A Proanthocyanidins-Rich Cili (Rosa roxburghii) Fruit Extract Protects CCl4-Induced Mouse Hepatic Fibrosis via Modulation of Ferroptosis and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Preparation of PACs-Enriched Cili Fruit Extract
2.3. Measurement of PACs Content
2.4. UPLC-Q-TOF-MS/MS Analysis
2.5. Cell Culture and Cell Viability Assay
2.6. Mitochondrial Morphology Measurement
2.7. Mouse Model of Liver Fibrosis and Sample Collection
2.8. Zebrafish Liver Fibrosis Model
2.9. Serum Biochemistry and Histopathology
2.10. Gut Microbiota Analysis
2.11. Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
3.1. Proanthocyanidins Composition of PACs-CFE
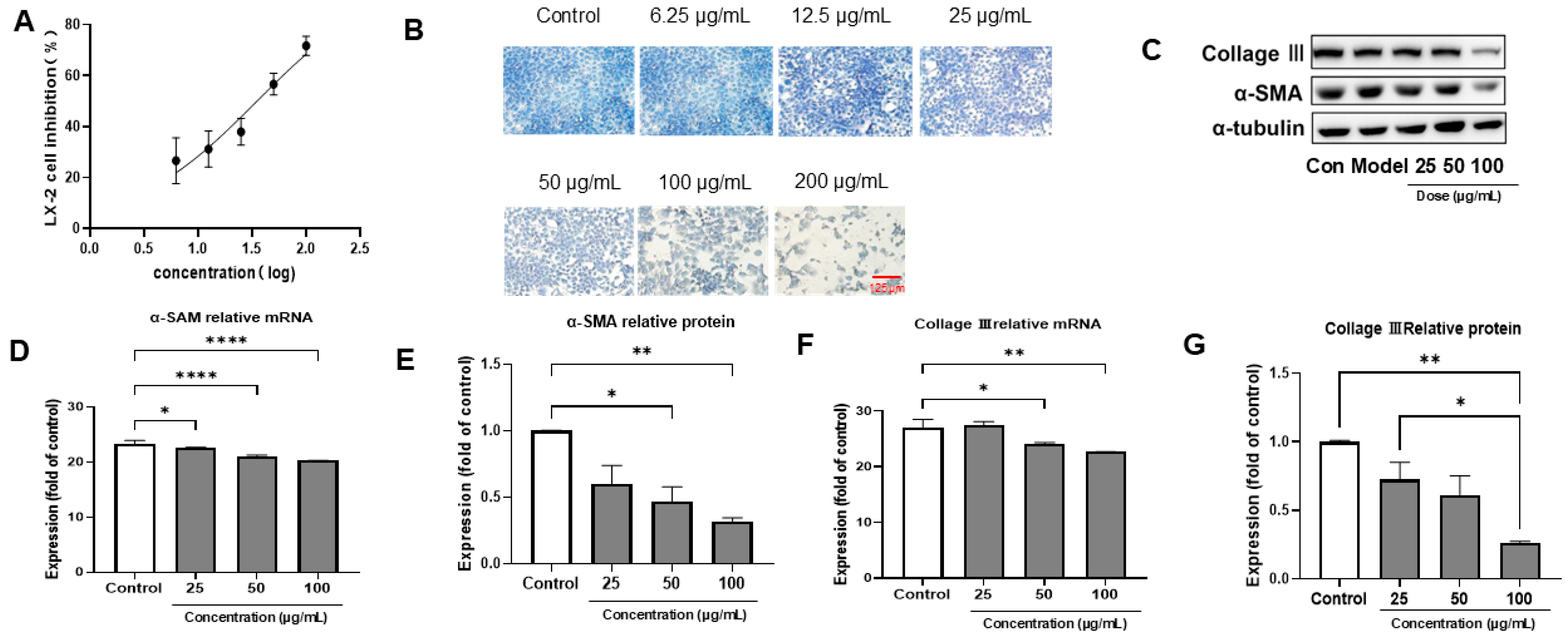
3.2. PACs-CFE Inhibits LX-2 Cell Activation
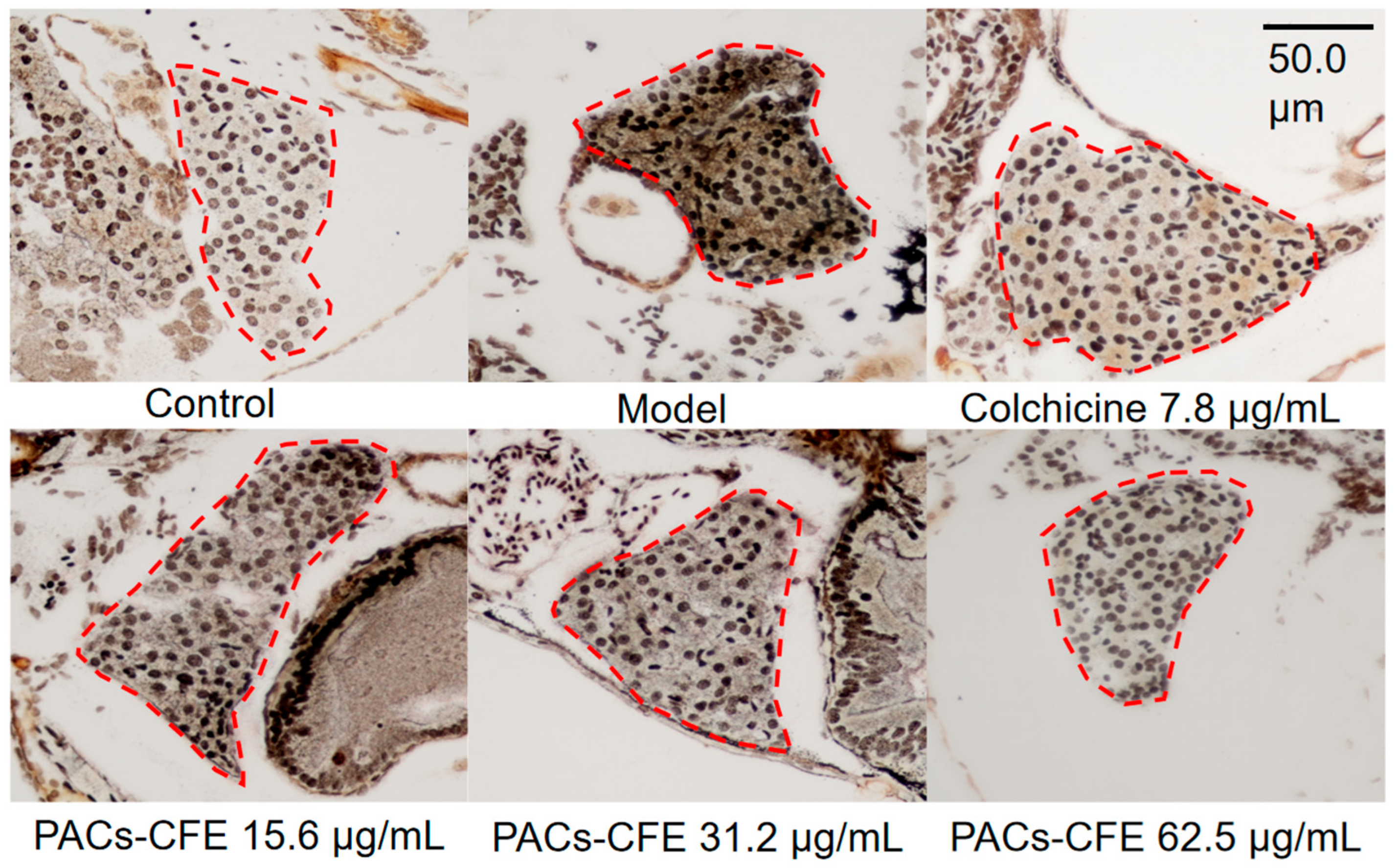
3.3. PACs-CFE Alleviates Liver Fibrosis in Zebrafish
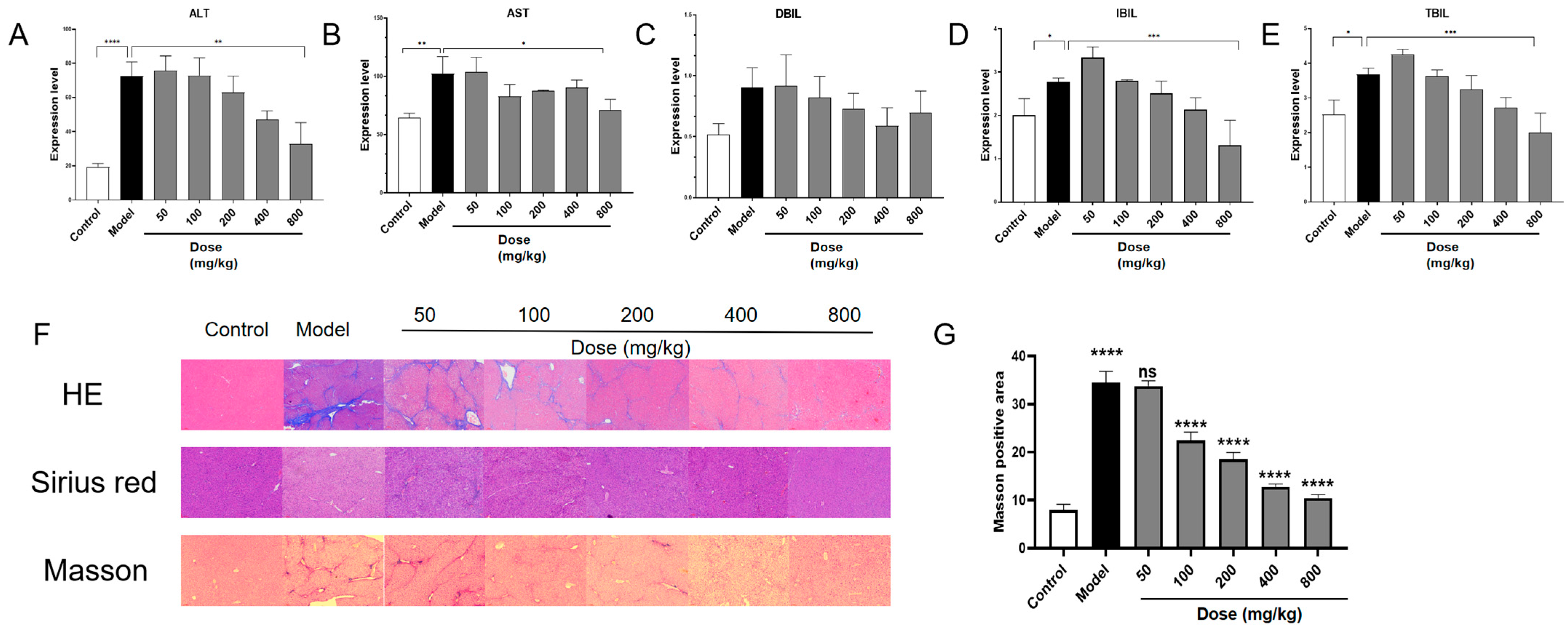
3.4. PACs-CFE Ameliorates Hepatic Fibrosis in Mice
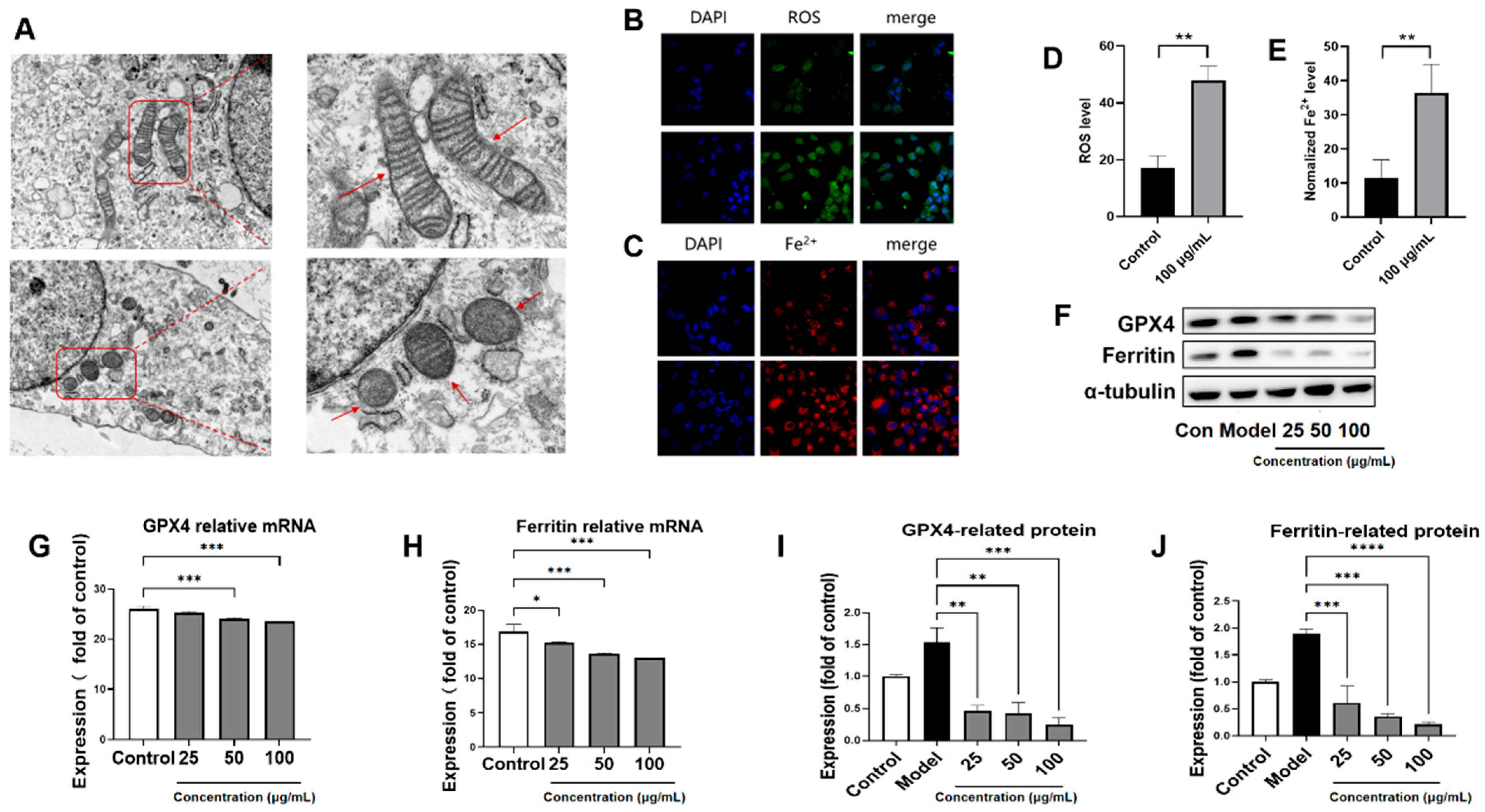
3.5. PACs-CFE Induces Ferroptosis in LX-2 Cells
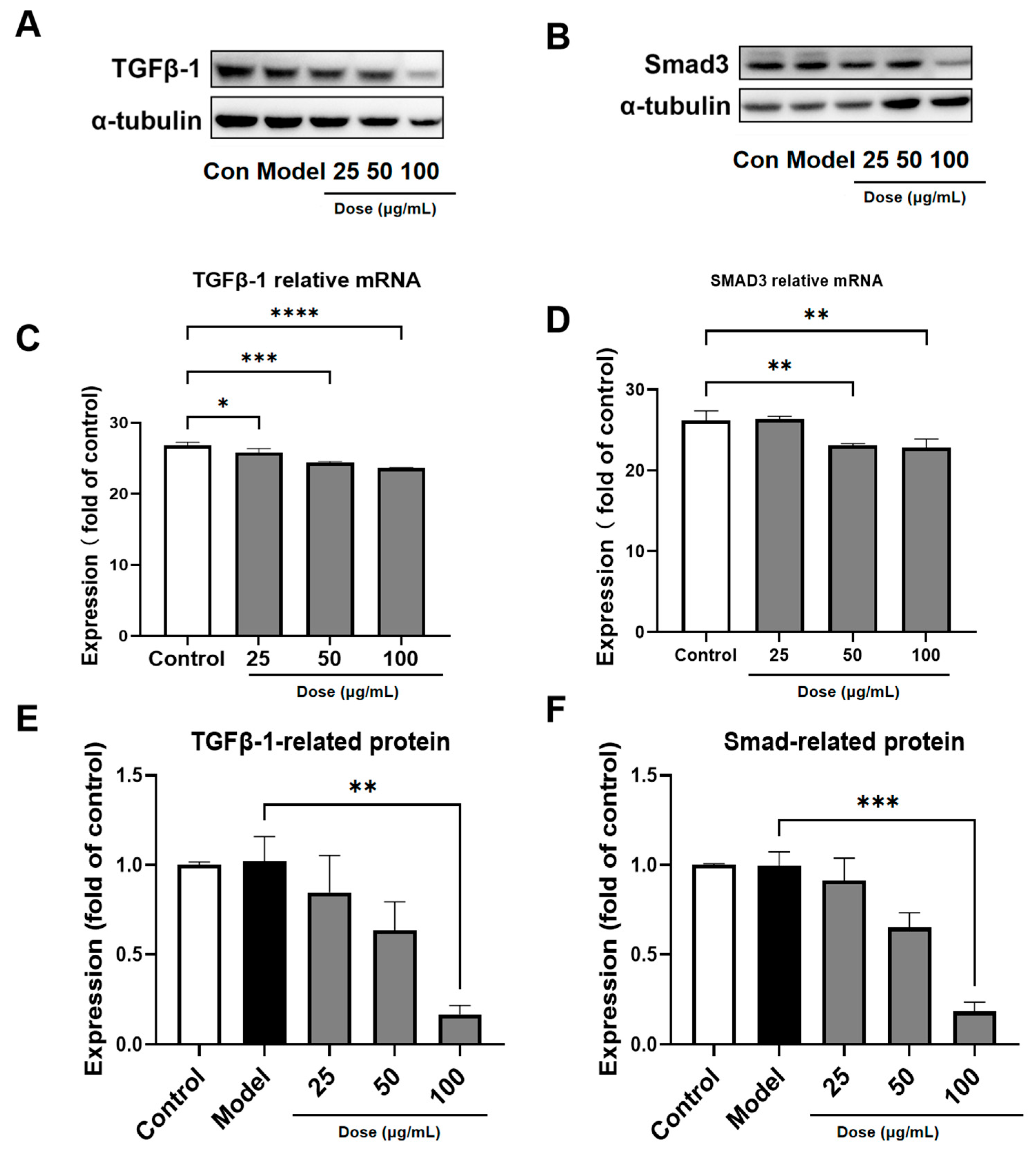
3.6. PACs-CFE Modulates the TGF-β1/Smad3 Pathway
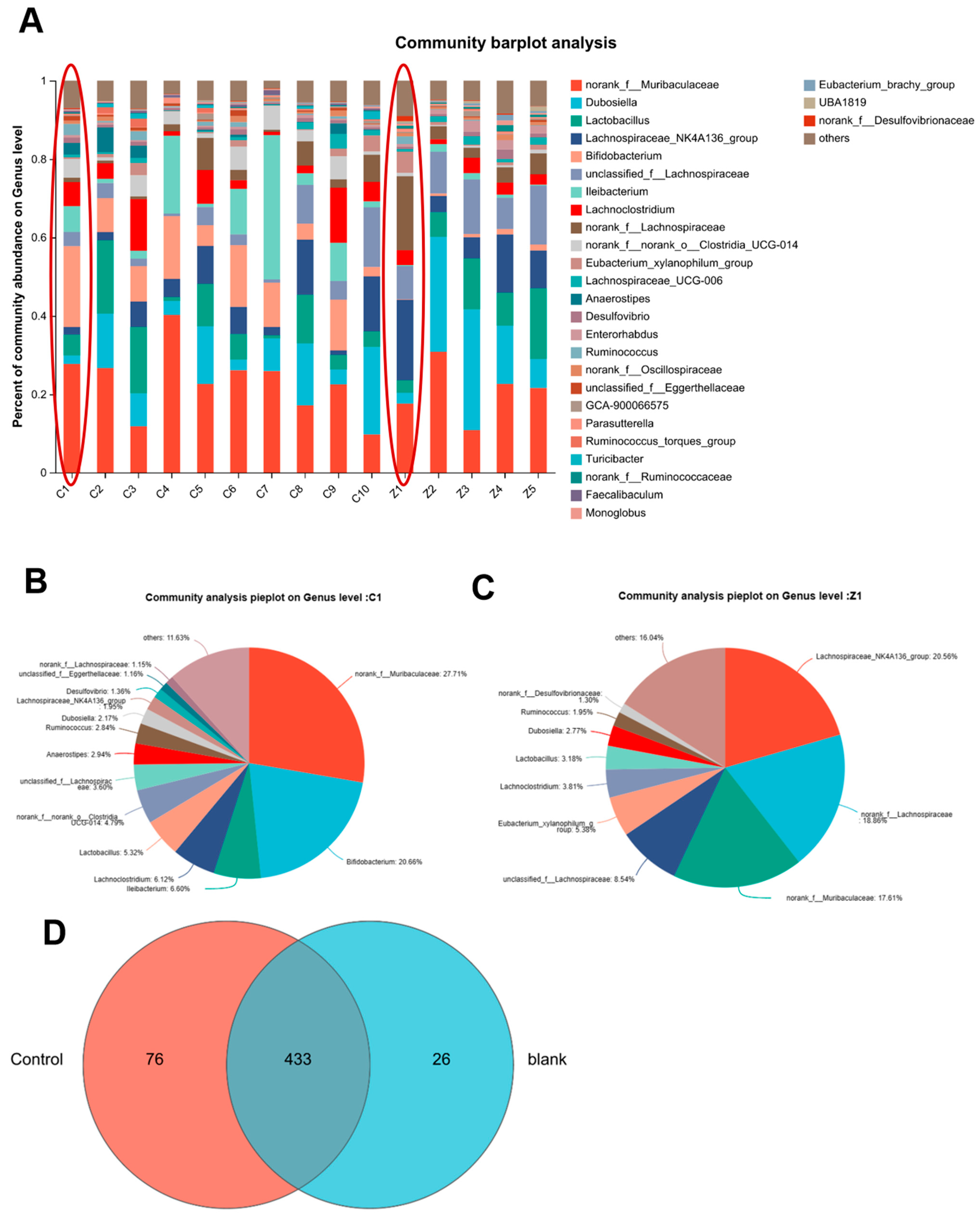
3.7. PACs-CFE Modulates Intestinal Microbiota in Mice with Liver Fibrosis
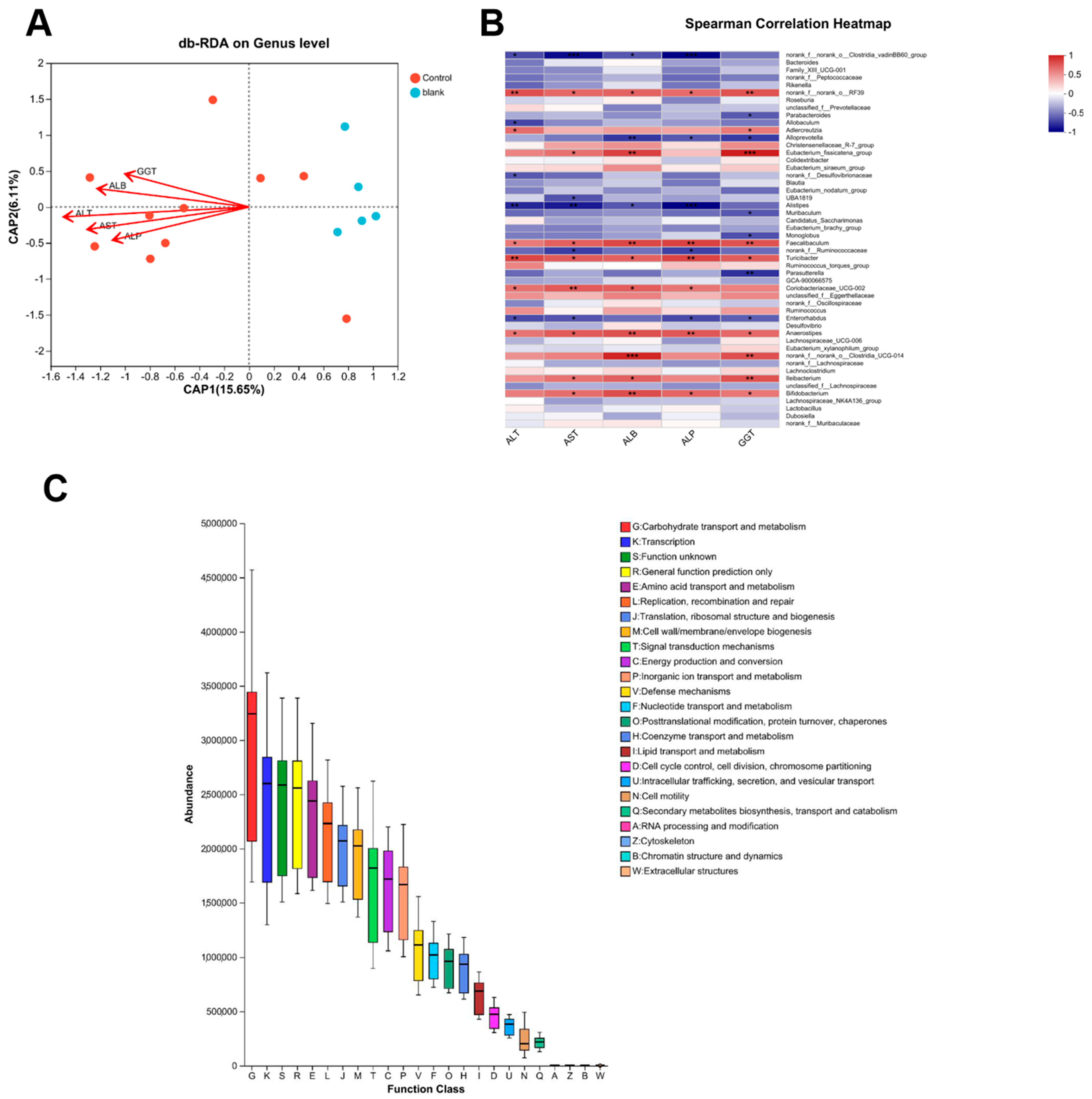
3.8. Functional Prediction of Gut Microbiota and Its Association with Anti-Fibrotic Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, C.; Zhang, Y.; Zhang, L.; Tian, Y.; Zhong, X.; Fang, X.; Yang, Y.; Tao, A. Exploring Rosa roxburghii Tratt polysaccharides: From extraction to application potential in functional products—An in-depth review. Int. J. Biol. Macromol. 2024, 280 Pt 1, 135543. [Google Scholar] [CrossRef]
- Hou, Z.; Yang, H.; Zhao, Y.; Xu, L.; Zhao, L.; Wang, Y.; Liao, X. Chemical characterization and comparison of two chestnut rose cultivars from different regions. Food Chem. 2020, 323, 126806. [Google Scholar] [CrossRef]
