Abstract
Background/Objectives: Eleutherococcus senticosus (Siberian ginseng) is an adaptogenic plant widely recognized for its antioxidant and immunomodulatory properties; however its hepatoprotective potential properties are unexplored. This study aimed to evaluate whether the fruit extract of E. senticosus contains chemical constituents with hepatoprotective effects in a paracetamol-induced liver injury model in mice. Methods: Female BALB/c mice were randomized into five groups: control, paracetamol (300 mg/kg, IP), E. senticosus extract (750 or 1500 mg/kg, PO) + paracetamol, and silymarin (50 mg/kg) + paracetamol. Extracts were administered for seven days before paracetamol challenge. Biochemical markers (ALT, AST, urea, creatinine, protein, albumin) and hematological parameters were assessed, and organs were subjected to histopathological examination. Phytochemical characterization of the extract was performed using UHPLC-DAD-MS and ICP-OES. Results: The 750 mg/kg dose of E. senticosus extract maintained ALT, AST, urea, and creatinine levels close to control values, while the higher dose (1500 mg/kg) was less effective and showed an increase in serum urea. Both extract doses and silymarin attenuated creatinine elevation induced by paracetamol. No histopathological changes were detected in the kidneys or brains of treated animals. Phytochemical analysis revealed high contents of phenolic acids (chlorogenic and dicaffeoylquinic acids), flavonoids, amino acids, and essential minerals. Conclusions: E. senticosus fruit extract demonstrated a hepatoprotective effect at an optimal dose (750 mg/kg), indicating a potential dose-dependent effect. The absence of histopathological alterations in key organs supports the fruit extract’s safety.
1. Introduction
Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. (ES), commonly known as Siberian ginseng, has been gaining interest for decades as a natural remedy supporting immunity and the body’s adaptation to stress. Its action as an adaptogen and immunostimulant has been used in traditional Chinese and Russian medicine for centuries, where the roots were used as a tonic to strengthen, prolong life, and improve overall well-being [1,2]. In Europe, research on this plant intensified in the second half of the 20th century, and E. senticosus is now widely used as an ingredient in supplements that support immunity and improve physical and mental performance. Its rich phytochemical composition includes eleutherosides, flavonoids, lignans, and phenolic acids, which are believed to have anti-inflammatory, antioxidant, and immunomodulatory properties [3].
The fruit of Eleutherococcus senticosus is a berry that can be soaked in water or processed into products such as fruit wine and fruit vinegar. These preparations are valued for their contribution to daily health care [4]. Recent research has highlighted the nutritional and functional potential of E. senticosus fruits. Besides the well-characterized eleutherosides, the fruits are a natural source of dietary polyphenols (including lignans (sesamin, syringaresinol), phenolic acids (chlorogenic acid, caffeic acid), polysaccharides, coumarins, and flavonoids (quercetin, kaempferol)), which contribute to their antioxidant activity and may provide protection against oxidative stress associated with liver injury [5,6,7]. The fruits also contain high levels of minerals, including calcium (3730–4495 mg/kg), magnesium (1430–1540 mg/kg), iron (35.4–53 mg/kg), manganese (75.2–88.3 mg/kg), zinc (18.9–41.0 mg/kg), copper (3.34–13 mg/kg), and selenium (0.19–0.61 mg/kg) [8]. Notably, significant amounts of myo-inositol and D-mannitol were detected [8,9,10]. Compositional analyses of ES fruits have shown that, alongside polyphenols, they also contain fatty acids and tocopherols. This combination not only supports their antioxidant properties but also expands their potential applications in pharma-nutrition [11]. Among the best-known active compounds obtained from the ES extracts, eleutheroside B (syringin) and eleutheroside E (syringaresinol) are the best known, and have been shown to influence enzymatic activity and cell membrane stability. Furthermore, studies on plant extracts containing these compounds suggest their ability to regulate ion transport in epithelial cells and alleviate oxidative stress and inflammation [12,13].
Eleutherococcus senticosus has wide-raging spectrum of health-promoting properties, including potential hepatoprotective effects. Its beneficial results on the liver have not yet been as extensively studied as other hepatoprotective herbs, such as Silybum marianum L. (milk thistle), which contains silymarin, a flavonolignan witch gargantuan hepatoprotective properties. It has been extensively studied in animal models, such as mice, to protect the liver from a variety of damages, including those induced by toxins such as acetaminophen (paracetamol) [14,15,16,17].
In our study, we used intractum, an extract obtained by macerating fresh plant material in water-ethanol mixture. Such a method of preparation ensures the preservation of a broader spectrum of active compounds, including volatile and easily degradable substances, which may be lost during drying. This allows the extract to better reflect the natural composition and biological activity of the fresh plant. To date, there is a lack of studies conducted to confirm the E. senticosus fruit extract’s hepatoprotective properties. Currently, available research suggests certain mechanisms that may support liver protection. In vitro studies have shown that E. senticosus fruit extract contains chemicals that may have beneficial effects on liver cells. Furthermore, the extract exerted an immunomodulatory effect by proliferating peripheral blood leukocytes, reducing their susceptibility to VSV infection [9]. In addition, compounds found in both the fruits and roots of E. senticosus have been shown to influence the activity of enzymes such as hyaluronidase, suggesting their potential role in managing conditions associated with inflammation [18]. E. senticosus has also been reported to decrease cardiovascular responses to stress, supporting its role in stress adaptation [19]. Moreover, leaf extracts of E. senticosus have shown antidiabetic activity and improvement of lipid metabolism, suggesting potential applications in diabetes management [20].
Therefore, the aim of the present study was to determine whether the fruit extract of Eleutherococcus senticosus exhibits hepatoprotective properties in a mouse model of paracetamol-induced liver damage. And finally, whether the fruits can be used as food ingredients.
2. Results and Discussion
In preclinical studies, mice are often used as an animal model to study the hepatotoxicity of paracetamol. Typically, they are given appropriate doses of paracetamol to induce toxic effects similar to those observed in humans. Paracetamol is metabolized in the liver, primarily by the cytochrome P450 enzyme system, particularly the CYP2E1 isoform. A small portion of this metabolism leads to the formation of a toxic metabolite, NAPQI (N-acetyl-p-benzoquinoneimine). At high concentrations, NAPQI binds to proteins in liver cells (hepatocytes), causing damage and necrosis. This leads to acute liver damage. Accumulation of NAPQI also leads to oxidative stress, which further damages hepatocytes and enhances inflammatory processes. Over time, hepatocyte damage leads to an inflammatory response and the development of liver necrosis, which is a clinical manifestation of hepatotoxicity [14,17,21]. Silymarin, a flavonolignan extracted from milk thistle (Silybum marianum), is known for its strong hepatoprotective properties. Its effectiveness has been extensively documented in animal models, including mice, where it has been shown to protect the liver from damage caused by toxins such as acetaminophen. The action of silymarin is based primarily on its antioxidant properties–this compound effectively neutralizes free radicals and reduces oxidative stress, which plays a key role in the pathogenesis of liver damage, especially in the presence of toxic metabolites such as NAPQI. Additionally, silymarin supports the restoration of glutathione, which is essential for detoxification processes in the liver. Through these mechanisms, it may limit liver damage even in the event of a paracetamol overdose [15,16,21]. The roots of Eleutherococcus senticosus, known as an adaptogen, exhibit a wide range of beneficial health properties, including potential liver protection. However, a hepatoprotective potential of the fruits has not yet been studied as thoroughly as that of the roots or silymarin. Available data suggest mechanisms that may support liver protection.
The study was performed in mice, which are commonly used preclinical models due to physiological similarities to humans. While direct extrapolation to humans should be performed with caution, the observed protective effects of Eleutherococcus senticosus extract provide valuable insights into its potential activity. These findings may help guide further research in other models and inform possible relevance to human health.
2.1. Evaluation of the Hepatoprotective Effect of the Eleutherococcus senticosus Fruit Extract in a Model of Liver Damage with Paracetamol
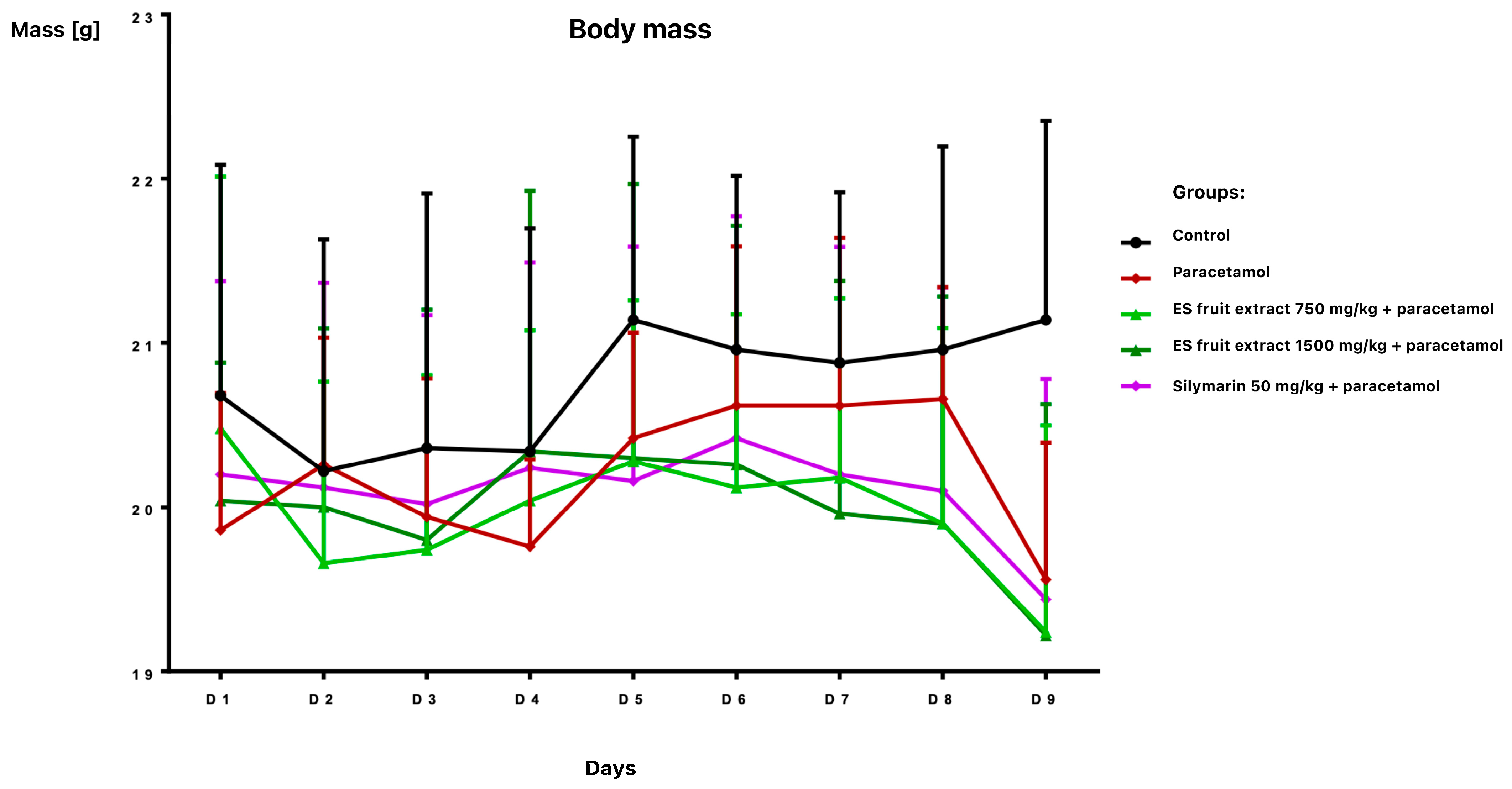
The study provided significant new evidence for the hepatoprotective properties of Eleutherococcus senticosus fruit extract in a mouse model of paracetamol-induced liver damage. The effects of Eleutherococcus senticosus fruit extract (750 and 1500 mg/kg) and silymarin (50 mg/kg) on paracetamol-induced alterations in hematological and biochemical parameters in mice were evaluated. Oral administrations were well tolerated, with no adverse effects observed in any group during the 7-day pre-treatment period. Body weight remained stable until day 8 (Figure 1).
