Novel Anthropometric Indices and Probability of Adequate Nutrient Intake in the Older Polish Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Recruitment
2.2. Data Collection
2.3. Dietary Assessment
- if D/SDD is greater than 1, then there is a lot of confidence that the usual intake of a nutrient is adequate,
- if D/SDD is lower than −1, then it is certain that the usual intake of a nutrient for the analyzed person is inadequate,
- if D/SDD is between −1 and 1, then it cannot be determined with certainty whether the intake of an individual is adequate or inadequate [25].
2.4. Anthropometric Variables
2.5. Statistical Analysis
3. Results
3.1. Probability of Adequate Nutrient Intake
3.2. BRI Patterns and General Characteristics by Clusters
3.3. BRI Patterns, Age, Energy Intake, and Micronutrients Score by Clusters
3.4. BRI Patterns, Socio-Demographic, Physical Activity Level, and Self-Rated Health Status by Clusters
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathers, J.C. Impact of Nutrition on the Ageing Process. Br. J. Nutr. 2015, 113, S18–S22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Senior, A.M.; Legault, V.; Lavoie, F.B.; Presse, N.; Gaudreau, P.; Turcot, V.; Raubenheimer, D.; Le Couteur, D.G.; Simpson, S.J.; Cohen, A.A. Multidimensional Associations between Nutrient Intake and Healthy Ageing in Humans. BMC Biol. 2022, 20, 196. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. Int. Rev. J. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in Older Adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing Is Associated with Decreases in Appetite and Energy Intake—A Meta-Analysis in Healthy Adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Marzetti, E. Anorexia of Aging: Metabolic Changes and Biomarker Discovery. Clin. Interv. Aging 2022, 17, 1761–1767. [Google Scholar] [CrossRef]
- Roberts, H.C.; Lim, S.E.R.; Cox, N.J.; Ibrahim, K. The Challenge of Managing Undernutrition in Older People with Frailty. Nutrients 2019, 11, 808. [Google Scholar] [CrossRef]
- Salari, N.; Darvishi, N.; Bartina, Y.; Keshavarzi, F.; Hosseinian-Far, M.; Mohammadi, M. Global Prevalence of Malnutrition in Older Adults: A Comprehensive Systematic Review and Meta-Analysis. Public Health Pract. 2025, 9, 100583. [Google Scholar] [CrossRef]
- Stoś, K.; Rychlik, E.; Woźniak, A.; Ołtarzewski, M.; Jankowski, M.; Gujski, M.; Juszczyk, G. Prevalence and Sociodemographic Factors Associated with Overweight and Obesity among Adults in Poland: A 2019/2020 Nationwide Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2022, 19, 1502. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity Paradox and Aging. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Morgan, P.T.; Smeuninx, B.; Breen, L. Exploring the Impact of Obesity on Skeletal Muscle Function in Older Age. Front. Nutr. 2020, 7, 569904. [Google Scholar] [CrossRef]
- Puzianowska-Kuznicka, M.; Kurylowicz, A.; Wierucki, L.; Owczarek, A.J.; Jagiello, K.; Mossakowska, M.; Zdrojewski, T.; Chudek, J. Obesity in Caucasian Seniors on the Rise: Is It Truly Harmful? Results of the PolSenior2 Study. Nutrients 2022, 14, 4621. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Tylutka, A.; Morawin, B.; Walas, Ł.; Michałek, M.; Gwara, A.; Zembron-Lacny, A. Assessment of Metabolic Syndrome Predictors in Relation to Inflammation and Visceral Fat Tissue in Older Adults. Sci. Rep. 2023, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between Body Roundness with Body Fat and Visceral Adipose Tissue Emerging from a New Geometrical Model: Body Roundness with Body Fat & Visceral Adipose Tissue. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, Z.; Gong, H.; Xu, T. The Association between Body Roundness Index and Sarcopenia in Older Adults: A Population-Based Study. Front. Public Health 2025, 13, 1554491. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Chen, M.; Lin, Z.; Hu, Y.; Luo, X. Association between Body Roundness Index and Frailty in Older Americans: A Cross-Sectional Study of NHANES 2007–2018. Front. Nutr. 2025, 12, 1534464. [Google Scholar] [CrossRef]
- Yang, W.; Chen, L.; Tong, L.; He, W.; Lin, H. Association between Body Roundness Index and Depression Among Middle-Aged and Older Adults in Chinese Communities: An Empirical Analysis Based on CHARLS Data. PLoS ONE 2025, 20, e0320139. [Google Scholar] [CrossRef]
- Guo, D.; Li, T.; Yang, Q.; Yang, C.; Yang, Y.; Liu, F.; Ma, J.; Tu, J.; Ning, X.; Wang, J.; et al. Relationship between Body Roundness Index and Cognitive Impairment in Middle-Aged and Older Adults: A Population-Based Cross-Sectional Study. Front. Aging Neurosci. 2025, 17, 1522989. [Google Scholar] [CrossRef]
- Kong, Y.; Luo, Q.; Zhang, Q.; Wei, Q. Association of the Body Roundness Index with New-Onset Cardiovascular Disease in Middle-Aged and Older Adults with and without Diabetes: Evidence from the China Health and Retirement Longitudinal Study. Diabetol. Metab. Syndr. 2025, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Bledowski, P.; Mossakowska, M.; Chudek, J.; Grodzicki, T.; Milewicz, A.; Szybalska, A.; Wieczorowska-Tobis, K.; Wiecek, A.; Bartoszek, A.; Dabrowski, A.; et al. Medical, Psychological and Socioeconomic Aspects of Aging in Poland. Exp. Gerontol. 2011, 46, 1003–1009. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Gronowska-Senger, A. Methodical Guide to Nutrition Habits Research; Human Nutrition Committee of the Polish Academy of Sciences: Warsaw, Poland, 2013. (In Polish) [Google Scholar]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M.; Charzewska, J. (Eds.) Instructions for the Use of the 5.0 Diet Program for Planning and Ongoing Evaluation of Individual and Collective Nutrition in Methodical Guide of Dietary Research; National Food and Nutrition Institute: Warsaw, Poland, 2015. (In Polish) [Google Scholar]
- Banna, J.C.; McCrory, M.A.; Fialkowski, M.K.; Boushey, C. Examining Plausibility of Self-Reported Energy Intake Data: Considerations for Method Selection. Front. Nutr. 2017, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojska, H. Dietary Reference Values for the Polish Population; National Institute of Public Health NIH—National Institute of Public Health: Warsaw, Poland, 2024. (In Polish) [Google Scholar]
- WHO Expert Committee on Physical Status. The Use and Interpretation of Anthropometry Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio. In Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-Height Ratio Is a Better Screening Tool than Waist Circumference and BMI for Adult Cardiometabolic Risk Factors: Systematic Review and Meta-Analysis: Waist-to-Height Ratio as a Screening Tool. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Ashwell, M.; Hsieh, S.D. Six Reasons Why the Waist-to-Height Ratio Is a Rapid and Effective Global Indicator for Health Risks of Obesity and How Its Use Could Simplify the International Public Health Message on Obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, J.; Liang, W.; Yang, X.; Ning, W.; Zhao, Z.; Chen, J.; He, Q. Association of Body Roundness Index with Cardiovascular Disease in Patients with Cardiometabolic Syndrome: A Cross-Sectional Study Based on NHANES 2009–2018. Front. Endocrinol. 2025, 16, 1524352. [Google Scholar] [CrossRef]