- Chen, C.; Tan, S.; Ren, T.; Wang, H.; Dai, X.; Wang, H. Polyphenol from Rosaroxburghii Tratt Fruit Ameliorates the Symptoms of Diabetes by Activating the P13K/AKT Insulin Pathway in db/db Mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Manna, K.; Khan, Z.S.; Saha, M.; Mishra, S.; Gaikwad, N.; Bhakta, J.N.; Banerjee, K.; Das Saha, K. Manjari Medika Grape Seed Extract Protects Methotrexate-Induced Hepatic Inflammation: Involvement of NF-κB/NLRP3 and Nrf2/HO-1 Signaling System. J. Inflamm. Res. 2023, 16, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, W.B.; Cai, X.H.; Lu, D.D.; He, X.Y.; Qiu, P.Y.; Wu, J. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 8171–8175. [Google Scholar] [CrossRef]
- Zhai, B.W.; Zhao, H.; Zhu, H.L.; Huang, H.; Zhang, M.Y.; Fu, Y.J. Triterpene acids from Rosa roxburghii Tratt fruits exert anti-hepatocellular carcinoma activity via ROS/JNK signaling pathway-mediated cell cycle arrest and mitochondrial apoptosis. Phytomed. Int. J. Phytother. Phytopharmacol. 2023, 119, 154960. [Google Scholar] [CrossRef]
- He, R.; Lian, Z.; Cheng, Z.; Liu, Y.; Peng, X.; Wang, Y.; Ma, H.; Zhou, X.; Ge, F. The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects. Foods 2025, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Zikela, L.; Yu, Z.; Han, J.; Zhu, H.; Wang, D.; Wang, X.; Li, S.; Han, Q. Protective effects of fermented Rosa roxburghii Tratt juice against ethanol-induced hepatocyte injury by regulating the NRF2-AMPK signaling pathway in AML-12 cells. Mol. Med. Rep. 2024, 30, 1–13. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef]
- Iredale, J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Investig. 2007, 117, 539–548. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Elpek, G. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014, 20, 7260–7276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, K.; Jia, R.; Xie, J.; Ma, L.; Hao, Z.; Zhang, W.; Mo, J.; Ren, F. Ferroportin-dependent ferroptosis induced by ellagic acid retards liver fibrosis by impairing the SNARE complexes formation. Redox Biol. 2022, 56, 102435. [Google Scholar] [CrossRef]
