Abstract
Background/Objectives: Protein–energy wasting (PEW) is a common complication in patients with chronic kidney disease (CKD) receiving renal replacement therapy by dialyses. This condition is associated with higher morbidity, mortality, and poorer quality of life. The aim of this systematic review was to evaluate the effectiveness of different nutritional strategies—such as oral nutritional supplements and intra-dialytic parenteral nutrition—in improving the nutritional status of these patients. Methods: A systematic review was carried out in accordance with the PRISMA statement. Searches were performed in PubMed, BVS, and Scopus between January and March 2025. Randomised or controlled clinical trials published in English or Spanish, available in full text, involving adults on haemodialysis (HD) or peritoneal dialyses (PD) were included. Fourteen studies met the inclusion criteria. Results: The nutritional interventions assessed produced consistent benefits in biochemical markers (e.g., serum albumin), muscle mass, inflammatory indices, and perceived quality of life. Intra-dialytic supplementation and multidisciplinary management were particularly effective in patients with moderate-to-severe malnutrition. Conclusions: Malnutrition is frequent and clinically significant in dialyses patients. Nutritional strategies—including oral supplementation, IDPN, and personalised counselling—effectively prevent and treat PEW. Early, tailored, evidence-based, and multidisciplinary implementation could decisively improve clinical prognosis and quality of life in this population.
1. Introduction
Chronic kidney disease (CKD) constitutes a major global public health problem, with an estimated prevalence of 10% among the adult population [1]—particularly in older adults and in those with type 2 diabetes mellitus or arterial hypertension [2,3]. In addition to older age, type 2 diabetes, and arterial hypertension, selected congenital kidney disorders—most notably autosomal dominant polycystic kidney disease—can also contribute to the progression to advanced CKD, potentially leading to the need for dialyses [4]. Progression to advanced stages leads to an irreversible decline in renal function, frequently requiring renal replacement therapy (RRT) by haemodialysis (HD) or peritoneal dialyses (PD). HD involves extracorporeal blood purification through a semipermeable membrane, whereas PD uses the peritoneal membrane as a natural filter to remove toxins and excess fluid [5].
Patients receiving RRT have a high-risk of protein–energy wasting (PEW), affecting 18–56% of this population [6,7,8]. Recent studies from diverse regions of the world confirm that malnutrition remains highly prevalent among dialyses patients, particularly in settings with limited resources, underscoring the need for nutritional interventions tailored to the local context [9,10]. PEW is associated with increased morbidity and mortality, infections, cardiovascular events, and a marked reduction in quality of life [11,12]. Its aetiology is multifactorial, comprising iatrogenic factors such as nutrient losses during dialyses, treatment-induced inflammation, metabolic acidosis, or inadequate dialyses dose, and non-iatrogenic factors such as anorexia, taste alterations, insulin resistance, hormonal disturbances (leptin, ghrelin), and disturbances in mineral and bone metabolism (e.g., calcium–phosphate imbalance and secondary hyperparathyroidism), as well as psychosocial factors including depression or low social support [13]. In PD, additional protein losses across the peritoneum may reach 20 g per day during peritonitis episodes [14].
Inflammation plays a central role in the pathophysiology of malnutrition in CKD. Elevated pro-inflammatory cytokines (TNF-α, IL-6) promote proteolysis, loss of muscle mass, and appetite suppression [15,16], while raised C-reactive protein (CRP) correlates with poorer nutritional status and greater cardiovascular risk [17].
Sarcopenia—progressive loss of skeletal muscle mass and strength—is highly prevalent in HD patients and closely linked to PEW. Its aetiology is multifactorial, involving inflammation, hormonal changes, metabolic acidosis, and nutrient deficiencies. Sarcopenia contributes to functional impairment, reduced exercise tolerance, and diminished quality of life, and has been associated with increased morbidity and mortality. Recognising and addressing sarcopenia at an early stage is essential to preserve functional capacity and improve clinical outcomes, highlighting the importance of timely and individualised nutritional interventions in this population [18].
Early assessment of nutritional status is essential for timely management of PEW. The KDOQI guidelines recommend a 3- to 7-day dietary record—including at least one HD day—as the reference method, although food frequency questionnaires or 24 h re-calls may also be employed [19]. Validated clinical tools include the Subjective Global Assessment (SGA) [20] and the Malnutrition–Inflammation Score (MIS), which incorporates biochemical parameters such as serum albumin and total iron-binding capacity [21].
Individualised dietary counselling is the first-line nutritional intervention, enhancing adherence, identifying barriers, and tailoring intake to patient needs. When counselling alone fails to reverse PEW, oral nutritional supplements (ONS) can meet protein–energy requirements without overloading residual renal function. In cases of digestive intolerance, severe malnutrition, or poor ONS adherence, intra-dialytic parenteral nutrition (IDPN) offers an effective alternative [22].
