Cohort B (20.7%) compared with Cohort A (13.6%) in this setting, while it became the predominant CT adopted in both cohorts in the second line setting (54.3% in Cohort A and 56.7 % in Cohort B). Only 1% of patients received FOLFOXIRI regimen in Cohort B, especially in the first line setting. Conversely, the number of patients receiving monotherapy was significantly lower in all the treatment lines in more recent years.
Differences were also noted in the administration of anti-EGFR and anti-VEGF antibodies and an increase between the two cohorts in both the first and the second line settings was observed. Details of treatment administration in the two cohorts are presented in Table 4.

Table 4.
Treatment characteristics.
2.3. Survival Analysis
The survival analysis was carried out on 788 patients (46.3% in Cohort A and 53.7% in Cohort B). The median follow-up time was 24.7 months (23.8 months in Cohort A and 25.6 months in Cohort B).
The mOS was 32.0 months (95% confidence interval (CI); 28.8 to 35.3 months). Patients’ survival in Cohort B was significantly longer compared with that in Cohort A (median 33.5 months vs. 29.2 months, respectively, HR 0.832; 95% CI 0.697–0.992; p = 0.041) (Figure 1).
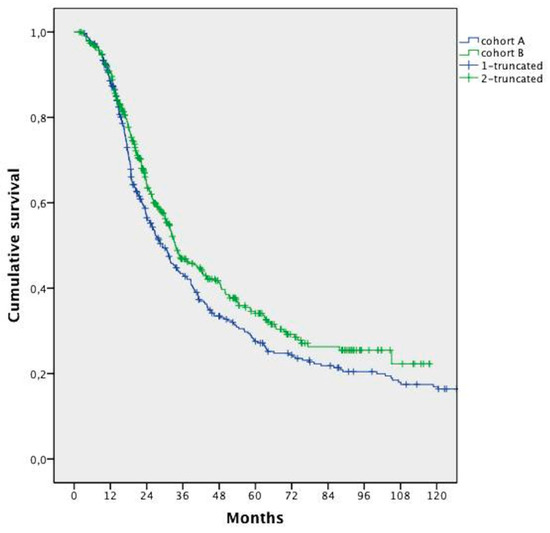
Figure 1.
Median overall survival analysis in the two cohorts.
Among patients included in the survival analysis, 456 underwent resection of hepatic and/or other metastatic sites, with a larger proportion of surgical patients in Cohort B (58% vs. 42%; p < 0.009). While hepatic resection rates remained similar through the years (20.3% Cohort A vs. 24.1% Cohort B), resection of metastases at other sites with or without hepatic surgery was performed with increasing frequency (21.4% Cohort A vs. 33.9% Cohort B; p < 0.005). In particular, the largest increase was in peritoneal surgery.
In the whole population, surgery in combination with CT allowed a significantly longer survival when compared with CT alone (median 58.5 months vs. 20.1 months, respectively, HR 0.262; 95% CI 0.216–0.316; p < 0.0001). This advantage was maintained even when distinguishing between liver surgery and surgery of other sites, with or without hepatic surgery (Figure 2).
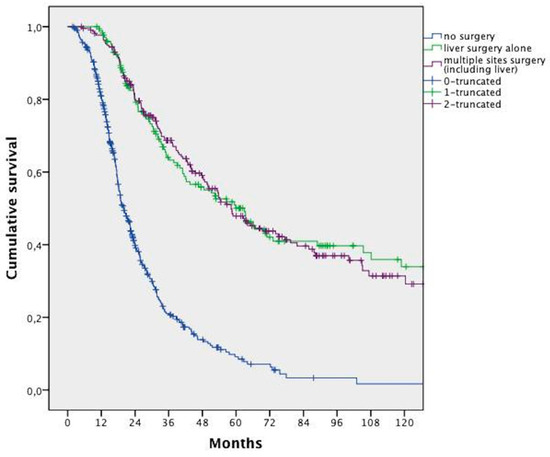
Figure 2.
Overall survival analysis of patients who underwent liver surgery vs. surgery of multiple sites (including liver) vs. no surgery.
No differences in survival of patients who underwent surgery—in addition to a systemic treatment—were detected between cohorts (median 58.9 months vs. 58.2 months, HR 1.033; 95% CI, 0.779–1.369; p = 0.822) (Figure 3A).