- Elrazik, N.a.A.; El-Mesery, M.; El-Shishtawy, M.M. Sesamol protects against liver fibrosis induced in rats by modulating lysophosphatidic acid receptor expression and TGF-β/Smad3 signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Akiyama, H.; Kanda, T.; Ohtake, Y.; Goda, Y. Apple procyanidin oligomers absorption in rats after oral administration: Analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 884–892. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical Diversity in Rhizomes of Three Reynoutria Species and their Antioxidant Activity Correlations Elucidated by LC-ESI-MS/MS Analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef]
- Sun, B.; Leandro, M.C.; De Freitas, V.; Spranger, M.I. Fractionation of red wine polyphenols by solid-phase extraction and liquid chromatography. J. Chromatogr. A 2006, 1128, 27–38. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, Y.; Xia, Q.; He, K. Ferroptosis and Liver Fibrosis. Int. J. Med. Sci. 2021, 18, 3361–3366. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, X.; Wang, L.; Fan, W.; Gao, S.; Zhang, D.; Ling, Z.; Zhang, Y.; Ma, L.; Bai, F.; et al. Salvianolic acid B attenuates liver fibrosis by targeting Ecm1 and inhibiting hepatocyte ferroptosis. Redox Biol. 2024, 69, 103029. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef]
- Jurado-Aguilar, J.; Barroso, E.; Bernard, M.; Zhang, M.; Peyman, M.; Rada, P.; Valverde, Á.M.; Wahli, W.; Palomer, X.; Vázquez-Carrera, M. GDF15 activates AMPK and inhibits gluconeogenesis and fibrosis in the liver by attenuating the TGF-β1/SMAD3 pathway. Metab. Clin. Exp. 2024, 152, 155772. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Bu, F.T.; Yang, Y.; Li, H.; Huang, C.; Meng, X.M.; Zhang, L.; Lv, X.W.; Li, J. LEFTY2 alleviates hepatic stellate cell activation and liver fibrosis by regulating the TGF-β1/Smad3 pathway. Mol. Immunol. 2020, 126, 31–39. [Google Scholar] [CrossRef]
- Xie, J.; Tian, S.; Liu, J.; Huang, S.; Yang, M.; Yang, X.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Combination Therapy with Indigo and Indirubin for Ulcerative Colitis via Reinforcing Intestinal Barrier Function. Oxid. Med. Cell Longev. 2023, 2023, 2894695. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, S.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Pine (Pinus massoniana Lamb.) Needle Extract Supplementation Improves Performance, Egg Quality, Serum Parameters, and the Gut Microbiome in Laying Hens. Front. Nutr. 2022, 9, 810462. [Google Scholar] [CrossRef]