- Pratt, J.; Narici, M.; Boreham, C.; De Vito, G. Dual-Energy X-Ray Absorptiometry Derived Body Composition Trajectories across Adulthood: Reference Values and Associations with Body Roundness Index and Body Mass Index. Clin. Nutr. 2025, 46, 137–146. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Fallahtafti, P.; Habibzadeh, A.; Ezzatollahi Tanha, A.; Alamdari, A.A.; Fallahtafti, P.; Shafi Kuchay, M. Effectiveness of Body Roundness Index for the Prediction of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2025, 24, 117. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H. The Association of Body Roundness Index and Body Mass Index with Frailty and All-Cause Mortality: A Study from the Population Aged 40 and above in the United States. Lipids Health Dis. 2025, 24, 30. [Google Scholar] [CrossRef]
- Gu, J.; Bao, Y.; Li, Y.; Chen, X.; Yuan, S.; Zhang, Y.; Pan, H.; Deng, X.; Han, L.; Ran, J. Body Roundness Index and Mental Health in Middle-Aged and Elderly Adults: A Prospective Cohort Study. Sci. Rep. 2025, 15, 22994. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; Lacey, S.; Arsenic, T.; McCarthy, A.L. The Nutritional Status and Dietary Intake of Free-Living Seniors: A Cross-Sectional Study. Clin. Nutr. ESPEN 2021, 43, 478–486. [Google Scholar] [CrossRef]
- Hengeveld, L.M.; Wijnhoven, H.A.; Olthof, M.R.; Brouwer, I.A.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Visser, M. Prospective Associations of Poor Diet Quality with Long-Term Incidence of Protein-Energy Malnutrition in Community-Dwelling Older Adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2018, 107, 155–164. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.Y. Identification of Hemoglobin Levels Based on Anthropometric Indices in Elderly Koreans. PLoS ONE 2016, 11, e0165622. [Google Scholar] [CrossRef] [PubMed]
- Sygnowska, E.; Waśkiewicz, A. Ocena Sposobu Żywienia Osób w Wieku 60-74 Lat. Badanie WOBASZ. Bromat.Chem.Toksykol. 2011, 44, 240–244. [Google Scholar]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping Low Intake of Micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef]
- Roman Viñas, B.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; De Groot, L.C.P.G.M.; Van’T Veer, P.; Matthys, C.; Serra Majem, L. Projected Prevalence of Inadequate Nutrient Intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Morales-Gurrola, G.; Preza-Rodríguez, L.; Gómez-Barrientos, A.; Olivas-Martínez, A.I.; Simental-Mendía, L.E. Magnesium Intake Is Associated with the Metabolically Healthy Obese Phenotype. J. Investig. Med. 2022, 70, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.; Ashton, L.M.; Lee, D.C.W.; Collins, C.E. Gender Differences in Diet Quality and the Association between Diet Quality and BMI: An Analysis in Young Australian Adults Who Completed the Healthy Eating Quiz. J. Hum. Nutr. Diet. 2024, 37, 943–951. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef]