Despite numerous studies on nutritional strategies in dialyses, methodological variability and heterogeneous interventions hinder direct comparison. This review addresses a specific evidence gap: prior syntheses have typically focused on single modalities (HD or PD) or isolated interventions and have seldom integrated recent randomised trials. We therefore map and compare the effects of oral nutritional supplements (ONS), intra-dialytic parenteral nutrition (IDPN), and selected dietary adjuncts across both HD and PD, with attention to inflammatory and functional endpoints and to implementation issues under-reported in earlier reviews. This systematic review therefore aimed to analyse the evidence on the impact of nutritional strategies—particularly ONS and IDPN—on nutritional status, body composition, biochemical markers, and quality of life in adult HD or PD patients.
2. Materials and Methods
2.1. Data Sources
This systematic review was conducted following PRISMA [23], and the research question was framed using the PICO model. Comprehensive searches of PubMed, Scopus, and the Virtual Health Library (BVS) were performed. The full PRISMA checklist is provided as Supplementary Material.
The search strategy combined controlled (MeSH/DeCS) and free terms in English and Spanish with the Boolean operator “AND”. Descriptors included are as follows: “Chronic kidney disease” OR “Enfermedad renal crónica”, “Malnutrition” OR “Desnutrición”, “Hemodialyses” OR “Hemodiálisis”, “Nutritional therapy” OR “Terapia nutricional”, “Dietary supplements” OR “Suplementos nutricionales”.
Searches were limited to January 2015–March 2025.
2.2. Eligibility Criteria
Studies were included if they met the following inclusion criteria:
- -
- Controlled or randomised clinical trials.
- -
- Adult participants (≥18 years) on HD or PD.
- -
- Articles in English or Spanish.
- -
- Free full-text availability.
- -
- Publication between 2015 and 2025.
- -
- Interventions evaluating nutritional strategies (ONS, IDPN, or combined approaches).
Exclusion criteria are as follows:
- -
- Systematic reviews, meta-analyses, or non-experimental designs.
- -
- Animal or in vitro studies.
- -
- Articles without full-text access.
- -
- Duplicates or publications in languages other than English/Spanish.
- -
- Studies unrelated to nutrition in dialyses patients.
2.3. Outcomes of Interest
Primary outcomes are as follows:
- -
- Changes in nutritional biomarkers (albumin, pre-albumin, transferrin).
- -
- Variations in muscle mass or body composition.
- -
- Improvement in Malnutrition–Inflammation Score (MIS).
- -
- Changes in health-related quality of life using validated questionnaires.
- -
- Secondary outcomes are as follows: Variations in inflammatory markers (CRP, IL-6) and immunological parameters.
Null Hypothesis
The null hypothesis of this systematic review states that there are no significant differences in nutritional, biochemical, inflammatory, or quality-of-life outcomes between dialyses patients receiving specific nutritional strategies (oral supplementation or intra-dialytic parenteral nutrition) and those receiving only conventional dietary counselling.
The review was designed to test this hypothesis and to determine whether nutritional interventions provide measurable clinical benefits compared with standard care.
2.4. Data Synthesis
Given the heterogeneity in study design, intervention type, duration, and outcome measures, a quantitative meta-analysis was not feasible. Results were synthesised qualitatively, comparing intervention effects by dialyses modality, intervention type, and evaluated parameters. Data were summarised in tables organised by intervention, study design, and outcomes.
3. Results
3.1. Study Selection
The systematic search yielded 1389 records: PubMed = 1153, BVS = 110, and Scopus = 126. After removing duplicates and screening titles/abstracts, 1320 records were excluded. Sixty-nine full-texts were assessed; forty-seven were excluded due to methodological issues or lack of access, leaving fourteen studies for qualitative synthesis (Figure 1).
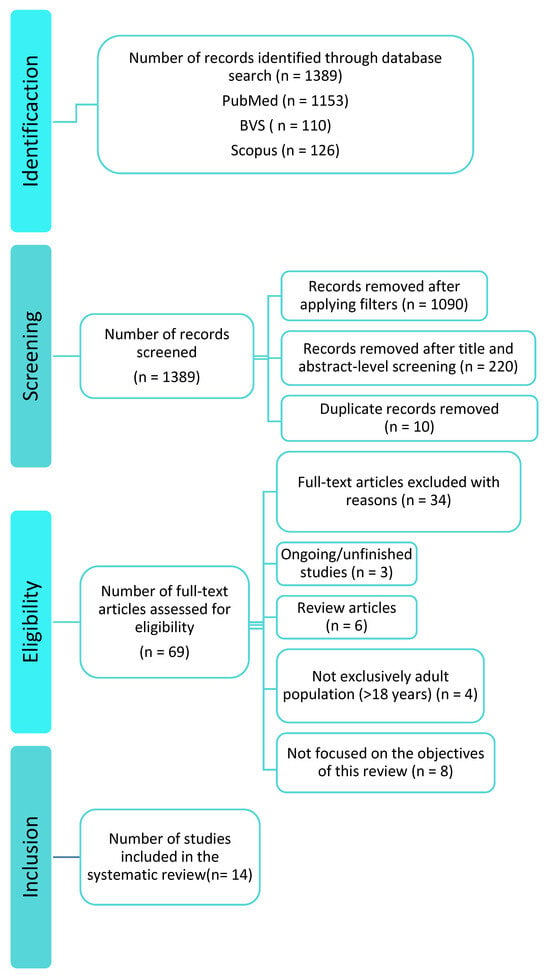
Figure 1.
