Abstract
This study examined the effects of ochratoxin A (OTA) exposure and graded dietary selenium (Se) supplementation on fatty acid (FA) composition and oxidative stress markers in the liver of broiler chickens. OTA is known to generate oxidative stress, promote lipid peroxidation, and affect the antioxidant system. Se, an essential trace element with antioxidant properties, may help counteract OTA-induced toxicity. In this short-term (5-day) in vivo feeding experiment, 21-day-old broiler chickens were divided into six groups, each with six birds: Control (diet free from Se), 0.3 mg/kg Se, 0.5 mg/kg Se, 2 mg/kg OTA, 2 mg/kg OTA + 0.3 mg/kg Se, 2 mg/kg OTA + 0.5 mg/kg Se. Our findings show that supplementing 0.3 mg/kg (p < 0.01) or 0.5 mg/kg Se (p < 0.001) in OTA-exposed birds significantly reduced the early oxidative stress markers (conjugated dienes and trienes) and significantly increased (0.3 mg/kg p < 0.01; 0.5 mg/kg p < 0.001) glutathione levels, indicating enhanced glutathione-dependent antioxidant protection. The treatments also significantly altered the ratio of monounsaturated and n6/n3 polyunsaturated FAs. OTA with 0.3 mg/kg Se supplementation significantly (p ˂ 0.05) reduced total unsaturation and FA average chain length. At a dose of 0.3 mg/kg, the interaction of Se and OTA altered the PUFA composition, while 0.5 mg/kg Se supplementation enhanced antioxidant defense and reduced lipid peroxidation. These results highlight the dual but separate role of Se, where inadequate doses may enhance OTA toxicity, while optimal supplementation may have a protective effect on hepatic lipid homeostasis. These findings can be used in the future progress of the mitigation strategy against OTA exposure in poultry nutrition.
Keywords:
ochratoxin A; selenium; broiler chicken; glutathione redox system; lipid peroxidation; fatty acid composition Key Contribution:
The selenium supplementation at 0.3 mg/kg did not prevent the ochratoxin A-induced disturbances in hepatic lipid metabolism and oxidative stress, while 0.5 mg/kg selenium enhanced antioxidant defense and preserves lipid integrity.
1. Introduction
Due to global warming, climate change within European temperate regions makes them more suitable for the growth and spread of toxigenic molds, including Aspergillus, Penicillium, and Fusarium. Rising temperature and periodic droughts expand fungal habitats, increasing mycotoxin contamination in crops []. Such contamination poses risks to agriculture, as contaminated animal feed can lead to production losses or toxic effects in livestock depending on the dose []. Ochratoxin A (OTA), a secondary metabolite of certain Aspergillus and Penicillium species, is one of the most prevalent nephro- and hepatotoxic mycotoxins contaminating poultry feeds worldwide []. Due to carry-over effects, OTA residues can also be found in animal-derived foods such as pork, poultry, milk, and cheese, contributing to human exposure [].
Broilers are markedly sensitive to OTA toxicity; exposure to 0.4–0.8 mg OTA/kg feed from day 1 to week 5 has been associated with significant reductions in body weight, feed intake, thymus weight, red and white blood cell counts, and immune response, along with increased mortality []. In the European Union, the recommended maximum level of OTA in cereal-based and poultry feeds is 0.10 mg/kg, as established by Commission Recommendation 2006/576/EC on the presence of OTA in products intended for animal feeding []. The toxic effect is the result of several mechanisms of action, such as the inhibition of protein synthesis, DNA and mitochondrial damage, and modulation of signaling pathways []. Besides these, OTA promotes the formation of reactive oxygen species, which can cause lipid peroxidation and oxidative damage to cellular components, including membrane lipids [,]. In liver (HepG2) cells, incubation with 100 μM OTA reduced cellular GSH levels, one of the most important intracellular antioxidants, and influenced ROS production in a dose-dependent manner, which may contribute to the hepatotoxic effects of OTA []. Alterations in the hepatic fatty acid (FA) composition and lipid peroxidation markers reflect these oxidative processes, disrupting membrane integrity, cellular permeability, and enzyme activity [], primarily altering the metabolism of the polyunsaturated FAs of the glycerophospholipidome. Lipid peroxidation depends on the rate of unsaturation of FAs because the higher number of unsaturated double bonds in the fatty acyl chain increases its susceptibility to peroxidation. Peroxidized FAs in the cell membranes, particularly in mitochondrial membrane, may cause the release of oxygen free radicals and induce oxidative stress in the liver [].
Consistent with these findings, studies have reported that low-molecular-weight antioxidants, such as tocopherol, ascorbic acid, and phytogenic bioactive feed additives can decrease lipid peroxidation and mitigate OTA-induced damage [,]. Selenium (Se) is an essential trace element that plays a key role in protecting against oxidative stress, both as a component of selenoproteins, particularly the glutathione peroxidase (GPx) isoenzymes, and by supporting non-enzymatic antioxidant mechanisms, such as vitamin E and reduced glutathione (GSH) []. Se has immunomodulatory effects similar to some bioactive plant extracts [,] and inhibits apoptosis []. Through these mechanisms, Se can reduce or even prevent the oxidative stress caused by OTA. Additionally, oxidative stress and Se modulate activation of liver progenitor cells through the VEGF-FGF signaling mechanism []
Se has an antagonistic effect against the toxicity of mycotoxins, such as aflatoxin A, T-2 toxin, deoxynivalenol, and OTA []. The protective effect of Se suggested provides its antioxidant properties through the Nrf2 signaling pathway. However, another mode of action of Se through the JAK1/STAT3 signaling has also been proposed and experimentally proven []. Selenomethionine could regulate the Nrf2/Keap1 and PI3K/AKT pathways to reduce the hepatotoxicity induced by OTA in chicken []. It means that selenomethionine activates signaling pathways that counteract OTA-induced oxidative stress. Moreover, nutritional factors such as Se supplementation can substantially modify the composition and metabolic capacity of the gut microbiota, which in turn may influence the severity and characteristics of OTA-induced liver injury []. There is no data in the literature about the effect of OTA on hepatocellular membrane FA composition, as well as in relation to selenomethionine. However, some studies propose potential FA alterations in meat and liver in OTA toxicity [,], but not about its interaction with Se supplementation.
