Abstract
The two venomous pit vipers, Trimeresurus macrops and T. hageni, are distributed throughout Thailand, although their abundance varies among different areas. No species-specific antivenom is available for their bite victims, and the only recorded treatment method is a horse antivenom raised against T. albolabris crude venom. To facilitate assessment of the cross-reactivity of heterologous antivenoms, protein profiles of T. macrops and T. hageni venoms were explored using mass-spectrometry-based proteomics. The results show that 185 and 216 proteins were identified from T. macrops and T. hageni venoms, respectively. Two major protein components in T. macrops and T. hageni venoms were snake venom serine protease and metalloproteinase. The toxicity of the venoms on human monocytes and skin fibroblasts was analyzed, and both showed a greater cytotoxic effect on fibroblasts than monocytic cells, with toxicity occurring in a dose-dependent rather than a time-dependent manner. Exploring the protein composition of snake venom leads to a better understanding of the envenoming of prey. Moreover, knowledge of pit viper venomics facilitates the selection of the optimum heterologous antivenoms for treating bite victims.
Keywords:
Trimeresurus macrops; Trimeresurus hageni; pit vipers; snake venom proteomics; cytotoxicity; U937 monocytes; fibroblasts Key Contribution:
This study revealed for the first time the venomic proteomes of the large-eyed pit viper (Trimeresurus macrops) and Hagen’s pit viper (T. hageni). Both snakes are widespread across Thailand and Southeast Asia. We quantitatively analyzed different protein clusters from these venoms. The cellular toxicity of the venoms on human fibroblasts and monocytes was also investigated.
1. Introduction
Venomous pit vipers are snakes of the Crotalinae subfamily, characterized by two movable fangs and heat-sensing pit organs located bilaterally between the eye and nostril. Trimeresurus is a prominent pit viper genus and comprises the greatest number of known species [1]. Trimeresurus snakes are endemic to Asia; they are widely distributed, ranging from deserts to rainforests and in terrestrial, arboreal, and aquatic habitats. Trimeresurus bite victims have been reported in several geographic regions, including Lao PDR [2], Hong Kong [3], Taiwan [4], Thailand [5], China [6], Sri Lanka [7], and Japan [8]. In addition, green pit vipers were responsible for 58% of snakebites reported in Vietnam in 2017 [9]. Trimeresurus venom varies in toxicity between species; prolonged clotting time is a significant symptom observed in humans [10], and tissue damage and hematotoxicity in bite victims have also been reported [11,12]. Since there is no species-specific antivenom available for Trimeresurus, except T. albolabris, the only treatment available for bite cases has been a hetero-specific antivenom [13]. Antivenoms raised in horses are the most common therapeutic agents for snakebite treatment; however, they can cause several side effects, such as anaphylactic shock and serum sickness [14]. Moreover, preparation of antivenom from horse blood is laborious and time-consuming with a low production yield [15]. According to proteomics studies, each specific venom contains a unique variety of toxins [16]. To date, T. insularis (Indonesian), T. borneensis (Borneo), T. stejnegeri (Taiwan), T. puniceus (Java), T. purpureomaculatus (Thailand), T. gramineus (India), T. nebularis (Malaysia), and T. alborabris (Thailand) have been investigated for their venom constituents [17,18,19]. However, there is no reported information on the venomic protein profile of the Southeast Asia endemic species T. macrops and T. hageni.
The large-eyed pit viper T. macrops can be distinguished from other green pit vipers by its relatively large eyes (Figure 1a). T. macrops bites frequently cause severe tissue damage in humans with the symptoms ranging from local swelling to severe systemic bleeding [20]. Its venom has a long half-life and can be retained within the human body for more than 14 days [21]. T. hageni, known as Hagen’s pit viper is also endemic to Southeast Asia (Figure 1b); however, there are only a few reports of the major symptoms of T. hageni venom. In addition, no T. macrops or T. hageni species-specific antivenoms are available. The antivenom raised from T. albolabris is currently used for neutralizing T. macrops and T. hageni venoms. In our study, a comparative proteomics approach was applied to the study of T. macrops and T. hageni venom protein composition. Moreover, we assessed the cytotoxicity of T. macrops and T. hageni venoms on monocytic cells (U937 cells) and skin fibroblasts (CRL-1474 cells) to clarify the effects on cellular physiology. The findings facilitate the analysis of cross-reactivity between these snake toxins and the available antivenoms. The identified toxins may be used for the development of inhibitory or neutralizing agents using molecular techniques to improve snakebite treatment. In addition, some proteins with beneficial activities could be further developed as novel pharmacological agents for human disease.

Figure 1.