PRISMA flow diagram of the literature search.
3.2. Characteristics of Included Studies
The 14 selected trials (2015–2025) evaluated nutritional interventions in adult HD or PD patients. Most investigated ONS or IDPN with or without dietary counselling. Outcomes included serum albumin, muscle mass, MIS, quality of life, and inflammatory markers. Key characteristics and findings are summarised in Table 1 and Table 2.

Table 1.
Summary of the results and characteristics of the selected articles on haemodialysis.

Table 2.
Summary of the results and characteristics of the selected articles on peritoneal dialyses.
3.3. Oral Nutritional Supplementation in Haemodialysis Patients
Figure 2 provides a detailed comparative overview of the nutritional interventions analysed, grouped into three categories, as follows: classic nutritional supplements, dietary adjuncts/bioactives, and probiotics. For each intervention, the figure summarises the main mechanism of action, route of administration, clinical utility, efficacy, improved outcomes, and bibliographic reference.
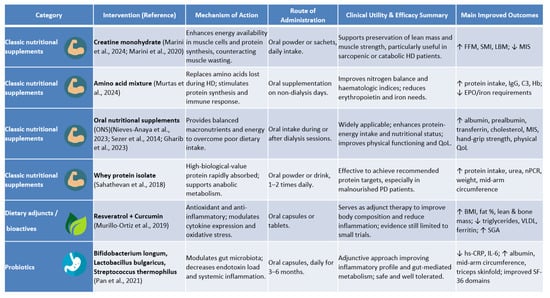
Figure 2.
Updated comparative overview of nutritional interventions assessed in this review, grouped by type (classic nutritional supplements, dietary adjuncts/bioactives, and probiotics). Each intervention is described according to mechanism of action, route of administration, clinical utility, and evidence of efficacy [6,24,25,27,28,29,30,35,36].
Seven studies evaluated ONS in HD, demonstrating significant improvements in nutritional, biochemical, body composition, and quality-of-life parameters, while effects on inflammation were less consistent.
Marini et al. [24] showed that long-term oral creatine improved fat-free mass and skeletal muscle index without altering MIS, whereas short-term supplementation reduced MIS and prevented lean mass loss [30], suggesting benefits for high-risk patients.
ONS combined with dry weight adjustment via bioelectrical impedance vector analysis (BIVA) improved serum albumin, hand-grip strength, and quality of life in the pilot study by Nieves-Anaya et al. [25]. Satirapoj et al. [26] reported that intra-dialytic ONS reduced MIS more effectively than inter-dialytic administration.
Sustained ONS for six months [6] increased serum albumin, BMI, and triceps skinfold thickness, whereas Gharib et al. [27] demonstrated improved albumin, pre-albumin, and PEW scores with reduced hs-CRP.
Amino acid replacement [28] enhanced protein intake, immunological parameters, and haematological status, while combined antioxidant supplementation with curcumin and resveratrol [29] improved muscle and bone mass and decreased ferritin.
Quality-of-life gains were documented by Nieves-Anaya et al. [25] and Gharib et al. [27], particularly in physical domains, although mental health components were unchanged.
3.4. Intra-Dialytic Parenteral Nutrition
Three trials investigated IDPN. Marsen et al. [31] found thrice-weekly IDPN for 16 weeks significantly increased pre-albumin, especially in moderately malnourished patients (SGA B). Kabasawa et al. [32] observed increased spontaneous protein intake and metabolic safety (fewer hypoglycaemic events) with ENEFLUID® over 12 months, despite unchanged transthyretin. Kittiskulnam et al. [33] reported improved albumin, weight, MIS, and intake in patients intolerant to ONS.
IDPN therefore appears effective for moderate–severe malnutrition or when oral intake is limited, supporting recovery of nutritional status and appetite when combined with ongoing counselling.
3.5. Nutritional Strategies in Peritoneal Dialyses
Three PD studies assessed multidisciplinary MNT, probiotics, or whey protein supplementation. All reported beneficial effects on nutritional, inflammatory, or quality-of-life outcomes. Liang et al. [34] showed that MNT improved albumin, anaemia markers, and reduced inflammation. Pan et al. [35] conducted a randomised controlled trial in which patients received probiotic capsules containing Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for several months. This intervention significantly reduced hs-CRP and IL-6, while improving serum albumin, mid-arm circumference, and triceps skinfold thickness. Moreover, participants reported improvements in selected domains of the SF-36 quality-of-life questionnaire, particularly those related to physical function. Sahathevan et al. [36] found that whey protein supplementation achieved recommended protein intake and improved nutritional markers in responders.
4. Discussion
A close examination of the studies included in this systematic review shows that several nutritional strategies—oral supplementation, intra-dialytic parenteral nutrition (IDPN), and multidisciplinary approaches—exert a beneficial effect on nutritional status, body composition, biochemical markers, and quality of life in patients undergoing dialyses. Malnutrition is highly prevalent in this population, with reported rates of 18–56% [6,7,8], arising both from the pathophysiology of chronic kidney disease itself and from dialyses-related factors such as protein losses, dietary restrictions, and persistent chronic inflammation.
