Abstract
The Mediterranean Diet (MD) is a lifestyle that involves not only dietary habits, well known for their effectiveness in preventing health risks by supplying well-balanced foods rich in bioactive compounds, but also daily habits that improve the quality of life. Older adults represent a segment of the population that can particularly benefit from this dietary pattern. However, the specific characteristics and needs of older individuals require a critical analysis of aspects that may limit adherence to the MD principles, including physical impairments related to eating, sensory and cultural aspects, accessibility of food sources, and the social context. The objective of this study was to review the potential benefits of the MD in relation to the needs, capacities and eating behaviors of older adults, focusing on the beneficial effects of plant-based food metabolites and their suitability for older adult diets. The results demonstrate how the MD can be tailored to meet the nutritional and functional needs of older adults, supporting healthy aging. Therefore, the Mediterranean lifestyle could be an effective tool in public health policies to promote healthy habits, thereby improving the quality of life in vulnerable population categories.
1. Introduction
The MD is a dietary lifestyle that emerged from studies conducted in the early 1950s by Ancel Keys and his team on the eating habits of countries bordering the Mediterranean Sea []. The original MD has undergone significant changes over time, influenced by shifts in culture, society, economy, and work-related transformations, leading to a reduction in adherence to its core principles []. Nevertheless, the benefits of such a dietary regimen in maintaining health, preventing communicable diseases, and improving longevity are widely recognized [,,]. In 2013, MD was inscribed on the UNESCO Representative List of Intangible Cultural Heritage of Humanity (UNESCO). The MD is characterized by a high consumption of fresh or dried fruits and vegetables, legumes, and whole-grain cereals. It also includes moderate consumption of fish and dairy products and a limited intake of red meat. Olive oil represents the main source of fat []. These features make the MD particularly suitable for promoting healthy aging []. Moreover, it can help mitigate age-related diseases in later life [] and counteract the onset of frailty []. A greater adherence to a plant-based diet, studied a posteriori in a group of European elders, is associated with lower all-cause mortality [], indicating the relevant impact of lifelong habits. However, actions aimed at maintaining or possibly increasing adherence to MD at a later age can positively affect health status and survival expectations. The correlation between health and adherence to balanced diets is well established across all stages of life but becomes particularly significant in later life due to its impact on healthy aging []. Finally, the MD adoption stimulates respect for natural resources and seasonality, playing an important role in preserving the environment. Indeed, the MD has been demonstrated to be the most sustainable diet considering several factors such as the environment, nutrition, the economy and socio-cultural aspects [].
In 2025, the Italian National Institute of Health published the Italian MD Guidelines [], developed based on the systematic reviews and meta-analyses of 3839 clinical studies, involving approximately 2 million participants, followed for an average of 13 years. The guidelines are based on 108 evidence-based recommendations on how the MD can help in disease prevention and management. Key outcomes supported by research include the possibility of lowering overall and cardiovascular mortality, reducing cancer incidence, and improving metabolic and cognitive health, with remarkable positive effects on the aging process. The guidelines underline the fact that the MD is recognized not only as a dietary pattern but as a comprehensive lifestyle that promotes health, prevents chronic diseases, and supports environmental sustainability in terms of natural resource consumption and environmental impact. It emphasizes not only balanced, mostly plant-based nutrition, but also regular physical activity and rest, conviviality, consumption of seasonal and local foods, short supply chains and minimally processed ingredients. Finally, a key aspect of the guidelines also concerns the economic impact of the MD. Several studies suggest that adopting this dietary pattern could significantly reduce healthcare costs, particularly at older age.
This review aims to highlight the scientific foundations underlying the beneficial effects of the MD, while also exploring its cultural and social implications. Although several reviews are available on the MD’s advantages, the present work addresses a specific gap in the literature by providing a comprehensive overview of the main benefits associated with its adoption, focusing on both the metabolites derived from dietary intake and the socio-cultural aspects that underpin the healthy lifestyle, which could enhance well-being in vulnerable population groups such as older adults in later life.
2. Methods
This narrative review investigates the health benefits of the MD on a specific population group, the older adults, based on the evidence currently available in the literature. The narrative approach was chosen due to the complexity and multidimensional nature of the topic. The literature search was carried out using multiple databases, including Web of Science, PubMed, Scopus and Google Scholar between November 2024 and October 2025. The primary search terms included “Mediterranean Diet” and “aging,” along with combinations of words such as “Mediterranean Diet + sustainable,” “Mediterranean Diet + healthy aging + bioactive compounds,” and “dietary + lifestyle + aging.” The previous terms were also used in combination with specific food categories. The search was limited to English-language publications, giving priority to papers from the past ten years to ensure the inclusion of the most recent and relevant studies. However, older references were also considered when updated data were not available.
Additional articles were identified from the references cited in the original papers. The selection process involved several stages: initial screening based on titles and abstracts, followed by full-text analysis. The following exclusion criteria were applied to the search: (a) inappropriate topics, not pertinent to the specific themes addressed in each section of the review, and (b) PhD dissertations, conference proceedings, abstracts, and unpublished studies. Finally, a total of 178 articles were selected for the review.
In the section regarding the MD bioactive molecules, both human studies (prospective cohorts, randomized trials, and meta-analyses) and translational or mechanistic research were included when they contributed to understanding the biological plausibility of observed health effects.
3. MD for Aged People
The aging process involves multiple events during which cells are stressed by endogenous and exogenous elements, causing DNA damage, mutations, dysfunctional protein accumulation (heat shock protein), oxidative stress, mitochondrial dysfunction and inflammation []. The phytochemical compounds, minerals and vitamins present in MD food can counteract these processes, promoting healthy aging and longevity, and slowing down the aging process by acting as antioxidants and/or anti-inflammatories [,].
3.1. MD in Disease Prevention
Adherence to MD can contribute to preventing and/or mitigating the main diseases affecting older people, such as diabetes, cardiovascular diseases, and cancer [,]. Indeed, evidence on the effectiveness of the MD against the most common chronic diseases is constantly growing [].
MD provides defense against cardiovascular diseases in both the general population and in patients already affected by these diseases. Moreover, it has been reported that the MD adherence decreases the risk of heart attacks, various types of coronary artery disease, stroke, and cardiovascular mortality [].
There is a direct association between the MD pattern and the reduced risk of diabetes. Particularly, it has been shown that even modest adherence to the MD likely decreases the incidence of type 2 diabetes [].
Recently, the European Prospective Investigation into Cancer and Nutrition conducted a study in 23 centers of 10 European countries, declaring that the MD exerts protective action against the four most frequent cancers in the European population (colorectal, breast, lung, and prostate cancer). High consumption of fruit, vegetables, fish and yogurt, coupled with low consumption of alcohol and red and processed meat, lowers the risk of cancer development [].
3.2. MD in Comorbidities Prevention
Mental decline and dementia are the main comorbidities and among the most common causes of death in the elderly []. Following the MD lifestyle lowers the risk of cognitive impairments, improving mental health [] and protecting against mental illnesses such as Alzheimer’s disease [,]. Indeed, phytochemical compounds present in Mediterranean food are active against oxidative stresses, protecting the brain and the nervous system from inflammation and the accumulation of AB plaques []. Other studies found adherence to MD effective in mitigating depression, anxiety and psychological distress [].
Following MD can also support physical performance maintenance, counteracting the age-related muscle mass and mineral bone density reductions, preserving sexual capacity, and preventing immune system dysregulation [].
3.3. MD in Sarcopenia Prevention
Sarcopenia is a neuromuscular degeneration caused by loss of physical function, particularly in older adults. Its progression can lead to various dysfunctions such as metabolic, osteoarticular, and cognitive disorders, which may result in malnutrition, physical inactivity, falls, depressive symptoms, and death []. Nutrition and dietary interventions are effective approaches to treat and prevent sarcopenia. The MD lifestyle positively influences physical function and helps prevent sarcopenia through typical foods such as extra-virgin olive oil, fruits, vegetables, and fish, and their anti-inflammatory and antioxidant properties, as well as the promotion of a healthy lifestyle that includes social and physical activity []. In fact, recent recommendations to prevent sarcopenia involve reducing sedentary time and increasing protein intake above the RDA (0.8 g/kg/day) to mitigate muscle loss [], since sarcopenic older adults tend to consume less protein []. Accordingly, a total daily protein intake of around 1.6–1.8 g/kg/day, with three main meals containing 0.6 g/kg of high-quality protein sources such as wheat protein—particularly abundant in the MD []—is an effective strategy to optimize nutrition and maintain muscle mass during aging []. MD meets these recommendations, aligning sensory satisfaction, palatability, and protein distribution across meals, promoting healthy nutrition.