Two prominent species of Trimeresurus snakes in Thailand. Adult large-eyed pit viper (T. macrops) with its noticeably large eyes (a) and the Hagen’s pit viper (T. hageni) perching on a tree branch (b).
2. Results
2.1. Proteomics Analysis of T. macrops and T. hageni Venom
After preparing venom from T. macrops and T. hageni, the proteins were separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 2). Bands at 15, 25, 35, 50, and 55 kDa were the most abundant in T. macrops venom. Whereas, bands at 15, 16, 20, 22, 32, 55, and 66 kDa were the most intense in T. hageni venom. Each gel lane was excised into 10 pieces. Peptides were extracted from the gel by in-gel digestion and further subjected to liquid chromatography–mass spectrometry (LC-MS/MS) analysis. After MASCOT searching against NCBI (Taxonomy: Chordata), the results revealed that the T. macrops and T. hageni venoms contained 185 and 216 proteins, respectively (Supplementary Table S1). The identified proteins were classified according to their gene ontology, including molecular function, biological process, and cellular component terms (Table 1).
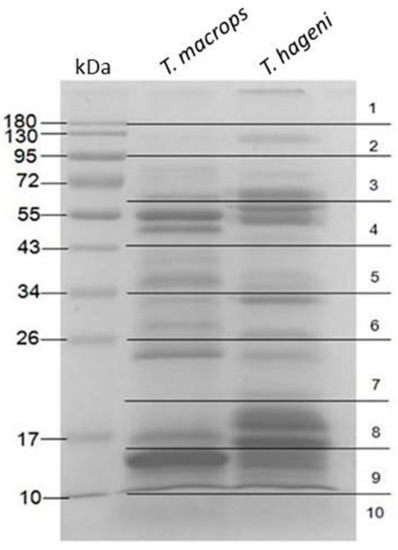
Figure 2.
Coomassie blue-stained 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis of T. macrops and T. hageni venoms (30 µg) under reducing conditions.

Table 1.
Gene ontology classification of Trimeresurus macrops and T. hageni crude venom proteins.
In terms of molecular function, most T. macrops (75%) and T. hageni (44.4%) venom proteins were involved in catalytic activity. Structural molecule activity proteins were observed only in T. macrops venom and represented 12.5% of the proteins. Whereas, molecular function regulators comprised 22.2% of T. hageni venom proteins. In terms of biological processes, proteins involved in biological regulation and cellular processes were the largest classes present in T. macrops (36.4%) and T. hageni (26.7%) venoms, respectively. While immune system process, response to stimulus, and localization proteins were found only in T. hageni venom. In terms of cellular processes, organelle proteins were found in both T. macrops (37.5%) and T. hageni (50%) venoms. Whereas, membrane and protein-containing complex molecules were presented only in T. macrops venom. Phospholipase A2 (PLA2), snake venom serine protease (SVSP), cysteine-rich secretory, snake venom metalloproteinase (SVMP), disintegrin, L-amino acid oxidase, and C-type lectin were common to the two snake venoms. These protein families contribute to the phenotypic effects of venom on victims. Therefore, all identified proteins were also classified according to the common properties of snake venom, as shown in Figure 3. PLA2, SVSP, cysteine-rich secretory, SVMP, and disintegrin were more abundant in T. macrops venom. Whereas, L-amino acid oxidase and C-type lectin were more abundant in T. hageni venom. Due to protein semi-quantification, the exponentially modified protein abundance index (emPAI) was used to estimate the amount of proteins. The 20 most abundant proteins in T. macrops and T. hageni venoms are displayed in Table 2 and Table 3, respectively.
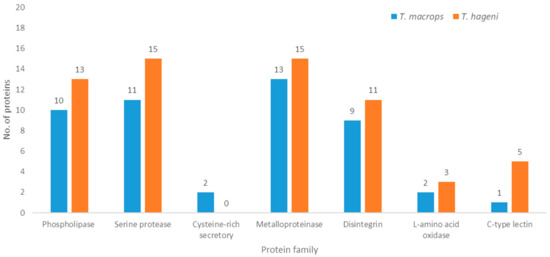
Figure 3.
Proteome classification of T. macrops (blue) and T. hageni (orange) venoms; proteomes were classified according to common functions of snake venom.

Table 2.
Top-20 unique proteins identified in T. macrops crude venom.

Table 3.
Top-20 unique identified proteins from T. hageni crude venom.