Based on these considerations, the aim of this research was to evaluate the changes in lipid peroxidation, efficiency of the glutathione redox system, and total phospholipid FA composition resulting from OTA-induced oxidative stress. Another aim was to assess the effectiveness of the Se supplementation in mitigating potential adverse effects caused by OTA exposure such as oxidative stress and FA composition.
2. Results
2.1. Body Weight, Liver Weight
Neither Se complementation, OTA separately, nor OTA + Se combinations significantly affected BW after 5 days. Liver weights (absolute and relative) were also similar among the groups (Table 1).

Table 1.
Body and liver weights of broiler chickens present as mean standard deviation (n = 6).
2.2. Fatty Acid Profile of Total Hepatic Phosphatides
The fatty acid profile of total hepatic phosphatides is shown in Table 2.

Table 2.
Hepatic dataset including phosphatide fatty acid of the 6 experimental groups (means of 6 individual data ± standard deviation).
As evaluated with ANOVA and Tukey’s post hoc test, within the saturated FAs, the lower level of Se supplementation (0.3 Se/kg feed) increased the C20:0 (arachidic acid) proportion above the control, while both levels of Se supplementation increased the C22:0 (behenic acid) proportion above the OTA mean. The Se-supplemented groups generally exhibited higher C22:0 levels, whereas the OTA-exposed groups showed a decrease in its content. Interestingly, total saturation level was lowest in the Se 0.5 group, while the highest value was shown in the OTA-Se 0.5 group.
Among the monounsaturated FAs, the proportion of C16:1 n-7 (palmitoleic acid) was higher in the Se 0.3 group as compared to the control, while the proportion of C18:1 n-9 (oleic acid) in the OTA-Se 0.3 group exceeded the control. An increase in total monounsaturated FAs was observed in both the Se 0.3 and OTA–Se 0.3 groups, merging the effects of both treatments when compared to the control.
In the n-6 FA family, the arachidonic acid (C20:4 n-6) proportion was lowered by Se 0.3 alone and in combination with OTA as well, as compared to the control, while the adrenic acid (C22:4 n-6) proportion was only lowered by the combination of Se 0.3 and OTA. Docosapentaenoic acid (C22:5 n-6) was decreased by the treatments Se 0.3, Se 0.5, and OTA-Se 0.3 below the control. Only the OTA-Se 0.3 group showed a substantial decrease in the sum of all n-6 FAs compared to the control (Table 3).

Table 3.
Summary of fatty acid classes and lipid quality indices in broiler chicken hepatic total PLs of the 6 experimental groups (means of 6 individual data ± standard deviation).
In the n-3 FA family, OTA-Se 0.3 lowered the docosapentaenoic acid (C22:5 n-3) proportion below the control, while for docosahexaenoic acid (C22:6 n-3), the Se 0.3, Se 0.5, and OTA-Se 0.3 treatments had the same effect. With respect to the total n-3 FAs, both the low-dose Se and OTA-Se 0.3 treatments lowered the proportion compared to the control.
The unsaturation index (UI) was lowered by OTA-Se 0.3, as compared to the control. A fully identical pattern was found for the average FA chain length (ACL).
Analyzing the Se and OTA effects as factors with the GLM model, the cases where Se did not provide a significant effect were C20:0 and the sum of saturated FAs, while the effect of OTA was more diverse on the FA profile (Table 3); it significantly affected C22:0, C20:4 n-6, total n-6, PUFA, and UI. Significant interactions among Se and OTA were not found on the lipid profile, indicating the agents’ separate effects.
2.3. Antioxidant and Lipid Peroxidation Parameters
The GSH concentration was elevated by OTA exposure alone and in combination with both low and high Se complementation above the control. However, Se supplementation did not change the GSH content. Se supplementation at both levels did not change the GPx activity. OTA exposure alone and in combination with Se revealed higher GPx activity than the control, and this difference was also significant as compared to Se-supplemented groups (Figure 1).
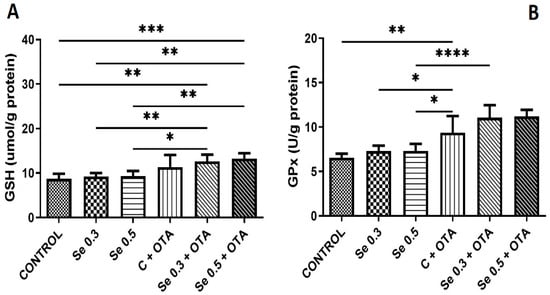
Figure 1.
Development of markers of glutathione-based antioxidant protection by day 5 of the experiment (means of 6 individual data ± standard deviation). (A) Reduced glutathione; (B) glutathione peroxidase (means of 6 individual data ± standard deviation). Control: no OTA or Se; Se 0.3: 0.3 mg/kg Se; Se 0.5: 0.5 mg/kg Se; OTA: 2 mg/kg OTA OTA-Se 0.3: 0.3 mg/kg Se and 2 mg/kg OTA; OTA-Se 0.5: 0.5 mg/kg Se and 2 mg/kg OTA. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
While 0.5 mg/kg Se supplementation and OTA exposure alone did not alter CD and CT levels, their combined application resulted in significant reduction compared to the control, Se 0.3, Se 0.5, and OTA alone groups. A reduction in CD and CT levels was also observed in the group receiving 0.3 mg/kg Se + OTA compared to the control and, in the case of CT, even compared to the Se 0.3 group. Both 0.3 mg/kg Se supplementation and OTA exposure alone reduced MDA concentrations relative to the control (Figure 2).