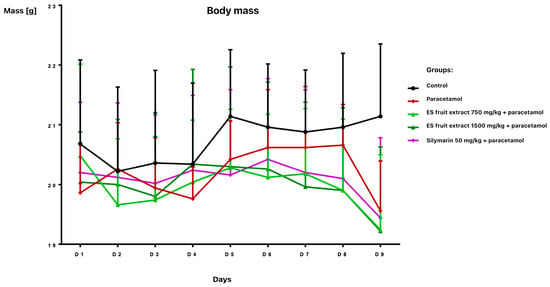
Figure 1.
Body weight [g] of mice receiving the test preparations.
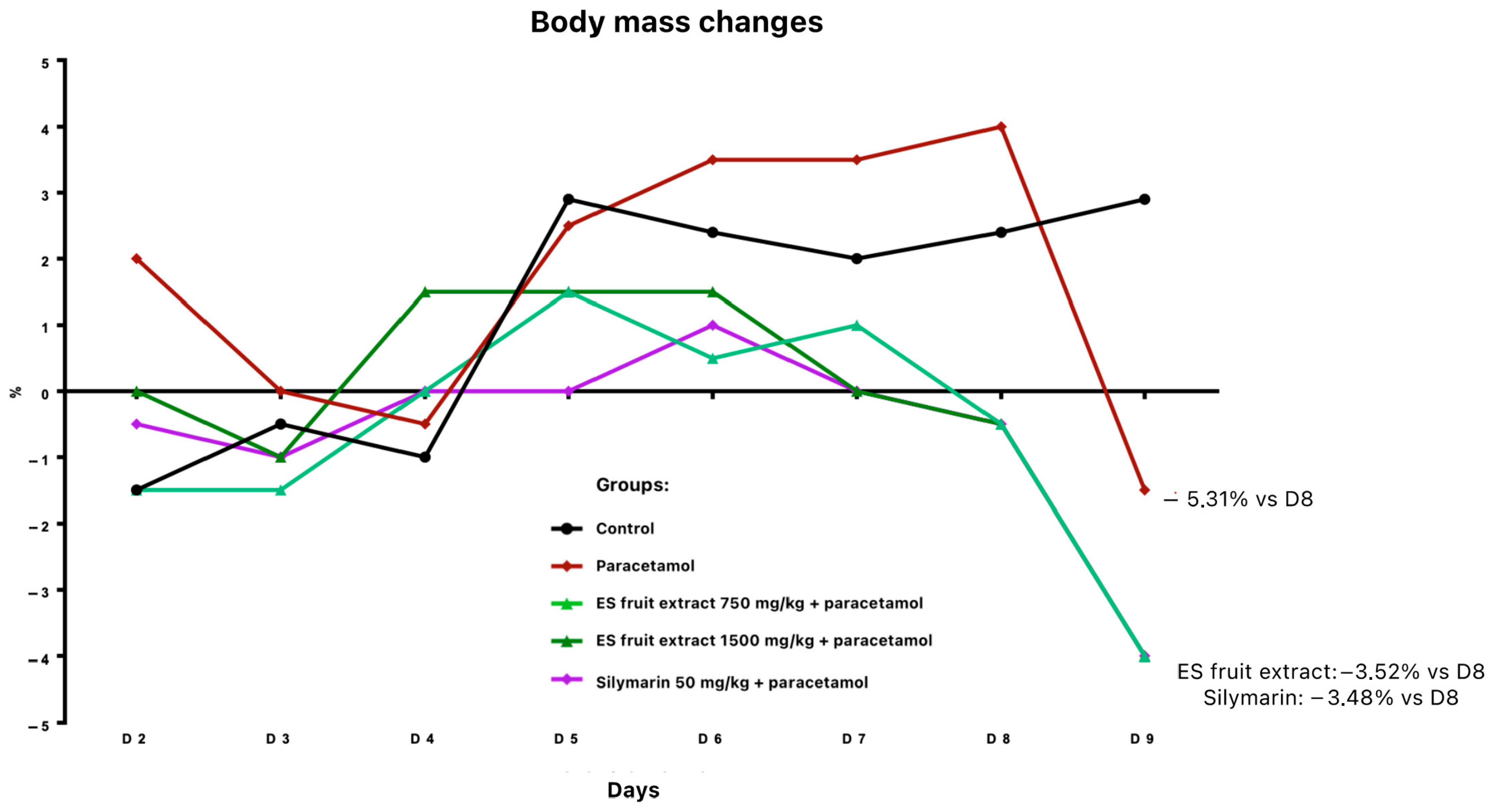
Twenty-four hours after paracetamol administration, a body weight reduction was noted: 3.5% in groups pre-treated with fruit extract or silymarin, and 5.3% in the paracetamol-only group (relative to day 8) (Figure 2). All paracetamol-treated animals exhibited ruffled fur.
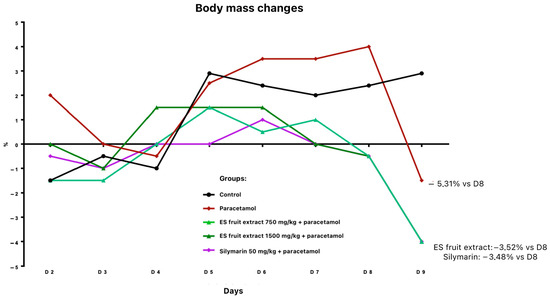
Figure 2.
Changes in body weight [%] receiving the test preparations.
Total organ weights suggested a trend towards decreased liver and spleen weights in pre-treated groups; However, when organ weights were adjusted relative to body weight, no statistically significant differences were observed. Lung weights were slightly higher in paracetamol-treated mice (Table 1). This suggests that preventive administration of an adaptogen or silymarin partially alleviates the acute metabolic stress induced by paracetamol overdose, which may be due to maintaining better water-electrolyte homeostasis and general condition of the mice.

Table 1.
Organ mass per body mass [%].
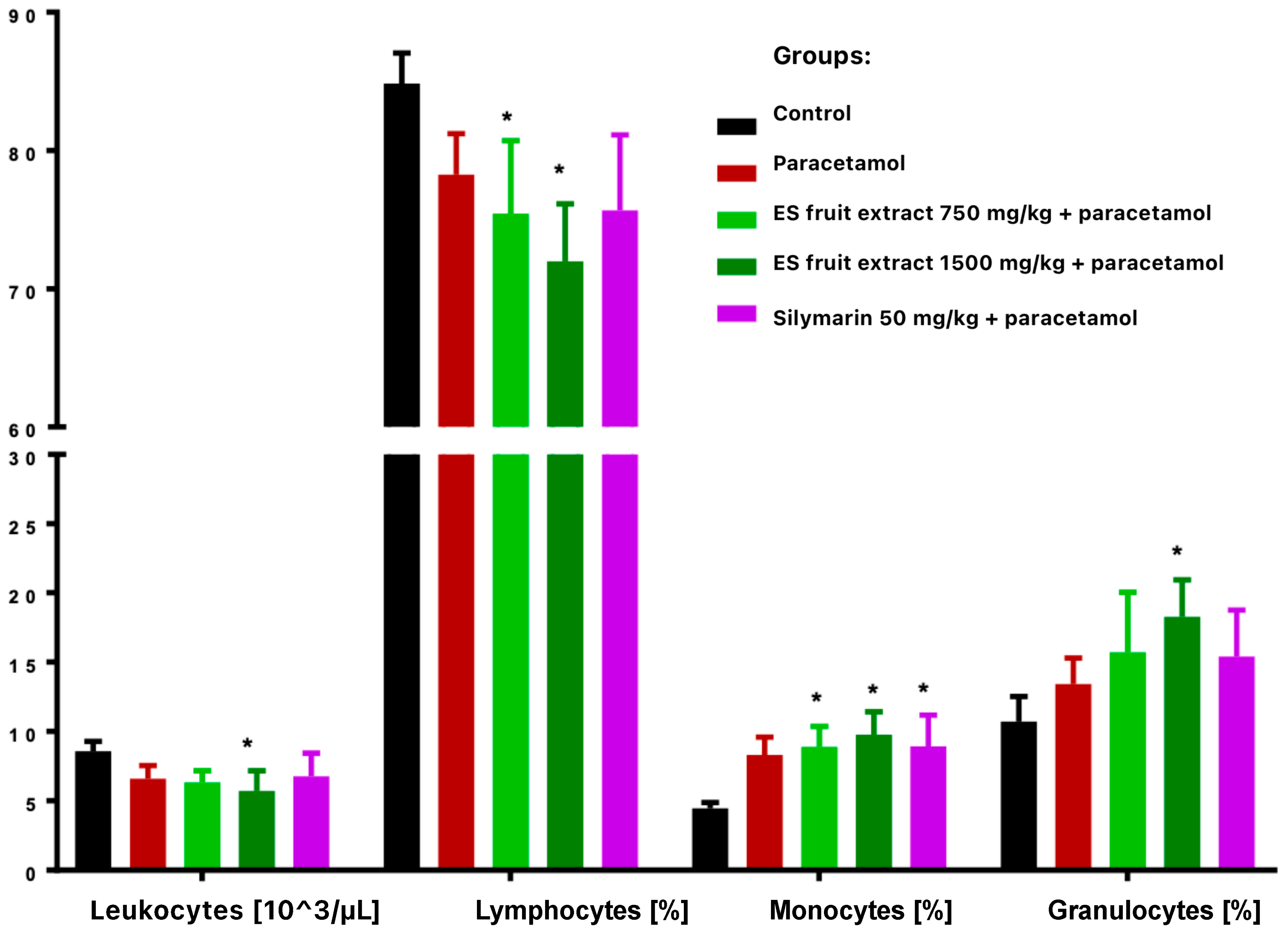
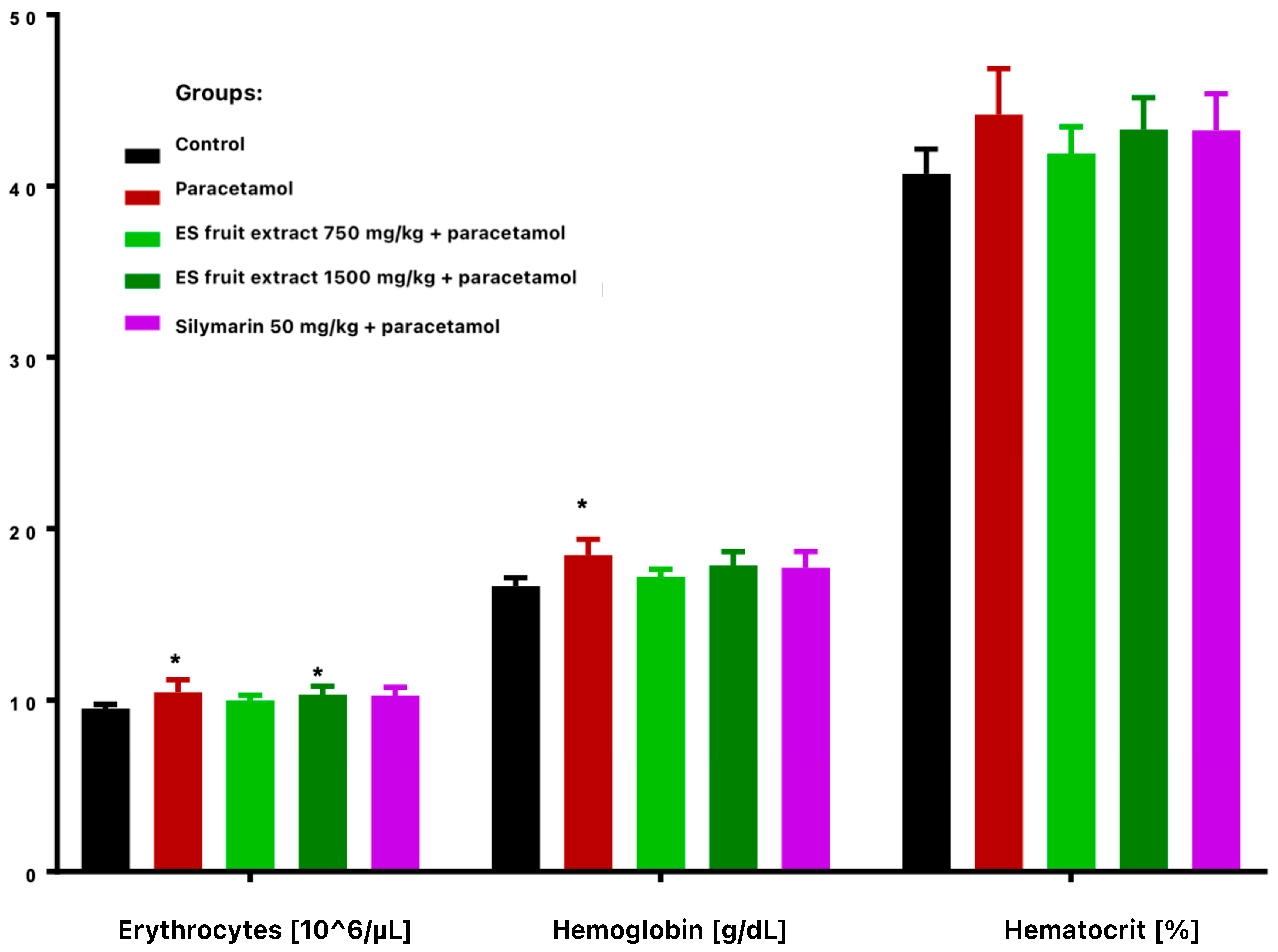