2.2. Cellular Toxicity of T. macrops and T. hageni Venoms on U937 Monocytic Cells and CRL-1474 Skin Fibroblasts
At the physiological level, Trimeresurus venoms possess a hematotoxic effect; however, the influence of these venoms at the cellular level on immunopotent, blood-inhabited mononuclear cells remains unknown. The cytotoxic effects of T. macrops and T. hageni venoms on U937 monocytes was examined using the 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Figure 4). There was no remarkable cytotoxic of T. macrops venom on U937 cells (Figure 4a,b). Monocytic cells seemed to be more susceptible to T. hageni than T. macrops venoms, as a sharp decrease in viable cells could be detected at 24 h for 0.5 mg/mL (p < 0.001) (Figure 4c). The cytotoxicity increased as the concentration of T. hageni venom increased, indicating a concentration-dependent action. In addition, a similar toxicity pattern was found when U937 cells were exposed to this species’ venom for 72 h, and the LC50 values of T. hageni were 0.66 and 0.90 mg/mL at 48 and 72 h, respectively.
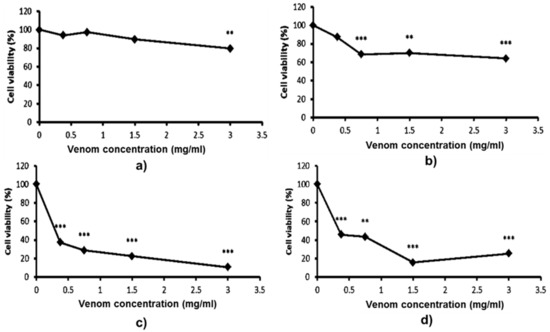
Figure 4.
Cytotoxicity of Trimeresurus macrops and T. hageni venoms on human monocytic cells determined by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. U937 cells were treated with 0 to 3 mg/mL T. macrops venom for 48 (a) and 72 h (b) or with 0 to 3 mg/mL of T. hageni venom for 48 (c) and 72 h (d). Percentage U937 cell viability of venom-treated compared with non-venom treated cells (control). Data represent the mean ± standard error of mean (SEM) from three independent experiments, ** p < 0.01, *** p < 0.001.
Because skin is the first organ to encounter the venom after a snakebite and severe dermal tissue damage is frequently reported for Trimeresurus cases, the effects of these pit viper venoms on skin fibroblasts were investigated (Figure 5). In comparison with monocytic cells, fibroblasts were more sensitive to both T. macrops and T. hageni venoms. Fibroblast cell viability substantially reduced after exposure to 0.1 mg/mL of T. macrops at 48 h (p < 0.05), and the decline persisted as the concentration of venom increased (Figure 5a), and there was a comparable cytotoxicity at 72 h (Figure 5a,b). LC50 values of T. macrops were 0.23 mg/mL at both 48 and 72 h. Compared with T. macrops, T. hageni venom showed negligible toxicity to fibroblasts. (Figure 5c,d). Concerning the relatively high LC50 doses, it should be considered that crude venom have been used in these experiments and that there is possible low synergic biological effects among the enzymes and other proteins in the venom of both snakes.
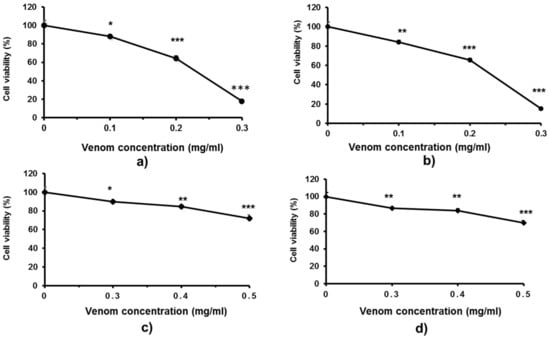
Figure 5.
Cytotoxicity of Trimeresurus macrops and T. hageni venoms on human skin fibroblasts determined by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. CRL-1474 skin fibroblasts were treated with 0 to 0.3 mg/mL T. macrops venom for 48 (a) and 72 h (b) or with 0 to 0.5 mg/mL T. hageni venom for 48 (c) and 72 h (d). Percentage CRL-1474 fibroblast cell viability of venom-treated compared with non-venom treated cells (control). Data represent the mean ± standard error of mean (SEM) from two independent experiments, * p < 0.05, ** p < 0.01 and *** p < 0.001.