- Zhong, Y.-B.; Kang, Z.-P.; Wang, M.-X.; Long, J.; Wang, H.-Y.; Huang, J.-Q.; Wei, S.-Y.; Zhou, W.; Zhao, H.-M.; Liu, D.-Y. Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J. Funct. Foods 2021, 86, 104716. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Q.; Xin, F.; Cao, B.; Qian, L.; Dong, Y. Neonatal Milk Fat Globule Membrane Supplementation During Breastfeeding Ameliorates the Deleterious Effects of Maternal High-Fat Diet on Metabolism and Modulates Gut Microbiota in Adult Mice Offspring in a Sex-Specific Way. Front. Cell. Infect. Microbiol. 2021, 11, 621957. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Maher, T.; Schambelan, M.; Kurtz, I.; Hulter, H.N.; Jones, J.W.; Sebastian, A. Amelioration of metabolic acidosis by dietary potassium restriction in hyperkalemic patients with chronic renal insufficiency. J. Lab. Clin. Med. 1984, 103, 432–445. [Google Scholar] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Zhang, R.; Zhao, L.; Yang, H.; Liao, X. CiLi (Rosa roxburghii Tratt.) polyphenols improve colitis via gut microbiota-lipid mediator-immunity axis. Food Res. Int. 2025, 209, 116257. [Google Scholar] [CrossRef]
- Yang, S.; Huang, X.Y.; Zhou, N.; Wu, Q.; Liu, J.; Shi, J.S. RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice. Nutrients 2022, 14, 1974. [Google Scholar] [CrossRef]
- Ma, L.; Li, C.; Lian, S.; Xu, B.; Lv, H.; Liu, Y.; Lu, J.; Ji, H.; Li, S.; Guo, J.; et al. Procyanidin B2 alleviates liver injury caused by cold stimulation through Sonic hedgehog signalling and autophagy. J. Cell. Mol. Med. 2021, 25, 8015–8027. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, C.; Liu, T.; Li, J.; Wu, L.; Yu, Q.; Li, S.; Zhou, Y.; Zhang, J.; Chen, J.; et al. Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis. J. Cell. Mol. Med. 2019, 23, 6479–6493. [Google Scholar] [CrossRef] [PubMed]