- Huang, J.-H.; Lu, Y.-F.; Cheng, F.-C.; Lee, J.N.-Y.; Tsai, L.-C. Correlation of Magnesium Intake with Metabolic Parameters, Depression and Physical Activity in Elderly Type 2 Diabetes Patients: A Cross-Sectional Study. Nutr. J. 2012, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.; Chan, T.Y.; Chu, Y.K.; Ling, J.; He, J.; Leung, K.; Ma, R.C.W.; Chan, J.C.N.; Chow, E. Higher Dietary Magnesium and Potassium Intake Are Associated with Lower Body Fat in People with Impaired Glucose Tolerance. Front. Nutr. 2023, 10, 1169705. [Google Scholar] [CrossRef]
- Wang, Q.; Si, K.; Xing, X.; Ye, X.; Liu, Z.; Chen, J.; Tang, X. Association between Dietary Magnesium Intake and Muscle Mass among Hypertensive Population: Evidence from the National Health and Nutrition Examination Survey. Nutr. J. 2024, 23, 37. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.-T.; Welch, A.A. Cross-Sectional Associations of Dietary and Circulating Magnesium with Skeletal Muscle Mass in the EPIC-Norfolk Cohort. Clin. Nutr. 2019, 38, 317–323. [Google Scholar] [CrossRef]
- Mlodzik-Czyzewska, M.A.; Malinowska, A.M.; Chmurzynska, A. Low Folate Intake and Serum Levels Are Associated with Higher Body Mass Index and Abdominal Fat Accumulation: A Case Control Study. Nutr. J. 2020, 19, 53. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Lacher, D.A.; Zhang, M.; Johnson, C.L.; Hamner, H.C.; Bailey, R.L.; Rader, J.I.; Yamini, S.; et al. Folate Status and Concentrations of Serum Folate Forms in the US Population: National Health and Nutrition Examination Survey 2011–2. Br. J. Nutr. 2015, 113, 1965–1977. [Google Scholar] [CrossRef]
- Mahabir, S.; Ettinger, S.; Johnson, L.; Baer, D.J.; Clevidence, B.A.; Hartman, T.J.; Taylor, P.R. Measures of Adiposity and Body Fat Distribution in Relation to Serum Folate Levels in Postmenopausal Women in a Feeding Study. Eur. J. Clin. Nutr. 2008, 62, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; Zenke-Philippi, C.; Neuhäuser-Berthold, M. Dynamics and Interactions of Cobalamin and Folate Status during Advanced Aging—A Longitudinal Study in a Community-Dwelling Cohort with Multiple Follow-Ups. Nutr. J. 2020, 9, 64. [Google Scholar] [CrossRef]
- Soysal, P.; Smith, L.; Capar, E.; Kalan, U.; Arik, F.; Isik, A.T. Vitamin B12 and Folate Deficiencies Are Not Associated with Nutritional or Weight Status in Older Adults. Exp. Gerontol. 2019, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oliai Araghi, S.; Braun, K.V.E.; Van Der Velde, N.; Van Dijk, S.C.; Van Schoor, N.M.; Zillikens, M.C.; De Groot, L.C.P.G.M.; Uitterlinden, A.G.; Stricker, B.H.; Voortman, T.; et al. B-Vitamins and Body Composition: Integrating Observational and Experimental Evidence from the B-PROOF Study. Eur. J. Nutr. 2020, 59, 1253–1262. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenic Obesity: The Confluence of Two Epidemics. Obes. Res. 2004, 12, 887–888. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, X.; Wang, X.; Huang, S.; Xu, J.; Xin, C.; Li, Z.; Wang, Y.; Ye, Y.; Liu, S.; et al. Prognostic Effect of Body Roundness Index on All-Cause Mortality among US Older Adults. Sci. Rep. 2025, 15, 17843. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Wells, J.C.K.; Smith, S.R.; Stephan, B.C.M.; Siervo, M. Sarcopenic Obesity: A Critical Appraisal of the Current Evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Kaluźniak-Szymanowska, A.; Kaczmarek, B.; Kujawska-Danecka, H.; Klich-Rączka, A.; Mossakowska, M.; Małgorzewicz, S.; Dworak, L.B.; Kostka, T.; et al. Socioeconomic Risk Factors of Poor Nutritional Status in Polish Elderly Population: The Results of PolSenior2 Study. Nutrients 2021, 13, 4388. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.M.; Bolland, M.J.; Gamble, G.D.; Leung, W.; Reid, I.R. Dietary Calcium Intake and Change in Bone Mineral Density in Older Adults: A Systematic Review of Longitudinal Cohort Studies. Eur. J. Clin. Nutr. 2022, 76, 196–205. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium Intake and Bone Mineral Density: Systematic Review and Meta-Analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef]