3.4. MD Adherence in Later Age
Only if the adherence to the MD has a consistent and foundational presence in daily eating habits and is supported by both food education and eating pleasure do the recognized health benefits have the potential to become effective. Interventions aimed at enhancing this aspect should align with the statement, ‘Make “good for you” taste good’ []. This means exploring and respecting individual choices and preferences. In this context, formulating suitable dietary strategies for older adults should consider their longstanding adherence to traditional eating practices but also their preferences for heritage eating habits []. According to this research, a convenient strategy is to explore alternative ingredients that enhance the health properties of traditional foods while maintaining most of their overall characteristics. Incorporating elements of MD into commonly consumed foods represents a promising approach that enhances their nutritional profile without compromising consumer acceptance. Indeed, Albergamo et al., in 2021 [], reformulated chicken burgers by incorporating typical MD ingredients, such as tomato, rosemary, basil, and thyme, together with powdered fortifying agents. A consumer test conducted with older adults demonstrated significantly higher levels of appreciation for the reformulated product compared to the standard burger. A similar approach was adopted by offering older adults’ fish-based sausages enriched with various combinations of vegetables and herbs []. In this case, senior consumers actively contributed to the development of acceptable recipes. A semi-trained panel of older adults evaluated each step of the product innovation process. This led to the creation of a healthy and nutritious novel food, well appreciated by older consumers. MD can also drive the formulation of the “Mediterranean” version of traditional foods, satisfying old people’s food habits while improving the healthiness of a typical recipe. Volpe et al., in 2021 [], involved aged consumers in the evaluation of MD-based pasta sauces and lasagna fillings as alternatives to red meat-based Bolognese ragù, showing that tailored-taste alternative ingredients can match the appreciation level of standard meat-based dishes.
4. Mediterranean Food Plants and Bioactive Molecules
The Mediterranean Diet model is grounded in a diverse range of foods with the predominance of plant-based foods, including fruits, vegetables, legumes, nuts, whole grains, and extra-virgin olive oil, which contribute to macronutrient balance, essential micronutrients and a diverse array of bioactive compounds, key mediators of the beneficial health outcomes associated with the MD. Bioactive molecules are non-nutrient secondary metabolites that exert regulatory effects on human physiology. In the context of the MD, the most relevant categories include polyphenols, carotenoids and terpenoids; vitamins with antioxidant properties; bioactive peptides; and sulfur-containing compounds (Figure 1). These compounds have been shown to modulate key biological processes linked to age-related pathologies, including oxidative stress, dysregulated lipid metabolism, endothelial dysfunction, and impaired immune responses. By targeting these mechanisms, they contribute to the prevention of cardiovascular and metabolic diseases, the maintenance of cognitive function, and the promotion of healthy aging in the elderly.
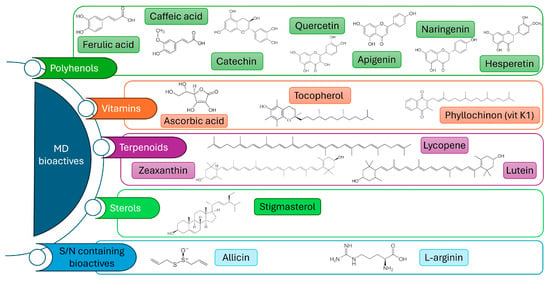
Figure 1.
Chemical structures of selected bioactive compounds found in plants characteristic of the Mediterranean diet. These include, but are not limited to, polyphenols, vitamins, terpenoids, sterols, S- and N-containing compounds, and phenolic acids, all of which contribute to the health-promoting properties of the diet. The structures are shown to illustrate the diversity of molecular classes derived from edible plants native to the Mediterranean region.
Whole grains such as wheat, rye, barley, and oats deliver alkylresorcinols (i.e., phenolic lipids that serve as biomarkers of whole-grain intake), β-glucans (i.e., soluble fibers), phenolic acids (e.g., ferulic, caffeic, and p-coumaric acids), flavonoids, lignans, tocols (i.e., vitamin E isomers), and phytosterols. Evidence from large prospective cohorts and meta-analyses supports their role in reducing the incidence of cardiovascular disease and type 2 diabetes in older adults [,,]. These compounds modulate lipid and glucose metabolism, attenuate systemic oxidative stress, and exert vasculoprotective actions [,,,,]. Mechanistic and translational studies further indicate favorable interactions with gut microbiota, enhancing the production of short-chain fatty acids and thereby influencing immune function, processes central to healthy aging []. Processing and cooking strongly influence the bioavailability. Milling into refined flour removes bran and germ, drastically reducing fiber, phenolic acids, and phytosterols [], whereas thermal treatments can degrade heat-sensitive tocols and flavonoids but may also increase the release of bound phenolic acids, enhancing colonic bioaccessibility after digestion [,]. Overall, according to the most relevant geriatric outcomes, the strength of the evidence is high, as the reported associations are consistently supported by findings from multiple cohort studies and randomized intervention trials (Table 1).

Table 1.
Plant-based foods typical of the MD and their bioactive compounds, mechanisms, and evidence intensity for healthy aging. Evidence type is indicated as “Strong” = supported by multiple large cohorts and meta-analyses or long-term RCTs; “Moderate” = consistent observational data and some RCTs/short-term interventions; “Translational” = mainly mechanistic, in vitro/animal or short-term human studies. The following abbreviations are used: “CVD”: Cardiovascular Disease; “T2D”: Type 2 Diabetes; “MUFA”: Monounsaturated Fatty Acids; “PUFA”: Polyunsaturated Fatty Acids; “BP”: Blood Pressure; “Mg”: Magnesium; “RCT”: Randomized Controlled Trial; “LDL”: Low-Density Lipoprotein.
Legumes provide not only plant protein (15–40% g/100 g dry basis depending on the species [,,]) and fermentable fiber (~12% g/100 g dry basis [,]) but also secondary metabolites such as flavonoids (e.g., catechin, epicatechin, quercetin, quercetin-3-O-glucoside, myricetin, kaempferol-3-O-rutinoside, and kaempferol-3-O-glucoside), especially abundant in legumes with colored seed coats, condensed tannins, saponins and tocopherols (δ- and γ-isoforms) [,]. These molecules have demonstrated antioxidant, antihypertensive, hypocholesterolemic, and anticancer activities, contributing to the prevention of sarcopenia and immune decline, conditions often exacerbated in later life [,,,,,,,]. Recent systematic reviews and meta-analyses indicate that higher legume intake is associated with reduced all-cause mortality and improved metabolic outcomes [,]. In addition, bioactive peptides and small proteins (e.g., lunasin, Bowman-Birk inhibitors) exert antioxidant, immunomodulatory and anti-inflammatory activities [,,]. Overall, according to the most relevant geriatric outcomes, the evidence can be considered moderate, as it is supported by consistent observational data and several intervention studies primarily addressing metabolic outcomes rather than long-term geriatric endpoints (Table 1).
Tomato, one of the most popular and consumed components of MD, is a unique source of bioactive molecules such as lycopene, polyphenols, phytosterols, and polyamines. Lycopene accumulates in the prostate, brain and vascular tissues, where it reduces oxidative DNA damage and supports cellular homeostasis []. Observational meta-analyses suggest an inverse association between tomato/lycopene intake and cardiovascular outcomes []. However, a recent meta-analysis of interventional trials [] found no consistent improvements in established risk factors such as blood pressure or lipids, highlighting the need for caution in extrapolating mechanistic findings to clinical outcomes. Processing strongly influences lycopene bioavailability. Heat treatment reduces vitamin C and some phenolics, but it also disrupts the plant cell matrix and enhances lycopene bioavailability, especially in the presence of olive oil [,]. In addition, tomatoes provide polyamines (e.g., spermidine, putrescine, and spermine), which have been recently linked to longevity [], and phytosterols (e.g., β-sitosterol, campesterol, stigmasterol) with cholesterol-lowering effects and improvement of cardiovascular outcomes, which is highly relevant in older adults [], and flavonoids such as quercetin, naringenin, and chlorogenic acid []. Tomato by-products, such as peels and seeds, also provide concentrated sources of carotenoids, polyphenols, dietary fiber, and anthocyanins, particularly in pigmented varieties, making them valuable raw materials for functional food formulations [,]. Finally, water-soluble compounds in tomatoes, including nucleosides, nucleotides, and phenolic acids, exhibit antiplatelet activity, thus showing antithrombotic effects [,]. Overall, according to the most relevant geriatric outcomes, the evidence is moderate, supported by findings from cohort studies and mechanistic research, although most interventional trials remain short-term (Table 1).