Paracetamol treatment reduced total leukocyte count and altered leukocyte distribution, with decreased lymphocytes and increased monocytes and granulocytes (Figure 3). These changes were more vivid and statistically significant in extract-treated groups compared with controls. Paracetamol alone significantly increased erythrocyte count and hemoglobin concentration (Figure 4), while hematocrit increase was not significant.
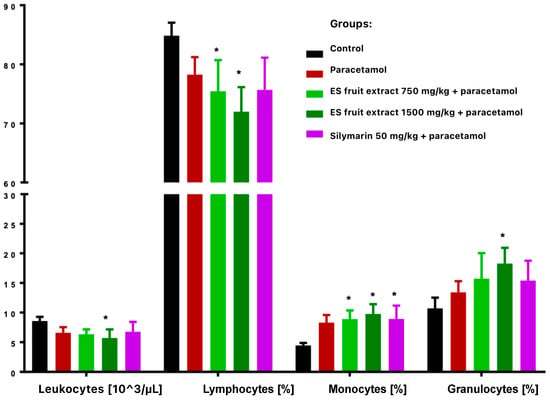
Figure 3.
White blood cells smear: leukocytes [103/µL], lymphocytes [%], monocytes [%], granulocytes [%]. * p < 0.05 nonparametric Kruskal–Wallis test, comparison vs. control.
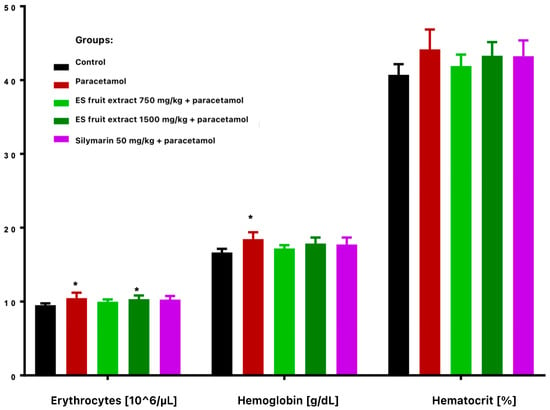
Figure 4.
Red blood cells parameters: erythrocytes [106/µL], hemoglobin [g/dL], hematocrit [%]. * p < 0.05 nonparametric Kruskal–Wallis test, comparison vs. control.
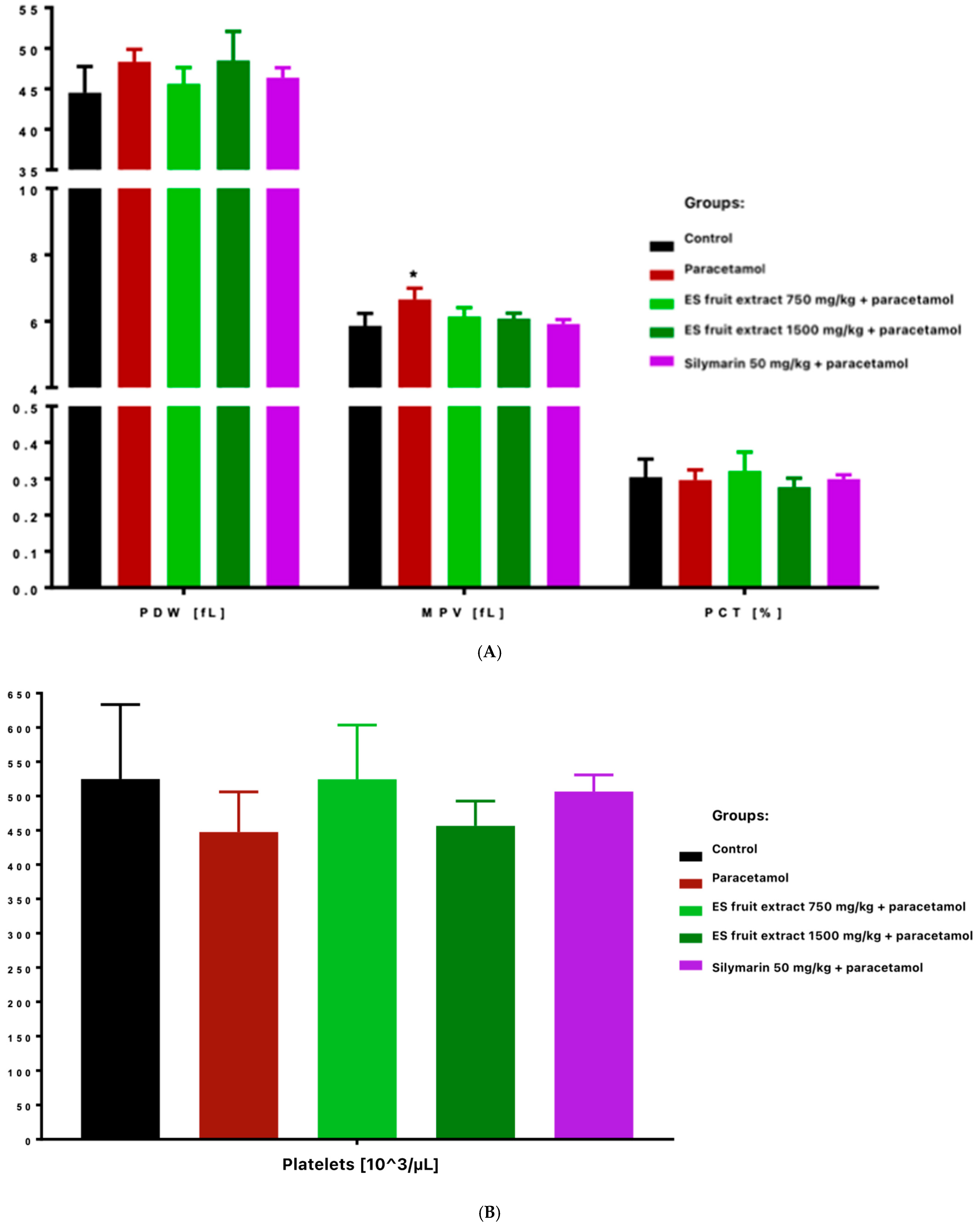
Platelet count decreased in paracetamol and high-dose ES fruit extract (1500 mg/kg) groups, accompanied by increased platelet anisocytosis (PDW) and mean platelet volume (MPV), and reduced plateletcrit (PCT) (Figure 5). Those results correspond to an acute stress and inflammatory reaction-paracetamol, especially in high doses, may cause the displacement of leukocytes (especially lymphocytes) into damaged tissues and the mobilization of neutrophils. In other words, the adaptogen did not prevent the decrease in leukocytes after paracetamol and even seemed to enhance it (these differences were statistically significant compared to the control group).