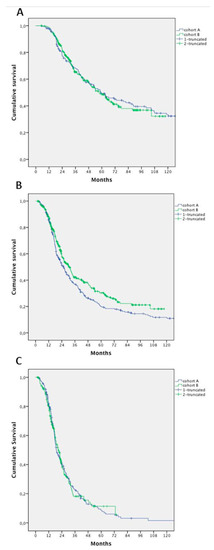
Figure 3.
Survival analysis according to treatment in Cohort A and B: (A) patients undergoing systemic treatment and surgery; (B) patients undergoing systemic treatment and surgery, excluding patients who had hepatic resection alone; and (C) patients undergoing exclusively systemic treatment.
After excluding patients who had hepatic resection alone, we found a significant improvement in survival in patients in Cohort B (25.7 months in Cohort A vs. 30.4 months in Cohort B; HR 0.796; 95% CI 0.656–0.967; p = 0.021) (Figure 3B).
We failed to demonstrate an improvement in OS in patients treated with CT alone (with or without targeted agents): mOS 18.9 months in Cohort A versus 20.7 months in Cohort B (HR 1.0; 95% CI 0.799–1.271; p = 0.948) (Figure 3C).
2.4. Prognostic Factors
The prognostic role of clinical characteristics was analysed by uni- and multivariate analysis (Table 5).

Table 5.
Univariate and multivariate analysis. CI—confidence interval.
At the multivariate analysis, a right-sided primary tumour and synchronous metastatic disease were found to be independent unfavorable prognostic factors. All these characteristics had a well-balanced distribution between cohorts (Table 6).

Table 6.
Unfavourable prognostic factors distribution.
In the whole population, a worse prognosis was observed for patients with right-sided compared with left-sided primary tumours—mOS 22.9 versus 38.6 months, respectively (HR 1.709; 95% CI 1.427–2.047; p < 0.0001) (Figure 4A).
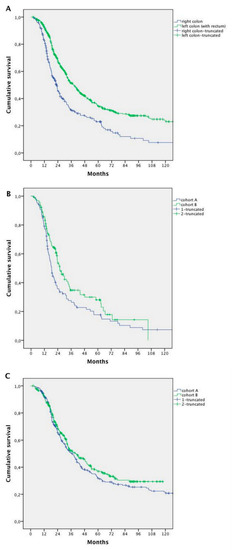
Figure 4.
Survival analysis according to the primary tumor site: (A) survival analysis of patients with right-sided primary tumor vs. left-sided primary tumor (whole population); (B) survival analysis for patients with right-sided primary tumor in Cohort A vs. Cohort B; and (C) survival analysis for patients with left-sided primary tumor in Cohort A vs. Cohort B.
Survival seemed to increase in both left- and right-sided patients in Cohort B compared with Cohort A. However, the difference was statistically significant for right-sided tumours only (18.5 months Cohort A vs. 25.8 months Cohort B, HR 0.738; 95% CI 0.546–0.998; p = 0.044), without changes in the left-sided ones (34.5 months Cohort A vs. 37.5 months Cohort B; HR 0.883; 95% CI 0.638–1.223; p = 0.455) (Figure 4B,C).
After excluding surgery, a significant improvement was achieved in patients treated with systemic therapy only (15.8 months Cohort A vs. 22.1 months Cohort B, p = 0.041), while mOS in resected patients remained stable over time for right-sided tumours (49.6 months Cohort A vs. 48.2 months Cohort B, p = 0.736).
Median survival was 36.2 months for patients with metachronous disease and 30.0 months for patients with synchronous disease (HR 0.718; 95% CI 0.592–0.870; p = 0.001). Patients with synchronous disease trended to show better rates of survival in Cohort B (median 26.0 months Cohort A vs. 31.9 months Cohort B; HR 0.814; 95% CI 0.660–1.005; p = 0.05), while no significant improvement was recorded in metachronous disease (34.5 months Cohort A vs. 37.5 months Cohort B; HR 0.883; 95% CI 0.638–1.223; p = 0.455) (Figure 5)
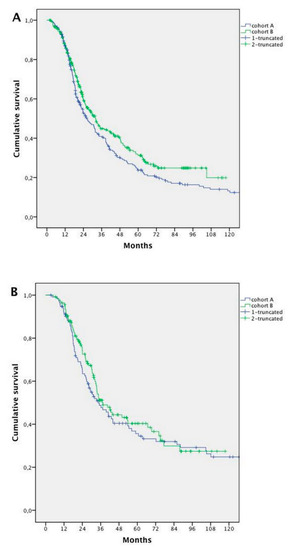
Figure 5.