Leafy vegetables such as spinach (Spinacia oleracea), kale (Brassica oleracea), lettuce (Lactuca sativa), chard (Beta vulgaris), arugula (Eruca sativa), and chicory (Cichorium intybus) and cucurbits such as zucchini (Cucurbita pepo), pumpkin (Cucurbita moschata), cucumber (Cucumis sativus), and melon (Cucumis melo) provide high levels of carotenoids like lutein and zeaxanthin, folates, vitamin K1, glucosinolates, and saponins and dietary fibers [,,,,]. Carotenoids are critical for visual health, particularly in the prevention of age-related macular degeneration, while folate status has been consistently linked to cognitive performance in older adults []. Vitamin K1 contributes to vascular, skeletal and neural protection []. Members of the Brassicaceae family, particularly kale, supply glucosinolates, precursors of isothiocyanates, which have been shown to regulate detoxification enzymes and exert anticancer effects []. Saponins can reduce intestinal cholesterol absorption, modulate immune responses, and display antitumor effects [,,]. Finally, both leafy greens and cucurbits are excellent sources of dietary fiber, which supports gut microbiota diversity, improves glycemic control, lowers cholesterol, and may reduce colorectal cancer risk []. Overall, according to the most relevant geriatric outcomes, the evidence is moderate, with high epidemiological consistency, although data from long-term intervention studies in older populations remain limited (Table 1).
Citrus fruits supply vitamin C, flavanones (e.g., hesperidin/hesperetin and naringin/naringenin), polymethoxylated flavones (nobiletin, tangeretin), and carotenoids [,,]. Meta-analyses report improved endothelial function and reduced risk of cardiovascular events []. Importantly, recent evidence highlights the role of citrus flavanones, including neohesperidin, hesperidin, and hesperetin, in vascular, cognitive and skeletal health. While mechanistic studies and short-term interventions support antioxidant, anti-inflammatory, and anti-apoptotic activities, clinical outcomes remain heterogeneous. In a 36-week randomized, placebo-controlled trial in older adults with subjective cognitive decline, citrus peel extract supplementation showed no significant improvement over placebo, underscoring the need for cautious interpretation of translational evidence []. Overall, according to the most relevant geriatric outcomes, the evidence can be considered moderate, showing consistency across human trials and mechanistic studies, although some results remain neutral. (Table 1).
Allium species, especially garlic, are widely recognized for their rich content of bioactive phytochemicals. Among these are sulfur-containing compounds (thiosulfinates, allicin, diallyl sulfides, S-allyl cysteine) with antimicrobial, antihypertensive, and cardioprotective activities [,,]. Meta-analyses consistently demonstrate that garlic supplementation can lower blood pressure and modestly reduce cholesterol in hypertensive adults [,]. Recent randomized trials also show the benefits of aged garlic extract on arterial stiffness and gut microbiota in hypertensive individuals []. Phenolic compounds, including β-resorcylic acid, pyrogallol, protocatechuic acid, gallic acid, rutin, and quercetin, further enhance antioxidant protection []. Overall, according to the most relevant geriatric outcomes, the evidence is moderate, supported by meta-analyses of randomized controlled trials, although some heterogeneity remains across studies. (Table 1)
Nuts, including almonds, walnuts, pistachios, and hazelnuts, are increasingly recognized as functional foods thanks to their richness in bioactive molecules. They provide a balanced profile of unsaturated fatty acids, including both monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA). Also, nuts supply tocopherols, especially vitamin E, found in high concentrations in almonds and hazelnuts; phytosterols; polyphenols (e.g., walnuts are particularly rich in ellagitannins, while almonds contain relevant amounts of flavan-3-ols); L-arginine; and minerals such as magnesium [,]. Numerous meta-analyses and large cohort studies confirm the inverse association between nut consumption and cardiovascular and all-cause mortality, supporting a dose–response effect up to about 30 g/day [,,]. Evidence remains strong for cardiovascular outcomes but moderate for cancer prevention. These findings align with the Mediterranean dietary pattern’s overall cardioprotective role [,,,,,,]. Overall, according to the most relevant geriatric outcomes, the evidence is strong, confirmed by multiple large cohort studies and intervention trials (Table 1).
Beyond staple foods, Mediterranean cuisine incorporates a wide array of seasonings and minor vegetables that further enrich its bioactive profile. Caper (Capparis spinosa L.), widely used as seasoning, and Mediterranean herbs (oregano, rosemary, sage, thyme, basil, parsley) provide terpenoids, essential oils, and polyphenols such as rosmarinic and apigenin acids [,,,,,,,,,,,,,,,]. These compounds improve cerebral blood flow, modulate cholinergic neurotransmission, and reduce neuroinflammation, supporting cognitive and vascular health in older adults []. Overall, according to the most relevant geriatric outcomes, the evidence is limited, as most data derive from mechanistic or preclinical studies, and randomized trials are lacking (Table 1).
Mediterranean Dietary Synergy
The MD represents a complex phytochemical network whose synergistic bioactive molecules directly target hallmarks of aging (e.g., oxidative stress, chronic inflammation, metabolic dysfunction, and neurodegeneration [,]). Evidence from large meta-analyses and prospective cohorts (MD overall, Table 2) consistently associates higher adherence to the MD with reduced all-cause mortality, cardiovascular disease, frailty and cognitive decline [,]. In addition, the NU-AGE one-year multicenter randomized controlled trial, conducted across five European countries, tested a personalized Mediterranean-like diet ensuring micronutrient adequacy and demonstrated improvements in gut microbiota composition, reductions in frailty, and favorable changes in immune and metabolic biomarkers related to inflammaging [,]. These findings provide mechanistic support for the biological plausibility of the MD’s benefits on healthy aging, although the generalizability remains limited by the high adherence and relatively healthy status of participants. Finally, polyphenol-rich dietary patterns, characterized by high consumption of fruits, vegetables, tea, cocoa, and red wine, have been systematically associated with reduced oxidative stress, inflammation, and vascular aging []. Overall, according to the most relevant geriatric outcomes, evidence for composite Mediterranean-style dietary patterns is strong, supported by multiple complementary study designs that together provide mechanistic, interventional, and epidemiological coherence (Table 2).

Table 2.
Mediterranean dietary patterns and synergistic effects on healthy aging. The following abbreviations are used: “CVD”: Cardiovascular Disease; “RCT”: Randomized Controlled Trial.
5. Sensory Perceptions and Food Preferences Among the Elderly
5.1. Sensory Perceptions in Aged People and MD
The aging process is accompanied by a decline in sensory acuity, as well as the onset of impairments affecting mastication, deglutition, and digestion []. When the perception of the sensory stimuli through the five senses is impaired by a physiological event, such as aging, the resulting loss and distortion of the sensory perception, paralleled by phenomena of sensory-specific satiety [], can lead to less food enjoyment and, in turn, less appetite and food intake, resulting in malnutrition []. The elderly can face undernutrition due to some food category deficiencies, such as protein deficiency; thus, although the age-related reduction in sensory-specific satiety favors a more monotonous diet, it is essential to maintain the variety and the richness in the products they consume []. This highlights the need to design elderly-friendly MD foods addressing such challenges while preserving food appreciation []. Olfactory loss is one of the most common phenomena observed. However, it was observed that older adults affected by this impairment tend to increase their consumption of MD basic food items such as fruits and vegetables while reducing high-fat foods []. A growing tendency to adopt MD principles has also been documented among elderly individuals in Italy [], with age-related increases in the intake of fruits, vegetables, and legumes, particularly pronounced among women.