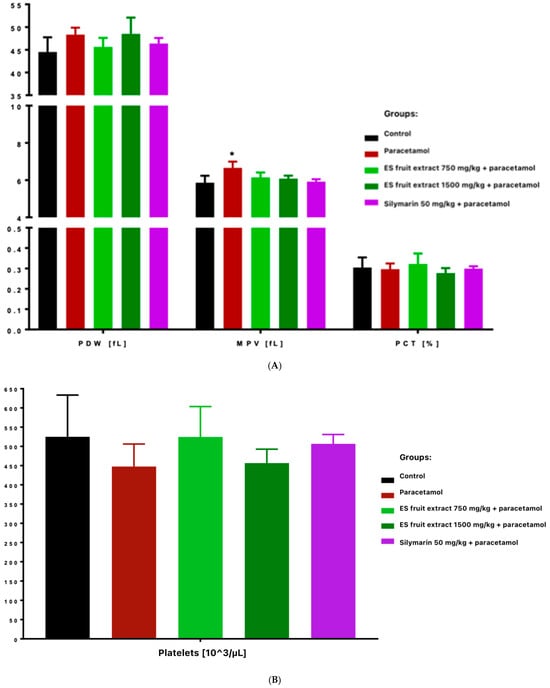
Figure 5.
Platelet parameters: platelet distribution width [fL], mean platelet volume [fL], plateletcrit [%] (A) and platelets [103/µL] (B). * p < 0.05 nonparametric Kruskal–Wallis test, comparison vs. control.
The most interesting results concern platelet parameters. The overdose of the paracetamol caused a significant reduction in platelet count. This was accompanied by an increase in platelet anisocytosis (PDW) and mean platelet volume (MPV) with a decrease in platelet permeability (PCT). This finding suggests increased platelet destruction or consumption (e.g., via microthrombi in the damaged liver) and simultaneous compensatory production of young, larger platelets by the bone marrow. Importantly, in the group protected with the lower dose of ES extract (750 mg/kg), no significant decrease in platelet count was observed, whereas with the high dose of ES extract (1500 mg/kg), thrombocytopenia was evident and similar to that in the paracetamol group. This indicates that the lower dose of the extract could protect megakaryocytes or inhibit platelet destruction (e.g., by limiting liver endothelial damage and microthrombi formation), whereas the higher dose did not produce this effect. Moreover, thrombocytopenia was even more pronounced in the high-dose ES group. It is possible that the surplus of some compounds in the high-dose ES had an adverse effect on platelet function or the coagulation system.
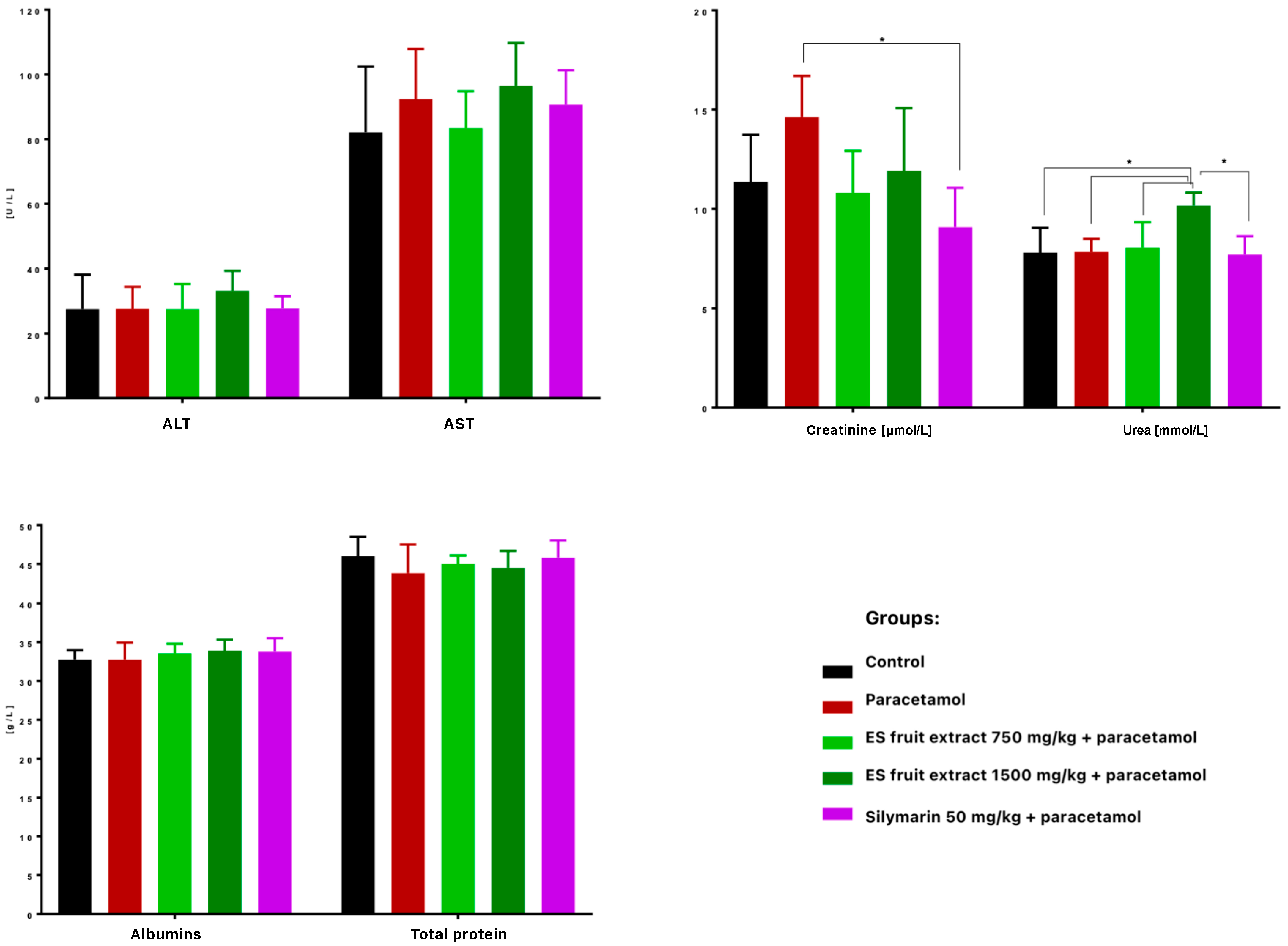
When it comes to the biochemical parameters, serum ALT and AST activities were not significantly elevated 24 h after paracetamol administration, suggesting no hepatotoxicity (Figure 6). Nonetheless, Eleutherococcus senticosus fruit extract at 750 mg/kg maintained ALT, AST, creatinine, and urea levels close to control values, whereas the 1500 mg/kg dose increased ALT (non-significant) and significantly raised serum urea. No significant changes were observed in total protein or albumin concentrations. Similarly, in a study on rats, a hepatoprotective effect was observed after administration of the root and rhizome extract. Lower doses (100, 300, and 500 mg/kg) led to a reduction in elevated AST and ALT levels induced by acetaminophen administration. Interestingly, in this case as well, a high dose of the extract (1000 mg/kg) caused an increase in the tested parameters. This confirms that the hepatoprotective effect of Eleutherococcus senticosus extract may be dose-dependent, and administration of excessively high doses may result in an opposite effect [22].
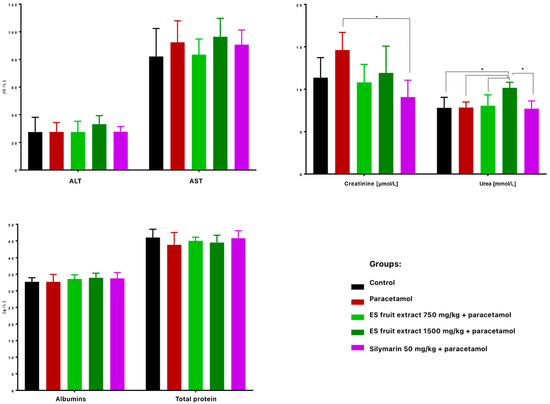
Figure 6.
Biochemical parameters in mouse serum after administration of the tested substances: AST [U/L], ALT [U/L], creatine [µmol/L], urea [mmol/L], albumins [g/L], total protein content [g/L]. * p < 0.05 nonparametric Kruskal–Wallis test, comparison vs. control.
Much more visible changes were observed in renal function indices. High-dose paracetamol can also cause kidney damage (e.g., acute renal tubular damage), as reflected by increased serum creatinine levels (Figure 6). A significant increase in creatinine levels was observed in the paracetamol group compared to the control group. Interestingly, the ES extract supplementation mitigated this increase–creatinine was lower at both doses than with paracetamol alone, with the effect at the 750 mg/kg dose (with levels similar to the control group) and slightly weaker at the 1500 mg/kg dose. Silymarin significantly prevented the increase in creatinine, so this could suggest that ES, like silymarin, may have a nephroprotective effect in the context of paracetamol poisoning.
Although there is limited data on the nephroprotective effects of Eleutherococcus senticosus extract, such an effect has previously been confirmed another adaptogenic plant-Panax ginseng. In a study on rat model, in which administration of hydroxyurea led to elevated levels of creatinine and urea, treatment with the drug in combination with Panax ginseng extract resulted in a reduction in these parameters (in the case of urea, even to levels lower than the control) [23]. Both Panax ginseng and Eleutherococcus senticosus are plants with adaptogenic properties. It is possible that ES extracts may also have a protective effect on the kidneys. However, further research is necessary to confirm this hypothesis.
Despite the absence of marked ALT/AST elevation after paracetamol (300 mg/kg, IP), extract at 750 mg/kg exhibited a protective trend on hepatic enzyme activity and renal function markers, as well as favorable effects on platelet parameters. The absence of significant elevation of ALT, AST, urea, and creatinine in the 750 mg/kg group compared to control indicates that the extract effectively prevented paracetamol-induced hepatotoxicity. Conversely, the higher ES fruit extract dose (1500 mg/kg) showed less benefit and some adverse trends (e.g., elevated urea). Both extract and silymarin potentiated the paracetamol-induced reduction in leukocytes and shift in leukocyte subpopulations.