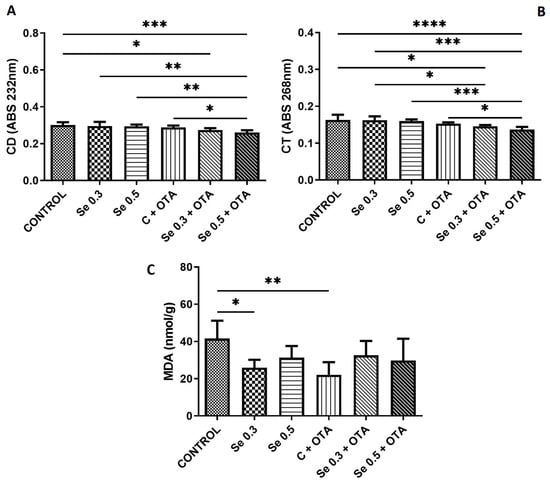
Figure 2.
Changes in the markers of lipid peroxidation by day 5 of the experiment (means of 6 individual data ± standard deviation). (A) Conjugated dienes; (B) conjugated trienes; (C) malondialdehyde. Control: no OTA or Se; Se 0.3: 0.3 mg/kg Se; Se 0.5: 0.5 mg/kg Se; OTA: 2 mg/kg OTA; OTA-Se 0.3: 0.3 mg/kg Se and 2 mg/kg OTA; OTA-Se 0.5: 0.5 mg/kg Se and 2 mg/kg OTA (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
Similarly to the effects’ analysis, GLM was invented to analyze possible interactions of Se and OTA. Effects are summarized in Table 4.

Table 4.
Effects of statistical interactions of OTA and Se on the hepatic antioxidant and lipid peroxidation parameters of broilers.
The results indicate the separate effects of Se and OTA, the latter significantly affecting all parameters, while Se had no effect on GSH and MDA. Remarkably, we did not find any interaction between the two agents.
2.4. Group Classification (sPLS-DA)
Performing sPLS-DA analysis revealed efficient spatial separation of the six experimental groups (Figure 3). In the figure, the OTA and Se groups’ effective spatial separation is clearly visible.
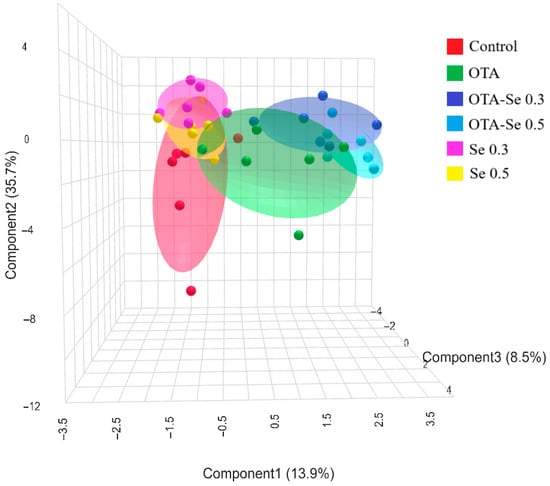
Figure 3.
3D sPLS-DA score plot of the experimental groups (data: phospholipid fatty acids, lipid peroxidation, and antioxidant markers). Control: no OTA or Se; Se 0.3: 0.3 mg/kg Se; Se 0.5: 0.5 mg/kg Se; OTA: 2 mg/kg OTA; OTA-Se 0.3: 0.3 mg/kg Se and 2 mg/kg OTA; OTA-Se 0.5: 0.5 mg/kg Se and 2 mg/kg OTA.
In the frame of the sPLS-DA analysis, we checked the top 10 most powerful loading items contributing to the spatial separations’ components. Figure 4 provides these loading plots with the most powerful biological variables. For component 1 the variables of the highest contribution to the separation were mostly antioxidants (GPx, GSH) and early lipid peroxidation markers (CD, CT); those for component 2 mostly contained FA groups (e.g., n3, MUFA), while those in component 3 were individual FAs and MDA.
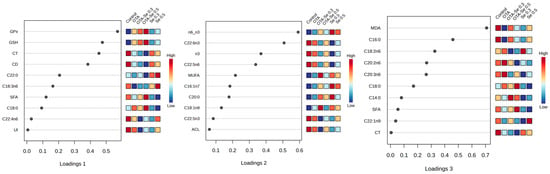
Figure 4.
Loading plots of the 3D sPLS-DA score plot, highlighting the biological components’ efficacy in group formation. Colors indicate variable abundance, with dark brown representing high values and blue representing low values.
3. Discussion
The 5-day exposure to 2 ppm OTA, a relatively high dose, was chosen to capture early oxidative stress responses and initial changes in lipid metabolism without causing severe systemic toxicity or secondary organ damage, allowing assessment of the primary interactions between OTA and Se. The current study indicates that dietary Se supplementation at both 0.3 and 0.5 mg/kg dosages modulates oxidative stress biomarkers and modifies the FA composition of hepatic membrane phospholipids in broiler chickens exposed to OTA. While the interaction between OTA and Se is known, its impact on membrane phospholipids remains unexplored. OTA modifies the hepatic xenobiotic biodetoxification mechanism and it may have an effect on OTA and Se interaction due to activation or suppression of different signaling pathways []. In addition, growing evidence suggests that the gut microbiota, through its close metabolic association with the liver, can influence hepatic phospholipid composition; thus, microbiota-mediated modulation of lipid metabolism may further interact with OTA and Se exposure [,]. Besides Se, other dietary strategies were proposed against OTA toxicity, such as vitamins and medicinal herb extract []. However, Se is a natural component of the regular diet of chicks, and its supplementation can be easily integrated into feeding practices.
