Abstract
Background: Inflammatory bowel disease (IBD) is a global disease with a considerable increase in prevalence and the impact on the health and well-being of patients suffering from this condition is vast. Diet has been suspected of being a contributor to IBD severity as well as intake of antibiotics. Methods: A literary search was conducted on the most recent studies on the subject of IBD, diet, and medical treatment to identify high-quality research findings within this area of research. Research published within the last decade was prioritized. Studies in English language were included in the search, and the knowledge gained was synthesized in the review. Results: Dietary patterns, specifically intake of Westernized diets, were associated with increased inflammation and increased disease severity in patients suffering from IBD, specifically patients suffering from Crohn’s disease (CD). A co-administration of pre- and probiotics was found to contribute to disease remission in ulcerative colitis patients, however, to a lesser extent in patients with CD. A bidirectional effect on the intestinal microbiome was seen as a result of intake of the medicines used for the treatment of IBD patients, which affects both bioavailability of the drug and efficacy of the treatment. The baseline composition of the intestinal microbiome in IBD patients dictates their response to the different treatments. Conclusions: Diet and medical treatment both have a large impact on the architecture of the intestinal Microbiome in IBD patients and are, as such, both essential to understand to enable individualized and optimized treatment.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract, which has traditionally been divided into Crohn’s disease (CD) and ulcerative colitis (UC) [1]. UC is a relapsing, chronic inflammatory disease that is restricted to the colon affecting only the mucosal membrane and is characterized by intermittent bloody diarrhea with pus, abdominal pain, weight loss, fatigue, and an urgent need of defecation. CD is a chronic, segmental, localized granulomatous disease that can affect the entire gastrointestinal tract, from the mouth to the anus, and is transmural. The symptoms are abdominal pain, chronic diarrhea—sometimes with blood—weight loss, ulcers in the mouth, and malabsorption. The etiology of IBD remains unknown; however, accumulating evidence suggests that a combination of dietary factors [2,3], intestinal dysbiosis [1,4], and abnormal immune response [5] may act as key triggers in disease development.
The annual incidence of IBD rates varies geographically, with 10.5 to 46.14 per 100,000 inhabitants in Europe, 1.37 to 1.5 per 100,000 inhabitants in Asia, 0.21 to 3.67 per 100,000 inhabitants in South America, and 7.3 to 30.2 per 100,000 inhabitants in North America [6]. The rising incidence of IBD among children and adolescents highlights the concern that oscillations in hormones may play a role as potential disease triggers [6]. A significantly higher regional prevalence of IBD has been observed in the Faroe Islands, with 106 cases per 100,000 inhabitants [7]. This raises the question of whether the differences in the IBD prevalence across regions are driven by diet, lifestyle, or genetic predisposition.
Human gut microbiota plays a central role in host metabolism, immunity, and neuroendocrine function [7]. The gut microbiome also influences the human hormone axis by modulating secretion of serotonin, insulin/leptin [8], cortisol, estrogen/testosterone [9], etc., which are speculated as a trigger of IBD among children and adolescents. It is well established that vaginally delivered newborns acquire their initial gut microbiota from their mother during birth and later through breastfeeding—both crucial for healthy gut development [10]. In contrast, infants delivered by cesarean section primarily acquire their early microbiota from the skin and surrounding hospital environment10 and are found to be more exposed to chronic diseases such as IBD and asthma [11].
Environmental factors—particularly diet [3] and medication [10]—play a major role in shaping the gut microbiota throughout life.
Although genetic predisposition is a well-established contributor to IBD pathogenesis—highlighted by variants in genes such as NOD2, ATG16L1 [12], and IL23R, which influence innate immunity, autophagy, and host–microbiome interactions [12], the present manuscript is intentionally focused on dietary factors and pharmacological therapies as modulators of the gut microbiome. Genetic factors shape disease susceptibility and can modify microbial composition or therapeutic response; however, an in-depth analysis of gene–microbiome interactions are beyond the scope of this review.
Diets rich in plant-based fibers promote the growth of beneficial bacterial taxa such as Faecalibacterium prausnitzii, Roseburia spp., Eubacterium rectale, and Bifidobacterium spp., which produce short-chain fatty acids (SCFAs) including butyrate, acetate, and propionate. These metabolites are essential for maintaining intestinal barrier integrity, regulating immune homeostasis, and supporting overall gut health [9]. In contrast, high-fat, high-sugar, and low-fiber Western diets reduce microbial diversity and favor pro-inflammatory bacterial species, contributing to dysbiosis and metabolic inflammation in the gut [13].
Additionally, medication such as antibiotics [10], proton-pump inhibitors [10], and corticosteroids [11] affects the host microbiome. This review explores the dual impact of diet and medication on gut microbiota in IBD, emphasizing the mechanisms underlying the beneficial and detrimental effects.
2. Materials and Methods
A comprehensive review of the international scientific literature was conducted to assess the role of dietary patterns and commonly used medications in shaping gut microbiota in patients with IBD. The research focused on identifying the most recent evidence describing how diet and pharmacological treatments act as modulators of the gut microbiome across different stages of the disease, including both the pre-diagnostic and post-diagnostic phases.
2.1. Literature Search
The literature review primarily drew on PubMed, Scopus, and ScienceDirect. Search strategies were based on the combinations of the following keywords, informed by previous work in the field: “gut microbiota,” “gut dysbiosis,” “inflammatory bowel disease,” “medication and gut microbiota in IBD,” “role of diet in gut microbiota in IBD,” “nutritional management of IBD,” and “fecal microbiota transplantation in IBD”. This approach ensured comprehensive coverage of studies addressing both dietary and pharmacological modulation of the gut microbiome in IBD.
2.2. Search Strategy and Eligibility Criteria
The search strategy for this review was guided by the PICO framework (Population, Intervention, Comparator, Outcomes). Studies were included only if participants were followed post-treatment with medication or nutritional interventions, or during the course of a nutritional intervention.
Population: Human participants of any age are diagnosed with IBD.
Intervention: All variations in diets and medications as modulators of the gut microbiome in IBD were included. Fecal microbiota transplantation (FMT) was defined as any procedure transferring a fecal microbiota suspension from a healthy donor to a recipient for therapeutic purposes.
Comparator: Studies with a control group were preferred, though studies without controls were also considered when relevant.
Outcomes: Primary outcomes focused on gut microbiota modulation, while secondary outcomes included related clinical or biochemical measures.
Time frame: Priority was given to research published in the past 10 years to capture the most current findings; however, 32 of 128 references are older than 10 years and were included due to their fundamental contributions to IBD research.
Language: Only English-language articles were considered.
Study types: Original research, clinical studies, and reviews were included. Foundational knowledge was supplemented with textbooks and peer-reviewed scientific reviews.
A total of 145 articles met these selection criteria. Results from the selected studies were synthesized into a translational discussion addressing clinical implications and future perspectives for human IBD management.
2.3. Critical Evaluation of Included Studies
The methodological quality of included studies was evaluated using standard criteria: the Cochrane Risk of Bias for RCTs and the Newcastle–Ottawa Scale for observational studies. Most RCTs were rated as low to moderate risk of bias, while observational studies showed moderate risk due to small sample sizes and potential confounding factors. Limitations such as inconsistent outcome measures, lack of blinding, and short follow-up were noted. Overall, the evidence is informative and was interpreted with caution.
Most human studies on diet and medication in IBD have robust sample sizes (>100 patients).
FMT studies have smaller patient cohorts due to the novelty of the therapy; more clinical evidence is needed.
Three relevant animal model studies are included and discussed to provide mechanistic insights.
Potential biases, variability in interventions, outcome measurements, and differences between human and animal studies are addressed.
The implications of these methodological factors for interpreting results and overall evidence strength are discussed.
3. Diet as a Modulator of Gut Microbiota in IBD
3.1. Beneficial Dietary Patterns
The high prevalence of IBD in Western countries and newly industrialized countries suggests that environmental exposure, particularly diet, might contribute to the risk of IBD. It is well known that diet shapes the composition of the human gut microbiota and that host cells use microbial metabolites as energy sources and immunomodulatory agents to maintain the intestinal homeostasis. The symbiotic relationship within the gut microbiota is essential for human health [14]. Disruption of this balance, for instance, through increased consumption of Westernized diets, which are rich in fat and poor in fiber, can result in microbial dysbiosis with subsequent gut inflammation [15].