Our synthesis complements current clinical guidance by integrating evidence across HD and PD and by comparing oral nutritional supplements, intra-dialytic parenteral nutrition, and selected dietary adjuncts within a single framework. This approach mirrors recommendations to individualise assessment and therapy, monitor inflammatory–nutritional phenotypes, and prioritise patient-centred outcomes [22,37]. It also addresses a persistent gap in prior reviews that typically examined single modalities or isolated interventions, thereby limiting applicability to real-world multidisciplinary care [38].
While not developed in detail here, dietary counselling is essential in the first steps of nutritional management. Oral supplementation is reserved for cases in which dietary measures prove inadequate, and IDPN should be considered only when both preceding strategies fail to improve nutritional status. This therapeutic escalation is taken for granted in routine clinical practice and constitutes the basis on which the evaluated interventions are built [19].
Within dietary counselling, one of the most widely used tools is the food record, which entails weighing and accurately recording everything consumed over three to fourteen days, including at least one weekend day. While this method is regarded as one of the most precise, it can modify patients’ eating habits during the recording period, leading to possible under-reporting of intake [39]. For a comprehensive appraisal, dietary records should be interpreted alongside paraclinical indices of systemic inflammation—such as C-reactive protein (CRP), interleukin-6 (IL-6), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), serum albumin, pre-albumin, and ferritin—and composite tools like the Malnutrition–Inflammation Score (MIS) or the Patient-Generated Subjective Global Assessment (PG-SGA) [14,17,21,22]. In addition to the parameters already discussed, it is worth mentioning the Prognostic Inflammatory and Nutritional Index (PINI), which integrates markers of inflammation and nutritional status to provide a more comprehensive assessment of their balance. This index has shown prognostic value in dialyses patients and could serve as a complementary tool to the MIS or PG-SGA for improving the detection of malnutrition–inflammation and guiding more individualised interventions [40].
With regard to oral nutritional supplementation, the included studies show that this strategy improves key parameters such as serum albumin, muscle mass, and body composition. Marini et al. [30] observed an increase in muscle mass after creatine administration without significant changes in inflammatory markers—highly relevant to the management of sarcopenia, a common complication in haemodialysis (HD) characterised by loss of muscle mass and strength [41]. This phenomenon, linked to protein–energy wasting, is defined as the progressive loss of muscle and energy stores caused by an imbalance between intake and nutritional requirements [42], which justifies the recommendation to exceed 1.4 g/kg/day of protein in patients with this clinical profile [22].
Clinically, these findings support a stepwise strategy: first-line dietary counselling, followed by ONS when intake remains insufficient, and IDPN for moderate–severe PEW, and intolerance or poor adherence to oral measures [20,38]. Protein targets should align with guideline ranges (≥1.0–1.2 g/kg/day in stable HD/PD; higher intakes in PEW or catabolic states), with systematic monitoring using MIS/PG-SGA and paraclinical indices (e.g., CRP, IL-6) [22]. Given the high prevalence of sarcopenia in HD, strength-oriented interventions (e.g., creatine or amino acid supplementation) may be considered adjuncts to exercise-based programmes, using EWGSOP2 criteria to track strength and muscle mass trajectories [41].
Intra-dialytic supplementation, by contrast, has proved more effective than inter-dialytic administration [26] because it not only improves nutritional status but also facilitates therapeutic adherence, probably owing to closer medical supervision during dialyses sessions—an element that has been shown to be crucial in other chronic diseases to enhance adherence [43,44]. Nevertheless, implementing this strategy is not free of operational obstacles, such as economic cost, the need for specific infrastructure in dialyses centres [45], and sociocultural factors that may influence patient acceptance [46]. Its indication should therefore be confined to specific clinical profiles, such as patients without family support or with difficulties in maintaining autonomous feeding [11].
Several factors help explain the heterogeneity that precluded a formal meta-analysis in our review: variability in baseline nutritional phenotype (e.g., SGA A/B/C or MIS thresholds), dialyses modality (HD vs. PD) and adequacy, intervention composition and dose (calorie–protein ONS, amino acid mixtures, creatine, bioactives, probiotics), timing (intra- vs. inter-dialytic), duration (4 weeks to 12 months), adherence/supervision, and outcome selection (albumin vs. pre-albumin, MIS vs. SGA, functional indices, inflammatory markers). Divergent results in trials combining intra-dialytic nutrition with exercise or counselling further illustrate this variability, with some studies showing improvement in MIS while others report neutral effects on functional performance [26,35,47].
Implementation should consider centre-level resources (e.g., capacity for supervised intra-dialytic feeding), reimbursement, product palatability/tolerance, and patient preference, all of which influence adherence and scalability. Guidance documents emphasise that parenteral routes are reserved for patients in whom oral strategies fail or are not feasible, and that ongoing dietetic follow-up is essential to sustain benefits and minimise costs [37,45,46].