- Bredariol, A.N.M.; Rossato, L.T.; De Branco, F.M.S.; Nahas, P.C.; Orsatti, F.L.; De Oliveira, E.P. Calcium Intake Is Inversely Associated with Body Fat in Postmenopausal Women. Clin. Nutr. ESPEN 2020, 39, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.J.; Shaw, S.E.; Carothers, L. Association of Calcium Intake and Adiposity in Postmenopausal Women. J. Am. Coll. Nutr. 2008, 27, 260–266. [Google Scholar] [CrossRef]
- Choi, M.-K.; Bae, Y.-J. Dietary Calcium, Phosphorus, and Osteosarcopenic Adiposity in Korean Adults Aged 50 Years and Older. Arch. Osteoporos. 2021, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of Increasing Dietary Calcium through Supplements and Dairy Food on Body Weight and Body Composition: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2015, 114, 1013–1025. [Google Scholar] [CrossRef]
- Vázquez-Lorente, H.; Ni, J.; Babio, N.; García-Arellano, A.; Romaguera, D.; Martínez, J.A.; Estruch, R.; Sánchez, V.M.; Vidal, J.; Fitó, M.; et al. Dietary Vitamin D Intake and Changes in Body Composition over Three Years in Older Adults with Metabolic Syndrome. J. Nutr. Health Aging 2025, 29, 100467. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Kang, H.-T.; Lee, D.-C.; Lee, H.-R.; Lee, Y.-J. Body Composition and Its Association with Cardiometabolic Risk Factors in the Elderly: A Focus on Sarcopenic Obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kondo, Y.; Fukuda, T.; Kitagawa, N.; Okada, H.; et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients 2021, 13, 2335. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Ebeling, P.R.; Sanders, K.M.; Aitken, D.; Winzenberg, T.; Jones, G. Vitamin D and Physical Activity Status: Associations With Five-Year Changes in Body Composition and Muscle Function in Community-Dwelling Older Adults. J. Clin. Endocrinol. Metab. 2015, 100, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Murata, E.M.; Yu, C.; Danik, J.; Kotler, G.; Cook, N.R.; Bubes, V.; Mora, S.; Chandler, P.D.; Tobias, D.K.; et al. Effects of Vitamin D3 Supplementation on Body Composition in the VITamin D and OmegA-3 TriaL (VITAL). J. Clin. Endocrinol. Metab. 2021, 106, 1377–1388. [Google Scholar] [CrossRef]
- Mertens, E.; Kuijsten, A.; Dofková, M.; Mistura, L.; D’Addezio, L.; Turrini, A.; Dubuisson, C.; Favret, S.; Havard, S.; Trolle, E.; et al. Geographic and Socioeconomic Diversity of Food and Nutrient Intakes: A Comparison of Four European Countries. Eur. J. Nutr. 2019, 58, 1475–1493. [Google Scholar] [CrossRef]
- Rippin, H.; Hutchinson, J.; Jewell, J.; Breda, J.; Cade, J. Adult Nutrient Intakes from Current National Dietary Surveys of European Populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Men n = 779 | Women n = 753 | p-Value 1 | 55–65 Years n = 283 | 66–75 Years n = 570 | >75 Years n = 679 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Macronutrient | |||||||
| Energy (kcal) | 1761 ± 517.9 | 1494 ± 432.9 | <0.001 | 1744 ± 541.1 a | 1640 ± 498.0 b | 1573 ± 465.8 b | <0.001 |