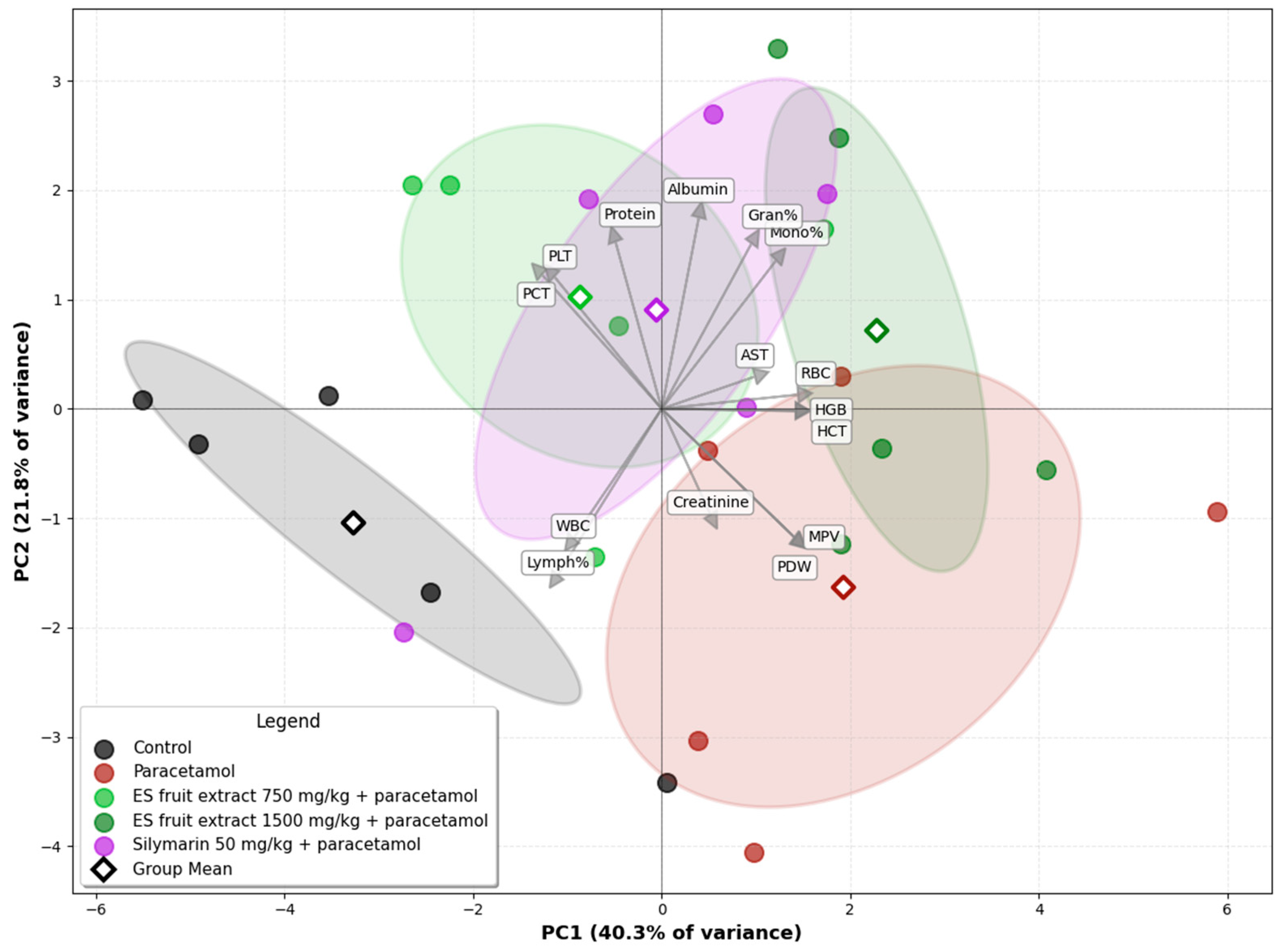
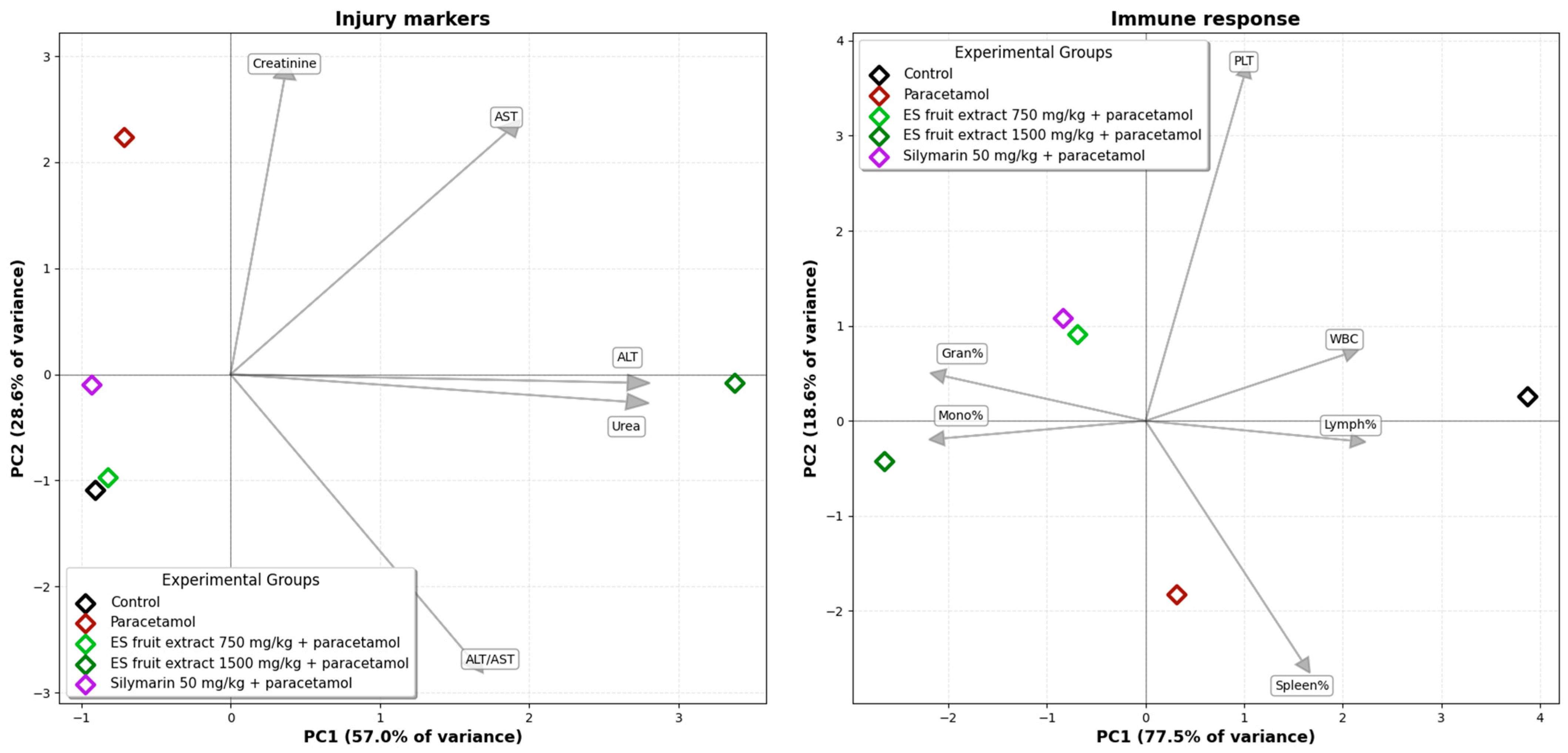
The Principal Component Analysis (PCA) of the experimental groups, based on biochemical and hematological parameters, illustrates the relationships among them (Figure 7). A clear separation is evident on the biplot between the control group (left side) and the paracetamol-only group (right side), along the PC1 axis. This axis accounts for the largest proportion of the data variance (40.3%) and can be interpreted as the axis of paracetamol-induced injury. The second principal component (PC2) explains 21.8% of the data variance. The position of the paracetamol group is associated with the vectors for variables such as creatinine and AST, as well as platelet parameters indicative of an inflammatory state (MPV, PDW), which graphically confirms the multi-organ toxicity of paracetamol. The group treated with the E. senticosus extract at a dose of 750 mg/kg (+paracetamol) is shifted towards the control group, and its group mean is located in close proximity to the mean of the reference silymarin group. The position of the ES fruit extract 750 mg/kg + paracetamol group between the Control and Paracetamol groups along the PC1 axis suggests that the lower extract dose partially mitigates the negative effects of paracetamol. Furthermore, this group’s confidence ellipse overlaps with that of the Silymarin 50 mg/kg + paracetamol group, suggesting that the overall effect of the 750 mg/kg ES fruit extract is similar to that of silymarin. The position of the ES fruit extract 1500 mg/kg + paracetamol group confirms that the higher dose of the extract did not exhibit a protective effect and, in fact, may have intensified the negative effects.
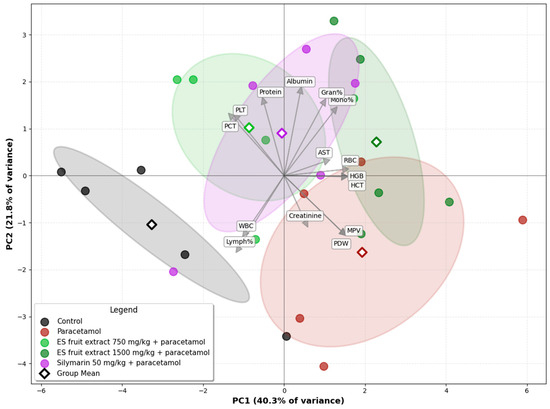
Figure 7.
Principal Component Analysis (PCA) of the experimental groups based on biochemical and hematological parameters. Ellipses: 95% confidence interval for the group mean.