IBD profoundly affects nutrient metabolism and nutritional requirements, often leading to altered body composition such as low body mass index (BMI), reduced lean body mass [16], malnutrition [17], or in some cases, obesity and hypermetabolism [18]. Many IBD patients report that certain foods exacerbate their symptoms, although the triggers are highly individual [19]. Restricting fermentable carbohydrates could help alleviate non-inflammatory gastrointestinal symptoms for some patients. One of the largest long-term studies on diet and IBD—a 26-year prospective cohort involving 170,776 women—was conducted by researchers Ananthakrishnan et al. (2013) [20]. In this study, participants in the ’Nurses’ Health Study’ completed detailed dietary questionnaires every four years. The results showed that long-term dietary fiber intake, particularly fiber derived from fruit, was associated with a significantly lower risk of developing CD, but not UC. Another study by Lopes et al. (2023) investigated the relationship between lifestyle factors—including body mass index (BMI), smoking, physical activity, and the intake of fruits, vegetables, and dietary fiber—and the risk of developing IBD in adults [21]. Participants were drawn from three large U.S. prospective cohorts: the Nurses’ Health Study (year-1976, n = 72,290), the Nurses’ Health Study II (year-1989, n = 93,909) [22], and the Health Professionals Follow-up Study (n = 41,871). The findings demonstrated that adherence to a healthy lifestyle (based on healthy diet, increased physical activity, no smoking) was associated with approximately a 50% reduction in the incidence of IBD. These results were largely confirmed in three independent European cohorts, suggesting that a substantial proportion of IBD cases could potentially be prevented through lifestyle modifications [21].
Mediterranean Diet
The Mediterranean diet is based on a traditional diet from Mediterranean countries such as Spain, Italy, France, and Greece, which focuses on fresh non-boiled or non-fried food, high in unprocessed plant-based foods such as fruits, vegetables, nuts, wholegrain cereals, olive oil (saturated fat), moderate fish, and shellfish consumption. Furthermore, it contains low to moderate dairy such as cheese and yogurt, limited red meat, and processed foods [23] (see Figure 1).
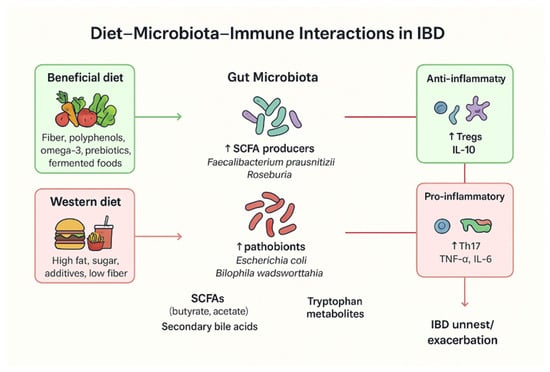
Figure 1.
Overview of diet–microbiota–immune interactions in IBD. A beneficial diet—often associated with the Mediterranean diet and rich in omega-3 fatty acids, prebiotics, and other anti-inflammatory components—can increase the abundance of SCFA-producing bacteria, which in turn regulate anti-inflammatory markers such as Interleukin-10 (IL-10). In contrast, a Western diet high in fat and sugar is linked to an increased abundance of pathobionts such as Escherichia coli, which elevate pro-inflammatory factors including Tumor Necrosis Factor-alpha (TNF-α) and IL-6, thereby promoting IBD relapses.
Khalili et al., (2020) [24] conducted a 17-year prospective study involving 164 patients with CD and 395 patients with UC. The findings indicate that adherence to a Mediterranean diet was particularly beneficial for individuals with CD, as was associated with a significantly reduced risk of later CD onset [24].
Haskey et al. 2023 [25] conducted a study with 15 UC patients who were randomized to a Mediterranean diet, and 13 UC patients who were assigned to a Canadian habitual diet for 12 weeks. The study showed how the group randomized to the Mediterranean diet had reduced levels of fecal calprotectin and higher levels of short-chain fatty acids (SCFA) in stool samples [26]. In addition, the results showed that UC patients randomized to the Mediterranean diet had alterations in beneficial microbiota species such as Alistipes finegoldi, Flavonifractor plauti, and SCFA-producing Ruminococcus bromii, which are usually associated with healthy intestinal microbiomes.
Dietary items associated with anti-inflammatory effects include carrots, sweet potatoes, spinach, iceberg lettuce, romaine lettuce, potato chips, corn chips, popcorn, crackers, apples, oranges, grapes, prune juice, beer, wine, tea, and coffee. However, the anti-inflammatory impact of these foods on the intestinal microbiome appears to be highly individual among IBD patients [27]. A study of 194 UC patients in remission indicated that a diet consisting of low intake of meat products and reduced consumption of alcoholic beverages is linked to disease remission in UC patients [28].
3.2. Prebiotics and Probiotics
The efficacy of probiotics in treating patients suffering from IBD depends on the viability, stability, and ability of the bacteria to survive the acidic gastric environment in the ventricle and reach the intestine for colonization [29]. Combining treatment of IBD patients with both probiotics and prebiotics—such as psyllium, inulin, resistant starches, and dietary fibers—enhances the bacteria’s ability of survival and activity, promoting short-chain fatty acid (SCFA) production in the gut and will result in lowering of the luminal pH to support beneficial bacterial growth [30,31].
In UC patients, alterations in the gut environment, including elevated small intestinal pH (≈7.5 vs. 6.0 in healthy individuals) and variable colonic pH (2.3–5.5 vs. 6.7), contribute to a dysbiosis state [32,33]. This shift favors increased Escherichia coli expansion and reduces the levels of Clostridiales—which are both important SCFA producers—which will contribute to an increased disturbance of the microbial balance [31,33].
Clinical and experimental studies have evaluated the efficacy of several probiotics for treatment of UC patients, notably E. coli Nissle 1917 and VSL#3 (a mixture of the Lactobacillus, Bifidobacterium, and Streptococcus strains) [29]. In UC patients, treatment with E. coli Nissle 1917 showed immunomodulatory effects, including reduced mucosal T-cell infiltration and pro-inflammatory cytokines, while enhancing IL-10 secretion in the gut [4,34,35,36]. However, as this bacterium is a B2-phylogroup strain, it harbors colibactin genes linked to genotoxicity in the gut and possesses a colorectal cancer risk [37,38].
Randomized controlled trials of UC patients suggest that E. coli Nissle 1917 may be as effective as the conventionally used drug in UC patients mesalazine in maintaining UC disease remission [39,40,41]. Although the results in this study were inconsistent, larger studies are needed to confirm these findings [38]. VSL#3 has shown efficacy in sustaining disease remission in UC patients for more than six months [42,43], but long-term outcomes in the long-term health of UC patients remain uncertain.
Lactic acid bacteria (Lactobacillus spp.) produce bacteriocins with antimicrobial and immunoregulatory effects [44,45,46], while Bifidobacterium—a dominant genus in breast-fed infants—supports immune maturation and inhibits the invasion of enteric pathogens via organic acids and antimicrobial peptides [47,48]. Overall, microbiota-targeted nutritional strategies combining pre- and probiotics show considerable promise as adjunctive therapies in IBD patients, but further controlled validation is required (Table 1).
Evidence on the role of probiotics in treating patients suffering from CD remains limited. Some studies suggest that treatment with probiotic supplementation, particularly with Bifidobacterium species or multi-strain formulations, may help maintain disease remission when administered to the patients for three to six months [49].
The evidence for probiotics in IBD remains mixed, reflecting substantial heterogeneity in strain selection, dosing, and clinical endpoints. Strain-specific effects are common: combinations such as VSL#3 have shown benefit in ulcerative colitis and pouchitis [50], whereas probiotics effects in CD often yield inconsistent results [50]. Dose–response relationships are poorly defined, and many studies use widely differing colony-forming unit (CFU) doses, making cross-study comparison challenging [50]. Although probiotics are generally considered safe, adverse events, including bloating, bacteremia in severely immunocompromised individuals, and rare systemic infections, have been reported [50]. More importantly, several well-controlled trials have reported no significant benefit compared with placebo, emphasizing that probiotics cannot yet be considered a reliable therapy for IBD management [50]. The variability in clinical outcomes underscores the need for standardized strain characterization, targeted indications, and mechanistic studies that can guide personalized microbial interventions.
3.3. Fecal Microbiota Transplantation (FMT)
FMT is an ancient therapy where beneficial microorganisms and substances from healthy donor stool are transferred to patients to restore balanced gut microbiota [51]. It contains not only bacteria but also viruses, metabolites like short-chain fatty acids (SCFAs), antimicrobial compounds, and other beneficial products that help repair the diseased intestinal environment.
The main challenges are ensuring safe donor screening [51,52,53,54,55], preserving anaerobic bacteria, and maintaining all beneficial components during production. Many current manufacturing methods such as exposure to oxygen [56], excessive dilution [57], centrifugation [57], or freeze-drying [58] can damage key microbes and reduce treatment effectiveness. Additives like cryoprotectants may also pose risks to the host [57].
FMT can be delivered by capsules, enema, endoscopy, or nasojejunal tube, depending on the disease location. Capsules are the most patient-friendly option for long-term treatment, especially in chronic conditions like UC and CD. However, high FMT capsule cost, inconsistent manufacturing methods, and safety concerns limit widespread use.