When both dietary counselling and oral supplementation are insufficient—owing to intolerance, poor adherence, or the severity of malnutrition—IDPN emerges as an effective alternative. Marsen et al. [31] and Kittiskulnam et al. [33] recorded significant improvements in pre-albumin and serum albumin, particularly when IDPN was accompanied by ongoing nutritional follow-up. This mode of support has also shown benefits in other high-risk settings, such as oncology [48] and intensive care [49], where the oral route is inadequate to meet requirements.
Among patients on peritoneal dialyses (PD), nutritional interventions likewise produced favourable effects on nutritional status and inflammatory markers. A randomised controlled trial from Asia led by Liang et al. [34] demonstrated that multidisciplinary medical nutrition therapy (MNT) reduced C-reactive protein (CRP) and improved protein and energy intake. Pan et al. [35] reported that probiotic supplementation markedly reduced CRP and IL-6, mirroring findings in inflammatory bowel diseases such as ulcerative colitis, where probiotics modulate the intestinal microbiota and decrease epithelial permeability, thereby reducing systemic inflammation [50].
Quality-of-life improvements mainly affected physical domains, underscoring the need for integrated psychological support within multidisciplinary teams [21].
Limitations include potential publication bias due to restricted database coverage, exclusion of observational studies, and small sample sizes with attrition. Most trials enrolled small cohorts, had short follow-up (<6 months), and rarely reported hard clinical endpoints such as hospitalisations, cardiovascular events, or mortality, precluding meta-analysis and limiting inferences on hospitalisations, cardiovascular events, or mortality. Additional limitations include the exclusion of older (>10 years) studies, variability in intervention protocols, and heterogeneity of outcome measures, which further complicate comparisons across studies. Taken together, these constraints substantially weaken the overall strength of the available evidence and warrant caution in interpreting the observed benefits. Emphasising these limitations highlights the gaps in current evidence and the need for adequately powered, long-term RCTs that assess clinically meaningful outcomes.
Future research should focus on standardising the design of nutritional intervention trials in dialyses patients, defining homogeneous inclusion criteria, adequate follow-up duration, and comparable outcome measures to facilitate quantitative synthesis and meta-analysis. It is particularly relevant to include inflammatory and functional biomarkers (such as CRP, IL-6, MIS, or PINI) as secondary endpoints to better characterise the malnutrition–inflammation complex and its response to therapy.
In addition, studies addressing cost-effectiveness, patient preferences, and adherence are needed to strengthen the development of patient-centred nutritional protocols. Integrating these findings into clinical guidelines (e.g., KDOQI, ESPEN) could help promote a more individualised, evidence-based practice. Finally, long-term multicentre clinical trials are required to determine whether nutritional interventions translate into improved survival, reduced hospitalisation, and enhanced overall quality of life in dialyses patients.
5. Conclusions
Malnutrition is frequent and clinically significant in dialyses patients. Nutritional strategies—including oral supplementation, IDPN, and personalised counselling—effectively prevent and treat PEW. Early, tailored, evidence-based, and multidisciplinary implementation could decisively improve clinical prognosis and quality of life in this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17213478/s1, PRISMA 2020 Checklist. Reference [51] is cited in the supplementary materials.
Author Contributions
Conceptualisation, P.A.-S. and M.L.S.-T.; methodology, P.A.-S., R.A.-D. and M.L.S.-T.; software, P.A.-S., M.L.S.-T. and S.M.-F.; validation, R.A.-D., E.G.-P. and M.L.S.-T.; formal analysis, P.A.-S., R.A.-D. and M.L.S.-T.; investigation, P.A.-S., R.A.-D. and M.L.S.-T.; resources, R.A.-D. and M.L.S.-T.; data curation, P.A.-S., M.L.S.-T., E.G.-P. and S.M.-F.; writing—original draft preparation, P.A.-S. and M.L.S.-T.; writing—review and editing, P.A.-S., R.A.-D., E.G.-P., S.M.-F. and M.L.S.-T.; visualisation, P.A.-S., E.G.-P., S.M.-F. and M.L.S.-T.; supervision, M.L.S.-T.; project administration, M.L.S.-T.; funding acquisition, E.G.-P., S.M.-F. and M.L.S.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. M.L. Sánchez-Tocino’s academic salary is financed by the University of Salamanca (USAL). The research groups of S.M.-F. and E.G.-P. and the APC are supported by projects PI21/01430 and PI24/01630, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this systematic review are included in this published article or referenced in publicly accessible databases.