5.2. The Sensory Science to Support Adherence to MD
Sensory science offers valuable methods for investigating older consumers’ attitudes and preferences and can support the development of satisfactory and palatable foods tailored to this population []. Further improvements can be achieved through advanced technologies that adapt food texture to older adults’ needs. Liu et al., in 2022 [], reported that soft, smooth, and moist foods are more suitable for the elderly. However, despite the expected decrease in olfactory acuity, odorous stimuli should also be considered. A survey conducted on over 65 Italian consumers asked participants to indicate their preferred sensory features for a functional food. The most appealing attributes reported were pleasant odor and flavor, as well as soft, warm, liquid, and thick textures []. This co-creation approach, based on in-depth interviews integrated with on-site testing adapted to older adults’ skills [], can support the introduction of MD foods appreciated by elderly consumers. The age-driven loss of sensitivity generally hurts eating pleasure, an essential component affecting food intake []. Nevertheless, a decreased sensitivity to bitterness with age may improve the appreciation of healthy vegetables such as Brassicaceae (e.g., cabbage, Brussels sprouts) and salad greens []. MD also includes a common use of herbs and spices, which can stimulate sensory perception and increase food appreciation while providing phenolic compounds and other bioactive substances [,]. Adherence to MD was reported to be correlated with aromatic plant consumption, but not with spices []. As reported by Laureati et al. in 2008 [], the elderly show a high interest in foods with enhanced taste and flavor stimuli. Adding flavor enhancers to the cooked meals was found to be effective for improving dietary intake and body weight in elderly nursing home residents []. Mediterranean herbs, including parsley, oregano, dill, and rosemary, were effective when tested for improving flavor intensity and liking of protein-rich foods [], although not clearly correlated to the protein intake. Spices may be effective in improving sensory perception, allowing the potential reduction of salt [,].
Visual stimuli can also help increase food appeal and appetite. Even observing simple food pictures can activate brain regions, near the gustatory areas, involved in taste perception []; moreover, the brilliant colors of fresh Mediterranean products may contribute to making food experiences more enjoyable for older people.
6. The MD Lifestyle as a Social Driver for Age-Friendly Communities
The MD was recently recognized as a healthy and sustainable dietary pattern [] based on local seasonal food, conviviality, food sustainable production and lifestyle []. These principles can be particularly relevant for older adults, who may engage in post-retirement activities such as urban horticulture, providing multiple sensory stimulation [,] and home cooking [], both of which offer recognized health benefits, especially for mental well-being. Horticultural activity in later life can contribute to preserving biodiversity and social sustainability, while also providing individuals with fresh, seasonal products. A garden can serve as a supplement to a self-subsistence strategy for fresh fruit and vegetables [] and implement their consumption []. The MD encourages the cultivation of indigenous species, local cereals, fruit and vegetables, which help preserve the environment and its biodiversity, while sustaining the traditional knowledge of their use [], usually passed down through gardening activities by the elderly. It has been reported that gardening intervention programs for older adults are effective in improving vegetable and fruit consumption in the population []. Moreover, improving access to local food markets that offer MD products may enhance older adults’ ability to maintain a nutritionally adequate dietary pattern. The relationship between the availability of healthy food options within residential neighborhoods and the prevalence of disability and cardiovascular diseases has been investigated [,]; however, further research is needed to study its actual impact, with particular emphasis on MD foods and related environmental factors.
Adherence to the MD principles entails a healthy lifestyle that has been proven to enhance self-realization, control, life enjoyment, and autonomy, essential elements for healthy aging []. MD retains a high socio-cultural food value [] as it is closely linked to the traditions and the cultural, social, and economic value that food has for Mediterranean people. Eating is not just about meeting nutritional and energy needs but also encompasses the care of food preparation, the pleasure in convivial moments, and the enjoyment of family meals. Mediterranean meals are an opportunity for social exchange and communication [], which reduces loneliness, thus promoting psychological well-being and general health []. These are central aspects in elderly nutrition; indeed, eating is strictly connected to the psycho-affective dimension in which the meal is served and strongly impacts food intake and meal pleasure [].
Public interventions aimed at providing affordable and accessible MD foods could positively impact the health of older populations and support broader social sustainability. Indeed, some strategies could be adapted to individuals affected by serious mental illnesses to encourage healthier eating habits []. These interventions involved motivational elements, such as familiarization with local farmers’ markets and guided visits to specific grocery stores. Such initiatives could be delivered through seminars and community events led by healthcare professionals, emphasizing diet, lifestyle, health, and conviviality—core pillars of the MD that contribute to promoting well-being and enhancing quality of life in older adults.
Barriers and Policy Opportunities for Supporting Mediterranean Diet Adherence in Older Populations
The accessibility of MD foods for older adults is often influenced by social and economic constraints. Irz et al., in 2014 [], investigated this issue across four European countries (Italy, Sweden, the United Kingdom, and Finland) and reported no positive association between nutritional health and economic resources. According to the authors, this finding may be considered encouraging, as it underscores the potential of public health policies, particularly those focused on information and education, over purely economic measures. Informational and educational interventions aimed at raising awareness of the health benefits of a balanced diet represent an effective strategy to enhance dietary quality among older populations.
A 12-year scientific literature review (2013–2024) by Colaprico et al. in 2024 [] further highlighted the health benefits associated with adopting the MD, indicating potential savings in healthcare expenditures and therefore suggesting that national public health programs should promote policies that support and encourage this lifestyle.
Additional research by Alves and Perelman, in 2022 [], reported a general increase in MD adherence among the European elderly population, mainly due to a reduction in animal protein consumption and an increase in legume intake. The authors speculated that this trend—more evident among affluent, educated, and healthy individuals—may be related to a more favorable economic background that enables greater consideration of health and environmental factors in food choices. However, these positive developments may also exacerbate dietary and health inequalities, highlighting the need for public interventions to mitigate this risk and, more specifically, to address social deprivation [].
A rational and evidence-based use of institutional catering can also play a significant role in supporting older adults’ adherence to the MD within residential settings, as demonstrated by the HECTOR project []. Subsequent studies have explored approaches to influence food choices among older adults toward more plant-based options, in line with the core principles of the MD []. Among these, the implementation of a nudging strategy—specifically, the introduction of a “dish of the day” option in catering services targeting older consumers in Denmark, France, Italy, and the United Kingdom—has been proven effective, with sensory appreciation emerging as one of the primary determinants of food selection. Overall, the nudging approach appears to be a promising strategy for fostering sustainable and health-oriented dietary behaviors in institutional catering environments.
Furthermore, Zhou et al., in 2018 [], in a broad overview of interventions promoting healthy eating among older adults in Europe and other countries, reported that dietary education, meal-service interventions, and multicomponent strategies are effective in increasing fruit and vegetable intake, diversifying food choices, and improving overall nutritional status. However, a recent literature review by Turner et al. in 2023 [] noted that most research on lifestyle–diet–health interconnections focuses on developing interventions and evaluating their effects on clinical markers, while much less attention is devoted to translating research outcomes into population-level implementation. These findings highlight a clear need for stronger collaboration between researchers, who generate evidence and devise intervention strategies, and the stakeholders, responsible for implementing these measures in real-world settings, to enhance population well-being.
7. Strengths and Limitations
The present study is a narrative review that examines the main benefits associated with adherence to the Mediterranean dietary pattern and lifestyle. Its key strength lies in the interdisciplinary approach adopted, which combines socio-cultural perspectives with biochemical and sensory aspects. Therefore, despite consistent evidence linking Mediterranean dietary patterns to improved aging trajectories, several limitations persist. Definitions of adherence and scoring systems for MD indices vary considerably, introducing heterogeneity across cohorts. Most data derive from observational designs subject to residual confounding, while long-term randomized trials in older adults with multimorbidity are scarce. Pragmatic studies in nursing homes and long-term care facilities are urgently needed to evaluate real-world applicability. Future research should standardize exposure metrics and integrate biomarkers of adherence and functional outcomes.
8. Conclusions
Due to the increase in life expectancy, public health services are becoming increasingly involved in improving the quality of life and extending the number of healthy years. This goal is pursued not only through general healthcare provision but also through specific preventive actions, such as creating physical and institutional environments that support a happy and healthy life []. In this context, eating habits play a crucial role, as an adequate diet was effective in maintaining good mental health in old age [,]. Owing to substantial scientific advances, it is now widely accepted that healthy aging in humans is closely associated with proper nutrition, which often entails avoiding unhealthy foods []. This review showed how the MD principles represent a comprehensive and evidence-based framework for mitigating age-related health decline and promoting well-being in later life while simultaneously fostering international scientific collaboration on the influence of traditional dietary practices on population health outcomes [].