3.1. Alterations in Hepatic Membrane Lipid Profile
The physiological dose of Se (0.3 mg/kg feed) addition modified the saturated and monounsaturated FA composition by increasing levels of arachidic acid (C20:0) and palmitoleic acid (C16:1 n-7). OTA exposure selectively reduced behenic acid (C22:0) levels. This decrease has also been reported in the liver of rats following zearalenone exposure [] and in rabbits treated with fumonisin B1 [], the latter being attributed to the inhibition of ceramide synthesis, as behenic acid is an essential component of sphingomyelins. In the case of OTA, such an effect has not been described yet; however, by influencing the transcription of several proteins [], it may disrupt ceramide or sphingomyelin metabolism, which could indirectly affect behenic acid levels. While 0.5 mg/kg Se alone reduced total SFA content, combining this dose with OTA resulted in an increase, suggesting that OTA counteracts the Se-induced reduction of specific SFAs, possibly by modulating elongase and desaturase activity, as observed in Se-treated [] and GPx1-overexpressing mice []. This suggests a complex, potentially antagonistic interaction (p = 0.007) between OTA and Se in the regulation of hepatic lipid metabolism and membrane integrity [].
While OTA exposure alone did not significantly affect PUFA levels, the combined treatment with 0.3 mg/kg Se led to decreases in both n-6 and n-3 FAs. This result suggests that OTA exposure influenced PUFA profiles in the liver membrane, but only during 0.3 mg/kg Se supplementation, potentially by affecting susceptibility to lipid oxidation []. These results indicate that the simultaneous presence of OTA and Se exerted marked effects on lipid metabolism, leading to enhanced PUFA degradation, as reflected by the decreased unsaturation index (UI) and average chain length (ACL) observed in the OTA–Se (0.3 mg/kg) group, in line with the recognized vulnerability of long-chain PUFAs to oxidative damage [].
3.2. Changes in Oxidative Stress Markers
A 5-day exposure to 2 mg/kg OTA increased GPx activity compared to the control group, which is consistent with a previous study reporting significantly higher GPx activity in broilers treated with 1 mg/kg OTA []. Additionally, elevated GPx activity was observed in the groups treated with 2 mg/kg OTA + 0.3 mg/kg Se, while increased GSH concentrations were found in both the 2 mg/kg OTA + 0.3 and 0.5 mg/kg Se groups. These findings suggest that Se supplementation in the presence of OTA enhances the glutathione redox response, likely by increasing Se-dependent GPx activity [], as part of a specific adaptive response to OTA-induced oxidative stress. Moreover, OTA-induced oxidative and ER stress pathways may also be modulated by certain gut-derived microbial and cellular processes, which can exert effects similar to those of Se observed in the current study []. In the case of another hepatotoxic mycotoxin, supplementation with 0.4 mg/kg Se similarly mitigated AFB1-induced oxidative stress in the broiler liver by enhancing GPx activity and elevating GSH content [].
The levels of early lipid peroxidation markers (CD and CT) did not change after feeding 0.2 mg/kg OTA compared to the control group. Similarly, in a previous study, these parameters showed no significant changes in the liver after 21 days of exposure to a 1126 μg OTA/kg feed []. However, supplementation with 0.5 mg/kg Se in addition to OTA resulted in a significant decrease in CD and CT levels compared to the OTA-only group. This effect is likely attributable to the antioxidant properties of Se, as further supported by the elevated GSH concentrations observed in this treatment group compared to the control. The level of the terminal phase marker of lipid peroxidation (MDA) decreased in the low Se (0.3 mg/kg) and OTA-treated group. The effect of Se on MDA formation is due to the well-known antioxidant effect of Se []. This effect was similar in a study with chicken breast muscle when MDA was reduced with 0.4 mg/kg Se, compared to controls []. In the OTA-treated group, this decrease was a consequence of reduced unsaturation of FAs (mainly C22:0). The altered FA composition may influence the intensity of lipid peroxidation processes due to different susceptibility to oxidation [].
Based on the sPLS-DA analysis, Se and OTA treatments in broilers primarily affect antioxidant defenses and early lipid peroxidation markers, followed by changes in FA composition and lipid peroxidation end products. These findings reflect a complex interaction between oxidative stress and lipid metabolism, further highlighting their biological significance as key differentiating variables among treatment groups. Metabolomic analysis of the effect of OTA and its interaction with aflatoxin M1 was investigated previously, but not with Se [].