Acknowledgments
The authors would like to express their gratitude to the Department of Nursing and Physiotherapy at the University of Salamanca for its institutional support and for providing the necessary resources to carry out this work. Their commitment to research and academic excellence has been essential to the development of this systematic review.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CKD | Chronic kidney disease |
| RRT | Renal replacement therapy |
| HD | Haemodialysis |
| PD | Peritoneal dialyses |
| PEW | Protein–energy wasting |
| ONS | Oral nutritional supplement |
| IDPN | Intra-dialytic parenteral nutrition |
| MIS | Malnutrition–inflammation score |
| SGA | Subjective global assessment |
| KDOQI | Kidney disease outcomes quality initiative |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| BVS | Biblioteca Virtual en Salud (virtual health library) |
| nPCR | Normalised protein catabolic rate |
| BMI | Body mass index |
| FFM | Fat-free mass |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
| TNF-α | Tumour necrosis factor-alpha |
| EPO | Erythropoietin |
| Hb | Haemoglobin |
| BIVA | Bioelectrical impedance vector analysis |
| ESRD | End-stage renal disease |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| VLDL | Very low-density lipoprotein |
| PG-SGA | Patient-Generated Subjective Global Assessment |
References
- Llisterri, J.L.; Micó-Pérez, R.M.; Velilla-Zancada, S.; Rodríguez-Roca, G.C.; Prieto-Díaz, M.Á.; Martín-Sánchez, V.; Barquilla, A.; Polo-García, J.; Segura-Fragoso, A.; Cinza-Sanjurjo, S.; et al. Prevalence of Chronic Kidney Disease and Associated Factors in the Spanish Population Attended in Primary Care: Results of the IBERICAN Study. Med. Clin. 2021, 156, 157–165. [Google Scholar] [CrossRef]
- Salvador González, B.; Rodríguez Pascual, M.; Ruipérez Guijarro, L.; Ferré González, A.; Cunillera Puertolas, O.; Rodríguez Latre, L.M. Chronic kidney disease in Primary Health Care: Prevalence and associated risk factors. Aten. Primaria 2015, 47, 236–245. [Google Scholar] [CrossRef][Green Version]
- Gorostidi, M.; Sánchez-Martínez, M.; Ruilope, L.M.; Graciani, A.; de la Cruz, J.J.; Santamaría, R.; Del Pino, M.D.; Guallar-Castillón, P.; de Álvaro, F.; Rodríguez-Artalejo, F.; et al. Chronic Kidney Disease in Spain: Prevalence and Impact of Accumulation of Cardiovascular Risk Factors. Nefrologia 2018, 38, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Okpechi, I.G.; Caskey, F.J.; Yang, C.-W.; Tonelli, M.; Jha, V. Perspectives on Early Detection of Chronic Kidney Disease: The Facts, the Questions, and a Proposed Framework for 2023 and Beyond. Kidney Int. 2023, 103, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Gorostidi, M.; Santamaría, R.; Alcázar, R.; Fernández-Fresnedo, G.; Galcerán, J.M.; Goicoechea, M.; Oliveras, A.; Portolés, J.; Rubio, E.; Segura, J.; et al. Spanish Society of Nephrology Document on KDIGO Guidelines for the Assessment and Treatment of Chronic Kidney Disease. Nefrologia 2014, 34, 302–316. [Google Scholar] [PubMed]
- Sezer, S.; Bal, Z.; Tutal, E.; Uyar, M.E.; Acar, N.O. Long-Term Oral Nutrition Supplementation Improves Outcomes in Malnourished Patients with Chronic Kidney Disease on Hemodialysis. JPEN J. Parenter. Enteral Nutr. 2014, 38, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Leinig, C.E.; Moraes, T.; Ribeiro, S.; Riella, M.C.; Olandoski, M.; Martins, C.; Pecoits-Filho, R. Predictive Value of Malnutrition Markers for Mortality in Peritoneal Dialysis Patients. J. Ren. Nutr. 2011, 21, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Pérez-Gómez, M.V.; Mahíllo, I.; Egido, J.; Ortiz, A.; Carrero, J.J. Prevalence of Protein-Energy Wasting Syndrome and Its Association with Mortality in Haemodialysis Patients in a Centre in Spain. Nefrología 2013, 33, 495–505. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Elkazaz, A.M. The effect of different timings of protein supplementation on variable outcomes in hemodialysis patients: A randomized clinical trial. Clin. Exp. Nephrol. 2025, 29, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Fotiadou, E.; Georgianos, P.I.; Chourdakis, M.; Zebekakis, P.E.; Liakopoulos, V. Eating during the Hemodialysis Session: A Practice Improving Nutritional Status or a Risk Factor for Intradialytic Hypotension and Reduced Dialysis Adequacy? Nutrients 2020, 12, 1703. [Google Scholar] [CrossRef]