Author Contributions
Conceptualization, S.P., R.T. and S.M.; writing—original draft preparation, S.P., R.T. and C.M.; writing—review and editing, R.V., S.M., M.C., C.M. and R.T.; supervision, S.P. and S.M.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NUTRAGE (Nutrizione, Alimentazione and Invecchiamento Attivo, FOE-2019, DSB. AD004. 271) project of the Italian National Research Council (CNR) and also partially funded by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of the Italian Ministry of University and Research funded by the European Union—Next GenerationEU. Project code PE00000003, Project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The Diet and 15-Year Death Rate in the Seven Countries Study. Am. J. Epidemiol. 2017, 185, 1130–1142. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Bach-Faig, A.; Raidó-Quintana, B. Nutritional and Cultural Aspects of the Mediterranean Diet. Int. J. Vitam. Nutr. Res. 2012, 82, 157–162. [Google Scholar] [CrossRef]
- Caprara, G. Diet and Longevity: The Effects of Traditional Eating Habits on Human Lifespan Extension. Mediterr. J. Nutr. Metab. 2018, 11, 261–294. [Google Scholar] [CrossRef]
- Giuffrè, D.; Giuffrè, A.M. Mediterranean Diet and Health in the Elderly. AIMS Public Health 2023, 10, 568–576. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Ognenoska, V.; Trajkovska-Broach, A. Mediterranean Diet: From Ancient Traditions to Modern Science—A Sustainable Way Towards Better Health, Wellness, Longevity, and Personalized Nutrition. Sustainability 2025, 17, 4187. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Critselis, E.; Panagiotakos, D. Adherence to the Mediterranean Diet and Healthy Ageing: Current Evidence, Biological Pathways, and Future Directions. Crit. Rev. Food Sci. Nutr. 2020, 60, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, G.; Capurso, A.; Capurso, C. Benefits of the Mediterranean Diet in the Elderly Patient; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Rizzoli, R.; Vaona, A.; Demurtas, J.; Crepaldi, G.; Maggi, S. Adherence to a Mediterranean Diet Is Associated with Lower Incidence of Frailty: A Longitudinal Cohort Study. Clin. Nutr. 2018, 37, 1492–1497. [Google Scholar] [CrossRef]
- Bamia, C.; Trichopoulos, D.; Ferrari, P.; Overvad, K.; Bjerregaard, L.; Tjønneland, A.; Halkjær, J.; Clavel-Chapelon, F.; Kesse, E.; Boutron-Ruault, M.-C.; et al. Dietary Patterns and Survival of Older Europeans: The EPIC-Elderly Study (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr. 2007, 10, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, S.; Montalcini, T.; Pujia, A. Mediterranean Diet In Healthy Aging. J. Nutr. Health Aging 2021, 25, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavska, P.; Silva, M.L.; Vicente, F.; Pereira, P. Mediterranean Dietary Pattern for Healthy and Active Aging: A Narrative Review of an Integrative and Sustainable Approach. Nutrients 2024, 16, 1725. [Google Scholar] [CrossRef]
- La Dieta Mediterranea. Available online: https://www.iss.it/-/dieta-mediterranea (accessed on 14 October 2025).
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.-W.; Lee, S.-J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef]
- Capurso, C.; Bellanti, F.; Lo Buglio, A.; Vendemiale, G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients 2019, 12, 35. [Google Scholar] [CrossRef]
- Laffond, A.; Rivera-Picón, C.; Rodríguez-Muñoz, P.M.; Juárez-Vela, R.; Ruiz de Viñaspre-Hernández, R.; Navas-Echazarreta, N.; Sánchez-González, J.L. Mediterranean Diet for Primary and Secondary Prevention of Cardiovascular Disease and Mortality: An Updated Systematic Review. Nutrients 2023, 15, 3356. [Google Scholar] [CrossRef] [PubMed]
- Sarsangi, P.; Salehi-Abargouei, A.; Ebrahimpour-Koujan, S.; Esmaillzadeh, A. Association between Adherence to the Mediterranean Diet and Risk of Type 2 Diabetes: An Updated Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2022, 13, 1787–1798. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Vaziri, Y. The Mediterranean Diet: A Powerful Defense against Alzheimer Disease–A Comprehensive Review. Clin. Nutr. ESPEN 2024, 64, 160–167. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Parisi, A.; Seminara, F.; Vernuccio, L.; Catanese, G.; Barbagallo, M. Mediterranean Diet and Lifestyle in Persons with Mild to Moderate Alzheimer’s Disease. Nutrients 2024, 16, 3421. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. MIND Diet and Cognitive Performance in Older Adults: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2019, 24, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Civille, G.V.; Oftedal, K.N. Sensory Evaluation Techniques—Make “Good for You” Taste “Good”. Physiol. Behav. 2012, 107, 598–605. [Google Scholar] [CrossRef]
- Laureati, M.; Pagliarini, E.; Calcinoni, O.; Bidoglio, M. Sensory Acceptability of Traditional Food Preparations by Elderly People. Food Qual. Prefer. 2006, 17, 43–52. [Google Scholar] [CrossRef]
- Albergamo, A.; Vadalà, R.; Metro, D.; Nava, V.; Bartolomeo, G.; Rando, R.; Macrì, A.; Messina, L.; Gualtieri, R.; Colombo, N.; et al. Physicochemical, Nutritional, Microbiological, and Sensory Qualities of Chicken Burgers Reformulated with Mediterranean Plant Ingredients and Health-Promoting Compounds. Foods 2021, 10, 2129. [Google Scholar] [CrossRef]
- Predieri, S.; Conte, A.; Danza, A.; Gatti, E.; Magli, M.; Maria, D.G. Senior Consumers Involvement in Developing New Fish-Based Foods Through Sequential Hedonic Tests. Curr. Res. Nutr. Food Sci. J. 2017, 5, 66–74. [Google Scholar] [CrossRef]
- Volpe, R.; Predieri, S.; Cianciabella, M.; Daniele, G.M.; Gatti, E.; Magli, M.; Rodinò, P.; Schiavetto, E.; Sotis, G.; Urbinati, S. EWHETA (Eat Well for a HEalthy Third Age) Project: Novel Foods to Improve the Nutrition in the Elderly People. Aging Clin. Exp. Res. 2021, 33, 1353–1358. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole Grain Consumption and Risk of Cardiovascular Disease, Cancer, and All Cause and Cause Specific Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes, Cardiovascular Disease, and Weight Gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R. The Potential Role of Phytochemicals in Wholegrain Cereals for the Prevention of Type-2 Diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Åman, P. Alkylresorcinols as Biomarkers of Whole-Grain Wheat and Rye Intake: Plasma Concentration and Intake Estimated from Dietary Records. Am. J. Clin. Nutr. 2008, 87, 832–838. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive Phytochemicals in Barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Shvachko, N.A.; Loskutov, I.G.; Semilet, T.V.; Popov, V.S.; Kovaleva, O.N.; Konarev, A.V. Bioactive Components in Oat and Barley Grain as a Promising Breeding Trend for Functional Food Production. Molecules 2021, 26, 2260. [Google Scholar] [CrossRef]
- Tian, W.; Zheng, Y.; Wang, W.; Wang, D.; Tilley, M.; Zhang, G.; He, Z.; Li, Y. A Comprehensive Review of Wheat Phytochemicals: From Farm to Fork and Beyond. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2274–2308. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.P.; Kumar, S.; Pandey, A.; Khan, M.K.; Singh, S.K.; Singh, G.P. Wheat Science: Nutritional and Anti-Nutritional Properties, Processing, Storage, Bioactivity, and Product Development; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-000-92349-0. [Google Scholar]