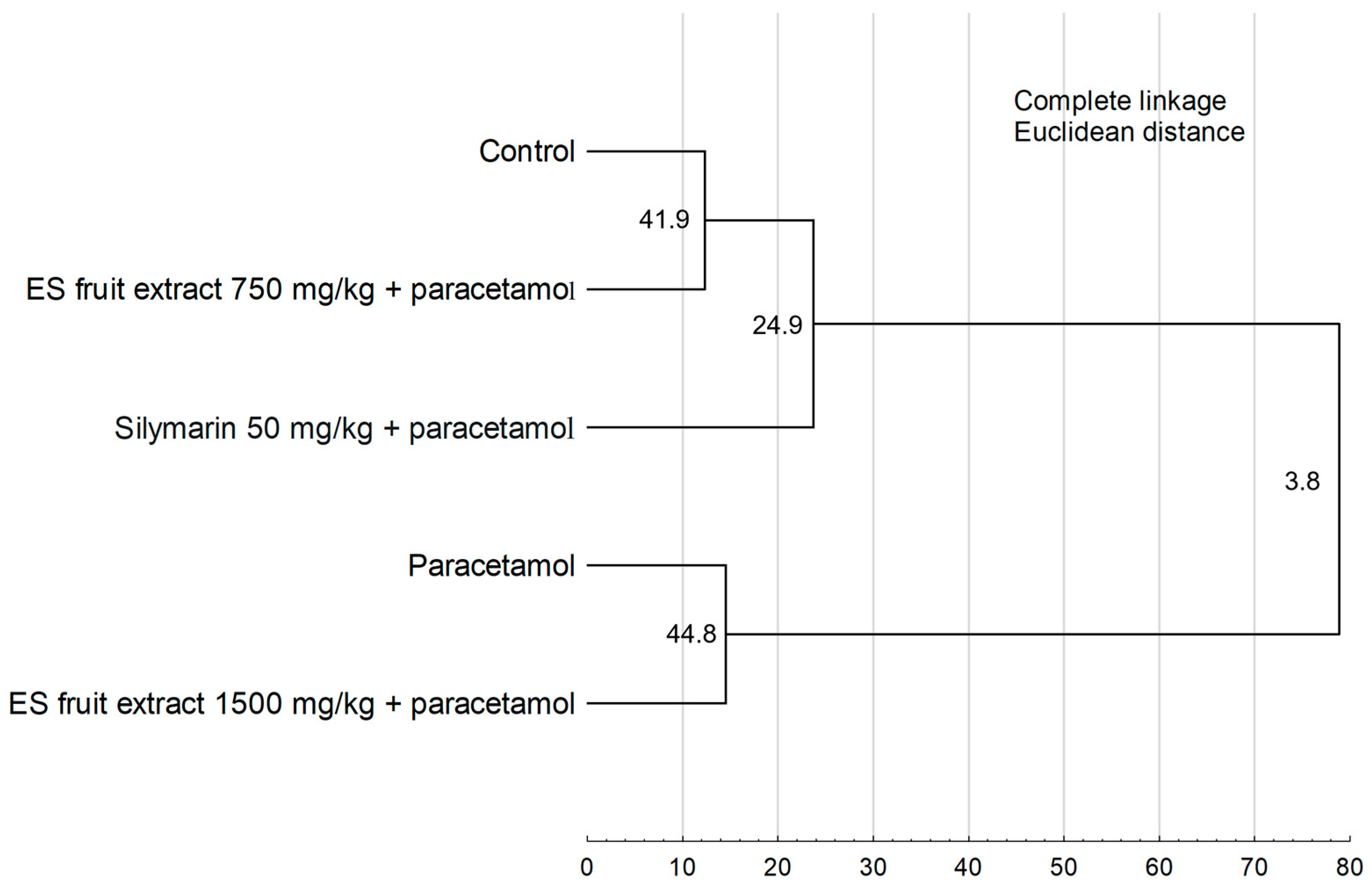
The results of the Hierarchical Cluster Analysis (Figure 8) confirm the findings from the PCA, revealing two main, distinctly separated clusters. One cluster group, the control group, the group treated with the 750 mg/kg ES extract, and the silymarin group. The second cluster consists of the paracetamol-only group and the group treated with the higher 1500 mg/kg ES extract dose. The close similarity between these latter two groups demonstrates that the 1500 mg/kg dose failed to provide a protective effect.
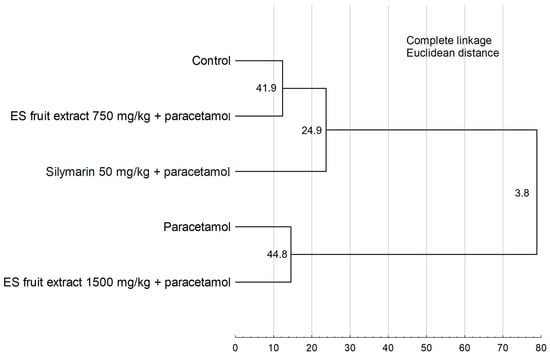
Figure 8.
Hierarchical cluster analysis of the experimental groups. Numbers at the nodes represent bootstrap support values (from 1000 replicates). Cophenetic correlation coefficient = 0.9548.
The PCA for injury markers (Figure 9) (ALT, AST, creatinine, urea) explains a total of 85.6% of the data variance across the first two axes. The group receiving the 750 mg/kg E. senticosus extract and the reference silymarin group are located in close proximity to the control group, indicating an effective protective action. The PCA for hematological parameters explains a total of 96.1% of the variance for the first two axes. In this analysis, the 750 mg/kg E. senticosus extract group clusters together with the silymarin group, indicating a similar protective effect.
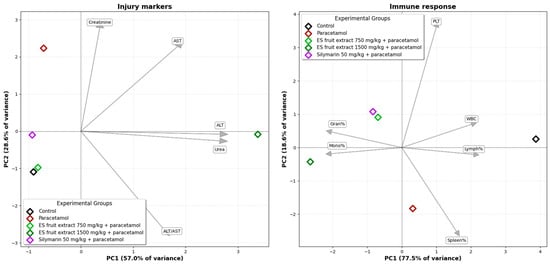
Figure 9.
Principal Component Analysis (PCA) of the studied groups based on injury markers and on immune response parameters.
The results suggest a multifaceted effect of ES, encompassing enzymatic protection, immune modulation, and antioxidant activity. The observed hematological changes, such as a decrease in leukocyte count and a shift in leukocyte populations (reduction in lymphocyte counts with an increase in monocytes and granulocytes), indicate a solid immune response induced by the toxic metabolite of paracetamol (NAPQI). Similar experiences have been previously documented [17] and interpreted as a proinflammatory and immune-damaging effect. The simultaneous enhancement of these changes by ES and silymarin may suggest an immunomodulatory effect of these substances, involving the regulation of proinflammatory cytokines and an adaptive immune response. The obtained biochemical parameters showed that ES extract at the optimal dose (750 mg/kg) stabilized liver enzyme activity (ALT, AST) and beneficially affected markers of renal function (creatinine and urea). This mechanism is likely related to the ability of ES active constituents to neutralize oxidative stress and inhibit lipid peroxidation. Similar hepatoprotective effects were also observed with silymarin, a known hepatoprotector with antioxidant properties [16].
The hepatoprotective activity observed after the administration of E. senticosus fruit extract at the dose of 750 mg/kg has not been fully determined, it may be associated with the antioxidant activity of the extract and it is likely to result from several complementary biological mechanisms. This mechanism could be similar to that induced by the administration of Panax ginseng extract, another member of the Araliaceae family known for its adaptogenic properties. The possible hepatoprotective effect of Black ginseng (sun-dried ginseng via repeated steaming) is primarily attributed to its antioxidant, anti-inflammatory, and antiapoptotic properties. Pretreatment with P. ginseng significantly attenuated acetaminophen-induced hepatotoxicity by restoring hepatic glutathione (GSH) levels and reducing malondialdehyde (MDA) accumulation, thereby mitigating lipid peroxidation and oxidative stress. Moreover, the extract downregulated cytochrome P450 2E1 (CYP2E1) expression, which limited the generation of reactive oxygen species (ROS) and toxic acetaminophen metabolites. In addition, P. ginseng decreased nitrative stress, as evidenced by reduced hepatic 3-nitrotyrosine (3-NT) formation, and suppressed the expression of proinflammatory enzymes, including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). At the cellular level, the extract modulated apoptosis-related proteins by decreasing Bax expression, increasing Bcl-2 expression, and lowering the Bax/Bcl-2 ratio, collectively contributing to reduced hepatocellular apoptosis and necrosis [24].
Given that Eleutherococcus senticosus also belongs to the Araliaceae family and exhibits comparable adaptogenic and antioxidant activities, it is plausible that its hepatoprotective mechanism may involve similar molecular pathways. The fruits contain a range of bioactive compounds, flavonoids (such as quercitin and its derivatives), phenolic acids (e.g., chlorogenic acid, dicaffeoylquinic acids), lignans, triterpenic acids (including oleanolic and ursolic acids) as well as volatile constituents [9,25,26]. These constituents have been reported to exhibit pronounced antioxidant and anti-inflammatory effects. Their activity may involve the enhancement of enzymatic antioxidant defenses, including superoxide dismutase, catalase, and glutathione peroxidase, as well as the restoration of the reduced glutathione pool and the reduction in lipid peroxidation in hepatic tissue. Su et al. [27] proved that E. senticosus flavonoids can significantly reduce the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in RAW cells treated with hydrogen peroxide (H2O2), thereby increasing the antioxidant capacity of cells. By improving the cellular redox balance, these compounds may protect hepatocytes from oxidative damage induced by the reactive metabolite of paracetamol. Another study showed that E. senticosus has a beneficial effect in treating liver injury and enhancing the antioxidant capacity, by regulating antioxidant and antiapoptotic-related gene expression level [28]. Song et al. [29] used Edwardsiella ictaluri infection model to demonstrate that the ES extract suppressed the expression of proinflammatory cytokines, including IL-1. The infection with E. ictaluri led to an upregulation of IL-1 and activation of the NF-κB/MyD88 signaling pathway, whereas treatment with the E. senticosus resulted in a significant downregulation of this pathway. Moreover, myo-inositol, which is also one of the components of the ES fruit extract [11], exhibits hepatoprotective activity as well. It has been proven that its administration led to an improvement in the AST/ALT ratio in patients with NAFLD, which are indicators of liver damage [30]. Myo-inositol supplementation improves liver enzymes concentrations, which is consistent with the results provided by Pan et al. [31] Zhao et al. [32] and Lee et al. [33].
Interestingly, a higher dose of ES extract (1500 mg/kg) showed a less beneficial effect, supporting the hypothesis of a paradoxical, potentially pro-oxidant effect when phytopreparations are exceeded at optimal concentrations. Available publications provide strong evidence that polyphenolic compounds and other phytoextracts—while beneficial in specific, moderate doses—can paradoxically exhibit pro-oxidant effects at higher concentrations. In this case with ES extract (1500 mg/kg) fits that dose-effect pattern, proving that at this concentration, it exhibits opposite, rather than hepatoprotective, effects. This phenomenon should be further analyzed in the context of the safe use of adaptogens, with lower but regular dosing recommended [34,35,36,37].