Survival analysis in synchronous vs. metachronous metastases: (A) survival analysis of patients with synchronous metastases in Cohort A vs. Cohort B; and (B) survival analysis in the two Cohorts for patients with metachronous metastases in Cohort A vs. Cohort B.
3. Discussion
Recent studies in mCRC have shown that new drug combinations allow patients to achieve mOS rates of around 30 months or more [5,6,7]. However, these results might overestimate real world data because clinical study subjects generally represent a selected population with better prognostic characteristics compared with the general population. When currently used combinations were published in the early 2000s [8,9,10], it became evident that mOS of mCRC patients could reach 21 to 24 months with CT and monoclonal antibodies (mAb). However, any comparison of those results with more recent data would be hindered by a number of biases.
In recent years, widespread awareness of the potential for cure has led to intensified treatment of mCRC, including multidisciplinary discussion and multimodality approach. Furthermore, molecular selection has gained an unquestionable role in exploiting EGFR inhibition and, in keeping with the rules in force, anti-EGFRs have also been introduced in first line treatments.
In this study, we looked into whether changes in practice might have influenced survival rates in an unselected population. The patients were treated at a single institute according to current guidelines and were retrospectively analysed. Two periods, for Cohort A until 2006, and for Cohort B from 2007 onwards, were compared.
The results of our analyses revealed that patients in Cohort B lived longer than patients in Cohort A. Kopetz et al. [11] demonstrated that in patients diagnosed from 1998 to 2004, improvements in outcomes were mainly associated with an increase in surgery, whereas in the period from 2004 to 2006, the rise was related to advances in medical therapy. In our series, the percentage of patients who underwent surgery increased from 42% in Cohort A to 48% in Cohort B, while liver resection was stable at around 22%. This percentage was nearly the same as that of Kopetz et al.; however, they did not analyse extra-hepatic surgery data. A previous report from a multicenter study, including our own, highlighted the role of surgery of lung metastases in improving survival [12]. Moreover, surgical management of mCRC has developed in recent years to include cytoreductive surgery of peritoneal metastases and HIPEC. These approaches are now evolving as new standards of treatment in highly specialized centers [13]. In our study, the percentage of patients who underwent extra-hepatic surgery increased from 21% in Cohort A to 34% in Cohort B and the survival curves of these patients were similar to those of patients who had liver surgery alone. It is of note that, whereas the survival rates of patients receiving liver surgery alone did not differ between cohorts, there was a significant increase of survival in the subgroup of patients who had extra-hepatic surgery in recent years.
The main change observed in medical treatment practice, along with the introduction of mAb, was the adoption in the first-line of a two-drug in a larger percentage of patients in Cohort B, leaving the monotherapy to a subgroup of patients with poor prognosis. This reflects the updating of international guidelines, which previously considered asymptomatic patients with low burden disease as suitable for sequential schedules of monotherapy [4]. More recently, a general strategy has evolved to consider a CT doublet with mAb as an appropriated choice, where the goal is disease control for patients for whom intensive treatment is not necessary [14]. Even in unfit patients, capecitabine plus bevacizumab or a reduced-dose doublet of cytotoxics or anti-EGFR-containing therapy can be considered [3].
Despite the evolution of medical strategies, if we compare survival rates of patients treated with CT alone, the increase of survival between the two cohorts was not significant in the whole population. However, considering patients with right-sided primary tumour, an independent negative prognostic factor, the survival of patients in Cohort B was significantly longer than in Cohort A. The prognostic role of tumour sideness has been known for a long time, however, recent studies have shed light on the biological causes of these differences [15,16]. By separately analyzing patients with right-sided or left-sided tumours, we found that while survival increased in both groups, the difference was statistically significant in the right-sided group only. Furthermore, considering synchronous and metachronous metastatic groups, the improvement of survival in Cohort B was more evident for patients with synchronous metastatic disease.
4. Materials and Methods
4.1. Study Design
The study is a retrospective, single institution experience, conducted on mCRC patients attending the Candiolo Cancer Institute from 1999 to 2016. All patients were treated, after informed consent was signed, according to institutional and national guidelines.
The aim of this study was to describe the evolution of survival of mCRC patients followed at a single institution over the past 17 years. All the patients were screened using the International Classification of Diseases 9th revision Clinical modification (ICD-9-CM) codes for colon and rectal cancer. Only patients receiving at least one course of therapy for mCRC were included. Both metachronous and synchronous metastatic disease presentations were taken into account. Metastatic disease was considered synchronous when diagnosed within six months after the initial diagnosis of CRC. For each patient, demographic and clinicopathological data were collected. We defined as a line of treatment all the therapies administered between the evidence of disease progression until further progression, even in the case of multimodality treatment.