3. Discussion
The protein patterns differed between T. macrops and T. hageni venoms, which was indicative of the non-identical protein composition of the two venoms. T. macrops and T. hageni venoms also demonstrated different protein patterns from T. borneensis, T. gramineus, T. puniceus, T. purpureomaculatus, T. stejnegeri, and Protobothrops flavoviridis [22]. All proteins of both venoms were classified according to their gene ontologies. In terms of molecular function, proteins involved in catalytic activity were the most abundant in both venoms, which corroborates the gene ontology analysis of platypus venom [23]. This protein class includes hemolytic and proteolytic proteins that play important roles during prey acquisition. The main functions of snake venom are to lubricate and immobilize prey. Several protein families have been found to be common in snake venoms, including phospholipase, serine protease, cysteine-rich secretory, metalloproteinase, disintegrin, L-amino acid oxidase, and c-type lectin. Our data show that in T. hageni venom, the abundance of proteins of the phospholipase, serine protease, metalloproteinase, disintegrin, L-amino acid oxidase, and c-type lectin families was higher than in T. macrops venom. However, no cysteine-rich secretory proteins were observed in T. hageni venom.
The two snake species produce biologically active substances in their venom capable of weakening prey to facilitate their capture. Phospholipase can hydrolyze glycerophospholipids to fatty acids and other lipophilic compounds [24]. This protein family from dangerous snake venoms has been reported to affect the peripheral neuromuscular system [25]. In addition, purified phospholipase A2 originating from various snake venoms have been shown to possess anticoagulant properties [26]. Agkistrodon halys blomhoffii [27] and A. palas [28] phospholipase A2 show antibacterial activity. The acidic phospholipase A2 5 (gi|13959432), acidic phospholipase A2 6 (gi|20177994), and basic phospholipase A2 2 (gi|13959429) were one of the twenty most abundant proteins in T. macrops crude venom. While, acidic phospholipase A2 2 (gi|3914268), acidic phospholipase A2 5 (gi|13959432), basic phospholipase A2 homolog Ts-R6 (gi|82201344), acidic phospholipase A2 1 (gi|129417), and D1E6b phospholipase A2 (gi|59727030) were among the 20 most abundant proteins in T. hageni crude venom. Although phospholipases were abundance proteins identified in both T. macrops and T. hageni. Their biological activities such as neuromuscular, anticoagulant, and antibacterial require further experiment for characterization.
Serine proteases have been reported to effect blood coagulation systems, e.g., the fibrinolytic system [29]. Some venom serine proteases are not susceptible to hirudin, heparin, or the most endogenous serine protease inhibitors. A few venom serine proteases can activate coagulation factor V, protein C, plasminogen, and platelets [30]. Snake venom serine protease 2B (gi|13959619) and snake venom serine protease 1 (gi|13959617) were found in abundant proportions in T. macrops and T. hageni crude venoms, respectively, and similarly to serine proteases of other snake venoms, they may disturb prey blood coagulation, resulting in severe bleeding. Venom metalloproteinases are also exceedingly toxic; these enzymes interfere with blood coagulation and hemostatic plug formation and destroy cell membranes and the extracellular matrix [31]. Snake venom metalloproteinase (gi|123894851) was abundant in T. macrops crude venom and may fulfil the same role as in other snake venoms.
Disintegrins are cysteine-rich peptides ranging from 45 to 84 amino acids in length and are non-enzymatic proteins found in the venom of numerous snake families. Their influence on platelet aggregation and integrin-dependent cell adhesion has been reported [32]. The proteolysis resistance of these proteins results in a sustained half-life in the blood of prey [33]. Disintegrin trigramin-gamma (gi|67462321) was predominantly found in T. macrops crude venom and plays a similar role as in other snake venoms. L-amino acid oxidase accelerates the oxidation of amino acids [34] and can cause plasma clotting disorders as well as inducing apoptosis in various cell lines [35]. L-amino acid oxidase was found in high concentrations in T. macrops and T. hageni and may contribute to the toxicity of the venoms. C-type lectin protein is a non-enzymatic, calcium dependent protein that binds sugar residues [36]. Many snake venom c-type lectin-like proteins target coagulation factors [37]. The Southeast Asian T. albolabris c-type lectin protein binds directly to the von Willebrand factor receptor, which has key functions in both the hemostatic and thrombotic pathways, leading to platelet agglutination [38]. In contrast, c-type lectin protein found in the Asian viper Echis carinatus inhibits platelet agglutination [39]. C-type lectin-like proteins were found in T. macrops and T. hageni venoms and may have similar functions in blood coagulation and platelet activation.
Cysteine-rich secretory protein is a single-chain bioactive polypeptide identified in several organisms, including snakes [40], reptiles [41], and mammals [42]. Snake venom cysteine-rich secretory protein can block smooth muscle contraction by blocking l-type calcium channels [43]. Moreover, this protein inhibits cyclic nucleotide-gated ion channels, which are significant in various sensory pathways, including vision and olfaction, as well as in other key cellular functions, such as hormone release and chemotaxis [44]. The cysteine-rich secretory protein family was identified in T. hageni, but not T. macrops, venom. The biological activity of these proteins may include effects on prey vision and olfaction.