2.2. Histopathological Studies
In our previous study [11], histopathological evaluation of liver tissue revealed no pathological changes in either control animals or those treated with concentrations of Eleutherococcus senticosus intractum. Hepatocytes maintained normal morphology and architecture, and the overall tissue structure was preserved, indicating an absence of hepatotoxic effects. These findings are consistent with other reports confirming the safety of E. senticosus extracts in traditional medicine, including studies showing protective effects against liver damage induced by diabetes or heavy metal exposure [38,39].
Based on these previously established results, liver histology was not re-examined in the present study to avoid redundant publication of the same data. Instead, we focused on the brain and kidneys, as these organs represent important targets for systemic toxicity and oxidative stress. This approach allowed us to investigate potential protective effects of E. senticosus in other critical organs while acknowledging the previously confirmed safety of the extract in the liver.
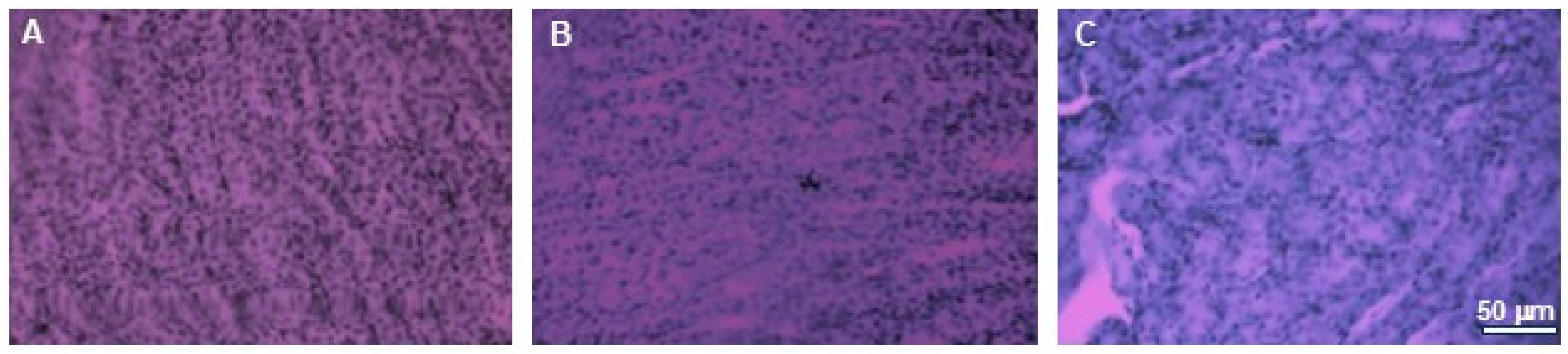
To analyze the effect on other tissues in the body, we examined the effect of extracts supply on the induction of cytotoxic effects in organs such as the kidneys and brains. Histopathological examination did not reveal any pathological changes in those organs obtained from mice in any experimental group. Minor changes to the kidneys were observed in one mouse in the group treated with 750 mg/kg b.w. of the intractum. Areas containing cells with irregular morphology and slight tubule degeneration were present. This animal also exhibited areas of lightening in H&E staining of the liver. However, the liver cells displayed normal morphology and arrangement [11]. The other animals in the study group did not show any changes indicating kidney damage. Their kidneys showed proper cell morphology and architecture (Figure 10).
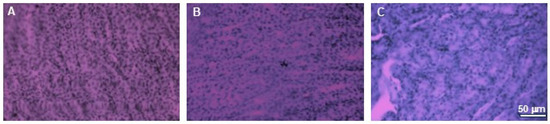
Figure 10.
H&E staining of kidneys. Microphotographs of representative kidneys sections obtained from control mouse (A) and animals treated with 750 (B) or with 1500 mg/kg b.w. of the intractum (C). See the proper morphology and cell arrangement within all the sections. Magnification: 400×, bar 50 μm.
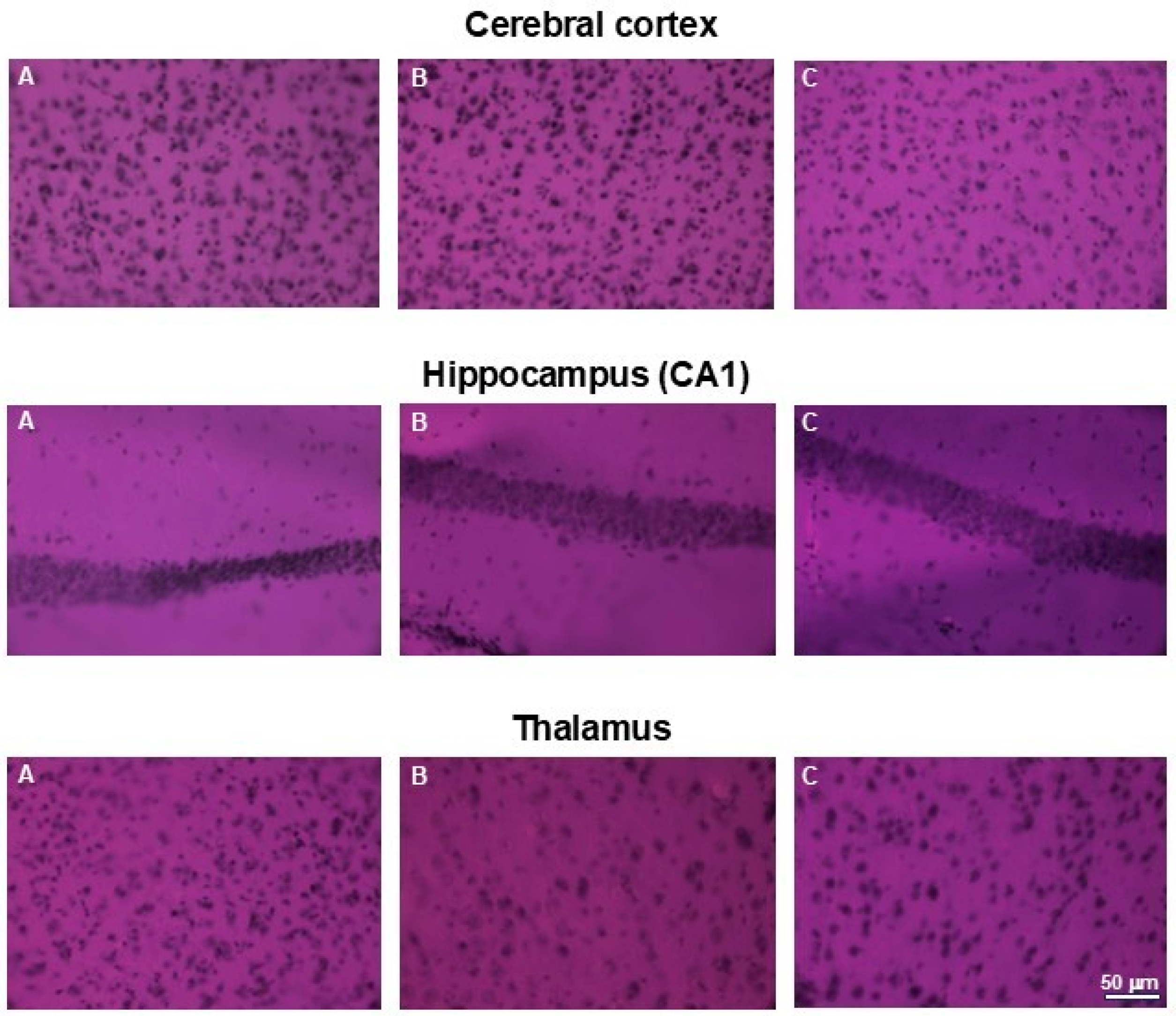
Similarly, no abnormalities in cell morphology or arrangement were observed in the brains of the examined animals. Figure 11. presents representative areas of the cerebral cortex and subcortical regions from animals in the control and both treated groups.
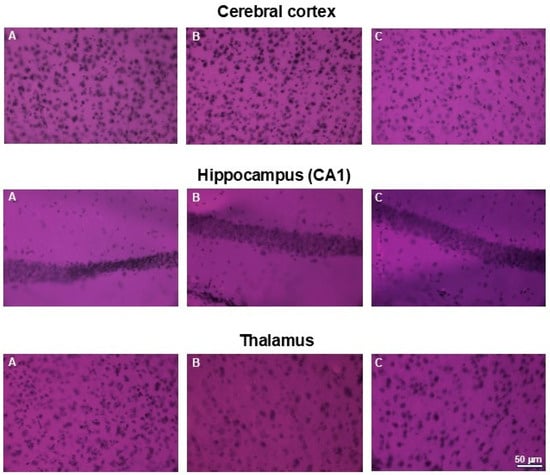
Figure 11.
H&E staining of brains. Microphotographs of representative brain sections obtained from control mouse (A) and animals treated with 750 (B) or with 1500 mg/kg b.w. of the intractum (C). Upper panel shows cerebral cortex, middle and bottom panels show selected subcortical structures: CA1 region of hippocampus and thalamus (respectively). Note the proper cell arrangement and morphology within all the sections. Magnification: 400×, bar 50 mm.
There were no reports of histopathological changes in the brain or kidneys caused by the administration of Eleutherococcus senticosus. In a study in rats with a cerebral ischemia model (4-VO), doses of 3, 30, and 300 mg/kg of ES ethanol bark extract protected hippocampal cells (CA1), reduced apoptosis, and triggered a beneficial immunohistochemical effect (reduction in COX-2, GFAP, and OX-42 antibody)—meaning no pathological changes. Immunohistochemical findings suggest that this effect may be linked to its anti-inflammatory activity, achieved through the suppression of COX-2, as well as reduced activation of microglia and astrocytes [40]. No histopathological changes were observed; on the contrary, a neuroprotective effect was observed.
Adaptogens as a group of medicinal plants, should demonstrate low levels of cytotoxicity in tissues, even with long-term use. For instance, the safety impact of long-term Panax ginseng supplementation was examined. No negative changes were observed during the 12-month treatment period in rat experiment model, no significant organ changes were observed, histopathological, hematological and serum biochemical analyses did not show any significance modifications proving the safety of using this raw material from the adaptogenic group [41].