Overall, 5 days of 2 mg/kg OTA exposure alone did not markedly alter the FA profile or antioxidant responses in broilers. However, in combination with the generally used 0.3 mg/kg Se content of feeds, OTA induced significant changes in lipid metabolism, as reflected in a decrease in both n-6 and n-3 FAs, as well as in a decrease in the unsaturation index and average chain length. These alterations were accompanied by increased GSH content and GPx activity, suggesting that the simultaneous presence of OTA and Se triggers parallel effects: while enhancing antioxidant responses, it also promotes lipid profile disturbances, compromising liver membrane integrity and redox balance. Based on the GLM analysis, the effects of OTA and selenium appeared independent, which may be due to the activation of different pathways. In another short-term experiment [], OTA stimulated the expression of xenobiotic detoxification enzymes (e.g., CYP1A2), whose metabolic by-products can promote oxidative membrane damage. In contrast, Se acted through selenoprotein-dependent antioxidant mechanisms, activating the glutathione redox system (GPX4, GSS, GSR), enhancing cellular redox homeostasis, rather than modulating OTA detoxification pathways (AHR–CYP1A2). These different mechanisms may explain the lack of a strong interaction and the limited protective efficacy of Se at physiological levels against OTA-induced hepatic alterations. Multivariate analysis further highlighted antioxidant capacity and lipid peroxidation markers as key discriminating factors between treatments, underlining the central role of redox balance in the effects of OTA and Se. The results differ from previous studies [], which may be due to short-term exposure and Se supplementation at the regulatory limit (0.3 and 0.5 mg/kg) concentrations, but highlight a possible negative dose-dependent effect of Se supplementation in OTA-contaminated feed.
4. Conclusions
The present study demonstrates that ochratoxin A (OTA) and selenium (Se) exert complex and dose-associated effects, but no proven interactions in broiler chickens, particularly in relation to lipid metabolism and oxidative stress responses. Separate OTA and Se effects’ parallel onset refers to divergent modes of action of these agents in the liver. An amount of 0.3 mg/kg Se was found to be ineffective against the effects of 0.2 mg/kg OTA in terms of hepatic PUFA levels, while 0.5 mg/kg Se supplementation effectively mitigated the effects of OTA by enhancing antioxidant defense, thus supporting membrane lipid integrity.
5. Materials and Methods
5.1. The Experimental Conditions
A total of 36 newly hatched Cobb 500 broiler cockerels were obtained from a hatchery (Babádi Hatchery Ltd., Ócsa-Felsőbabád, Hungary) and divided into six groups (n = 6, in 2 replicates). The birds in each group were housed in pens bedded with pine wood shavings. The short-term OTA exposure trial lasted for 5 days and was conducted on 21-day-old birds. The short-term exposure was selected based on our previous protocol. The short period allowed us to follow the early changes in OTA exposure and Se supplementation, and ageing did not modify the effects. Experimental groups were formed at 20 days of age, during which the birds were individually weighed to ensure that the average body weight within each group did not differ by more than 5%. Feed and drinking water were provided ad libitum under a natural light regimen (15 h light–9 h dark). The nutritional composition of the diet was as follows: 88.76% dry matter, 21.25% crude protein, 4.25% crude fiber, 1.10% calcium, 1.12% lysine, 0.38% methionine, 0.89% methionine + cysteine, 0.48% available phosphorus, 0.25% sodium, and 12.66 MJ/kg metabolizable energy (Table S1). The feed excluded mycotoxin binders and coccidiostats. The following groups were established: control (diet free from OTA and Se), Se groups (received diets supplemented with organic Se at either 0.3 mg/kg or 0.5 mg/kg concentrations), and OTA groups (fed diets contaminated with 2 mg OTA/kg, as well as the same OTA level with either 0.3 or 0.5 mg Se/kg. Se levels in broiler chicken feed differ across countries, typically ranging from 0.1 to 0.5 mg/kg, reflecting regional regulatory differences and evolving nutritional recommendations. Among these different concentrations, the 0.3 mg/kg dietary Se level was selected based on earlier studies that showed optimal performance at this concentration [,]. Moreover, the maximum permitted Se concentration in feed according to EU regulations is 0.5 mg/kg [], so this higher concentration was also used in this experiment to enhance antioxidant defense mechanisms. Liver samples were collected from six birds per treatment group within 120 h of the start of OTA exposure and the samples were then stored frozen at −70 °C for at least one month until further analysis.
5.2. Ochratoxin A Contamination and Selenium Supplementation
OTA was produced by inoculating sterile ground corn substrate with Aspergillus albertensis strain SZMC 22107, obtained from the Microbiological Collection of the University of Szeged, Hungary. The fungal strain was cultured on potato dextrose agar (PDA) plates at 25 °C for eight days. Subsequently, the surface of the PDA was rinsed with deionized (DI) water to prepare the inoculum suspension. For substrate infection, 1 kg of corn matrix was moistened with 400 mL of DI water, sterilized, and cooled. Then, 2 mL of the fungal inoculum, adjusted to an optical density (OD) of 2.0, was thoroughly mixed into the corn substrate. The inoculated substrate was incubated at 25 °C for 30 days. Following incubation, OTA concentration in the substrate was quantified in triplicate by high-performance liquid chromatography (HPLC) with fluorescence detection, following immunoaffinity cleanup using OchraStar® IAC columns (RomerLabs, Tulln, Austria), as described by Stroka et al. []. The calculated amount of OTA-contaminated substrate was mixed into the experimental feed based on its measured OTA content, and the final OTA content of the experimental feeds was also determined in triplicate (Table 5).

Table 5.
The OTA and Se levels in the experimental feed (mg/kg).
An organic Se (selenomethionine) preparation, Selisseo® (Adisseo, Antony, France), containing 100% hydroxy-selenomethionine (OH-SeMet), was used. The Se concentration of the feed sample was determined in triplicate by inductively coupled plasma mass spectrometry (ICP-MS) (Perkin Elmer NEXION 2000, Perkin Elmer, Waltham, MA, USA). For sample preparation, 0.5 g of homogenized sample was weighed into Teflon digestion vessels (CEM MARS XPreSS, CEM Corp., Matthews, NC, USA). Each sample was treated with 5 mL of nitric acid (HNO3) and 5 mL of hydrogen peroxide (H2O2). The vessels were sealed and subjected to microwave digestion according to the following program: 35 min heating time to a final temperature of 200 °C, followed by 50 min holding time at a maximum power of 1700 W. After digestion, the samples were cooled and diluted with ultrapure water to a final volume of 25 mL. A fivefold dilution was performed before analysis. Blank and quality control samples underwent identical preparation. Teflon digestion vessels were cleaned with a 0.15 M HCl solution between runs to ensure analytical accuracy. The amount of Se preparation was mixed into the experimental feed, and the final Se content of the experimental feeds was determined in triplicate (Table 5).