- Wang, B.; Nie, C.; Li, T.; Zhao, J.; Fan, M.; Li, Y.; Qian, H.; Wang, L. Effect of Boiling and Roasting on Phenolic Components and Their Bioaccessibilities of Highland Barley. Food Res. Int. 2022, 162, 112137. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health Benefits of Whole Grain: Effects on Dietary Carbohydrate Quality, the Gut Microbiome, and Consequences of Processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of Nuts and Legumes and Risk of Incident Ischemic Heart Disease, Stroke, and Diabetes: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Glenn, A.J.; Nishi, S.K.; Chiavaroli, L.; Seider, M.; Khan, T.; Bonaccio, M.; Iacoviello, L.; Mejia, S.B.; Jenkins, D.J.A.; et al. Associations between Dietary Pulses Alone or with Other Legumes and Cardiometabolic Disease Outcomes: An Umbrella Review and Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, S308–S319. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of Dietary and Supplemental Lycopene on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased Dietary and Circulating Lycopene Are Associated with Reduced Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds From Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin—Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- O’Connor, D.M.A.; Scarlett, S.; De Looze, C.; O’Halloran, A.M.; Laird, E.; Molloy, A.M.; Clarke, R.; McGarrigle, C.A.; Kenny, R.A. Low Folate Predicts Accelerated Cognitive Decline: 8-Year Follow-up of 3140 Older Adults in Ireland. Eur. J. Clin. Nutr. 2022, 76, 950–957. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin Contributes to the Vascular Protective Effects of Orange Juice: A Randomized Crossover Study in Healthy Volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic Consumption of Flavanone-Rich Orange Juice Is Associated with Cognitive Benefits: An 8-Wk, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Jalili, F.; Moradi, S.; Talebi, S.; Mehrabani, S.; Ghoreishy, S.M.; Wong, A.; Jalalvand, A.R.; Kermani, M.A.H.; Jalili, C.; Jalili, F. The Effects of Citrus Flavonoids Supplementation on Endothelial Function: A Systematic Review and Dose–Response Meta-Analysis of Randomized Clinical Trials. Phytother. Res. 2024, 38, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Galluzzi, S.; Marizzoni, M.; Gatti, E.; Bonfiglio, N.S.; Cattaneo, A.; Epifano, F.; Frisoni, G.B.; Genovese, S.; Geviti, A.; Marchetti, L.; et al. Citrus Supplementation in Subjective Cognitive Decline: Results of a 36-Week, Randomized, Placebo-Controlled Trial. Nutr. J. 2024, 23, 135. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P.; Fakler, P.; Sullivan, T. Effect of Garlic on Blood Pressure: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2008, 8, 13. [Google Scholar] [CrossRef]
- Reinhart, K.M.; Talati, R.; White, C.M.; Coleman, C.I. The Impact of Garlic on Lipid Parameters: A Systematic Review and Meta-Analysis. Nutr. Res. Rev. 2009, 22, 39–48. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front. Nutr. 2018, 5, 122. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut Consumption and Risk of Cardiovascular Disease, Total Cancer, All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of Nut Consumption with Total and Cause-Specific Mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.L.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for Dementia Therapy: Review of Pharmacological Activity and Pilot Tolerability Clinical Trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis spinosa L.): An Updated Review on Its Phytochemistry, Nutritional Value, Traditional Uses, and Therapeutic Potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef] [PubMed]
- Pengelly, A.; Snow, J.; Mills, S.Y.; Scholey, A.; Wesnes, K.; Butler, L.R. Short-Term Study on the Effects of Rosemary on Cognitive Function in an Elderly Population. J. Med. Food 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Faridzadeh, A.; Salimi, Y.; Ghasemirad, H.; Kargar, M.; Rashtchian, A.; Mahmoudvand, G.; Karimi, M.A.; Zerangian, N.; Jahani, N.; Masoudi, A.; et al. Neuroprotective Potential of Aromatic Herbs: Rosemary, Sage, and Lavender. Front. Neurosci. 2022, 16, 909833. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shen, A.; Zhang, T.; Jiang, L.; El-Seedi, H.; Zhang, G.; Sui, X. Legumes as an Alternative Protein Source in Plant-Based Foods: Applications, Challenges, and Strategies. Curr. Res. Food Sci. 2024, 9, 100876. [Google Scholar] [CrossRef]
- Mehany, T.; Olawoye, B.; Popoola-Akinola, O. Exploring Bioactive Compounds and Biological Functions of Underutilized Legumes: Advancing the Development of Ideal Plant-Based Milks. J. Future Foods, 2025; in press. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Grdeń, P.; Jakubczyk, A. Health Benefits of Legume Seeds. J. Sci. Food Agric. 2023, 103, 5213–5220. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-Chemical and Functional Properties of Legume Protein, Starch, and Dietary Fiber—A Review. Legume Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Angeloni, S.; Borsetta, G.; Caprioli, G.; Nzekoue, F.K.; Sagratini, G.; Vittori, S. Polyphenols, Saponins and Phytosterols in Lentils and Their Health Benefits: An Overview. Pharmaceuticals 2022, 15, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-S.; Contador, C.A.; Ng, M.-S.; Yu, J.; Chung, G.; Lam, H.-M. The Effects of Domestication on Secondary Metabolite Composition in Legumes. Front. Genet. 2020, 11, 581357. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, R.G.; Olivares-Ochoa, X.C.; Salinas-Varela, Y.; Guajardo-Espinoza, D.; Roldán-Flores, L.G.; Rivera-Leon, E.A.; López-Quintero, A. Phenolic Compounds and Anthocyanins in Legumes and Their Impact on Inflammation, Oxidative Stress, and Metabolism: Comprehensive Review. Molecules 2025, 30, 174. [Google Scholar] [CrossRef]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive Compounds in Legumes: Implications for Sustainable Nutrition and Health in the Elderly Population. Trends Food Sci. Technol. 2021, 117, 139–147. [Google Scholar] [CrossRef]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, R.; Kumar, S.; Saini, R.K.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A Concise Review on Food Related Aspects, Applications and Health Implications. Food Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Antioxidant, Anti-Aging and Organ Protective Effects of Total Saponins from Aralia Taibaiensis. Drug Des. Devel. Ther. 2021, 15, 4025–4042. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Tamburino, R.; Severino, V.; Sandomenico, A.; Ruvo, M.; Parente, A.; Chambery, A.; Maro, A.D. De Novo Sequencing and Characterization of a Novel Bowman–Birk Inhibitor from Lathyrus sativus L. Seeds by Electrospray Mass Spectrometry. Mol. BioSyst. 2012, 8, 3232–3241. [Google Scholar] [CrossRef]
- Losso, J.N. The Biochemical and Functional Food Properties of the Bowman-Birk Inhibitor. Crit. Rev. Food Sci. Nutr. 2008, 48, 94–118. [Google Scholar] [CrossRef]
- Birk, Y. The Bowman-Birk inhibitor. Trypsin- and chymotrypsin-inhibitor from soybeans. Int. J. Pept. Protein Res. 1985, 25, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of Lycopene as Antioxidant Carotenoid in the Prevention of Chronic Diseases: A Review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato Lycopene and Its Role in Human Health and Chronic Diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Dalbeni, A.; Treggiari, D.; Tagetti, A.; Bevilaqua, M.; Bonafini, S.; Montagnana, M.; Scaturro, G.; Minuz, P.; Fava, C. Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients 2018, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, W.; Li, D.; Chan, A.S.C. Evaluation of Nutritional Compositions, Bioactive Components, and Antioxidant Activity of Three Cherry Tomato Varieties. Agronomy 2023, 13, 637. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato Pomace Food Waste from Different Variants as a High Antioxidant Potential Resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Whelan, S.; Luther, V.; Horgan, G.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of Tomato Extract on Platelet Function: A Double-Blinded Crossover Study in Healthy Humans. Am. J. Clin. Nutr. 2006, 84, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, K.; Crosbie, L.; Margaret, J.; Gordon, A. Effects of Tomato Extract on Human Platelet Aggregation In Vitro. Platelets 2001, 12, 218–227. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and Bioactives in Green Leafy Vegetables and Cognitive Decline. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, G.-S. Potential Role of Inflammasomes in Aging. Int. J. Mol. Sci. 2025, 26, 6768. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Li, F.; Lei, X.; Ge, L.; Li, H.; Zhao, N.; Ming, J. The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods 2024, 13, 3507. [Google Scholar] [CrossRef]