All the descriptive analyses of the patients’ characteristics included patients treated between January 1999 and November 2016. However, in order to minimize the risk of bias related to a small group of patients treated before 2001 (the date of the setting up of a multidisciplinary team for mCRC treatment) and to obtain a minimum follow-up period of two years, the survival analysis did not include patients diagnosed before 1 January 2001 or after 31 December 2014.
Overall survival was calculated from the date of diagnosis of metastatic disease until death, as notified by the registry office, or censored at the last follow-up visit. The median follow-up period was calculated on the basis of median survival without considering life status. In order to detect survival changes, patients were divided into two groups according to the year of metastatic disease diagnosis: Cohort A (between 2001 and 2006) and Cohort B (between 2007 and 2014). The cut off point was set at 2006 as this date coincided with the introduction of the molecular targeted agents in clinical practice.
4.2. Statistical Analysis
SPSS for Windows 20.0 (IBM SPSS, Chicago, IL, USA) was used for all statistical analyses. The differences between proportions were evaluated by the chi-square test with Yates correction, when appropriate. The Cox proportional hazards regression model was used and survival curves were plotted using Kaplan–Meier and compared by log-rank test. Overall survival was calculated from the date of diagnosis of metastatic disease until death, or censored at the last follow-up visit.
4.3. Ethics Approval and Consent to Participate
The study was performed in accordance with the Declaration of Helsinki. The development and the publication of this report was approved by the Institutional Review Board. The Medical Ethical Committee of the IRCCS Candiolo Cancer Institute confirmed that formal ethical approval was not required given the retrospective and observational nature of the study.
5. Conclusions
We realise that our study is limited by various biases related to the retrospective nature of our analyses. However, our results suggest that in current clinical practice, unless patients are classified as unfit for therapy, the therapeutic strategy is moving towards intensive treatment where at least two cytotoxic therapies are combined together with biological agents, and a multimodal approach in which surgery of metastatic sites is considered feasible. This approach seems appropriate to increase patient survival. In particular, it is likely that poor prognostic subgroups of mCRC patients would benefit from an integration of medical and surgical treatments in a ‘continuum of care’ strategy.
Author Contributions
Conceptualization, E.F., F.C. and F.L.; methodology, E.F.; software, F.C.; validation, F.L.; formal analysis, E.F. and F.C.; investigation, E.F.; resources, E.F., F.C., M.G.C., R.F. (Roberto Filippi), I.D., G.C., C.C., R.F. (Renato Ferraris) and M.V.; data curation, E.F. and F.C.; writing—original draft preparation, E.F.; writing—review and editing, F.C., F.L., M.G.C., R.F. (Roberto Filippi), I.D., G.C., P.L., D.M., C.C., M.V., M.A. and F.L.; visualization, E.F.; supervision, F.L; project administration, E.F., F.C. and F.L.; funding acquisition, M.A and F.L. All authors have reviewed, discussed, and agreed on their individual contributions.
Funding
This work was supported by grants from Fondazione Piemontese per la Ricerca sul Cancro-Onlus (FPRC) 5X1000–; “Identification of new druggable pathways in intrahepatic cholangiocarcinoma”; Ministero della Salute 2010; Fondo per la ricerca locale (Linea B), LEOF_RIC_LOC_14_01 project title: “Transcriptomic and genetic analysis of paired primary and recurrent intrahepatic cholangiocarcinoma”; Progetto di Rete–NET2011-02352137 “Genomic base triage for target therapy in colorectal cancer” Ricerca Sanitaria Finalizzata 2011.
Acknowledgments
We would like to thank Andrew Martin Garvey for the native English speaker revision of the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5- FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (WT) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J. Clin. Oncol 2014, 32, LBA3. [Google Scholar]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Tampellini, M.; Ottone, A.; Bellini, E.; Alabiso, I.; Baratelli, C.; Bitossi, R.; Brizzi, M.P.; Ferrero, A.; Sperti, E.; Leone, F.; et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: Results from a large retrospective study. Oncologist 2012, 17, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat. Rev. 2016, 48, 42–49. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.; Rho, Y.S.; Kuruba, G.; Mamo, A.; Gilabert, M.; Kavan, T.; Panasci, L.; Melnychuk, D.; Batist, G.; Kavan, P. Clinical Practice Patterns in Chemotherapeutic Treatment Regimens for Metastatic Colorectal Cancer. Clin. Colorectal. Cancer 2016, 15, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic Survival Associated with Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).