Several studies have demonstrated the cytotoxicity of crude snake venom on human cell lines using in vitro assays, e.g., Lachesis muta venom on keratinocytes [45], and Ophiophagus hannah and Echis carinatus on pancreatic tumor cells [46]. Both T. macrops and T. hageni showed cytotoxicity towards human monocytes and skin fibroblasts. However, the latter exhibited a greater sensitivity to both Trimeresurus venoms, indicating a greater abundance of molecular targets for proteolytic enzymes. In addition, Foxo-3-mediated oxidative stress DNA damage leading to cellular senescence was recently reported in fibroblasts exposed to non-lethal doses of Naja siamensis, Naja melanoleuca, and Dendroaspis viridis venoms [47]. Such effects could also be stimulated by pit viper venoms.
There is an agreement between the monocyte toxicity and the proteomics results, as T. hageni contained more venomous biological substances, and the venom contained members of the cysteine-rich secretory protein family. Indeed, the discrepancy in fibrinogen pseudo-procoagulant clotting potency and Green Pit Viper antivenom neutralization efficacy between T. macrops and T. hageni was recently revealed [48], reflecting the fundamental evolutionary differences among the different Trimeresurus species. An in-depth study of other relevant physiologic effects would be beneficial in the continuing characterizations of the clinical effects of T. macrops and T. hageni venoms. The information would also facilitate the use of antivenom for treating bite victims. In addition, some venom components may be useful in the development of novel therapeutic agents for human diseases.
4. Materials and Methods
4.1. Venom Collection
The study has been reviewed and approved by the committee in accordance with Queen Saovabha Memorial Institute regulations and policies governing the care and use of laboratory animals (QSMI-ACUC-01-2018). The venoms of T. macrops and T. hageni were extracted from individual snakes and kept in a 1.5 mL microcentrifuge tubes. After being weighed, the fresh (liquid) venoms were immediately frozen at −20 °C and lyophilized. The dry (lyophilized) venoms were pooled (from at least three snakes) and stored at −20 °C for use in this study.
4.2. T. macrops and T. hageni Venom Protein Separation
Crude venoms of T. macrops and T. hageni were mixed with lysis buffer (containing 1% Triton X-100 (Merck, Germany), 1% sodium dodecyl sulfate (SDS) (Merck, Darmstadt, Germany), and 1% NaCl (Merck, Germany). The venoms were analyzed for their estimated protein concentration by Quick Start™ Bradford Protein Assay (Bio-Rad, Berkeley, CA, USA). A 30 µg sample of T. macrops and T. hageni venom solutions were separated by 12% SDS-PAGE (Bio-Rad, CA, USA) and stained by Coomassie R-250 solution (Bio-Rad, CA, USA). A whole lane of each venom was excised into 10 pieces and further subjected to in-gel digestion.
4.3. In-Gel Digestion
A 50% acetonitrile (ACN) in 50 mM ammonium bicarbonate solution was used for destaining the blue color from gel slides. Venom proteins were reduced by 4 mM dithiothreitol and incubated at 60 °C for 15 min. The reduced proteins were further alkylated by 250 mM iodoacetamine (Sigma-Aldrich, Saint Louis, MO, USA) and incubated at room temperature for 30 min in the dark. The gel pieces were dehydrated by removing all solution and adding 100% ACN (Thermo Scientific, Waltham, MA, USA). For tryptic digestion, trypsin (Sigma-Aldrich, USA, T6567) in 50 mM ammonium bicarbonate (Sigma-Aldrich, USA) was added to rehydrate the gels and incubated overnight at 37 °C. The peptide was extracted by adding 100% ACN and incubating for 15 min. The solution was transferred into a new microcentrifuge tube and dried using centrifugal concentrator (TOMY, Katsushika, Japan). The peptide mixtures were stored at −20 °C prior to mass spectrometric (MS) analysis.
4.4. Mass Spectrometric Analysis
Venom peptides were dissolved in 0.1% formic acid (Sigma-Aldrich, USA) and subjected to an Ultimate® 3000 Nano-LC system analysis (Thermo Scientific, USA). The peptides were eluted and infused with a microTOF-Q II (Bruker, Bremen, Germany). The acquisition was operated by HyStar™ version 3.2 (Bruker, Germany). The data were processed and converted to mascot generics files (.mgf) using Compass DataAnalysis™ software version 3.4 (Bruker, Germany). The database search was performed using Mascot Daemon software (Matrix Science, Boston, MA, USA) against Chordata NCBI database with the following parameters: one missed cleavage site, variable modifications of carbamidomethyl (C) and oxidation (M), 0.8 Da for MS peptide tolerance, and 0.8 Da for MS/MS tolerance. The significance threshold was set at 95%. Three biological replications were performed for protein identification. The obtained proteins were classified according to their gene ontology using Blast2Go software.