- Justet, A.; Klay, D.; Porcher, R.; Cottin, V.; Ahmad, K.; Molina Molina, M.; Nunes, H.; Reynaud-Gaubert, M.; Naccache, J.M.; Manali, E.; et al. Safety and efficacy of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis and carrying a telomere-related gene mutation. Eur. Respir. J. 2021, 57, 2003198. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, M.; Pan, J.; Xu, X.; Zhang, Y.; Han, X.; Yin, L.; Chen, L.; Ren, J.; Yu, J.; et al. Doxofylline ameliorates liver fibrosis by regulating the ferroptosis signaling pathway. Front. Pharmacol. 2023, 14, 1135366. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Song, Y.; Wei, J.; Li, R.; Fu, R.; Han, P.; Wang, H.; Zhang, G.; Li, S.; Chen, S.; Liu, Z.; et al. Tyrosine kinase receptor B attenuates liver fibrosis by inhibiting TGF-β/SMAD signaling. Hepatology 2023, 78, 1433–1447. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wang, Y.; Fang, L.; Guo, C.; Sang, T.; Peng, H.; Zhao, Q.; Chen, S.; Lin, X.; et al. Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomed. Int. J. Phytother. Phytopharmacol. 2023, 110, 154626. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.; Zhang, S.; Zhai, J.; Wu, S.; Wang, G. The evaluation of hepatoprotective effects of flavonoids from Scorzonera austriaca Wild against CCl4-induced acute liver injury in vitro and in vivo. Drug Chem. Toxicol. 2022, 45, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
| NO | RT/min | m/z [M-H]- | Identification | Molecular Formula | MS2 Ions (m/z) | ppm |
|---|---|---|---|---|---|---|
| 1 | 1.88 | 865.1974 | B-type proanthocyanidin trimer | C45H38O18 | 865.1976, 739.1680, 695.1403, 577.1363, 407.0780, 287.0564 | −1.3 |
| 2 | 4.12 | 865.1978 | B-type proanthocyanidin trimer | C45H38O18 | 865.1985, 739.1697, 695.1416, 577.1350, 407.0778, 287.0564 | −0.9 |
| 3 | 5.44 | 577.1344 | B-type proanthocyanidin dimer | C30H26O12 | 577.1346, 451.1033, 425.0876, 407.0766, 289.0712, 255.0301, 125.0242 | 1.3 |
| 4 | 6.39 | 865.1975 | B-type proanthocyanidin trimer | C45H38O18 | 865.2011, 739.1680, 695.1409, 577.1373, 407.0776, 287.0564 | −1.2 |