5.3. The Analysis of Oxidative Stress Markers
Conjugated dienes (CD) and trienes (CT) were determined in triplicate by absorption spectra after extraction of liver lipid content in trimethylpentane []. Malondialdehyde (MDA) concentrations were determined in triplicate by complexation with 2-thiobarbituric acid in an acidic medium at high temperature []. GSH levels were measured in triplicate by the method of Rahman et al. [], and GPx activity was measured in triplicate by the method of Lawrence and Burk []. GSH content and GPx activity were normalized to the protein concentration of the supernatant fraction, which was determined using the Folin–Ciocalteu phenol reagent method [].
5.4. Fatty Acid Profiling of Tissue Phospholipids
Liver samples were homogenized in a chloroform–methanol (2:1, v/v) solution at a 20-fold volume, followed by lipid extraction according to the method of Folch et al. []. High-purity solvents (Merck-Sigma-Aldrich, Schnelldorf, Germany) were used, with 0.01% w/v butylated hydroxytoluene added to prevent FA oxidation. Following Leray et al. [], 10 mg of total extracted lipids was applied onto glass chromatographic columns packed with 300 mg silica gel (230–400 mesh) for lipid fractionation. Neutral lipids were eluted using 10 mL chloroform, followed by 15 mL acetone–methanol (9:1, v/v), whereas total phospholipids (PLs) were eluted with 10 mL pure methanol. The collected PL fraction was evaporated under a nitrogen stream and subsequently methylated via the base-catalyzed sodium methoxide (NaOCH3) method described by Christie []. The determination of FA methyl esters (FAMEs) was performed as previously described []. Results are expressed as weight percentages of total FAMEs.
5.5. Statistical Evaluation
Intergroup differences were tested with analysis of variance and Tukey’s post hoc test. The selective effects of Se and OTA on biological variables were analysed with GLM, and OTA and Se were enrolled as fixed factors into the models. IBM SPSS 29 (2024) and GraphPad Prism 9.5.2 (GraphPad Software, San Diego, CA, USA) were used for the analysis. Furthermore, by involving FA, lipid peroxidation, and antioxidant data in a joint approach, sparse Partial Least Squares Discriminant Analysis (sPLS-DA) was performed with the Metaboanalyst GUI (version 2) [].
5.6. Ethical Issues
During the experiment, the guidelines set by the European Communities Council Directive (86/609 EEC) were followed. The experimental protocol was approved by the Food Chain Safety, Land Use, Plant and Soil Protection and Forestry Directorate of the Pest County Governmental Office (PE/EA/1964-7/2017) with the lowest number of animals possible for an accurate statistical analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17120568/s1, Table S1: Broiler diet composition and nutrient content (Calculated).
Author Contributions
Conceptualization: S.K., M.M. and A.S.; methodology: S.K., K.B., E.Z., E.A., O.A. and Á.F.-N.; investigation: S.K., K.B., E.Z. and B.K. original draft preparation: S.K., M.M., O.A. and E.A.; review and editing: S.K., A.S. and M.M.; supervision: M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the EKÖP-MATE/2024/25/K university research scholarship Programme of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. This work was partially funded by the Hungarian Academy of Sciences (HUN-REN-MATE, Mycotoxins in the Food Chain research group) and by the Hungarian National Laboratory project RRF-2.3.1-21-2022-00007, and further by the Flagship Research Groups Programme and the Research Excellence Programme of the Hungarian University of Agriculture and Life Sciences.