Although FMT has shown promising results in subsets of patients with IBD, safety concerns remain central to its clinical evaluation. Documented risks include pathogen transmission, particularly from multi-drug-resistant organisms (MDROs), which has led to safety alerts and stricter donor-screening requirements [53]. Rigorous donor selection protocols now involve extensive medical history review, serology, stool pathogen testing (including MDRO screening), and repeated assessments [55]. Long-term consequences of altering the gut microbiome remain uncertain, with concerns regarding metabolic, immunological, and oncological outcomes that may emerge years after treatment. Regulatory frameworks differ internationally: in the EU, FMT is regulated variably at the national level (ranging from tissue-based regulation to investigational medicinal product status), whereas the U.S. FDA currently classifies FMT as an investigational product except for recurrent C. difficile infection under enforcement discretion [50]. These issues highlight the need for standardized protocols, harmonized regulatory oversight, and high-quality randomized trials before widespread adoption of FMT in IBD. Under the upcoming EU Regulation on Substances of Human Origin (SoHO; Regulation (EU) 2024/1938) [59], which enters into force on 7 August 2027, stool banks and FMT treatment facilities will be classified as SoHO entities and regulated similarly to blood and tissue establishments.
The challenge with FMT is finding healthy stool donors and regularly screening for pathogenic microorganisms to avoid the risk of infection. Therefore, it is very important to follow the national or international guidelines regarding donor banking and donor screening [52,53,54,55].
Clinical studies show FMT can induce remission in UC patients, but results vary [60,61,62,63]. Long-term, repeated treatment with correct administration—using colonoscopy or oral capsule—seems more effective than single administrations using enema. It is also essential to consider the manufacturing method [64], as it can have a significant impact on clinical outcomes. The efficacy of FMT as any other medical treatment depends strongly on proper production processes, accurate dosing, and an appropriate duration of administration, which should be personalized according to the patient’s disease type and disease severity ((ICH Q7 (International Council for Harmonization), Good Manufacturing Practice for Active Pharmaceutical Ingredients), (WHO Essential Medicines (2019) Administration Frequency) [65,66].
High-quality clinical trials—especially using FMT capsules—are needed. Safe, standardized production in hospitals under strict guidelines is essential to make FMT reliable, accessible, and effective.
3.4. Harmful Dietary Patterns
The Westernized diet is linked to high-income, industrialized nations like the US and Western Europe, and it is rapidly spreading to developing and middle-income countries. A Westernized diet is based highly on ultra-processed beverages (UPBs), processed and ultra-processed foods (UPFs), red and processed meats, saturated fats, refined sugars, and high-calorie-density foods, while being low in dietary fiber, whole grains, fruits and vegetables, and other plant-based foods [67].
The consumption of ultra-processed foods (UPFs) including ready-to-eat soups, meals, sweets, breakfast cereals, baked goods, processed meats, ice creams, frozen desserts, and ultra-processed beverages (UPBs) such as flavored waters, concentrates, sports and energy drinks, coffee beverages, fruit juices, and carbonated soft drinks have all been associated with an increased risk of various non-communicable diseases, including IBD [68].
Despite these health risks, global consumption of UPFs and UPBs continues to rise, driven by major shifts in food production, processing, manufacturing, marketing, retail, and consumption practices, as well as the growing economic and political influence of large food corporations (“Big Food”) [68]. This trend is particularly pronounced in high-income countries, where increasing sales of UPF products parallel the rising prevalence of non-communicable diseases such as IBD. Increased consumption of UPBs and UPFs, which are high in salt, sugar, and fat, was observed to be associated with the development of CD cases in a meta-analysis comprising 1,068,425 participants each divided into five cohorts, where scientific findings were published between 2020 and 2022 [69] (see Figure 1).
The Westernized diet is strongly associated with the development of autoimmune and inflammatory diseases through multiple mechanisms:
(A) High fat intake contributes to obesity and modulates T-cell responses, promoting immune dysregulation [70].
(B) Excess sodium consumption (particularly from processed foods) induces pro-inflammatory immune phenotypes via osmotic stress pathways, including enhanced Th17 cell responses [71].
(C) Diets rich in fat and refined sugars alter the composition and structure of the gut microbiota, shifting it from a state of symbiosis to dysbiosis. This disruption triggers host immune activation and increase intestinal epithelial permeability [67]. The commensal intestine microbiota is crucial for elimination of pathogens in the gut. Alteration of the intestinal microbiota/dysbiosis leads to increased Bacteroidetes levels and a decreased beneficial bacteria/Firmicutes ratio [72].
Dietary items that have been associated with promoting inflammatory responses include various processed and red meats (such as hotdogs, bacon, hamburgers, beef, pork, lamb dishes, liver, canned tuna, shrimp, breaded fish, and other seafoods), as well as certain vegetables and plant foods (including corn, mixed vegetables, eggplant, celery, mushrooms, peppers, cucumbers, and fresh tomatoes, tomato juice, and tomato sauce). In addition, sugar-sweetened and artificially sweetened beverages such as cola, caffeine-free cola, Pepsi, other carbonated drinks containing caffeine and sugar, and low-calorie versions of these beverages have also been linked to increased inflammatory activity [27]. However, the impact of disease manifestations associated with the intake of above-mentioned inflammatory diets are highly individual among IBD patients.
Modulation of the gut microbiota in IBD alters the bioavailability of key vitamins and minerals, including vitamin K [73]. Reduced abundance of butyrate-producing Clostridiales species in active IBD leads to lower levels of fecal SCFA, diminishing the epithelial energy supply and the anti-inflammatory signaling while permitting the overgrowth of E. coli [74,75,76,77]. Micronutrient deficiencies (particularly iron, zinc, folate, and vitamin B12) contribute to anemia, which is one of the most common complications associated with IBD [78]. Zinc supports DNA synthesis and immune regulation [79], while iron, folate, and vitamin B12 are essential for erythropoiesisVitamin D deficiency is common in IBD, affecting gut barrier integrity via E-cadherin induction and promoting antimicrobial peptide production [80,81,82]. Nutritional therapy has been shown to result in superior mucosal healing compared with treatment with corticosteroids [83], and it may exert prebiotic effects by modulating the gut microbiota and restoring metabolic and immune balance, Table 1.
While inulin is well known and used as a prebiotic fiber that promotes Bifidobacteria growth and SCFA production, excessive intake of this can result in dysbiosis or inflammation in the gut, which was associated with hepatic inflammation and cholestasis in animal models with a high intake [84]. Dietary emulsifiers and food additives (e.g., polysorbate-80, carboxymethylcellulose) can disrupt the intestinal mucus layer in the gut, which can alter gut microbiota composition, and promote colitis-like inflammation in experimental animal models, Table 1 [85].

Table 1.
Summary of dietary components and microbiota effects in inflammatory bowel disease (IBD).
Table 1.
Summary of dietary components and microbiota effects in inflammatory bowel disease (IBD).
| Dietary Component | Microbiota Effects | Mechanistic/Metabolic Pathways | Impact on IBD Pathogenesis | Key References |
|---|---|---|---|---|
| Dietary fiber (prebiotics) | ↑ Faecalibacterium prausnitzii, Roseburia, Bifidobacterium; ↑ microbial diversity | Fermentation → ↑ short-chain fatty acids (SCFAs), esp. butyrate; enhances epithelial integrity | Anti-inflammatory; promotes mucosal healing; ↓ flare frequency | Makki et al., 2018 [86]; Parada Venegas et al., 2019 [87] |
| High-fat (Western) diet | ↓ Bacteroidetes; ↑ Firmicutes, Proteobacteria, Bilophila wadsworthia | ↑ bile acids and endotoxins → triggers Th1/Th17 activation | Promote dysbiosis, intestinal permeability, chronic inflammation | Agus et al., 2018 [88]; Devkota et al., 2013 [89] |
| High-sugar diet | ↓ Bacteroidetes, Akkermansia muciniphila; ↑ Enterobacteriaceae | Alters mucus layer, ↑ oxidative stress | Exacerbates colitis; increases gut permeability | Khan et al., 2020 [90]; Martinez-Medina et al., 2014 [91] |
| Animal protein (red/processed meat) | ↓ Bacteroides, ↓ Lactobacillus | ↑ Sulfide, ammonia, and nitroso compounds → epithelial damage | Correlated with increased relapse risk in UC | Jantchou et al., 2010 [92]; David et al., 2014 [93] |
| Polyunsaturated fatty acids (PUFAs) | Modulates Bacteroidetes/Firmicutes ratio | ω-3: anti-inflammatory; ω-6: pro-inflammatory eicosanoid production | ω-3 reduces inflammation; ω-6 aggravates it | Calder, 2015 [94]; Marion-Letellier et al., 2016 [95] |
| Polyphenols (plant-based foods) | ↑ Lactobacillus, Bifidobacterium; ↓ Clostridium perfringens | Antioxidant, prebiotic effects; enhances SCFA production | Decreases inflammation, protects mucosa | Cardona et al., 2013 [96]; Etxeberria et al., 2013 [97] |
| Artificial sweeteners (sucralose, saccharin) | ↓ SCFA producers; ↑ Bacteroides, Clostridiales | Alters microbial signaling and insulin response | Associated with dysbiosis, potential flare risk | Suez et al., 2014 [98]; Ruiz-Ojeda et al., 2019 [99] |
| Probiotic foods (yogurt, kefir, fermented vegetables) | ↑ Lactobacillus, Bifidobacterium; competitive inhibition of pathobionts | Competitive inhibition, modulation of immune signaling | Shown to maintain remission and reduce symptoms | Ianiro et al., 2018 [100]; Derwa et al., 2017 [101] |
↑ indicates an increase; ↓ indicates a decrease. Italicized text indicates bacterial taxa.