4.5. Cell Culture
Human monocytic cells (U937 cell line from CLS cell line service) and normal human skin fibroblast cell line (CRL-1474 from ATCC) were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) and D-MEM (Invitrogen), respectively. Media were supplemented with 10% fetal calf serum (Sigma-Aldrich, Saint Louis, MO, USA), 2 mM L-glutamine, penicillin (100 units/mL), and streptomycin (100 µg/mL). The cells were incubated in 5% CO2 at 37 °C and sub-cultured every 3 days. The morphology was monitored under an inverted microscope (CX1 Olympus, Tokyo, Japan).
4.6. Venom Cytotoxicity on Monocytic Cell (U937 Cell) and Skin Fibroblasts (CRL-1474)
U937 cells at a density of 2.5 × 105 cells/mL and fibroblasts at 105 cells/mL were seeded into a well of 96-well plate and cultured for 24 h. The solution was discarded from each well. T. macrops and T. hageni crude venoms were added to U937 cells (at concentration ranging from 0 to 3 mg/mL), and fibroblasts (at concentration ranging from 0 to 0.5 mg/mL) and further incubated for 48 and 72 h. To examine cell viability, 10 µL of 5 mg/mL MTT was added to each well. The reaction was incubated in 5% CO2 at 37 °C for 4 h. The absorbance of formazan product was measured at 570 nm by an Infinite M200 PRO microplate reader (Tecan, Mannedorf, Switzerland). The percentage of cell viability was calculated relative to the untreated control cells.
4.7. Statistical Analysis
Quantitative data are presented as mean ± SEM. Statistical significance between groups was analyzed using standard t-tests or two-way ANOVA followed by the Bonferroni test. Significant p-values are indicated within the figure panels. Error bars indicate SEM.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/12/1/54/s1, Supplementary Table S1. T. macrops and T. hageni venom proteins identified by proteomics (XLSX 558 KB).
Author Contributions
Conceptualization S.K., L.C., J.N., O.K., T.V., S.S., N.C. and O.R.; Methodology, S.K., L.C., T.T., J.N., O.K. and O.R.; Software, T.T. and O.R.; Validation, L.C. and O.R.; Formal analysis, S.K., J.N. and O.R.; Investigation, S.K., T.T. and J.N.; Resources, L.C., P.L. and T.V.; Data curation, S.K., L.C., J.N., P.L. and O.R.; Writing—original draft preparation, O.R.; Writing—review and editing, S.K. and O.R.; supervision, S.S. and N.C. project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Center of Excellence on Biodiversity, grant number BDC-PG4-161009.
Acknowledgments
We thank the Central Equipment Unit, Faculty of Tropical Medicine, Mahidol University for facilitating the equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arruda, L.K.; Santos, A.B. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 399–402. [Google Scholar] [CrossRef]
- Malhotra, A.; Thorpe, R.S. A phylogeny of the Trimeresurus group of pit vipers: New evidence from a mitochondrial gene tree. Mol. Phylogenet. Evol. 2000, 16, 199–211. [Google Scholar] [CrossRef]
- Kurohmaru, M.; Matsui, T.; Igarashi, H.; Hattori, S.; Hayashi, Y. Distribution of actin filaments in the seminiferous epithelium of the Habu, Trimeresurus flavoviridis. Anat. Histol. Embryol. 2019, 48, 505–507. [Google Scholar] [CrossRef]
- Blessmann, J.; Nguyen, T.P.N.; Bui, T.P.A.; Krumkamp, R.; Vo, V.T.; Nguyen, H.L. Incidence of snakebites in 3 different geographic regions in Thua Thien Hue province, central Vietnam: Green pit vipers and cobras cause the majority of bites. Toxicon 2018, 156, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Blessmann, J.; Khonesavanh, C.; Outhaithit, P.; Manichanh, S.; Somphanthabansouk, K.; Siboualipha, P. Venomous snake bites in Lao PDR: A retrospective study of 21 snakebite victims in a provincial hospital. Southeast Asian J. Trop. Med. Public Health 2010, 41, 195–202. [Google Scholar] [PubMed]
- Hon, K.L.; Kwok, L.W.; Leung, T.F. Snakebites in children in the densely populated city of Hong Kong: A 10-year survey. Acta Paediatr. 2004, 93, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.Z. Taiwan’s venomous snakebite: Epidemiological, evolution and geographic differences. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 96–101. [Google Scholar] [CrossRef]