- Baldelli, S.; Lombardo, M.; D’Amato, A.; Karav, S.; Tripodi, G.; Aiello, G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods 2025, 14, 912. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierro, J.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The Gastrointestinal Behavior of Saponins and Its Significance for Their Bioavailability and Bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2024, 29, 113. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Zaborska, A.; Łaptaś, A.; Olech, Z. A Study of the Inhibition of Jack Bean Urease by Garlic Extract. Food Chem. 2004, 85, 553–558. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Tang, Y.; Lv, D.; Tao, Y.; Wang, J. The Therapeutic Effects of Natural Organosulfur Compounds on Atherosclerosis and Their Potential Mechanisms: A Comprehensive Review. Front. Cardiovasc. Med. 2025, 12, 1599154. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R., Jr. Health Benefits of Nuts: Potential Role of Antioxidants. Br. J. Nutr. 2006, 96, S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of Nuts and Their Potential Health Benefits—An Overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Arnesen, E.K.; Thorisdottir, B.; Bärebring, L.; Söderlund, F.; Nwaru, B.I.; Spielau, U.; Dierkes, J.; Ramel, A.; Lamberg-Allardt, C.; Åkesson, A. Nuts and Seeds Consumption and Risk of Cardiovascular Disease, Type 2 Diabetes and Their Risk Factors: A Systematic Review and Meta-Analysis. Food Nutr. Res. 2023, 67, 10.29219/fnr.v67.8961. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Lorenzon dos Santos, J.; Schaan de Quadros, A.; Weschenfelder, C.; Bueno Garofallo, S.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Youssef, R.; Najm, D.B.; Al-Bourji, M.; Boutros, P.H. Association of the Mediterranean Diet with Mental Well-Being: A Cross-Sectional Study among Adults Living in Lebanon. BMC Public Health 2025, 25, 2689. [Google Scholar] [CrossRef]
- Scholey, A.B.; Tildesley, N.T.J.; Ballard, C.G.; Wesnes, K.A.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An Extract of Salvia (Sage) with Anticholinesterase Properties Improves Memory and Attention in Healthy Older Volunteers. Psychopharmacology 2008, 198, 127–139. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia Spp. Plants-from Farm to Food Applications and Phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- de Macedo, L.M.; dos Santos, É.M.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia Rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of Ultrastructural Changes and the Anticarcinogenic Effects of Thymol and Carvacrol on Ovarian Cancer Cells: Which Is More Effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol Promotes Cell Cycle Arrest and Apoptosis through PI3K/AKT Signaling Pathway in MCF-7 Breast Cancer Cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Tapsell, L.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.; et al. Health Benefits of Herbs and Spices: The Past, the Present, the Future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Jackson, P.A.; Wightman, E.L.; Veasey, R.; Forster, J.; Khan, J.; Saunders, C.; Mitchell, S.; Haskell-Ramsay, C.F.; Kennedy, D.O. A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. Nutrients 2020, 12, 2254. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R.; Walasek-Janusz, M. Chemical Composition, Biological Activity, and Potential Uses of Oregano (Origanum vulgare L.) and Oregano Essential Oil. Pharmaceuticals 2025, 18, 267. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Mena, P.; Meschi, T. The Interaction between Mediterranean Diet and Intestinal Microbiome: Relevance for Preventive Strategies against Frailty in Older Individuals. Aging Clin. Exp. Res. 2024, 36, 58. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean Diet and Health Status: An Updated Meta-Analysis and a Proposal for a Literature-Based Adherence Score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Berendsen, A.A.M.; Van de Rest, O.; Feskens, E.J.M.; Santoro, A.; Ostan, R.; Pietruszka, B.; Brzozowska, A.; Stelmaszczyk-Kusz, A.; Jennings, A.; Gillings, R.; et al. Changes in Dietary Intake and Adherence to the NU-AGE Diet Following a One-Year Dietary Intervention among European Older Adults—Results of the NU-AGE Randomized Trial. Nutrients 2018, 10, 1905. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Doets, E.L.; Kremer, S. The Silver Sensory Experience—A Review of Senior Consumers’ Food Perception, Liking and Intake. Food Qual. Prefer. 2016, 48, 316–332. [Google Scholar] [CrossRef]
- Rolls, B.; McDermott, T. Effects of Age on Sensory-Specific Satiety. Am. J. Clin. Nutr. 1991, 54, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Chen, J. The Eating Capability: Constituents and Assessments. Food Qual. Prefer. 2016, 48, 345–358. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, L.; Dong, Q.; Hu, X.; Yang, Y.; Liu, T.; Wu, B.; Shan, B.; Yin, C.; et al. Sensory Insights in Aging: Exploring the Impact on Improving Dietary Through Sensory Enhancement. Food Sci. Nutr. 2025, 13, e70074. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Wang, J.; Xu, X. Food for the Elderly Based on Sensory Perception: A Review. Curr. Res. Food Sci. 2022, 5, 1550–1558. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The Influence of Olfactory Loss on Dietary Behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Predieri, S.; Sotis, G.; Rodinò, P.; Gatti, E.; Magli, M.; Rossi, F.; Daniele, G.M.; Cianciabella, M.; Volpe, R. Older Adults’ Involvement in Developing Satisfactory Pasta Sauces with Healthy Ingredients. Br. Food J. 2018, 120, 804–814. [Google Scholar] [CrossRef]
- Maitre, I.; Symoneaux, R.; Sulmont-Rossé, C. 23-Sensory Testing in New Product Development: Working with Older People. In Rapid Sensory Profiling Techniques; Delarue, J., Lawlor, J.B., Rogeaux, M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; pp. 485–508. ISBN 978-1-78242-248-8. [Google Scholar]
- Daniele, G.M.; Medoro, C.; Lippi, N.; Cianciabella, M.; Magli, M.; Predieri, S.; Versari, G.; Volpe, R.; Gatti, E. Exploring Eating Habits, Healthy Food Awareness, and Inclination Toward Functional Foods of Italian Elderly People Through Computer-Assisted Telephone Interviews (CATIs). Nutrients 2024, 16, 762. [Google Scholar] [CrossRef]
- Grunert, K.G.; Dean, M.; Raats, M.M.; Nielsen, N.A.; Lumbers, M. A Measure of Satisfaction with Food-Related Life. Appetite 2007, 49, 486–493. [Google Scholar] [CrossRef]
- Subar, A.F.; Heimendinger, J.; Patterson, B.H.; Krebs-Smith, S.M.; Pivonka, E.; Kessler, R. Fruit and Vegetable Intake in the United States: The Baseline Survey of the Five a Day for Better Health Program. Am. J. Health Promot. 1995, 9, 352–360. [Google Scholar] [CrossRef]
- Delgado, A.; Gonçalves, S.; Romano, A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods 2023, 12, 840. [Google Scholar] [CrossRef]
- Bower, A.; Marquez, S.; de Mejia, E.G. The Health Benefits of Selected Culinary Herbs and Spices Found in the Traditional Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef]
- Louro, T.; Simões, C.; Castelo, P.M.; Capela e Silva, F.; Luis, H.; Moreira, P.; Lamy, E. How Individual Variations in the Perception of Basic Tastes and Astringency Relate with Dietary Intake and Preferences for Fruits and Vegetables. Foods 2021, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Laureati, M.; Pagliarini, E.; Calcinoni, O. Does the Enhancement of Chemosensory Stimuli Improve the Enjoyment of Food in Institutionalized Elderly People? J. Sens. Stud. 2008, 23, 234–250. [Google Scholar] [CrossRef]