Dietary composition strongly influences gut microbial diversity and function. Fiber-rich and plant-based diets enhance the abundance of short-chain fatty acid (SCFA)-producing bacteria, promoting anti-inflammatory immune pathways and epithelial integrity. In contrast, Western-style diets high in fat and sugar reduce beneficial taxa and increase pro-inflammatory metabolites, contributing to dysbiosis and mucosal inflammation.
4. Medication and Gut Microbiota Interactions in IBD
Since nearly one in four adults worldwide use proton pump inhibitors (PPIs), PPI exposure represents an important confounder or effect modifier when examining the gut microbiota in patients with IBD [102]. Prolonged use of PPIs has been associated with reduced intestinal microbial diversity and reduced levels of beneficial anaerobes, while favoring enrichment of oral and upper gastrointestinal taxa such as Streptococcus, Enterococcus, and Enterobacteriaceae due to a less acidic environment in the upper GI tract. PPI-induced intestinal dysbiosis, which is associated with elevated gastric pH, may predispose to small intestinal bacterial overgrowth (SIBO) and altered metabolic activity [103,104], which is linked to dysbiosis.
The usage of non-steroidal anti-inflammatory drugs (NSAIDs) is very common but varies widely depending on age group, region, and indication. The studies show that NSAIDs promote a shift toward proinflammatory taxa by reducing mucosa-associated beneficial bacteria, which leads to increased permeability and barrier dysfunction [105]. Therefore, when studying the IBD microbiome, it is important to be mindful of NSAID usage, given its potential effects on gut mucosa and the intestinal microbiota [106].
Therapeutic agents used in IBD—such as corticosteroids, aminosalicylates, immunomodulators, biologics, and antibiotics—can themselves influence the gut microbiota, and vice versa. Medications may alter microbial diversity, metabolic pathways and products, and antibiotic resistance patterns, while microbial composition on the other hand can affect drug metabolism, efficacy, and toxicity. Understanding these bidirectional interactions is critical for optimizing treatment strategies and developing microbiota-targeted adjunctive therapies.
Thus, the study of medication–microbiota interactions in IBD patients offers a promising avenue to improve personalized medicine approaches, enhance therapeutic outcomes, and identify novel microbial or metabolite-based interventions. In the following section, we will investigate medication used in IBD patients and its effect on gut microbiota.
4.1. Corticosteroids
Microbiome changes: Oral or intravenous treatment with corticosteroids rapidly suppress systemic inflammation by broadly inhibiting pro-inflammatory cytokine production and immune cell activation. Beyond direct immunosuppression, corticosteroids alter the colonic microenvironment and can shift gut microbial communities in both animal models and in humans (including changes in diversity and relative abundances of key taxa in the intestinal tract [107]). These microbiota changes may influence mucosal barrier function and metabolite production (e.g., SCFAs), with potential downstream effects on intestinal mucosal healing and generalized susceptibility to infection. Thus, while steroids are highly effective for induction of disease remission in IBD patients, their effects on the microbiome might contribute to variability in response and to infection risk during therapy [107].
Blesl et al., 2024 [108] performed a prospective study of UC patients in active disease states who received systemic corticosteroids, which was associated with a longitudinal restoration of microbial composition and metabolic capacity. The study showed successful steroid treatment tended to shift the microbiome toward a healthier composition [108]. Guo et al., 2022 [109] conducted a study where two induction therapies were compared: exclusive enteral nutrition (EEN) [110] versus treatment with systemic corticosteroids. The microbiome analysis of serial CD patients’ fecal samples collected before and after induction therapy showed how treatment with EEN and corticosteroids, respectively, resulted in different trajectories of changes in the gut microbiome. Patients who received treatment with EEN tended to have larger shifts in community composition in their intestinal microbiome compared with patients treated with corticosteroids. This finding indicates that treatment with systemic steroids does change the intestinal microbiome in humans, but the magnitude and direction differ from that of dietary therapy [109]. Comparison of the composition of the microbiotas in the UC patients before and after induction therapy with glucocorticoid vs. fecal microbiota transplantation (FMT, colonoscopy) showed how both therapies significantly reduced the intestinal levels of the inflammation markers TNF-α and IL-10 [111], while treatment with steroids modify the ecosystem different compared with FMT. Li et al., 2023 [112] performed a rat-experiment study which showed that long-term prednisone treatment can cause fungal microbiota dysbiosis (disturbances in the composition of the fungal intestinal microbiome), which affects the ecological interaction between gut mycobiome and bacteriome in the rats. More studies are needed to investigate the effect of glucocorticoid on the gut microbiome in humans.
Non-microbiome side effects caused by corticosteroid therapy are described in Table 2 and include metabolic and endocrine [113], musculoskeletal [114], immunosuppression and risk of infections [115], neuropsychiatric [116], gastrointestinal disorders [117], dermatologic disorders [118], and adrenal disorders [119] side effects.

Table 2.
Table shows corticosteroids associated extensive systemic side effects.
4.2. Aminosalicylates
Aminosalicylates such as mesalazine (5-ASA) are front-line therapies for mild-to-moderate UC cases and act primarily through topical anti-inflammatory effects in the colon. Animal studies show that treatment with 5-ASA can directly affect the gut bacteria, and the drug can be metabolized by the microbiota; conversely, microbiome composition appears to predict and can modulate the 5-ASA efficacy [120], Table 3. The microbial metabolism of 5-ASA may reduce its local bioavailability or alter the drug activity, and shifts in microbial community structure with 5-ASA treatment have been reported, Table 3. These bidirectional interactions suggest the gut microbiome both mediates and modifies the therapeutic effect of 5-ASA [121].

Table 3.
Mechanistic effect of after 5-ASA.
4.3. Immunomodulators
Thiopurines (azathioprine, 6-mercaptopurine) suppress the adaptive immunity, and the drugs are used for maintenance of disease remission in IBD patients. Recent research indicates that gut bacteria can influence the pharmacokinetics and efficacy of thiopurine [121]. For example, certain commensal bacteria decrease the conversion of the drug to active metabolites or otherwise reduce the drug bioavailability, which can potentially promote treatment failure [121]. Additionally, immunosuppression itself may alter microbiota composition indirectly via changes in the mucosal immunity. These interactions may partly explain interpatient variability in therapeutic response to various treatments, and it highlights the microbiome as a potential biomarker or interventional target to improve thiopurine treatment outcomes [121].
4.4. Biological Therapy
Biologic therapies (e.g., anti-TNF agents such as infliximab/adalimumab, anti-integrin vedolizumab) and anti-interleukin agents (Ustekinumab) [122] target specific inflammatory pathways in the intestinal tract and have transformed IBD management [122]. Multiple studies show that patients who respond to biological therapy often have distinct baseline intestinal microbiome features (higher α-diversity and greater abundance of butyrate-producing Clostridiales such as Faecalibacterium prausnitzii) compared with IBD patients classified as nonresponders, and it is reported how successful treatment with biological therapy can partially restore a “healthier” microbiome profile in the gut of IBD patients [123]. Conversely, the intestinal microbiome appears to influence biologic efficacy through the modulation [124] of mucosal immune activation, microbial metabolite production (short-chain fatty acid) [125], and intestinal mucosal healing capacity. Therefore, fecal microbial signatures are being investigated as predictive biomarkers for biologic response to various treatments and as targets for adjunctive microbiome therapies [126].
4.5. Janus Kinase (JAK) Inhibitors
JAK inhibitors [127] including tofacitinib (a pan-JAK inhibitor with predominant JAK1/3 activity) and upadacitinib (a selective JAK1 inhibitor) are increasingly used for moderate-to-severe ulcerative colitis (UC), particularly in biologic-exposed patients. Their impact on the gut microbiome has only recently been investigated, and although data remains limited, emerging findings indicate indirect microbiota-modulating effects mediated via inflammation control rather than direct antimicrobial action [127].
Early-phase multi-omics and sequencing studies suggest that clinical responders to JAK inhibition exhibit partial restoration of gut microbial diversity, especially increases in short-chain fatty acid (SCFA)-producing taxa such as Faecalibacterium prausnitzii and Roseburia spp., organisms typically depleted during active UC [127]. These improvements parallel reductions in inflammatory cytokines such as IFN-γ and IL-6, which may create a more permissive environment for beneficial anaerobes to recover [128].