- Viravan, C.; Looareesuwan, S.; Kosakarn, W.; Wuthiekanun, V.; McCarthy, C.J.; Stimson, A.F.; Bunnag, D.; Harinasuta, T.; Warrell, D.A. A national hospital-based survey of snakes responsible for bites in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 100–106. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, J.; Liang, X.; Wu, Y.; Yan, M.; Zhu, M.; Fu, Y. Acute cerebral infarction following a Trimeresurus stejnegeri snakebite: A case report. Medicine 2019, 98, e15684. [Google Scholar] [CrossRef]
- Witharana, E.W.R.A.; Gnanathasan, A.; Dissanayake, A.S.; Wijesinghe, S.K.J.; Kadahetti, S.C.L.; Rajapaksha, R.M.J.K. Sri Lankan green pit viper (Trimeresurus trigonocephalus) bites in Deniyaya: A clinico-epidemiological study. Toxicon 2019, S0041, 30414–30423. [Google Scholar] [CrossRef]
- Chotenimitkhun, R.; Rojnuckarin, P. Systemic antivenom and skin necrosis after green pit viper bites. Clin. Toxicol 2008, 46, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Rajaiah, R.; Angaswamy, N.; Krishna, S.; Bannikuppe, V.S. Biochemical and pharmacological characterization of Trimersurus malabaricus snake venom. J. Cell. Biochem. 2018, 119, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Galdamez, L.A.; Tomasheski, R. White-Lipped tree viper (Cryptelytrops albolabris) envenomation in an American viper keeper. J. Emerg. Med. 2017, 53, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- Sivaganabalan, R.; Ismail, A.K.; Salleh, M.S.; Mohan, K.; Choo, T.C.; Adnan, A.; Ariff, A.M.; Mohamed, Z.; Thevarajah, N.; Daud, R.; et al. Guideline: Management of Snakebite Ministry of Health Malaysia, 1st ed.; Ministry of Health Malaysia: Putrajaya, Malaysia, 2017.
- Tu, Y.Y.; Chen, C.C.; Chang, H.M. Isolation of immunoglobulin in yolk (IgY) and rabbit serum immunoglobulin (IgG) specific against bovine lactoferrin by immunoaffinity chromatography. Food Res. Int. 2001, 34, 783–789. [Google Scholar] [CrossRef]
- Ralidis, M.P. Medical treatment of reptile envenomation: A review of the current literature. Top. Emerg. Med. 2000, 22, 16–36. [Google Scholar]
- Calvete, J.J.; Sanz, L.; Angulo, Y. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef]
- Jones, B.K.; Saviola, A.J.; Reilly, S.B.; Stubbs, A.L.; Arida, E.; Iskandar, D.T.; McGuire, J.A.; Yates, J.R.; Mackessy, S.P. Venom Composition in a Phenotypically Variable Pit Viper (Trimeresurus insularis) across the Lesser Sunda Archipelago. J. Proteome Res. 2019, 18, 2206–2220. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Quah, E.S.H.; Ismail, A.K.; Khomvilai, S.; Sitprija, V.; Tan, N.H. Venomics of Trimeresurus (Popeia) nebularis, the Cameron Highlands Pit Viper from Malaysia: Insights into Venom Proteome, Toxicity and Neutralization of Antivenom. Toxins 2019, 11, 95. [Google Scholar] [CrossRef]
- Soogarun, S.; Sangvanich, P.; Chowbumroongkait, M.; Jiemsup, S.; Wiwanikit, V.; Pradniwat, P.; Palasuwan, A.; Pawinwongchai, J.; Chanprasert, S.; Moungkote, T. Analysis of green pit viper (Trimeresurus alborabris) venom protein by LC/MS-MS. J. Biochem. Mol. Toxicol. 2008, 22, 225–229. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Banjongkit, S.; Chantawibun, W.; Akkawat, B.; Juntiang, J.; Noiphrom, J.; Pakmanee, N. Intragumtornchai, Green pit viper (Trimeresurus albolabris and T. macrops) venom antigenaemia and kinetics in humans. Trop. Doct. 2007, 37, 207–210. [Google Scholar] [CrossRef]
- Wilson, D.; Daly, N.L. Venomics: A mini-review. High-Throughput. 2018, 7, 19. [Google Scholar] [CrossRef]
- Whittington, C.M.; Papenfuss, A.T.; Locke, D.P.; Mardis, E.R.; Wilson, R.K.; Abubucker, S.; Mitreva, M.; Wong, E.S.; Hsu, A.L.; Kuchel, P.W.; et al. Novel venom gene discovery in the platypus. Genome Biol. 2010, 11, R95. [Google Scholar] [CrossRef]