| 5 | 7.05 | 865.1989 | B-type proanthocyanidin trimer | C45H38O18 | 865.1953, 739.1672, 695.1398, 577.1337, 407.0770, 287.0556 | 0.4 |
| 6 | 7.07 | 1153.2619 | B-type procyanidin tetramers | C60H50O28 | 1153.2646, 1001.2092, 983.2072, 865.1980, 575.1198, 449.0911, 739.1715 | 0 |
| 7 | 7.8 | 577.1352 | B-type proanthocyanidin dimer | C30H26O12 | 577.1343, 451.1019, 425.0911, 407.0779, 289.0709, 287.0564, 245.0825, 125.0240 | 0.2 |
| 8 | 8.16 | 865.1974 | B-type proanthocyanidin trimer | C45H38O18 | 865.1953, 739.1672, 695.1398, 577.1337, 407.0770, 287.0556 | −1.3 |
| 9 | 8.52 | 1153.2621 | B-type procyanidin tetramers | C60H50O28 | 1153.2640, 1001.2169, 983.2226, 865.1972, 575.1209, 449.0897, 739.1673 | 0.2 |
| 10 | 9.49 | 865.1982 | B-type proanthocyanidin trimer | C45H38O18 | 865.1977, 739.1671, 695.1415, 7.1354, 407.0781, 287.0564 | −0.4 |
| 11 | 9.6 | 1153.2608 | B-type procyanidin tetramers | C60H50O28 | 1153.2585, 1001.2150, 983.2022, 865.1967, 575.1186, 449.0878, 739.1668 | −1 |
| 12 | 9.68 | 289.0719 | catechins | C15H14O6 | 245.0796, 205.0503, 203.0722 | 0.5 |
| 13 | 10.2 | 865.1979 | B-type proanthocyanidin trimer | C45H38O18 | 865.1957, 739.1660, 695.1402, 577.1344, 407.0767, 287.0559 | −0.7 |
| 14 | 10.67 | 1153.2627 | B-type procyanidin tetramers | C60H50O28 | 1153.2591, 1001.2169, 983.2085, 865.1984, 575.1204, 449.0885, 739.1670 | 0.7 |
| 15 | 11.17 | 577.1341 | B-type proanthocyanidin dimer | C30H26O12 | 577.1354, 451.1041, 425.0876, 407.0767, 289.0713, 299.0567, 125.0241 | 1.7 |
| 16 | 11.44 | 1153.2625 | B-type procyanidin tetramers | C60H50O28 | 1153.2602, 1001.2149, 983.2193, 865.1972, 739.1685, 575.1201, 449.0879 | 0.5 |
| 17 | 11.7 | 865.1987 | B-type proanthocyanidin trimer | C45H38O18 | 865, 1971, 739.1665, 695.1405, 577.1361, 407.0775, 287.0560 | 0.2 |
| 18 | 11.81 | 729.1456 | B-type proanthocyanidin dimeric monogallate | C52H42O22 | 729.1448, 577.1343, 425.0876, 407.0770, 287.0568 | 0.7 |
| 19 | 12.13 | 1153.2622 | B-type procyanidin tetramers | C60H50O28 | 1153.2621, 1001.2182, 983.2076, 865.2001, 739.1703, 575.1201, 449.0877 | 0.2 |