A longitudinal profiling study of UC patients treated with tofacitinib found that responders demonstrated microbiome trajectories resembling remission associated states, including increased alpha diversity and reduced abundance of pro-inflammatory Proteobacteria [129]. Notably, non-responders did not show this pattern, suggesting that microbial shifts may reflect disease control rather than a direct drug effect. Overall, current evidence shows that JAK inhibitors do not directly alter microbial composition but rather normalize dysbiosis through rapid and profound suppression of intestinal inflammation, promoting the recovery of SCFA-producing commensals. However, data are still sparse, sample sizes are small, and more controlled prospective microbiome studies are needed.
4.6. Sphingosine-1-Phosphate (S1P) Receptor Modulator
S1P receptor modulators such as ozanimod represent a newer class of oral therapies for ulcerative colitis. These agents reduce gut-homing lymphocyte trafficking, thereby dampening mucosal inflammation [130]. Although direct human evidence on microbiome modulation is currently limited, emerging preclinical and translational studies suggest that S1P signaling can indirectly influence the gut microbial environment through immune–epithelial pathways [131].
Clinical trials of ozanimod in UC primarily evaluate clinical and endoscopic outcomes, but investigators have hypothesized that reduced inflammation may secondarily promote a more favorable microbial profile [132]. However, no published clinical study to date has systematically characterized microbiome shifts during S1P-modulator therapy, making this an important area for future research.
4.7. Early-Life Exposures and Their Influence on Gut Microbiota in IBD
A growing body of evidence shows that early-life exposures profoundly influence microbial development and may increase subsequent risk of IBD. Antibiotics given during infancy disrupt gut microbiome maturation, reduce diversity, and alter long-term colonization patterns [133]. Multiple cohort studies have linked childhood antibiotic exposure to increased IBD risk, particularly Crohn’s disease [134].
Perinatal factors:
The mode of delivery strongly shapes initial microbial colonization. Cesarean delivery is associated with delayed establishment of enriched anaerobic communities [135] and persistence of skin-associated taxa [134].
Breastfeeding promotes Bifidobacterium-dominant profiles that support immune regulation and epithelial tolerance [134]. These factors may influence long-term immune development relevant to IBD susceptibility [136].
Pediatric IBD:
Children diagnosed with IBD display more severe dysbiosis than adults, including reduced microbial diversity, lower abundance of butyrate-producing taxa, and increased Proteobacteria [136]. Because pediatric patients have fewer accumulated environmental confounders, these findings help identify early biological signatures associated with disease development.
4.8. Antibiotics
Antibiotics profoundly and often persistently disrupt the gut microbial diversity and community composition; broad-spectrum agents can deplete beneficial commensals (including butyrate producing taxa such as Faecalibacterium prausnitzii and Roseburia spp.) while promoting overgrowth of opportunistic or resistant organisms [137]. In patients suffering from IBD, antibiotics are used selectively (e.g., for infections, pouchitis, or perianal disease), but the use is associated with both short-term symptomatic changes such as diarrhea and vomiting and longer-term risks such as long-lasting dysbiosis in the intestinal microbiome.
In general, antibiotic exposure has been linked to an increased risk of new-onset IBD in epidemiologic studies, and antibiotics can worsen or complicate microbiome-mediated metabolic and immune functions in patients who have already been diagnosed with IBD [138]. At the same time, targeted antibiotic regimens can be therapeutic in certain IBD-related complications, underscoring that antibiotic–microbiome interactions are highly context-dependent. Epidemiological studies show that early-life and repeated antibiotic exposure significantly increase the risk of developing IBD, most likely by perturbing immune tolerance and intestinal barrier function [139]. Furthermore, antibiotics can exacerbate intestinal dysbiosis and impair the metabolic and immunologic regulatory pathways [140]. Conversely, targeted antibiotic regimens, such as metronidazole or ciprofloxacin in patients suffering from perianal localized CD, can be therapeutically beneficial in specific contexts [141]. These findings emphasize that antibiotic–microbiome interactions in IBD are highly context-dependent, capable of both therapeutic and detrimental side effects depending on timing, spectrum, and host factors.
5. Discussion
Diet and commonly prescribed medications are among the strongest and most influential factors shaping the composition and function of the gut microbiota. Multiple studies indicate that both diet and medication can act as key environmental triggers in the development of IBD in genetically predisposed individuals. Evidence supporting this includes findings that identical twins are often discordant for IBD, demonstrating that genetics alone cannot explain disease onset [142]. Furthermore, higher rates of IBD have been observed among second-generation immigrants, highlighting the strong impact of environmental exposures (particularly diet, lifestyle, and medication use) on disease risk [143]. An increased prevalence of IBD is seen among individuals living in developing countries, which indicates environmental factors such as food and medicine play a significant role in the disease triggers of IBD. It is important to note that eating and drinking, beyond their biological necessity, serve as important psychological and social roles: providing pleasure, comfort, and a sense of belonging [144]. Food is deeply intertwined with social and cultural identity and plays a central part in how people interact, celebrate, and connect with family, friends, and colleagues [25]. Patients suffering from IBD use different food-related strategies to control/manage intestinal symptoms, such as identifying trigger foods, following restrictive diets, control of portion size, and shortening of eating intervals, which might have consequences on the intake of nutrition. As a result of these behavioral strategies and limited knowledge on the effect of dieting on the disease, IBD patients’ food-related quality of life is also affected. Known beneficial diets rich in fiber, omega-3, prebiotics, and fermented foods will promote SCFA producers such as Faecalibacterium prausnitzii and Roseburia, which are associated with a healthy gut microbiome.
However, dietary tolerance varies greatly among patients with IBD, even foods generally considered beneficial. This variability makes it challenging to recommend a single dietary approach for all patients with IBD. Instead, personalized nutrition plans—developed in collaboration with dietitians or nutrition specialists—may yield more favorable outcomes, particularly in patients with CD. It is important to be cautious when providing dietary recommendations to IBD patients as a physician and ensure that patients do not replace or rely on commonly consumed processed foods or beverages, such as cola, which have been shown to exert pro-inflammatory effects on the gut.
Maintaining a balance in dietary intake is important, as excessive consumption of otherwise beneficial foods or prebiotics may lead to adverse and unwanted effects in IBD patients. For instance, overconsumption of inulin has been associated with liver dysfunction in experimental studies [84]. The studies link a Westernized diet to increased pathobionts such as E. coli and Bilophila wadsworthia, decreased levels of SCFA-producing bacteria, and a reduction in SCFA, which are linked to most of the autoimmune diseases, including IBD.
The intake of medication plays a key role in shaping our gut microbiome, specifically in early childhood, as evidence indicates that antibiotic usage, such as broad-spectrum β-lactams, during the first year of a newborn is linked to autoimmune diseases such as the development of IBD [145].
The medication in focus, when studying the IBD intestinal microbiome, is that used to promote and maintain disease remission in IBD patients, such as corticosteroids (e.g., prednisone, budesonide). The studies show that corticosteroids induce alteration in the gut microbiota, including metabolic disturbances, fungal microbiota dysbiosis, increased susceptibility to infection, and neuropsychiatric manifestations [112]. Treatment with corticosteroids significantly reduces TNF-α and IL-10 [111], which promotes disease remission in IBD patients. However, given the established importance of the gut microbiome in maintaining overall health, it is reasonable to question if the non-microbiome-related side effects of corticosteroid therapy—such as metabolic and endocrine [113] disturbances, immunosuppression, and increased infection risk [115] (Table 2)—may in fact be mediated, at least in part, induced by corticosteroid-related alterations in gut microbial composition and function. This potential microbiome-dependent mechanism warrants further investigation.
Anti-inflammatory Aminosalicylates (5-ASA) are metabolized by the gut bacteria, therefore only IBD patients with the right composition of the intestinal microbiome will experience 5-ASA efficacy [120,121]. The efficacy of immunomodulators, such as azathioprine, is linked to the composition of the gut bacteria, as certain commensal bacteria decrease conversion of the drug derivatives to active metabolites or otherwise reduce drug bioavailability, which is followed by treatment failure [121]. The studies indicate that immunosuppression itself caused by treatment with immunomodulators may alter microbiota composition indirectly via changes in mucosal immunity [121].
The research shows how the baseline composition of the intestinal microbiome is important, when treating IBD patients with biological therapy such as anti-TNF, anti-integrin, and anti-IL agents. The intestinal microbiome appears to influence the efficacy of biological therapy through the modulation of mucosal immune activation and microbial metabolite production in the gut [123,125]. Overall, the research reviewed reports how the majority of IBD drugs both shape and are shaped by the intestinal microbiome, which secondly affects drug bioavailability, efficacy, safety, and long-term mucosal outcomes in IBD patients. The baseline microbial composition is a predictor of the efficacy of biological therapy and anti-inflammatory drugs such as aminosalicylates. This enables a new therapeutic opportunity to enhance drug response or reduce toxicity by modulating the gut microbiome through diet and fecal microbiota transplantation. Future randomized controlled studies are warranted to assess the potential of fecal microbiota transplantation (FMT) to beneficially reshape the gut microbiome and thereby enhance the clinical efficacy and durability of biologic therapies in patients with IBD.