Institutional Review Board Statement
The research obtained approval from the Institutional Animal Ethics Committee of the Hungarian University of Agriculture and Life Sciences (MKK-TAKT-003/2019, 27 February 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Casu, A.; Leggieri, M.C.; Toscano, P.; Paola, B.P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Tahir, M.A.; Abbas, A.; Muneeb, M.; Bilal, R.M.; Hussain, K.; Abdel-Moneim, A.M.E.; Farag, M.R.; Dhama, K.; Elnesr, S.S.; Alagawany, M. Ochratoxicosis in poultry: Occurrence, environmental factors, pathological alterations and amelioration strategies. World’s Poult. Sci. J. 2022, 78, 727–749. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Balasubramanian, B.; Park, S.; Jha, R.; Andretta, I.; Bakare, A.G.; Kim, I.H. Ochratoxin A: Carryover from animal feed into livestock and the mitigation strategies. Anim. Nutr. 2021, 7, 56–63. [Google Scholar] [CrossRef]
- Elaroussi, M.A.; Mohamed, F.R.; El Barkouky, E.M.; Atta, A.M.; Abdou, A.M.; Hatab, M.H. Experimental ochratoxicosis in broiler chickens. Avian Pathol. 2006, 35, 263–269. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of ochratoxin A in products intended for animal feeding. OJEU 2006, L229, 35–37. [Google Scholar]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Rahimtula, A.D.; Béréziat, J.C.; Bussacchini-Griot, V.; Bartsch, H. Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochem. Pharmacol. 1988, 37, 4469–4477. [Google Scholar] [CrossRef]
- Hameed, M.R.; Khan, M.Z.; Saleemi, M.K.; Khan, A.; Akhtar, M.; Hassan, Z.U.; Hussain, Z. Study of ochratoxin A (OTA)-induced oxidative stress markers in broiler chicks. Toxin Rev. 2017, 36, 270–274. [Google Scholar] [CrossRef]
- García-Pérez, E.; Ryu, D.; Lee, C.; Lee, H.J. Ochratoxin A induces oxidative stress in HepG2 Cells by impairing the gene expression of antioxidant enzymes. Toxins 2021, 13, 271. [Google Scholar] [CrossRef]
- Ali, O.; Szabó, A. Review of eukaryote cellular membrane lipid composition, with special attention to the fatty acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Pamplona, R.; Prat, J.; Cadenas, S.; Rojas, C.; Pérez-Campo, R.; López Torres, M.; Barja, G. Low fatty acid unsaturation protects against lipid peroxidation in liver mitochondria from long-lived species: The pigeon and human case. Mech. Ageing Dev. 1996, 86, 53–66. [Google Scholar] [CrossRef]
- Hoehler, D.; Marquardt, R.R. Influence of vitamins E and C on the toxic effects of ochratoxin A and T-2 toxin in chicks. Poult. Sci. 1996, 75, 1508–1515. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Peng, S.; Ou, Y.; He, B.; Li, Y.; Lin, Q. Effects of Artemisia argyi powder on egg quality, antioxidant capacity, and intestinal development of roman laying hens. Front. Physiol. 2022, 13, 902568. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2019, 98, 4231–4239. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Hassan, F.U.; Liu, C.; Mehboob, M.; Bilal, R.M.; Arain, M.A.; Siddique, F.; Chen, F.; Li, Y.; Zhang, J.; Shi, P.; et al. Potential of dietary hemp and cannabinoids to modulate immune response to enhance health and performance in animals: Opportunities and challenges. Front. Immunol. 2023, 14, 1285052. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, K.; Wang, M.; He, Z.; Yu, B.; Wang, X.; Pan, X.; Luo, Y.; Xu, S.; Lau, J.T.Y.; et al. VEGF-FGF signaling activates quiescent CD63+ liver stem cells to proliferate and differentiate. Adv. Sci. 2024, 11, 2308711. [Google Scholar] [CrossRef]
- Ren, Z.; Hongyi He, H.; Fan, Y.; Chen, C.; Zuo, Z.; Junliang Deng, J. Research progress on the toxic antagonism of selenium against mycotoxins. Biol. Trace Elem. Res. 2019, 190, 273–280. [Google Scholar] [CrossRef]
- He, J.; Feng, X.; Liu, Y.; Wang, Y.; Ge, C.; Liu, S.; Jiang, Y. Graveoline attenuates D-GalN/LPS-induced acute liver injury via inhibition of JAK1/STAT3 signaling pathway. Biomed. Pharmacother. 2024, 177, 117163. [Google Scholar] [CrossRef]
- Li, P.; Li, K.; Zou, C.; Tong, C.; Sun, L.; Cao, Z.; Yang, S.; Lyu, Q. Selenium yeast alleviates ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins 2020, 12, 143. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Zeng, T.; Wang, W.; Chen, Q.; Zhao, J.; Zhang, G.; Lu, L.; Yang, H.; Xiao, Y. Duck gut metagenome reveals the microbiome signatures linked to intestinal regional, temporal development, and rearing condition. Imeta 2024, 3, e198. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Ding, X.F.; Cao, H.J.; Ni, Q.Z.; Zhu, B.; Ma, N.; Zhang, F.K.; Wang, Y.K.; Xu, S.; Chen, T.W.; et al. Ochratoxin A induces steatosis via PPARγ-CD36 axis. Toxins 2021, 13, 802. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; da Silva, J.A.; Barroso, D.C.; Glória, E.M.; Mesadri, J.; Wagner, R.; Baldisserotto, B.; Val, A.L. Dietary ochratoxin A (OTA) decreases growth performance and impairs muscle antioxidant system and meat fatty acid profiles in juvenile tambaqui (Colossoma macropomum). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108803. [Google Scholar] [CrossRef]
- Wu, Z.; Shangguan, D.; Huang, Q.; Wang, Y. Drug metabolism and transport mediated the hepatotoxicity of Pleuropterus multiflorus root: A review. Drug Metab. Rev. 2024, 56, 349–358. [Google Scholar] [CrossRef]
- Yang, A.; Ye, Y.; Liu, Q.; Xu, J.; Li, R.; Xu, M.; Wang, X.; Fu, S.; Yu, R. Response of nutritional values and gut microbiomes to dietary intake of ω-3 polyunsaturated fatty acids in Tenebrio molitor larvae. Insects 2025, 16, 970. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Dong, L.; Li, Y.; Ding, H.; Wang, J.; Xu, Y.; Sun, W.; Li, L. Exploring the lipid-lowering effects of cinnamic acid and cinnamaldehyde from the perspective of the gut microbiota and metabolites. Food Funct. 2025, 16, 4399–4414. [Google Scholar] [CrossRef]