- Mathey, M.-F.A.M.; Siebelink, E.; de Graaf, C.; Van Staveren, W.A. Flavor Enhancement of Food Improves Dietary Intake and Nutritional Status of Elderly Nursing Home Residents. J. Gerontol. Ser. A 2001, 56, M200–M205. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Breen, J.A.; Pan, Z. Effects of Culinary Spices on Liking and Consumption of Protein Rich Foods in Community-Dwelling Older Adults. Nutrients 2023, 15, 1172. [Google Scholar] [CrossRef]
- Dougkas, A.; Vannereux, M.; Giboreau, A. The Impact of Herbs and Spices on Increasing the Appreciation and Intake of Low-Salt Legume-Based Meals. Nutrients 2019, 11, 2901. [Google Scholar] [CrossRef]
- Ghawi, S.K.; Rowland, I.; Methven, L. Enhancing Consumer Liking of Low Salt Tomato Soup over Repeated Exposure by Herb and Spice Seasonings. Appetite 2014, 81, 20–29. [Google Scholar] [CrossRef]
- Simmons, W.K.; Martin, A.; Barsalou, L.W. Pictures of Appetizing Foods Activate Gustatory Cortices for Taste and Reward. Cereb. Cortex 2005, 15, 1602–1608. [Google Scholar] [CrossRef]
- Real, H.; Dias, R.R.; Graça, P. Mediterranean Diet Conceptual Model and Future Trends of Its Use in Portugal. Health Promot. Int. 2021, 36, 548–560. [Google Scholar] [CrossRef]
- Koura, S.; Oshikawa, T.; Ogawa, N.; Snyder, S.M.; Nagatomo, M.; Nishikawa, C. Utilization of Horticultural Therapy for Elderly Persons in the Urban Environment. Acta Hortic. 2010, 881, 865–868. [Google Scholar] [CrossRef]
- Koura, S.; Okawa, H.; Oshikawa, T.; Ueda, T.; Nishikawa, C.; Ikeda, A.; Kamijyo, K. Dementia Protective Efficacy by the Combination of Active and Passive Horticultural Therapy for All Persons Concerned. Acta Hortic. 2018, 1215, 223–232. [Google Scholar] [CrossRef]
- Mills, S.; Brown, H.; Wrieden, W.; White, M.; Adams, J. Frequency of Eating Home Cooked Meals and Potential Benefits for Diet and Health: Cross-Sectional Analysis of a Population-Based Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Zschippig, C.; Kluss, T. Gardening in Ambient Assisted Living. Urban For. Urban Green. 2016, 15, 186–189. [Google Scholar] [CrossRef]
- Alaimo, K.; Packnett, E.; Miles, R.A.; Kruger, D.J. Fruit and Vegetable Intake among Urban Community Gardeners. J. Nutr. Educ. Behav. 2008, 40, 94–101. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; Vecchia, C.L.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean Diet with Four Sustainable Benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef]
- Sommerfeld, A.J.; McFarland, A.L.; Waliczek, T.M.; Zajicek, J.M. Growing Minds: Evaluating the Relationship between Gardening and Fruit and Vegetable Consumption in Older Adults. HortTechnology 2010, 20, 711–717. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Kingston, A.; Houlden, V.; Franklin, R. The Longitudinal Associations between Proximity to Local Grocery Shops and Functional Ability in the Very Old Living with and without Multimorbidity: Results from the Newcastle 85+ Study. Arch. Gerontol. Geriatr. 2022, 101, 104703. [Google Scholar] [CrossRef]
- Bhatia, R.; Hernandez, M.A.; Platt, J.; Newman, A.B.; Siscovick, D.S.; Mukamal, K.J.; Lovasi, G.S. Associations of Neighbourhood Food Retail with Disability and Death in Older Adults: Cardiovascular Health Study. BMJ Nutr. Prev. Health 2024, 7, e000646. [Google Scholar] [CrossRef]
- Maltarić, M.; Ruščić, P.; Kolak, M.; Bender, D.V.; Kolarić, B.; Ćorić, T.; Hoejskov, P.S.; Bošnir, J.; Kljusurić, J.G. Adherence to the Mediterranean Diet Related to the Health Related and Well-Being Outcomes of European Mature Adults and Elderly, with an Additional Reference to Croatia. Int. J. Environ. Res. Public Health 2023, 20, 4893. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Bernardi, E.; Visioli, F. Fostering Wellbeing and Healthy Lifestyles through Conviviality and Commensality: Underappreciated Benefits of the Mediterranean Diet. Nutr. Res. 2024, 126, 46–57. [Google Scholar] [CrossRef]
- Divert, C.; Laghmaoui, R.; Crema, C.; Issanchou, S.; Wymelbeke, V.V.; Sulmont-Rossé, C. Improving Meal Context in Nursing Homes. Impact of Four Strategies on Food Intake and Meal Pleasure. Appetite 2015, 84, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Farland, G.; Cohen, D.; Gerstler, C.; Margolies, P.; Pope, L.; Rotter, M.; Compton, M.T. A Group-Based, Six-Lesson Healthy Eating Curriculum for Individuals with Serious Mental Illnesses: Development and Implementation. Community Ment. Health J. 2024, 60, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Irz, X.; Fratiglioni, L.; Kuosmanen, N.; Mazzocchi, M.; Modugno, L.; Nocella, G.; Shakersain, B.; Traill, W.B.; Xu, W.; Zanello, G. Sociodemographic Determinants of Diet Quality of the EU Elderly: A Comparative Analysis in Four Countries. Public Health Nutr. 2014, 17, 1177–1189. [Google Scholar] [CrossRef]
- Colaprico, C.; Crispini, D.; Rocchi, I.; Kibi, S.; De Giusti, M.; La Torre, G. Cost and Cost-Effectiveness of the Mediterranean Diet: An Update of a Systematic Review. Nutrients 2024, 16, 1899. [Google Scholar] [CrossRef]
- Alves, R.; Perelman, J. European Mature Adults and Elderly Are Moving Closer to the Mediterranean Diet—A Longitudinal Study, 2013–2019. Eur. J. Public Health 2022, 32, 600–605. [Google Scholar] [CrossRef]
- Tatoli, R.; Lampignano, L.; Donghia, R.; Castellana, F.; Zupo, R.; Bortone, I.; De Nucci, S.; Campanile, G.; Lofù, D.; Vimercati, L.; et al. Dietary Customs and Social Deprivation in an Aging Population From Southern Italy: A Machine Learning Approach. Front. Nutr. 2022, 9, 811076. [Google Scholar] [CrossRef] [PubMed]
- Lachat, C.; Naska, A.; Trichopoulou, A.; Engeset, D.; Fairgrieve, A.; Marques, H.Á.; Kolsteren, P. Essential Actions for Caterers to Promote Healthy Eating out among European Consumers: Results from a Participatory Stakeholder Analysis in the HECTOR Project. Public Health Nutr. 2011, 14, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Perez-Cueto, F.J.A.; Dos Santos, Q.; Bredie, W.L.P.; Molla-Bauza, M.B.; Rodrigues, V.M.; Buch-Andersen, T.; Appleton, K.M.; Hemingway, A.; Giboreau, A.; et al. Promotion of Novel Plant-Based Dishes among Older Consumers Using the ‘Dish of the Day’ as a Nudging Strategy in 4 EU Countries. Food Qual. Prefer. 2019, 75, 260–272. [Google Scholar] [CrossRef]
- Zhou, X.; Perez-Cueto, F.J.A.; Santos, Q.D.; Monteleone, E.; Giboreau, A.; Appleton, K.M.; Bjørner, T.; Bredie, W.L.P.; Hartwell, H. A Systematic Review of Behavioural Interventions Promoting Healthy Eating among Older People. Nutrients 2018, 10, 128. [Google Scholar] [CrossRef]
- Turner, A.; LaMonica, H.M.; Flood, V.M. Behaviour Change Techniques Used in Mediterranean Diet Interventions for Older Adults: A Systematic Scoping Review. Nutrients 2023, 15, 1189. [Google Scholar] [CrossRef]
- Volpe, R.; Sotis, G.; Predieri, S.; Magli, M. Good Mental Health in Old Age Is A Real Possibility. J. Aging Sci. 2017, 5, 168. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kyrozis, A.; Rossi, M.; Katsoulis, M.; Trichopoulos, D.; La Vecchia, C.; Lagiou, P. Mediterranean Diet and Cognitive Decline over Time in an Elderly Mediterranean Population. Eur. J. Nutr. 2015, 54, 1311–1321. [Google Scholar] [CrossRef]
- Vissavajjhala, P. Chapter 1—Impact of Nutrition on Healthy Aging. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–10. ISBN 978-0-12-805376-8. [Google Scholar]
- Maggi, S.; Ecarnot, F.; Gianfredi, V.; Nucci, D.; Veronese, N.; Lei, L.; Hu, M.; Avart, C.; Capurso, A.; Chen, L.; et al. Mediterranean Diet and Cantonese Cuisine for Human Health: Report from a Sino-Italian Bilateral Meeting. Aging Clin. Exp. Res. 2025, 37, 295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).