| Protein (g) | 70.9 ± 20.2 | 59.7 ± 17.0 | <0.001 | 69.6 ± 20.7 a | 65.9 ± 19.3 b | 63.2 ± 18.8 c | <0.001 |
| Carbohydrate (g) | 238.7 ± 74.4 | 210.3 ± 64.5 | <0.001 | 234.7 ± 74.9 a | 227.3 ± 74.5 ab | 218.4 ± 65.9 b | 0.013 |
| Fat (g) | 64.9 ± 26.5 | 52.7 ± 20.6 | <0.001 | 65.7 ± 28.4 a | 59.1 ± 23.8 b | 55.9 ± 23.0 c | <0.001 |
| SFA (g) | 24.5 ± 10.6 | 20.0 ± 9.1 | <0.001 | 24.5 ± 11.3 a | 22.1 ± 10.2 b | 21.5 ± 9.4 b | <0.001 |
| MUFA (g) | 26.5 ± 12.1 | 21.1 ± 9.0 | <0.001 | 27.1 ± 13.3 a | 24.0 ± 10.4 b | 22.4 ± 10.2 c | <0.001 |
| PUFA (g) | 9.1 ± 4.6 | 7.6 ± 3.5 | <0.001 | 9.3 ± 5.0 a | 8.7 ± 4.1 a | 7.8 ± 3.8 b | <0.001 |
| Dietary fiber (g) | 18.8 ± 7.4 | 16.9 ± 6.0 | <0.001 | 19.0 ± 7.0 a | 18.8 ± 7.2 a | 16.6 ± 6.2 b | <0.001 |
| Nutrients | Sex | Age and Sex-Specific EAR | Dietary Intake | p-Value 1 | D/SDD > 1 2 | D/SDD ≤1 and ≥−1 3 | D/SDD < −1 4 | p-Value 5 |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n (%) | |||||||
| Vitamin A (µg) | M | 630 | 1050 ± 1024 | 0.289 | 196 (25.2) | 583 (74.8) | 0 (0) | 0.274 |
| W | 500 | 997 ± 882 | 208 (27.6) | 545 (72.4) | 0 (0) | |||
| Vitamin C (mg) | M | 75 | 65.6 ± 54.1 | 0.019 | 107 (13.7) | 366 (47.0) | 306 (39.3) | <0.001 |
| W | 60 | 69.6 ± 51.1 | 177 (23.5) | 441 (58.6) | 135 (17.9) | |||
| Thiamine (mg) | M | 1.1 | 1.13 ± 0.4 | <0.001 | 178 (22.8) | 412 (52.9) | 189 (24.3) | 0.046 |
| W | 0.9 | 0.95 ± 0.3 | 172 (22.8) | 436 (57.9) | 145 (19.3) | |||
| Riboflavin (mg) | M | 1.1 | 1.48 ± 0.5 | <0.001 | 369 (47.4) | 369 (47.4) | 41 (5.2) | 0.002 |
| W | 0.9 | 1.33 ± 0.5 | 393 (52.2) | 344 (45.7) | 16 (2.1) | |||
| Niacin (mg) | M | 12 | 16.27 ± 6.4 | <0.001 | 361 (46.3) | 384 (49.3) | 34 (4.4) | 0.001 |
| W | 11 | 13.94 ± 5.5 | 279 (37.1) | 431 (57.2) | 43 (5.7) | |||
| Vitamin B6 (mg) | M | 1.4 | 1.65 ± 0.5 | <0.001 | 312 (40.0) | 383 (49.2) | 84 (10.8) | 0.048 |
| W | 1.3 | 1.48 ± 0.5 | 256 (34.0) | 410 (54.4) | 87 (11.6) | |||
| Vitamin B12 (µg) | M | 2.0 | 4.45 ± 4.2 | <0.001 | 211 (27.1) | 568 (72.9) | 0 (0) | 0.816 |
| W | 2.0 | 3.45 ± 3.2 | 200 (26.6) | 553 (73.4) | 0 (0) | |||
| Folate (µg) | M | 320 | 228.73 ± 83.4 | <0.001 | 32 (4.1) | 148 (19.0) | 599 (76.9) | 0.973 |
| W | 320 | 211.76 ± 75.8 | 32 (4.2) | 140 (18.6) | 581 (77.2) | |||
| Calcium (mg) | M | 800 | 546.4 ± 266.2 | 0.010 | 32 (4.1) | 98 (12.6) | 649 (83.3) | <0.001 |
| W | 1000 | 504.2 ± 222.5 | 5 (0.7) | 53 (7.0) | 695 (92.3) | |||
| Phosphorus (mg) | M | 580 | 1129.9 ± 364.6 | <0.001 | 676 (86.8) | 101 (13.0) | 2 (0.2) | <0.001 |
| W | 580 | 978.1 ± 309.0 | 545 (72.4) | 202 (26.8) | 6 (0.8) | |||
| Magnesium (mg) | M | 350 | 275.2 ± 90.2 | <0.001 | 53 (6.8) | 215 (27.6) | 511 (65.6) | <0.001 |
| W | 265 | 246.4 ± 76.2 | 113 (15.0) | 394 (52.3) | 246 (32.7) | |||
| Zinc (mg) | M | 9.4 | 9.4 ± 2.9 | <0.001 | 166 (21.3) | 402 (51.6) | 211 (27.1) | <0.001 |
| W | 6.8 | 7.8 ± 2.3 | 253 (33.6) | 422 (56.0) | 78 (10.4) | |||
| Iron (mg) | M | 6.0 | 10.6 ± 4.0 | <0.001 | 569 (73.0) | 207 (26.6) | 3 (0.4) | <0.001 |
| W | 6.0 | 9.0 ± 3.1 | 422 (56.0) | 324 (43.0) | 7 (0.9) | |||
| Copper (mg) | M | 0.7 | 1.1 ± 0.4 | <0.001 | 513 (65.8) | 257 (33.0) | 9 (1.2) | <0.001 |
| W | 0.7 | 1.0 ± 0.3 | 399 (53.0) | 335 (44.5) | 19 (2.5) | |||
| Iodine (µg) | M | 95 | 160.0 ± 52.8 | <0.001 | 543 (69.7) | 228 (29.3) | 8 (1.0) | <0.001 |
| W | 95 | 143.6 ± 46.9 | 449 (59.6) | 290 (38.5) | 14 (1.9) | |||
| Nutrients | Sex | Reference Value (AI) | Dietary Intake | p-Value 1 | Adequate | p-Value 2 |
|---|---|---|---|---|---|---|