While our review initially discusses diet and pharmacotherapy separately, it is increasingly recognized that these factors interact in clinical practice, producing additive, synergistic, or competing effects on gut microbiota. For example, dietary interventions may modulate microbial composition in ways that enhance or counteract the effects of medications such as antibiotics, immunosuppressants, or biologics. These interactions can influence inflammation, metabolite production, and overall gut ecosystem resilience. Understanding the combined impact of diet and pharmacotherapy is crucial for designing personalized therapeutic strategies. Future studies should evaluate these interactions longitudinally in human cohorts to determine optimal combinations for restoring microbial balance and improving clinical outcomes in IBD.
6. Conclusions
Diet and medication are central modulators of gut microbiota in IBD, and their effects can be beneficial or detrimental depending on context, dosage, and timing. Understanding these mechanisms is essential for guiding precision nutrition and therapy. This review highlights the growing evidence that dietary interventions, pharmacotherapy, and fecal microbiota transplantation can modulate gut microbial composition and influence disease outcomes. However, significant knowledge gaps remain, particularly regarding integrative approaches that combine diet and medication, the limited clinical evidence for FMT in IBD, and the need for larger, well-controlled longitudinal studies. Future research should focus on personalized microbiota-targeted strategies, combined dietary and pharmacological interventions, and mechanistic studies in both human and animal models. The findings have practical implications for clinicians and dietitians, providing a scientific basis for optimizing dietary guidance, therapeutic decisions, and patient management in IBD.
Author Contributions
H.C.M.-L. and A.E.J. has conceived, designed and draft manuscript A.E.J. and M.W. has gathered relevant references, make the tables and figure H.C.M.-L. and B.H.J.; intellectual and specialist contributions, supervision, project administration, All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Innovation fonden”, grant number 4289-00312B.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| FMT | Fecal Microbiota Transplantation |
| PPI | proton pump inhibitor |
References
- Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M. Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2019, 32, e00060-18. [Google Scholar] [CrossRef]
- Hébuterne, X.; Filippi, J.; Al-Jaouni, R.; Schneider, S. Nutritional consequences and nutrition therapy in Crohn’s disease. Gastroentérologie Clin. Biol. 2009, 33, S235–S244. [Google Scholar] [CrossRef]
- Anneberg, O.M.; Petersen, I.S.B.; Jess, T.; De Freitas, M.B.; Jalili, M. The dietary inflammatory potential and its role in the risk and progression of inflammatory bowel disease: A systematic review. Clin. Nutr. 2025, 47, 146–156. [Google Scholar] [CrossRef]
- Yang, H.; Mirsepasi-Lauridsen, H.C.; Struve, C.; Allaire, J.M.; Sivignon, A.; Vogl, W.; Bosman, E.S.; Ma, C.; Fotovati, A.; Reid, G.S.; et al. Ulcerative Colitis-associated E. coli pathobionts potentiate colitis in susceptible hosts. Gut Microbes 2020, 12, 1847976. [Google Scholar] [CrossRef]
- Chandwaskar, R.; Dalal, R.; Gupta, S.; Sharma, A.; Parashar, D.; Kashyap, V.K.; Sohal, J.S.; Tripathi, S.K. Dysregulation of T cell response in the pathogenesis of inflammatory bowel disease. Scand. J. Immunol. 2004, 100, e13412. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Update on the incidence and prevalence of inflammatory bowel disease in the United States. Gastroenterol. Hepatol. 2016, 12, 704–707. [Google Scholar]
- Nielsen, K.R.; Midjord, J.; Lophaven, S.N.; Langholz, E.; Hammer, T.; Burisch, J. The Incidence and Prevalence of Inflammatory Bowel Disease Continues to Increase in the Faroe Islands—A Cohort Study from 1960 to 2020. J. Crohn’s Colitis 2024, 18, 308–319. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- D’afflitto, M.M.; Upadhyaya, A.; Green, A.M.; Peiris, M. Association between Sex Hormone Levels and Gut Microbiota Composition and Diversity—A Systematic Review. J. Clin. Gastroenterol. 2022, 56, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.B.M.; Jansson, S.M.; Wewer, V.; Malham, M. Early Life Oral Antibiotics Are Associated With Pediatric-Onset Inflammatory Bowel Disease—A Nationwide Study. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. Molecular and Cellular Endocrinology The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Rodríguez-Bores, L.; Fonseca, G.C.; Villeda, M.A.; Yamamoto-Furusho, J.K. Novel genetic markers in inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 5560–5570. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Leach, S.T. Role of Probiotics and Prebiotics in Gut Symbiosis. Nutrients 2024, 16, 238. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. iScience 2023, 26, 105905. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Trott, M.J.; Bartholomeusz, F.D.; Andrews, J.M. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 213–225. [Google Scholar] [CrossRef]
- Lomer, M.C.; Cahill, O.; Baschali, A.; Sarathy, P.P.; Sarantidou, M.; Mantzaris, G.J.; Gaya, D.R.; Katsanos, K.; Christodoulou, D.K.; Gerasimidis, K. A multicentre study of nutrition risk assessment in adult patient. Ann. Nutr. Metab. 2019, 74, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, R.; Marra, M.; Pagano, M.C.; Alfonsi, L.; Santarpia, L.; Cioffi, I.; Contaldo, F.; Pasanisi, F. Resting energy expenditure in adult patients with Crohn’ s disease. Clin. Nutr. 2017, 36, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Dhillon, A.; Zhang, D.; Me, S.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A Prospective Study of Long-term Intake of Dietary Fiber and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef]
- Lopes, E.W.; Chan, S.S.; Song, M.; Ludvigsson, J.F.; Håkansson, N.; Lochhead, P.; Clark, A.; Burke, K.E.; Ananthakrishnan, A.N.; Cross, A.J.; et al. Lifestyle Factors for the Prevention of Inflammatory Bowel Disease. Gut 2023, 72, 1093–1100. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Ananthakrishnan, A.N.; Higuchi, L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012, 61, 1686–1692. [Google Scholar] [CrossRef]
- Grad, S.R.; Diet, D.; Kouris-blazos, A.; Singh, M.F. Evolution of Mediterranean diets and cuisine: Concepts and definitions. Asia Pac. J. Clin. Nutr. 2017, 26, 749–763. [Google Scholar]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohn’s Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Betensky, J.D.; Robinson, D.G.; Gunduz-Bruce, H.; Sevy, S.; Lencz, T.; Kane, J.M.; Malhotra, A.K.; Miller, R.; McCormack, J.; Bilder, R.M.; et al. Patterns of stress in schizophrenia. Psychiatry Res. 2008, 160, 38–46. [Google Scholar] [CrossRef][Green Version]
- Potential, D.I.; Colitis, U. Dietary Inflammatory Potential and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2021, 159, 873–883. [Google Scholar]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Rok Orel, T.K.T.; Orel, R.; Trop, T.K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 11505–11524. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, D.; Kolios, G. Probiotics and prebiotics in inflammatory bowel disease: Microflora “on the scope”. Br. J. Clin. Pharmacol. 2008, 65, 453–467. [Google Scholar] [CrossRef]
- Mardini, H.E.; Grigorian, A.Y. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: A meta-analysis. Inflamm. Bowel Dis. 2014, 20, 1562–1567. [Google Scholar]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Mirsepasi-Lauridsen, H.C. Therapy Used to Promote Disease Remission Targeting Gut Dysbiosis, in UC Patients with Active Disease. J. Clin. Med. 2022, 11, 7472. [Google Scholar] [CrossRef]
- Helwig, U.; Lammers, K.M.; Rizzello, F.; Brigidi, P.; Rohleder, V.; Caramelli, E.; Gionchetti, P.; Schrezenmeir, J.; Foelsch, U.R.; Schreiber, S.; et al. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J. Gastroenterol. 2006, 12, 5978–5986. [Google Scholar] [CrossRef]
- Hibi, T.; Inoue, N.; Ogata, H.; Naganuma, M. Introduction and overview: Recent advances in the immunotherapy of inflammatory bowel disease. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar]
- Hogaboam, C.M.; Vallance, B.A.; Kumar, A.; Addison, C.L.; Graham, F.L.; Gauldie, J.; Collins, S.M. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J. Clin. Investig. 1997, 100, 2766–2776. [Google Scholar] [CrossRef]