- Ikizler, T.A. Optimal Nutrition in Hemodialysis Patients. Adv. Chronic Kidney Dis. 2013, 20, 181–189. [Google Scholar] [CrossRef]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Criteria for Classification of Protein-Energy Wasting in Dialysis Patients: Impact on Prevalence. Br. J. Nutr. 2019, 121, 1271–1278. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.-H.; Ng, H.-M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- Rodríguez-García, V.H.; López-Guerra, E.A.; Rodríguez-Castellanos, F.E. Association between Peritoneal Protein Excretion, Peritonitis and D/P Phosphate, in Patients on Peritoneal Dialysis. Nefrología 2013, 33, 204–213. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J.; Avesani, C.M.; Suliman, M.E.; Kato, S.; Bárány, P.; Snaedal-Jonsdottir, S.; Alvestrand, A.; Heimbürger, O.; et al. Comparison of Nutritional and Inflammatory Markers in Dialysis Patients with Reduced Appetite. Am. J. Clin. Nutr. 2007, 85, 695–701. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing Outcome Predictability of Markers of Malnutrition-Inflammation Complex Syndrome in Haemodialysis Patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.C.; Vogt, B.P.; Martin, L.C.; Caramori, J.C.T. Malnutrition Inflammation Score Cut-off Predicting Mortality in Maintenance Hemodialysis Patients. Clin. Nutr. ESPEN 2017, 17, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Delgado García de Polavieja, M.; Escribano Loma, S.; Manso de Real, P.; Sánchez Tocino, M.L.; Arenas Jiménez, M.D. ¿Qué novedades aportan en la práctica clínica las guías KDOQI de nutrición después de 20 años? Nefrología 2022, 14, 1–10. [Google Scholar]
- Tapiawala, S.; Vora, H.; Patel, Z.; Badve, S.; Shah, B. Subjective Global Assessment of Nutritional Status of Patients with Chronic Renal Insufficiency and End Stage Renal Disease on Dialysis. J. Assoc. Physicians India 2006, 54, 923–926. [Google Scholar] [PubMed]
- de Oliveira Santin, F.G.; Bigogno, F.G.; Dias Rodrigues, J.C.; Cuppari, L.; Avesani, C.M. Concurrent and Predictive Validity of Composite Methods to Assess Nutritional Status in Older Adults on Hemodialysis. J. Ren. Nutr. 2016, 26, 18–25. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.C.B.; Schincaglia, R.M.; Candow, D.G.; Pimentel, G.D. Effect of Creatine Supplementation on Body Composition and Malnutrition-Inflammation Score in Hemodialysis Patients: An Exploratory 1-Year, Balanced, Double-Blind Design. Nutrients 2024, 16, 615. [Google Scholar] [CrossRef]
- Nieves-Anaya, I.; Várgas, M.B.; García, O.P.; Biruete, A.; Kistler, B.; Atilano-Carsi, X. Effect of Oral Nutritional Supplementation Combined with Impedance Vectors for Dry Weight Adjustment on the Nutritional Status, Hydration Status and Quality of Life in Patients on Chronic Hemodialysis: A Pilot Study. Clin. Nutr. ESPEN 2023, 54, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B.; Apiyangkool, T.; Thimachai, P.; Nata, N.; Supasyndh, O. Intradialytic Oral Nutrition Effects on Malnourished Hemodialysis Patients: A Randomized Trial. Sci. Rep. 2024, 14, 21400. [Google Scholar] [CrossRef]
- Gharib, M.S.; Nazeih, M.S.; El Said, T.W. Effect of Intradialytic Oral Nutritional Supplementation on Nutritional Markers in Malnourished Chronic Hemodialysis Patients: Prospective Randomized Trial. BMC Nephrol. 2023, 24, 125. [Google Scholar] [CrossRef]
- Murtas, S.; Reggiardo, G.; Contu, R.; Cadeddu, M.; Secci, R.; Putzu, P.; Mocco, C.; Leoni, M.; Gigante Maria, V.; Marras, C.; et al. Replacement of the Massive Amino Acid Losses Induced by Hemodialysis: A New Treatment Option Proposal for a Largely Underestimated Issue. Clin. Nutr. ESPEN 2024, 63, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Murillo Ortiz, B.O.; Fuentes Preciado, A.R.; Ramírez Emiliano, J.; Martínez Garza, S.; Ramos Rodríguez, E.; de Alba Macías, L.A. Recovery of Bone and Muscle Mass in Patients with Chronic Kidney Disease and Iron Overload on Hemodialysis and Taking Combined Supplementation with Curcumin and Resveratrol. Clin. Interv. Aging 2019, 14, 2055–2062. [Google Scholar] [CrossRef]
- Marini, A.C.B.; Motobu, R.D.; Freitas, A.T.V.; Mota, J.F.; Wall, B.T.; Pichard, C.; Laviano, A.; Pimentel, G.D. Short-Term Creatine Supplementation May Alleviate the Malnutrition-Inflammation Score and Lean Body Mass Loss in Hemodialysis Patients: A Pilot Randomized Placebo-Controlled Trial. JPEN J. Parenter. Enteral Nutr. 2020, 44, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Marsen, T.A.; Beer, J.; Mann, H.; German IDPN-Trial Group. Intradialytic Parenteral Nutrition in Maintenance Hemodialysis Patients Suffering from Protein-Energy Wasting. Results of a Multicenter, Open, Prospective, Randomized Trial. Clin. Nutr. Edinb. Scotl. 2017, 36, 107–117. [Google Scholar] [CrossRef]