| Mean ± SD | n (%) | |||||
| Vitamin D (µg) | M | 15 | 3.24 ± 2.7 | <0.001 | 5 (0.6) | 0.275 |
| W | 15 | 2.53 ± 2.5 | 2 (0.3) | |||
| Vitamin E (mg) | M | 10 | 7.64 ± 4.1 | 0.009 | 163 (20.9) | <0.001 |
| W | 8 | 6.97 ± 3.3 | 245 (32.5) |
| Nutrients | Age | Dietary Intake | p-Value 1 | D/SDD > 1 2 | D/SDD ≤1 and ≥−1 3 | D/SDD < −1 4 | p-Value 5 |
|---|---|---|---|---|---|---|---|
| Mean ± SD | n (%) | ||||||
| Vitamin A (µg) | 55–65 | 1056 ± 953.4 a | 78 (27.6) | 205 (72.4) | 0 (0) | 0.128 | |
| 66–75 | 1056 ± 949.1 a | 0.001 | 164 (28.8) | 406 (71.2) | 0 (0) | ||
| >75 | 983 ± 964.9 b | 162 (23.9) | 517 (76.1) | 0 (0) | |||
| Vitamin C (mg) | 55–65 | 74.8 ± 53.1 a | 64 (22.6) | 159 (56.2) | 60 (21.2) | <0.001 | |
| 66–75 | 70.5 ± 54.3 a | <0.001 | 108 (18.9) | 316 (55.4) | 146 (25.6) | ||
| >75 | 62.2 ± 50.6 b | 112 (16.5) | 332 (48.9) | 235 (34.6) | |||
| Thiamine (mg) | 55–65 | 1.12 ± 0.4 a | 83 (29.3) | 168 (59.4) | 32 (11.3) | <0.001 | |
| 66–75 | 1.06 ± 0.4 b | <0.00 | 137 (24.0) | 320 (56.1) | 113 (19.8) | ||
| >75 | 0.99 ± 0.4 c | 130 (19.1) | 360 (53.0) | 189 (27.8) | |||
| Riboflavin (mg) | 55–65 | 1.44 ± 0.5 | 156 (55.1) | 123 (43.5) | 4 (1.4) | 0.044 | |
| 66–75 | 1.41 ± 0.5 | 0.085 | 285 (50.0) | 265 (46.5) | 20 (3.5) | ||
| >75 | 1.38 ± 0.5 | 321 (47.2) | 325 (47.9) | 33 (4.9) | |||
| Niacin (mg) | 55–65 | 16.41 ± 5.8 a | 147 (51.9) | 132 (46.6) | 4 (1.4) | <0.001 | |
| 66–75 | 15.61 ± 6.4 b | <0.001 | 253 (44.4) | 299 (52.4) | 18 (3.2) | ||
| >75 | 14.19 ± 5.7 c | 240 (35.3) | 384 (56.6) | 55 (8.1) | |||
| B6 (mg) | 55–65 | 1.65 ± 0.5 a | 129 (45.6) | 138 (48.8) | 16 (5.6) | <0.001 | |
| 66–75 | 1.61 ± 0.5 a | <0.001 | 224 (39.3) | 299 (52.5) | 47 (8.2) | ||
| >75 | 1.50 ± 0.5 b | 215 (31.7) | 356 (52.4) | 108 (15.9) | |||
| B12 (µg) | 55–65 | 3.90 ± 3.5 | 82 (29.0) | 201 (71.0) | 0 (0) | 0.252 | |
| 66–75 | 3.98 ± 3.9 | 0.502 | 161 (28.2) | 409 (71.8) | 0 (0) | ||
| >75 | 3.96 ± 3.8 | 168 (24.7) | 511 (75.3) | 0 (0) | |||
| Folate (µg) | 55–65 | 235.52 ± 79.9 a | 22 (7.8) | 53 (18.7) | 208 (73.5) | 0.009 | |
| 66–75 | 226.34 ± 82.0 b | <0.001 | 22 (3.8) | 115 (20.2) | 433 (76.0) | ||
| >75 | 209.09 ± 77.2 c | 20 (2.9) | 120 (17.7) | 539 (79.4) | |||
| Calcium (mg) | 55–65 | 564.1 ± 269.3 a | 12 (4.2) | 51 (18.0) | 220 (77.7) | <0.001 | |
| 66–75 | 516.2 ± 235.5 b | 0.022 | 10 (1.8) | 40 (7.0) | 520 (91.2) | ||
| >75 | 517.6 ± 244.6 b | 15 (2.2) | 60 (8.8) | 604 (89.0) | |||
| Phosphorus (mg) | 55–65 | 1117.9 ± 39.9 a | 239 (84.4) | 43 (15.2) | 1 (0.4) | 0.008 | |
| 66–75 | 1070.5 ± 64.3 b | <0.001 | 469 (82.3) | 99 (17.4) | 2 (0.3) | ||
| >75 | 1016.4 ± 29.7 c | 513 (75.6) | 161 (23.7) | 5 (0.7) | |||
| Magnesium (mg) | 55–65 | 281.1 ± 84.2 a | 48 (17.0) | 122 (43.1) | 113 (39.9) | <0.001 | |
| 66–75 | 268.3 ± 91.4 b | <0.001 | 67 (11.7) | 246 (43.2) | 257 (45.1) | ||
| >75 | 246.6 ± 76.6 c | 51 (7.5) | 241 (35.5) | 387 (57.0) | |||
| Zinc (mg) | 55–65 | 9.3 ± 2.9 a | 107 (37.8) | 148 (52.3) | 28 (9.9) | <0.001 | |
| 66–75 | 8.8 ± 2.9 b | <0.001 | 162 (28.4) | 312 (54.7) | 96 (16.8) | ||
| >75 | 8.1 ± 2.5 c | 150 (22.1) | 364 (53.6) | 165 (24.3) | |||
| Iron (mg) | 55–65 | 10.5 ± 3.5 a | 221 (78.1) | 61 (21.5) | 1 (0.4) | <0.001 | |
| 66–75 | 10.1 ± 3.9 b | <0.001 | 387 (67.9) | 182 (31.9) | 1 (0.2) | ||
| >75 | 9.2 ± 3.5 c | 383 (56.4) | 288 (42.4) | 8 (1.2) | |||
| Copper (mg) | 55–65 | 1.1 ± 0.3 a | 196 (69.3) | 85 (30.0) | 2 (0.7) | <0.001 | |