- Nougayrède, J.P.; Chagneau, C.V.; Motta, J.P.; Bossuet-Greif, N.; Belloy, M.; Taieb, F.; Gratadoux, J.J.; Thomas, M.; Langella, P.E.O. A Toxic Friend: Genotoxic and Mutagenic Activity of the Probiotic Strain Escherichia coli Nissle 1917. Am. Soc. Microbiol. 2021, 6, e00624-21. [Google Scholar] [CrossRef]
- Petersen, A.M.; Mirsepasi, H.; Halkjær, S.I.; Mortensen, E.M.; Nordgaard-Lassen, I.; Krogfelt, K.A. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: A double-blind randomized placebo controlled clinical trial. J. Crohn’s Colitis 2014, 8, 1498–1505. [Google Scholar] [CrossRef]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schütz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Rembacken, B.; Snelling, A.; Hawkey, P.; Chalmers, D.; Axon, A. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999, 354, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The Probiotic Preparation, VSL#3 Induces Remission in Patients With Mild-to-Moderately Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209.e1. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar]
- Iordache, F.; Iordache, C.; Chifiriuc, M.C.; Bleotu, C.; Pavel, M.; Smarandache, D.; Sasarman, E.; Laza, V.; Bucu, M.; Dracea, O.; et al. Antimicrobial and immunomodulatory activity of some probiotic fractions with potential clinical application. Zootechnica 2008, 11, 41–51. [Google Scholar]
- Garcia-Lafuente, A.; Antolin, M.; Guarner, F.; Crespo, E.; Salas, A.; Forcada, P.; Laguarda, M.; Gavalda, J.; Baena, J.A.; Vilaseca, J.; et al. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am. J. Physiol. Liver Physiol. 1997, 272, G10–G15. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Gut 2017, 5, 73–98. [Google Scholar] [CrossRef]
- Moroni, O.; Kheadr, E.; Boutin, Y.; Lacroix, C.; Fliss, I. Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl. Environ. Microbiol. 2006, 72, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Vakadaris, G.; Stefanis, C.; Giorgi, E.; Brouvalis, M.; Voidarou, C.; Kourkoutas, Y.; Tsigalou, C.; Bezirtzoglou, E. The Role of Probiotics in Inducing and Maintaining Remission in Crohn’ s Disease and Ulcerative Colitis: A Systematic Review of the Literature. Biomedicines 2023, 11, 494. [Google Scholar] [CrossRef]
- Grigoryan, Z.; Shen, M.J.; Twardus, S.W.; Beuttler, M.M.; Chen, L.A.; Bateman-House, A. Fecal microbiota transplantation: Uses, questions, and ethics. Med. Microecol. 2021, 6, 100027. [Google Scholar] [CrossRef]
- Gaines, S.; Alverdy, J.C. Fecal micobiota transplantation to treat sepsis of unclear etiology. Crit. Care Med. 2017, 45, 1106–1107. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Dahlerup, J.F.; Engberg, J.H.; Erikstrup, C.; Helms, M.; Juel, M.A.; Kjeldsen, J.; Nielsen, H.L.; Nilsson, A.C.; Rode, A.A.; et al. Danish national guideline for the treatment of Clostridioides difficile infection and use of faecal microbiota transplantation FMT. Scand. J. Gastroenterol. 2021, 56, 1056–1077. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Rode, A.A.; Bytzer, P.; Pedersen, O.B.; Engberg, J. Establishing a donor stool bank for faecal microbiota transplantation: Methods and feasibility. Eur. J. Clin. Microbiol. Infect Dis. 2019, 38, 1837–1847. [Google Scholar] [CrossRef]
- Svensson, C.K.; Cold, F.; Ribberholt, I.; Zangenberg, M.; Mirsepasi-Lauridsen, H.C.; Petersen, A.M.; Helms, M. The Efficacy of Faecal Microbiota Transplant and Rectal Bacteriotherapy in Patients with Recurrent Clostridioides difficile Infection: A Retrospective Cohort Study. Cells 2022, 11, 3272. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Zhao, B.; Lo, S.; Browne, P.D.; Günther, S.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef]
- Pourrat, A.; Baillieu, V.; Ansel, S.; Leonardi, M.; Poiron, P.; Bellais, S.; Paul, M.; Nebbad, B. Standardized freeze-dried FMT: Is the ideal protectant out there? Front. Microbiol. 2025, 16, 1618067. [Google Scholar] [CrossRef]
- Official Journal of the European Union, REGULATION (EU) 2024/1938 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/EC, EN L Series, 17.7. 2024. Available online: https://eur-lex.europa.eu/eli/reg/2024/1938/oj (accessed on 17 July 2024).
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Lauridse, H.C.M.; Mollerup, S.; Jensen, B.H.; Nielsen, H.V.; Helms, M.; Petersen, A.M. Fecal Microbiota Transplantation promotes disease remission in a patient with active Crohn’s disease: A Case Report. Med. Res. Arch. 2024, 12, 5. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Sood, A.; Mahajan, R.; Singh, A.; Midha, V.; Mehta, V.; Narang, V.; Singh, T.; Pannu, A.S. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients with Ulcerative Colitis: A Pilot Study. J. Crohn’s Colitis 2019, 13, 1311–1317. [Google Scholar] [CrossRef]
- Lauridsen, H.C. Capsule Comprising a Faecal Composition. WO2021130182A1, 1 July 2021. [Google Scholar]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Routledge: Abingdon, UK, 1982. [Google Scholar]
- Considerations, G. Guidance for Industry Bioavailability and Bioequivalence Guidance for Industry Bioavailability and Bioequivalence. Biopharmaceutics 2014, 2014, 1–26. Available online: https://www.fda.gov/media/88254/download (accessed on 29 April 2020).
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2015, 14, 404. [Google Scholar] [CrossRef]
- Moodie, R.; Bennett, E.; Kwong, E.J.L.; Santos, T.M.; Pratiwi, L.; Williams, J.; Baker, P. Ultra-Processed Profits: The Political Economy of Countering the Global Spread of Ultra-Processed Foods—A Synthesis Review on the Market and Political Practices of Transnational Food Corporations and Strategic Public Health Responses. Int. J. Health Policy Manag. 2021, 10, 968–982. [Google Scholar] [CrossRef]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.; Ananthakrishnan, A.N.; Chan, S.S.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2483–2495.e1. [Google Scholar] [CrossRef]
- Yang, F.; Xie, R.; Huang, M.; Hu, C.; Wu, Y.; Li, X.; Chen, H. High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model. Foods 2025, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Balan, Y.; Packirisamy, R.M.; Mohanraj, P.S. State of the art paper High dietary salt intake activates inflammatory cascades via Th17 immune cells: Impact on health and diseases. Arch. Med. Sci. 2022, 18, 459–465. [Google Scholar] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Grzybowska-Chlebowczyk, U.; Landowski, P.; Szaflarska-Poplawska, A.; Klincewicz, B.; Adamczak, D.; Banasiewicz, T.; Plawski, A.; Walkowiak, J. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci. Rep. 2014, 4, 4768. [Google Scholar] [CrossRef]
- Wolin, M.J. Volatile fatty acids and the inhibition of Escherichia coli growth by rumen fluid. Appl. Microbiol. 1969, 17, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Gnaedinger, A.; Hauck, W.; Breuer, R.I. Organic anions and the diarrhea of inflammatory bowel disease. Dig. Dis. Sci. 1988, 33, 1353–1358. [Google Scholar] [CrossRef]
- Fleming, L.L.; Floch, M.H. Digestion and absorption of fiber carbohydrate in the colon. Am. J. Gastroenterol. 1986, 81, 507–511. [Google Scholar]
- Segain, J.-P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.-P. Butyrate inhibits inflammatory responses through NF B inhibition: Implications for Crohn’ s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parenter. Enter. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Peterman, I.; Hübner, C.; Philpott, M.; Shellin, A.N. Uncoupling gene—Diet interactions in inflammatory bowel disease (IBD). Genes Nutr. 2007, 2, 71–73. [Google Scholar] [CrossRef]
- PálMer, H.G.; GonzálEz-Sancho, J.M.; Espada, J.; Berciano, M.T.; Puig, I.; Baulida, J.; Quintanilla, M.; Cano, A.; de Herreros, A.G.; Lafarga, M.; et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001, 154, 369–388. [Google Scholar] [CrossRef]
- Müller, K.; Ødum, N.; Bendtzen, K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol. Lett. 1993, 35, 177–182. [Google Scholar] [CrossRef]
- Wang, T.-T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet AloneVersus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; San Yeoh, B.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 404–413. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. Review The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Nat. Publ. Gr. 2018, 6, 19032. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.; Leone, V. Dietary fat-induced taurocholic acid production promotes pathobiont and colitis in IL-10−/−mice. Nature 2013, 487, 104–108. [Google Scholar] [CrossRef]