- Kabasawa, H.; Hosojima, M.; Kanda, E.; Nagai, M.; Murayama, T.; Tani, M.; Kamoshita, S.; Kuroda, A.; Kanno, Y. Efficacy and Safety of Intradialytic Parenteral Nutrition Using ENEFLUID® in Malnourished Patients Receiving Maintenance Hemodialysis: An Exploratory, Multicenter, Randomized, Open-Label Study. PLoS ONE 2024, 19, e0311671. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Banjongjit, A.; Metta, K.; Tiranathanagul, K.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. The Beneficial Effects of Intradialytic Parenteral Nutrition in Hemodialysis Patients with Protein Energy Wasting: A Prospective Randomized Controlled Trial. Sci. Rep. 2022, 12, 4529. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, F.; Guo, L.; Jiang, W.; Li, J.; Shu, P. Effect of Multidisciplinary Medical Nutrition Therapy on the Nutrition Status of Patients Receiving Peritoneal Dialysis: A Randomized Controlled Trial. Nutr. Clin. Pract. 2025, 40, 106–116. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, L.; Dai, B.; Lin, B.; Lin, S.; Lin, E. Effects of Probiotics on Malnutrition and Health-Related Quality of Life in Patients Undergoing Peritoneal Dialysis: A Randomized Controlled Trial. J. Ren. Nutr. 2021, 31, 199–205. [Google Scholar] [CrossRef]
- Sahathevan, S.; Se, C.-H.; Ng, S.; Khor, B.-H.; Chinna, K.; Goh, B.L.; Gafor, H.A.; Bavanandan, S.; Ahmad, G.; Karupaiah, T. Clinical Efficacy and Feasibility of Whey Protein Isolates Supplementation in Malnourished Peritoneal Dialysis Patients: A Multicenter, Parallel, Open-Label Randomized Controlled Trial. Clin. Nutr. ESPEN 2018, 25, 68–77. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN Guideline on Clinical Nutrition in Hospitalized Patients with Acute or Chronic Kidney Disease. Clin. Nutr. Edinb. Scotl. 2021, 40, 1644–1668. [Google Scholar] [CrossRef]
- Ren, S.; Yao, X.; Ren, S.; Feng, Y. Oral Nutritional Supplement Helps to Improve Nutritional Status of Dialysis Dependent Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2023, 10, 1294064. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.; Bonilla, D.A.; Almendra-Pegueros, R.; Pérez-López, A.; Gamero, A.; dos Santos Duarte Junior, M.A.; Peterman-Rocha, F.; Lozano-Lorca, M.; Camacho-López, S.; Kammar-García, A.; et al. Evaluación de La Ingesta Alimentaria: Una Reflexión Que Nos Acerque al Futuro. Rev. Esp. Nutr. Humana Dietética 2021, 25, 266–268. [Google Scholar] [CrossRef]
- Cordos, M.; Martu, M.A.; Vlad, C.E.; Toma, V.; Ciubotaru, A.D.; Badescu, M.C.; Goriuc, A.; Foia, L. Early Detection of Inflammation and Malnutrition and Prediction of Acute Events in Hemodialysis Patients through PINI (Prognostic Inflammatory and Nutritional Index). Diagnostics 2024, 14, 1273. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tocino, M.L.; Miranda-Serrano, B.; Gracia-Iguacel, C.; de-Alba-Peñaranda, A.M.; Mas-Fontao, S.; López-González, A.; Villoria-González, S.; Pereira-García, M.; Ortíz, A.; González-Parra, E. Sarcopenia Assessed by 4-Step EWGSOP2 in Elderly Hemodialysis Patients: Feasibility and Limitations. PLoS ONE 2022, 17, e0261459. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Barril-Cuadrado, G.; Sánchez, R.; Egido, J.; Ortiz-Arduán, A.; Carrero, J.J. Defining Protein-Energy Wasting Syndrome in Chronic Kidney Disease: Prevalence and Clinical Implications. Nefrología 2014, 34, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Wilczynski, N.; Navarro, T.; Hobson, N.; Jeffery, R.; Keepanasseril, A.; Agoritsas, T.; Mistry, N.; Iorio, A.; Jack, S.; et al. Interventions for Enhancing Medication Adherence. Cochrane Database Syst. Rev. 2014, 2014, CD000011. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.B.; Ackloo, E.; Sahota, N.; McDonald, H.P.; Yao, X. Interventions for Enhancing Medication Adherence. Cochrane Database Syst. Rev. 2008, 2008, CD000011. [Google Scholar] [CrossRef]
- Cano, N.J.M.; Aparicio, M.; Brunori, G.; Carrero, J.J.; Cianciaruso, B.; Fiaccadori, E.; Lindholm, B.; Teplan, V.; Fouque, D.; Guarnieri, G.; et al. ESPEN Guidelines on Parenteral Nutrition: Adult Renal Failure. Clin. Nutr. Edinb. Scotl. 2009, 28, 401–414. [Google Scholar] [CrossRef]
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition Prescription to Achieve Positive Outcomes in Chronic Kidney Disease: A Systematic Review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef]
- Ikizler, T.A. Intradialytic Nutrition and Exercise: Convenience versus Efficacy. Kidney Int. 2019, 96, 549–552. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. Edinb. Scotl. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. Edinb. Scotl. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, O.P.; Bueno, S.M.; Kalergis, A.M. Probiotics in Inflammatory Bowel Disease: Microbial Modulation and Therapeutic Prospects. Trends Mol. Med. 2025, 31, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).