| 66–75 | 1.1 ± 0.4 a | <0.001 | 357 (62.6) | 208 (36.5) | 5 (0.9) | ||
| >75 | 1.0 ± 0.3 b | 359 (52.9) | 299 (44.0) | 21 (3.1) | |||
| Iodine (µg) | 55–65 | 158.7 ± 55.6 a | 200 (70.7) | 78 (27.5) | 5 (1.8) | 0.026 | |
| 66–75 | 154.1 ± 50.2 a | 0.011 | 378 (66.3) | 187 (32.8) | 5 (0.9) | ||
| >75 | 147.3 ± 48.5 b | 414 (61.0) | 253 (37.2) | 12 (1.8) | |||
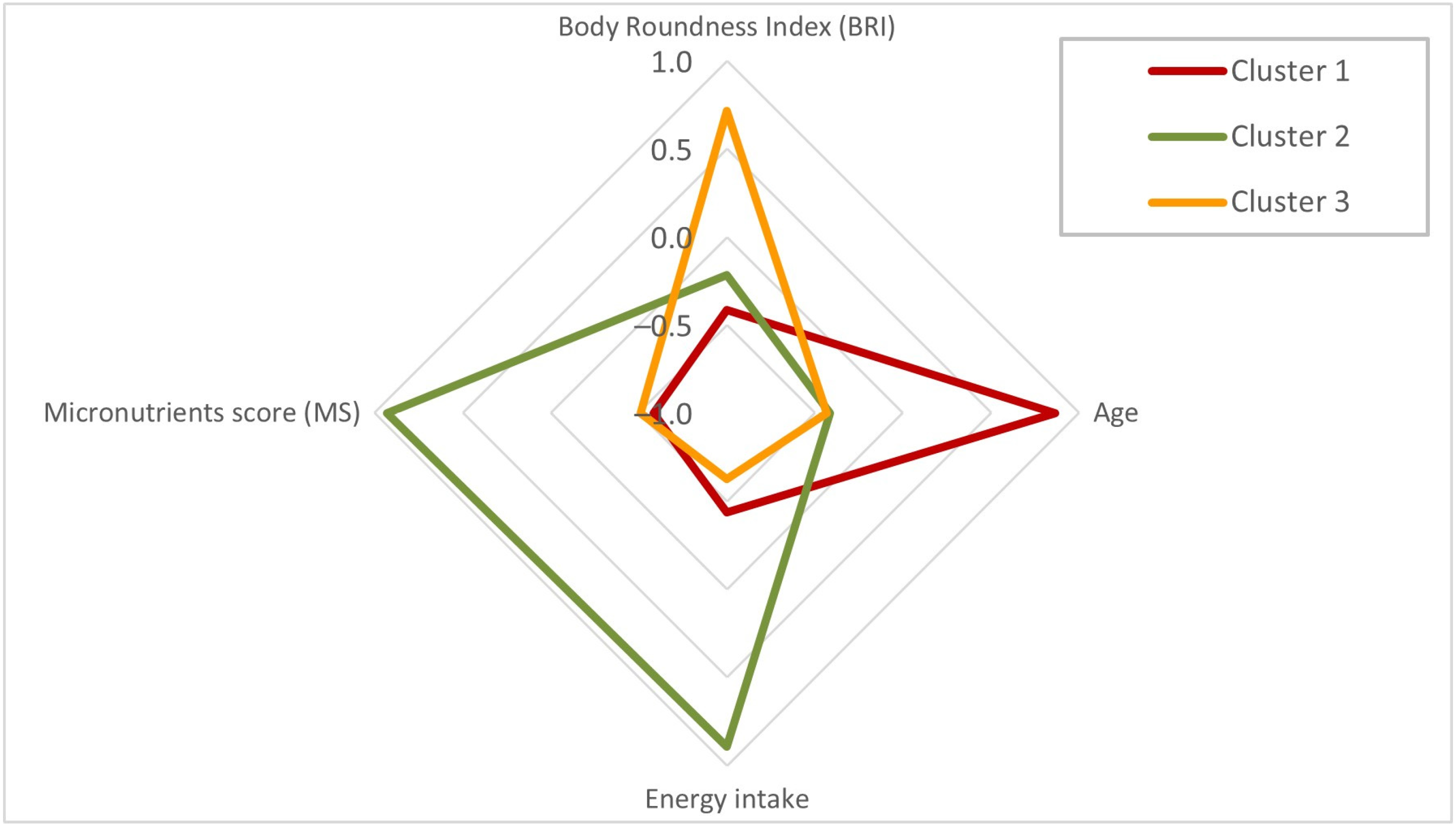
| Parameter | Cluster 1 n = 495 | Cluster 2 n = 557 | Cluster 3 n = 452 | p-Value 1 |
|---|---|---|---|---|
| BRI (Body Roundness Index) | 4.97 ± 1.37 a | 5.34 ± 1.57 b | 7.08 ± 1.96 c | <0.001 |
| Age (years) | 83.6 ± 7.5 a | 69.8 ± 9.7 b | 69.6 ± 8.4 b | <0.001 |
| Energy intake (kcal) | 1414 ± 325 a | 2076 ± 441 b | 1322 ± 270 a | <0.001 |
| Micronutrients score (index) | 7.3 ± 2.1 a | 11.5 ± 1.6 b | 7.5 ± 2.0 a | <0.001 |
| Parameter | Cluster 1 | Cluster 2 | Cluster 3 | p-Value 1 | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | n = 495 | n = 557 | n = 452 | ||||
| women | 222 | 44.8 | 245 | 44.0 | 269 | 59.5 | <0.001 |
| men | 273 | 55.2 | 312 | 56.0 | 183 | 40.5 | |
| Age group | n = 495 | n = 557 | n = 452 | ||||
| 55–65 years | 3 | 0.6 | 172 | 30.9 | 107 | 18.8 | <0.001 |
| 66–75 years | 88 | 17.8 | 234 | 42.0 | 238 | 37.2 | |
| >75 years | 404 | 81.6 | 151 | 27.1 | 107 | 44.0 | |
| Education | n = 492 | n = 556 | n = 451 | ||||
| None/incomplete primary | 94 | 19.1 | 17 | 3.1 | 22 | 4.9 | <0.001 |
| Primary | 169 | 34.3 | 142 | 25.5 | 187 | 41.5 | |
| Professional | 175 | 35.6 | 273 | 49.1 | 194 | 43.0 | |
| Secondary | 10 | 2.0 | 27 | 4.9 | 8 | 1.8 | |
| Higher | 44 | 8.9 | 97 | 17.4 | 40 | 8.9 | |
| Marital status | n = 493 | n = 555 | n = 451 | ||||
| Widowed/divorced/ unmarried (single) | 273 | 55.4 | 188 | 33.9 | 186 | 41.2 | <0.001 |
| Married; living with partner (not single) | 220 | 44.6 | 367 | 66.1 | 265 | 58.8 | |
| Self-rated health status (0—worst to 10—best) | n = 459 | n = 546 | n = 445 | ||||
| 0–3 | 41 | 8.9 | 19 | 3.5 | 31 | 7.0 | <0.001 |
| 4–5 | 165 | 35.9 | 163 | 29.9 | 151 | 33.9 | |
| 6–7 | 149 | 32.5 | 206 | 37.7 | 166 | 37.3 | |
| 8–10 | 104 | 22.7 | 158 | 28.9 | 97 | 21.8 | |
| Physical activity | n = 495 | n = 557 | n = 451 | ||||
| Low | 164 | 33.2 | 68 | 12.2 | 88 | 21.3 | <0.001 |
| Moderate | 330 | 66.8 | 489 | 87.7 | 363 | 80.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białecka-Dębek, A.; Wierzbicka, E.; Januszko, O.; Pietruszka, B.; Szybalska, A.; Mossakowska, M. Novel Anthropometric Indices and Probability of Adequate Nutrient Intake in the Older Polish Population. Nutrients 2025, 17, 3666. https://doi.org/10.3390/nu17233666
Białecka-Dębek A, Wierzbicka E, Januszko O, Pietruszka B, Szybalska A, Mossakowska M. Novel Anthropometric Indices and Probability of Adequate Nutrient Intake in the Older Polish Population. Nutrients. 2025; 17(23):3666. https://doi.org/10.3390/nu17233666
Chicago/Turabian StyleBiałecka-Dębek, Agata, Elżbieta Wierzbicka, Olga Januszko, Barbara Pietruszka, Aleksandra Szybalska, and Małgorzata Mossakowska. 2025. "Novel Anthropometric Indices and Probability of Adequate Nutrient Intake in the Older Polish Population" Nutrients 17, no. 23: 3666. https://doi.org/10.3390/nu17233666
APA StyleBiałecka-Dębek, A., Wierzbicka, E., Januszko, O., Pietruszka, B., Szybalska, A., & Mossakowska, M. (2025). Novel Anthropometric Indices and Probability of Adequate Nutrient Intake in the Older Polish Population. Nutrients, 17(23), 3666. https://doi.org/10.3390/nu17233666