- Khan, N.; Vallarino, C.; Lissoos, T.; Darr, U.; Luo, M. Risk of Infection and Types of Infection Among Elderly Patients With Inflammatory Bowel Disease: A Retrospective Database Analysis. Inflamm. Bowel Dis. 2020, 26, 462–468. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased e coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Marion-letellier, R.; Savoye, G.; Ghosh, S. IBD: In Food We Trust. J. Crohns Colitis 2016, 2016, 1351–1361. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 9, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Ruiz-ojeda, F.J.; Plaza-díaz, J.; Sáez-lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, 531–548. [Google Scholar] [CrossRef]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Shanika, L.G.T.; Reynolds, A.; Pattison, S.; Braund, R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023, 79, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Rogers, M.A.; Aronoff, D.M. The Influence of Nonsteroidal Anti-Inflammatory Drugs on the Gut Microbiome Mary. Clin. Microbiol. Infect. 2017, 22, 178.e1–178.e9. [Google Scholar] [CrossRef]
- Maseda, D.; Ricciotti, E. NSAID–Gut Microbiota Interactions. Front. Pharmacol. 2020, 11, 558924. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Inoue, T.; Leone, V.A.; Dalal, S.; Touw, K.; Wang, Y.; Musch, M.W.; Theriault, B.; Higuchi, K.; Donovan, S.; et al. Using Corticosteroids to Reshape the Gut Microbiome: Implications for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 21, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Blesl, A.; Wurm, P.; Waschina, S.; Gröchenig, H.P.; Novacek, G.; Primas, C.; Reinisch, W.; Kutschera, M.; Illiasch, C.; Hennlich, B.; et al. Prediction of Response to Systemic Corticosteroids in Active UC by Microbial Composition—A Prospective Multicenter Study. Inflamm. Bowel Dis. 2024, 30, 9–19. [Google Scholar] [CrossRef]
- Guo, D.; Fang, L.; Liu, R.; Li, Y.; Lv, L.; Niu, Z.; Chen, D.; Zhou, Y.; Zhu, W. Exploring Different Effects of Exclusive Enteral Nutrition (EEN) and Corticosteroids on the Gut Microbiome in Crohn ’ s Disease Based on a Three-Stage Strategy. Gastroenterol. Res. Pr. 2022, 2022, 6147124. [Google Scholar]
- Brown, S.C.; Wall, C.L.; Gearry, R.B.; Day, A.S. Exclusive Enteral Nutrition for the Treatment of Pediatric Crohn ’ s Disease: The Patient Perspective. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, Z.; Ding, L.; Fu, Y.; Fan, J.; Mei, Q.; Lou, L.; Wang, J.; Yin, N.; Lu, Y.; et al. Fecal microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J. Transl. Med. 2022, 20, 354. [Google Scholar] [CrossRef]
- Li, W.; Shu, Y.; Zhang, J.; Wu, M.; Zhu, G.-H.; Huang, W.-Y.; Shen, L.; Kang, Y. Long-term prednisone treatment causes fungal microbiota dysbiosis and alters the ecological interaction between gut mycobiome and bacteriome in rats. Front. Microbiol. 2023, 14, 1112767. [Google Scholar] [CrossRef]
- Oppenheimer, G.; Ginzburg, L. American Gastroenterological Association Institute Technical Review on Corticosteroids, Immunomodulators, and Infliximab in Inflammatory Bowel Disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef]
- Van Staa, T.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Oral corticosteroids and fracture risk: Relationship to daily and cumulative doses. Rheumatology 2000, 39, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.G.; Abrahamowicz, M.; Beauchamp, M.-E.; Ray, D.W.; Bernatsky, S.; Suissa, S.; Sylvestre, M.-P. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: A nested case—Control analysis. Ann. Rheum. Dis. 2012, 71, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Bostwick, J.M. Psychiatric Adverse Effects of Corticosteroids. Mayo Clin. Proc. 2006, 81, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Else, T.; Bancos, I.; Hahner, S.; Hamidi, O.; van Hulsteijn, L.; Husebye, E.S.; Karavitaki, N.; Prete, A.; Vaidya, A.; et al. European Society of Endocrinology and Endocrine Society Joint Clinical Guideline: Diagnosis and Therapy of Glucocorticoid-induced Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2024, 109, 1657–1683. [Google Scholar] [CrossRef]
- Wada, H.; Miyoshi, J.; Kuronuma, S.; Nishinarita, Y.; Oguri, N.; Hibi, N.; Takeuchi, O.; Akimoto, Y.; Lee, S.T.M.; Matsuura, M.; et al. 5-Aminosalicylic acid alters the gut microbiota and altered microbiota transmitted vertically to offspring have protective effects against colitis. Sci. Rep. 2023, 13, 12241. [Google Scholar] [CrossRef]
- Ford, A.C.; Achkar, J.P.; Khan, K.J.; Kane, S.V.; Talley, N.J.; Marshall, J.K.; Moayyedi, P. Efficacy of 5-Aminosalicylates in Ulcerative Colitis: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2011, 106, 601–616. [Google Scholar] [CrossRef]
- Doherty, M.K.; Ding, T.; Koumpouras, C.; Telesco, S.E.; Monast, C.; Das, A.; Brodmerkel, C.; Schloss, P.D. Fecal Microbiota Signatures Are Associated with Response to Ustekinumab Therapy among Crohn’s Disease Patients. mBio 2018, 9, e02120-17. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut microbiome function predicts response to anti-integrin biologic therapy in Inflammatory Bowel diseases. Cell Host Microbe 2018, 21, 603–610.e3. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B.; et al. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate With Efficacy of Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Veltkamp, S.H.C.; Voorneveld, P.W. The Cell-Specific Effects of JAK1 Inhibitors in Ulcerative Colitis. J. Clin. Med. 2025, 14, 608. [Google Scholar] [CrossRef]
- Khan, A.U.; Ali, M.; Wahab, M.A. Comparative efficacy of pharmacologic interventions in ulcerative colitis: A network meta analysis. Inflammopharmacology 2025, 33, 2679–2687. [Google Scholar] [CrossRef]
- Favaron, A.; Sangfuang, N.; McCoubrey, L.E.; Awad, A.; Ghyselinck, J.; Marzorati, M.; Verstrepen, L.; De Munck, J.; De Medts, J.; Basit, A.W.; et al. European Journal of Pharmaceutical Sciences Assessing the effects of tofacitinib on the gut microbiome in inflammatory bowel disease. Eur. J. Pharm. Sci. 2026, 216, 107365. [Google Scholar] [CrossRef] [PubMed]
- Bravo, G.Á.; Cedeño, R.R.; Casadevall, M.P.; Ramió-Torrentà, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Wang, S.; Xu, M.; Wu, Z.; Deng, F. The role of sphingosine-1-phosphate in the gut mucosal microenvironment and in fl ammatory bowel diseases. Front. Physiol. 2023, 14, 1235656. [Google Scholar] [CrossRef]
- Choon, X.Y.; Yeo, J.H.; White, C.; Sharma, E.; Samaan, M.A. The Current Sphingosine 1 Phosphate Receptor Modulators in the Management of Ulcerative Colitis. J. Clin. Med. 2025, 14, 3475. [Google Scholar] [CrossRef]
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’ re doing? Transl. Pediatr. 2019, 8, 42–55. [Google Scholar] [CrossRef]
- Hviid, A.; Svanström, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatments on strain-level diversity and stability. Sci. Transl. Med. 2017, 8, 343. [Google Scholar]
- Sabino, J.; Tarassishin, L.; Eisele, C.; Hawkins, K.; Barré, A.; Nair, N.; Rendon, A.; Debebe, A.; Picker, M.; Agrawal, M.; et al. Influence of Early Life Factors, including breast milk Composition, on the Microbiome of Infants Born to Mothers with and without Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 17, 1723–1732. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2017, 22, 458–478. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, C.; Li, G.; Li, J.; Duan, L. Antibiotic Exposure and Risk of New-Onset Inflammatory Bowel Disease: A Systematic Review and Dose-Response. Clin. Gastroenterol. Hepatol. 2025, 23, 45–58.e15. [Google Scholar] [CrossRef]
- Faye, A.S.; Allin, K.H.; Iversen, A.T.; Agrawal, M.; Faith, J.; Colombel, F.; Jess, T. Antibiotic use as a risk factor for inflammatory bowel disease across the ages: A population-based cohort study. Gut 2023, 72, 663–670. [Google Scholar] [CrossRef]
- Taitz, J.J.; Tan, J.; Ni, D.; Potier-villette, C.; Grau, G.; Nanan, R.; Macia, L. Antibiotic-mediated dysbiosis leads to activation of inflammatory pathways. Front. Immunol. 2025, 15, 1493991. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moller, F.T.; Knudsen, L.; Harbord, M.; Satsangi, J.; Gordon, H.; Christiansen, L.; Christensen, K.; Jess, T.; Andersen, V. Danish cohort of monozygotic inflammatory bowel disease twins: Clinical characteristics and inflammatory activity. World J. Gastroenterol. 2016, 22, 5050–5059. [Google Scholar] [CrossRef][Green Version]
- Agrawal, M.; Corn, G.; Shrestha, S.; Nielsen, N.M.; Frisch, M.; Colombel, J.-F.; Jess, T. Inflammatory bowel diseases among first-generation and second-generation immigrants in Denmark: A population-based cohort study. Gut 2021, 70, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M. Breaking Bread: The Functions of Social Eating. Adapt. Hum. Behav. Physiol. 2017, 3, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Theoklis, E.; Haynes, K. Antibiotic Exposure and IBD Development Among Children: A Population-Based Cohort Study. Pediatrics 2012, 77, 4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.