- Leslie, C.C. Properties and regulation of cytosolic phospholipase A2. J. Biol. Chem. 1997, 272, 16709–16712. [Google Scholar] [CrossRef]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 17, 2533–2571. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Wűster, W.; Cook, D.A.N.; Bolton, F.M.S. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.V.; Morhy, L.; Arni, R.K.; Ward, R.J.; Díaz, C.; Gutiérrez, J.M. Amino acid sequence of a myotoxic Lys49-phospholipase A2 homologue from the venom of Cerrophidion (Bothrops) godmani. Biochim. Biophys. Acta 1998, 1384, 204–208. [Google Scholar] [CrossRef]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 2000, 36, 1749–1800. [Google Scholar] [CrossRef]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim Biophys. Acta 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Iwanaga, S.; Takeya, H. Structure and function of snake venom metalloproteinase family. In Methods in Protein Sequence Analysis; Plenum Press: New York, NY, USA, 1993; pp. 107–115. [Google Scholar]
- Bjarnason, J.B.; Fox, J.W. Snake venom metalloendopeptidases: Reprolysins. Methods Enzymol. 1995, 248, 345–368. [Google Scholar]
- McLane, M.A.; Sanchez, E.E.; Wong, A.; Paquette-Straub, C.; Perez, J.C. Disintegrins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 327–355. [Google Scholar] [CrossRef]
- Normand, P. Snake Venom Disintegrins and Cell Migration. Toxins 2010, 2, 2606–2621. [Google Scholar]
- Krebs, H.A. The Enzymes, 1st ed.; Delmar Publishers: New York, NY, USA, 1933. [Google Scholar]
- Ande, S.R.; Fussi, H.; Knauer, H.; Murkovic, M.; Ghisla, S.; Fröhlich, K.U.; Macheroux, P. Induction of apoptosis in yeast by L-amino acid oxidase from the Malayan pit viper Calloselasma rhodostoma. Yeast 2008, 25, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Lu, Q.; Clemetson, J.M. Snake C-type lectin-like proteins and platelet receptors. Pathophysiol. Haemost. Thromb. 2005, 34, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Shikamoto, Y.; Fujimoto, Z.; Morita, T.; Mizuno, H. Crystallization and preliminary X-ray analysis of coagulation factor IX-binding protein from habu snake venom at pH 6.5 and 4.6. Acta Crystallogr. Sect. F Struct Biol. Cryst. Commun. 2005, 61, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Clemetson, J.M. Platelet GPIb complex as a target for anti-thrombotic drug development. Thromb. Haemost. 2008, 99, 473–479. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Peng, M.; Lu, W.; Beviglia, L.; Niewiarowski, S.; Kirby, E.P. Echicetin: A snake venom protein that inhibits binding of von Willebrand factor and alboaggregins to platelet glycoprotein Ib. Blood 1993, 81, 2321–2328. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Reddy, T.; Gibbs, G.M.; Merriner, D.J.; Kerr, J.B.; O’Bryan, M.K. Cysteine-rich secretory proteins are not exclusively expressed in the male reproductive tract. Dev. Dyn. 2008, 237, 3313–3323. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef]
- Brown, R.L.; Haley, T.L.; West, K.A.; Crabb, J.W. Pseudechetoxin: A peptide blocker of cyclic nucleotide-gated ion channels. Proc. Natl. Acad. Sci. USA 1999, 96, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Stransky, S.; Costal-Oliveira, F.; Lopes-de-Souza, L.; Guerra-Duarte, C.; Chávez-Olórtegui, C.; Braga, V.M.M. In vitro assessment of cytotoxic activities of Lachesis muta muta snake venom. PLoS Negl. Trop. Dis. 2018, 12, e0006427. [Google Scholar] [CrossRef] [PubMed]
- Kerkkamp, H.; Bagowski, C.; Kool, J.; van Soolingen, B.; Vonk, F.J.; Vlecken, D. Whole snake venoms: Cytotoxic, anti-metastatic and antiangiogenic properties. Toxicon 2018, 150, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Bocian, A.; Petrilla, V.; Adamczyk-Grochala, J.; Szymura, K.; Hendzel, W.; Kaleniuk, E.; Hus, K.; Petrillova, M.; Wnuk, M. Snake venoms promote stress-induced senescence in human fibroblasts. J. Cell. Physiol. 2019, 234, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal viperid snake genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).