| 20 | 12.42 | 1017.2082 | B-type proanthocyanidin trimeric monogallate | C52H42O22 | 1017.2063, 891.1785, 865.1923, 847.1709, 729.1449, 577.1339, 407.0770, 287.0554 | −1.3 |
| 21 | 12.47 | 577.135 | B-type proanthocyanidin dimer | C30H26O12 | 577.1334, 425.0869, 407.0759, 289.0712, 287.0555, 125.0244 | 0.3 |
| 22 | 13.2 | 1153.2618 | B-type procyanidin tetramers | C60H50O28 | 1153.2601, 1001.2159, 983.2059, 865.1971, 739.1668, 575.1210, 449.0888 | −0.1 |
| 23 | 14.24 | 1153.2611 | B-type procyanidin tetramers | C60H50O28 | 1153.2592, 1001.2146, 983.2041, 865.1991, 739.1683, 575.1199, 449.0882 | −0.7 |
| 24 | 15.27 | 1153.2611 | B-type procyanidin tetramers | C60H50O28 | 1153.2595, 1001.2140, 983.2060, 865.2001, 739.1672, 575.1186, 449.0874 | −0.7 |
| 25 | 15.73 | 729.1443 | B-type proanthocyanidin dimeric monogallate | C37H30O16 | 729.1442, 577.1326, 425.0862, 407.0756, 287.0707 | 2.5 |
| 26 | 16.55 | 577.1339 | B-type proanthocyanidin dimer | C30H26O12 | 577.1579, 407.0772, 301.0709, 289.0711, 245.0822, 125.0245 | 2.2 |
| 27 | 17.78 | 729.1465 | B-type proanthocyanidin dimeric monogallate | C37H30O16 | 729.1479, 577.1335, 425.0882, 407.0771, 287.0566, 289.0719 | 0.5 |
| 28 | 17.88 | 865.1976 | B-type proanthocyanidin trimer | C45H38O18 | 865.1969, 739.1683, 695.1398, 577.1354, 407.0770, 287.0559 | −1.1 |
| 29 | 18.67 | 1153.2612 | B-type procyanidin tetramers | C60H50O28 | 1153.2612, 1001.2169, 983.2032, 865.1998, 739.1681, 575.1200, 449.0866 | −0.6 |
| 30 | 18.97 | 1017.2103 | B-type proanthocyanidin trimeric monogallate | C52H42O22 | 1017.2092, 891.1809, 865.1638, 847.1553, 729.1460, 729.1460, 577.1375, 407.0823, 287.0563 | 0.8 |
| 31 | 19.21 | 865.197 | B-type proanthocyanidin trimer | C45H38O18 | 865.1950, 739.1665, 695.1412, 577.1360, 407.0764, 287.0560 | −1.8 |
| 32 | 19.65 | 729.146 | B-type proanthocyanidin dimeric monogallate | C37H30O16 | 729.1470, 577.1356, 425.0866, 407.0768, 289.0716, 287.0548 | 0.2 |
| 33 | 19.77 | 1153.2617 | B-type procyanidin tetramers | C60H50O28 | 1153.2602, 1001.2171, 983.2070, 865.2009, 739.168, 575.1202, 449.0862 | 0.2 |