- Galvano, F.; Piva, A.; Ritieni, A.; Galvano, G.J. Dietary strategies to counteract the effects of mycotoxins: A review. J. Food Prot. 2001, 64, 120–131. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and combined effects of fumonisin B1, deoxynivalenol and zearalenone on the hepatic and renal membrane lipid integrity of rats. Toxins 2017, 10, 4. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Romvári, R.; Kovács, M. Individual and combined haematotoxic effects of fumonisin B1 and T-2 mycotoxins in rabbits. Food Chem. Toxicol. 2014, 72, 257–264. [Google Scholar] [CrossRef]
- Vettorazzi, A.; van Delft, J.; de Cerain, A.L. A review on ochratoxin A transcriptomic studies. Food Chem. Toxicol. 2013, 59, 766–783. [Google Scholar] [CrossRef]
- Zhao, Z.; Kim, J.; Lei, X.G. High dietary fat and selenium concentrations exert tissue-and glutathione peroxidase 1–dependent impacts on lipid metabolism of young-adult mice. J. Nutr. 2020, 150, 1738–1748. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, K.; Feng, C.; Lei, X.G. Selenium-dependent glutathione peroxidase 1 regulates transcription of elongase 3 in murine tissues. Free Radic. Biol. Med. 2023, 208, 708–717. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006; Volume 974, ISBN 1-904761-16-X. [Google Scholar]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef]
- Kövesi, B.; Worlanyo, A.P.; Kulcsár, S.; Ancsin, Z.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Curcumin mitigates ochratoxin A-induced oxidative stress and alters gene expression in broiler chicken liver and kidney. Acta Vet. Hung. 2024, 72, 41–50. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, Z.; Tian, Q.; Wen, W.; Chen, F.; Huang, X.; Li, Y. Lactobacillus delbrueckii alleviates lipopolysaccharide-induced muscle inflammation and atrophy in weaned piglets associated with inhibition of endoplasmic reticulum stress and protein degradation. FASEB 2024, 38, e70041. [Google Scholar] [CrossRef]
- Wu, B.; Mughal, M.J.; Fang, J.; Peng, X. The protective role of selenium against afb1-induced liver apoptosis by death receptor pathway in broilers. Biol. Trace Elem. Res. 2019, 191, 453–463. [Google Scholar] [CrossRef]
- Kövesi, B.; Cserháti, M.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Long-term effects of ochratoxin A on the glutathione redox system and its regulation in chicken. Antioxidants 2019, 8, 178. [Google Scholar] [CrossRef]
- Surai, P.F.; Dvorska, J.E.; Sparks, N.H.; Jacques, K.A. Impact of mycotoxins on the body’s antioxidant defence. In Nutritional Biotechnology in the Feed and Food Industries, 2nd ed.; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK, 2002; pp. 131–141. [Google Scholar]
- Giamouri, E.; Fortatos, E.; Pappas, A.C.; Papadomichelakis, G. Comparative study between dietary nanoelemental, inorganic, and organic selenium in broiler chickens: Effects on meat fatty acid composition and oxidative stability. Sustainability 2023, 15, 9762. [Google Scholar] [CrossRef]
- Holman, R.T. Autoxidation of fats and related substances. In Progress in Chemistry of Fats and Other Lipids, 2nd ed.; Holman, R.T., Lundberg, W.O., Malkin, T., Eds.; Pergamon Press: London, UK, 1956; Volume 2, pp. 51–98. [Google Scholar]
- Gao, Y.-N.; Wu, C.-Q.; Wang, J.-Q.; Zheng, N. Metabolomic analysis reveals the mechanisms of hepatotoxicity induced by aflatoxin M1 and ochratoxin A. Toxins 2022, 14, 141. [Google Scholar] [CrossRef]
- Kövesi, B.; Kulcsár, S.; Ancsin, Z.; Erdélyi, M.; Zándoki, E.; Tóth, M.; Gömbös, P.; Freiler-Nagy, Á.; Balogh, K.; Mézes, M. Short-Term Effects of Dietary Selenomethionine Supplementation on Hepatic and Renal Transcriptomic Alterations Induced by Ochratoxin a in Broiler Chickens. Toxins 2025, 17, 460. [Google Scholar] [CrossRef]
- Sobolev, O.I.; Lisohurska, D.V.; Pyvovar, P.V.; Topolnytskyi, P.P.; Gutyj, B.V.; Sobolieva, S.V.; Borshch, O.O.; Liskovich, V.A.; Verkholiuk, M.M.; Petryszak, O.Y.; et al. Modeling the effect of different dose of selenium additives in compound feed on the efficiency of broiler chicken growth. Ukr. J. Ecol. 2021, 11, 292–299. [Google Scholar] [CrossRef]
- Elkhateeb, F.S.; Ghazalah, A.A.; Lohakare, J.; Abdel-Wareth, A.A. Selenium nanoparticle inclusion in broiler diets for enhancing sustainable production and health. Sci. Rep. 2024, 14, 18557. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Durjava, M.; Dusemund, B.; Kouba, M.; López-Alonso, M.; Puente, S.L.; et al. Consumer safety of feed additives containing selenium. EFSA J. 2024, 22, e8857. [Google Scholar] [CrossRef]
- Stroka, J.; Ambrosio, M.; Doncheva, I.; Lerda, D.; Mischke, C.; Breidbach, A. Validation of an analytical method to determine the content of ochratoxin A in animal feed. IRMM 2009, 32. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis (28.054), 14th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984; p. 243. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulphide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–956. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Leray, C.; Andriamampandry, M.; Gutbier, G.; Cavadenti, J.; Klein-Soyer, C.; Gachet, C.; Cazenave, J.P. Quantitative analysis of vitamin E, cholesterol and phospholipid fatty acids in a single aliquot of human platelets and cultured endothelial cells. J. Chromatogr. B 1997, 696, 33–42. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Kulcsár, S.; Turbók, J.; Kövér, G.; Balogh, K.; Zándoki, E.; Ali, O.; Szabó, A.; Mézes, M. Exposure to a combination of fusarium mycotoxins leads to lipid peroxidation and influences antioxidant defenses, fatty acid composition of phospholipids, and renal histology in laying hens. Toxins 2024, 16, 226. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37 (Suppl. S2), W652–W660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).