| 34 | 20.32 | 865.1972 | B-type proanthocyanidin trimer | C45H38O18 | 865.1972, 739.1679, 695.1400, 577.1354, 407.0770, 287.0564 | −1.5 |
| 35 | 21.01 | 577.1344 | B-type proanthocyanidin dimer | C30H26O12 | 577.1761, 407.0777, 289.0714, 287.0567, 245.0474, 125.0236 | 1.3 |
| 36 | 21.5 | 289.0719 | epicatechin | C15H14O6 | 245.0817, 205.0483, 203.0698 | 0.5 |
| 37 | 24.43 | 1017.2098 | B-type proanthocyanidin trimeric monogallate | C52H42O22 | 1017.2080, 891.1795, 865.1624, 847.1614, 729.1460, 577.1349, 407.0769, 287.0556 | 0.3 |
| 38 | 30.88 | 577.135 | B-type proanthocyanidin dimer | C30H26O12 | 577.1365, 425.0942, 289.0715, 287.0562, 125.0241, 245.0816, 407.0773 | 0.2 |
| PACs-CFE | |
|---|---|
| The total proanthocyanidin (%) | 84.2 |
| Proanthocyanidin B1 (%) | 1.9 |
| (+)-catechin (%) | 9.9 |
| (−)-epicatechin (%) | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zheng, J.; Zheng, X.; Zhou, D.; Ma, H.; Zhou, X.; Ge, F. A Proanthocyanidins-Rich Cili (Rosa roxburghii) Fruit Extract Protects CCl4-Induced Mouse Hepatic Fibrosis via Modulation of Ferroptosis and Gut Microbiota. Nutrients 2025, 17, 3463. https://doi.org/10.3390/nu17213463
Liu Y, Zheng J, Zheng X, Zhou D, Ma H, Zhou X, Ge F. A Proanthocyanidins-Rich Cili (Rosa roxburghii) Fruit Extract Protects CCl4-Induced Mouse Hepatic Fibrosis via Modulation of Ferroptosis and Gut Microbiota. Nutrients. 2025; 17(21):3463. https://doi.org/10.3390/nu17213463
Chicago/Turabian StyleLiu, Yang, Jingzhong Zheng, Xin Zheng, Dan Zhou, Hang Ma, Xue Zhou, and Fahuan Ge. 2025. "A Proanthocyanidins-Rich Cili (Rosa roxburghii) Fruit Extract Protects CCl4-Induced Mouse Hepatic Fibrosis via Modulation of Ferroptosis and Gut Microbiota" Nutrients 17, no. 21: 3463. https://doi.org/10.3390/nu17213463
APA StyleLiu, Y., Zheng, J., Zheng, X., Zhou, D., Ma, H., Zhou, X., & Ge, F. (2025). A Proanthocyanidins-Rich Cili (Rosa roxburghii) Fruit Extract Protects CCl4-Induced Mouse Hepatic Fibrosis via Modulation of Ferroptosis and Gut Microbiota. Nutrients, 17(21), 3463. https://doi.org/10.3390/nu17213463