2.3. Phytochemical Characterization-Amino Acid Profile
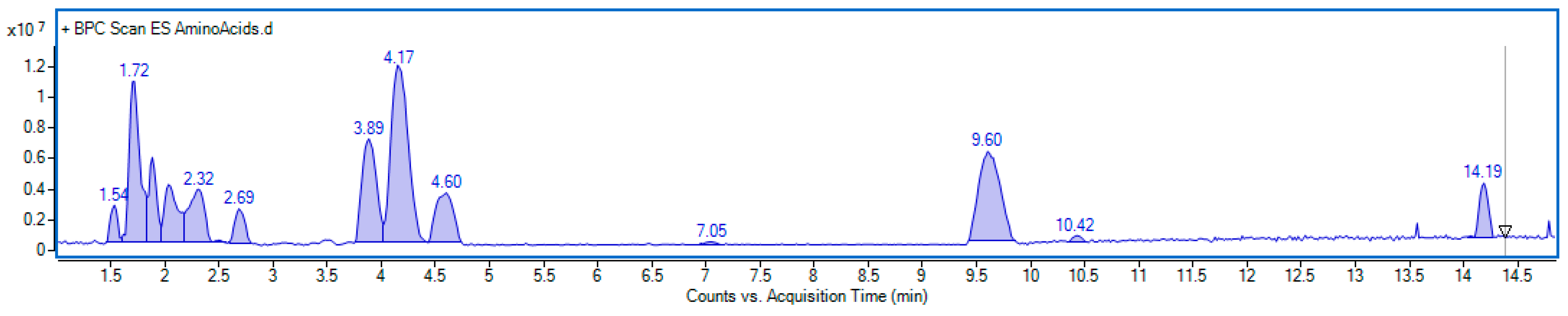
Our study showed that the amino acid profile of E. senticosus (ES) fruit extract was diverse and included twenty components (Figure 12).
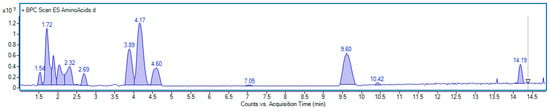
Figure 12.
Base peak chromatogram obtained in positive ionization mode of E. senticosus fruit extract analyzed for amino acid content. Identified compounds are listed in Table 1.
Ten compounds were identified based on their characteristic mass spectra, while the remaining ten were detected through extracted ion chromatograms within specific mass ranges. Quantitative analysis revealed that tyrosine, leucine, phenylalanine, and isoleucine were the predominant amino acids, with concentrations of 3.98, 3.55, 2.75, and 2.13 mg/g of dry weight, respectively. Furthermore, moderate amounts of serine, glutamic acid, arginine, tryptophan, valine, and norvaline were also detected, ranging from 1.05 to 1.54 mg/g. The detailed results of the quantification are summarized in Table 2. Examples of mass spectra of main amino acids and extracted ion chromatograms used for quantification are included in the Supplementary Material (Figures S1 and S2).

Table 2.
Results of quantification the amino acids identified in E. senticosus fruit extract.
Amino acids play a crucial role in hepatoprotective actions, supporting liver health and function. They are essential for the synthesis of proteins, including enzymes and detoxifying agents that aid in liver cell regeneration and the neutralization of toxins. Specific amino acids, such as glycine, glutamate, methionine and cysteine, contribute to the production of glutathione–a key antioxidant that protects liver cells from oxidative stress [42]. Thus, adequate dietary intake of amino acids can support detoxification processes and promote hepatic recovery.
2.4. Low Molecular Weight Organic Acid
Three low molecular weight organic acids were detected in the extract in significant amounts, including citric, malic, and gluconic acid (Figure S3). Among them, malic acid was predominant, followed by citric acid with the content of 25.1 and 8.4 µg/g, respectively. The results of the quantification are presented in Table 3.

Table 3.
Results of quantification of low molecular organic acid identified in E. senticosus fruit extract.
Low molecular weight organic acids, such as citric and malic acid, may play a supportive role in liver function through several biochemical mechanisms. As intermediates of the tricarboxylic acid (TCA) cycle, they contribute to cellular energy production, which is essential for the liver’s metabolic and detoxifying activities. Additionally, citric acid exhibits antioxidant and metal-chelating properties, potentially reducing oxidative stress in hepatic tissue [43,44].
2.5. Secondary Metabolites
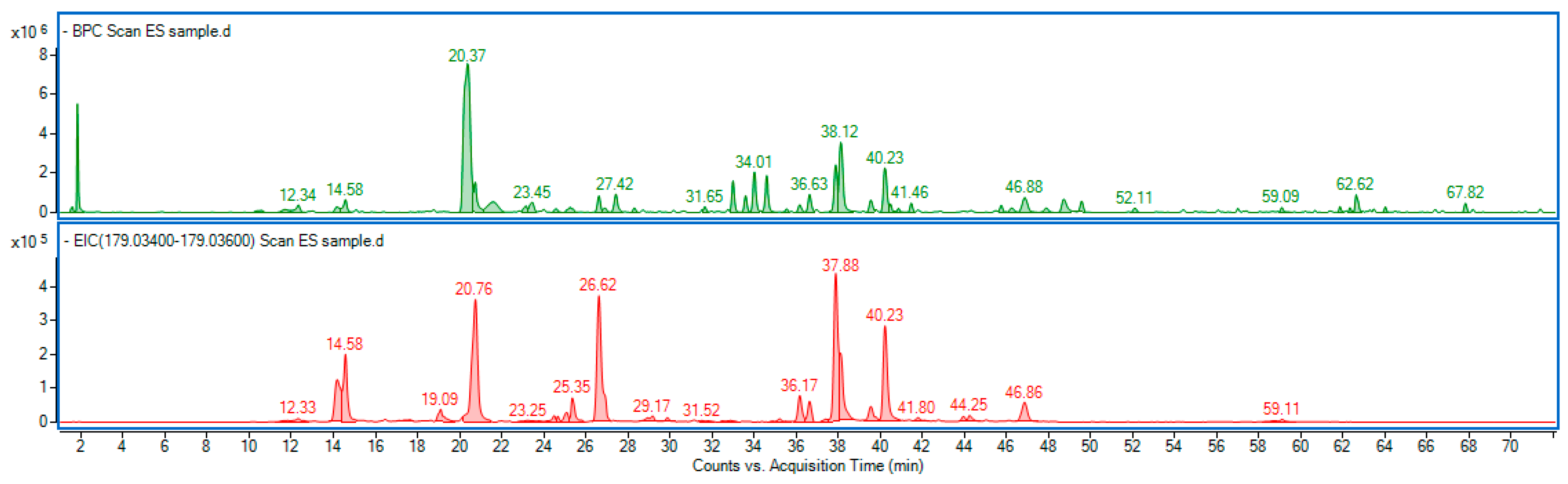
Our study showed that the ES extract is rich in phenolic compounds, particularly derivatives of caffeic acid, namely caffeoylquinic acids and dicaffeoylquinic acids (Figure 13). Among them, 3-O-caffeoylquinic acid (common name: chlorogenic acid) was predominant. The detailed chromatographic and mass spectrometric data of identified phenolic constituents are included in the Supplementary Material Table S1.
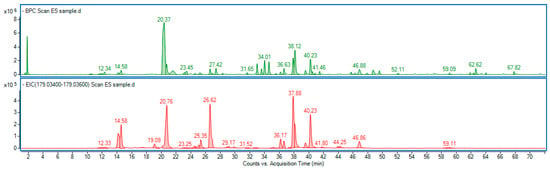
Figure 13.
Base peak chromatogram (green line) of Eleutherococcus senticosus fruit extract, along with the extracted ion chromatogram (red line), showing a mass range corresponding to caffeic acid (C9H8O4) a ppm error of −5.46 to 5.65. Identified compounds are listed in Table S1.
The analysis revealed a diverse profile of phenolic compounds, with phenolic acids being predominant. Among the phenolic acids, chlorogenic acids were the most abundant, with a content of 28.02 ± 1.21 mg/g, followed by dicaffeoylquinic acids (13.42 ± 0.76 mg/g). Other identified compounds included caffeoylshikimic acids, feruloylquinic acids, and protocatechuic acid. The total phenolic acid content was 43.77 mg/g. In contrast, flavonoid content was significantly lower, amounting to 2.22 mg/g. Among the flavonoids, quercetin 3-O-hexosides, including glucoside and galactoside forms, as well as quercetin O-rutinosides, were the most prominent. Catechin and its hexoside derivative were detected in smaller amounts. Free quercetin was also identified, with a content of 0.19 ± 0.01 mg/g. The results of phenolic acid and flavonoids quantification expressed as total amount of different isomeric forms are summarized in Table 4.

Table 4.
The results of quantification of identified phenolic acids and flavonoids found in E. senticosus fruit extract expressed as milligrams per gram of dried extract.
Anthocyanins in the ES extract were represented by one dominant constituent (2.79 ± 0.14 mg/g) at retention time 21.55 min, characterized by a typical UV-Vis absorption spectrum with a maximum in the range of 520–530 nm. The compound exhibited [m/z+H]+ = 581.15154 (estimated formula: C26H28O15, ppm error: 2.49), with a characteristic fragment ion at [m/z+H] = 287.05548 (estimated formula: C15H10O6, ppm error: 1.63), corresponding to the neutral aglycone form of cyanidin (Figure S4).
Furthermore, based on literature data regarding characteristic constituents of Eleutherococcus species [45], additional secondary metabolites were investigated based on extracted ion approach. Quinic acid (cyclohexanecarboxylic acid, formula: C7H12O6) was extracted within [m/z−H] range 191.055–191.057 (a mass error from −5.76 to 4.63 ppm) and it was found in significant amount of 11.56 ± 0.87 mg/g. Eleutheroside E (syringaresinol) belonging to the lignan family, identified by the characteristic adduct [m/z+HCOOH]− = 787.26391 and parent ion [m/z+H]− = 741.26173 (estimated formula: C34H46O18, mass error: 2.32 and 0.8 ppm, respectively) was detected at a concentration of 0.22 ± 0.02 mg/g. Triterpenic acids were also detected based on extracted ions corresponding to the mass range characteristic for these compounds ([M–H]− = 455.350–455.355). The analysis revealed two peaks: one corresponding to a mixture of oleanolic and ursolic acid (total content: 7.24 µg/g of extract), and another representing an unidentified triterpenic acid (content: 14.32 µg/g). Extracted ion chromatograms of mentioned compound are presented in Figure S5.
Secondary metabolites are organic compounds produced by plants that are not directly involved in their growth or development. They are classified as non-nutritional food components, as they are not essential nutrients like carbohydrates, proteins, or fats. However, they are important for human health and nutrition due to their wide range of biological activities. Among secondary metabolites, phenolic compounds—such as phenolic acids and flavonoids—are of particular interest. These compounds have been shown to exhibit strong antioxidant, anti-inflammatory, and antimicrobial properties. They help protect the body from oxidative stress, modulate enzyme activity, and influence signaling pathways involved in chronic diseases such as cardiovascular disorders, diabetes, and certain types of cancer. Therefore, their presence in the diet contributes significantly to the promotion of health and the prevention of disease.
2.6. Mineral Composition
The elemental composition of the extract is presented in Table 5. Among the analyzed elements, potassium (K) was the most abundant, with a concentration of 24,181 µg/g and a coefficient of variation (CV) of 0.27%, followed by calcium (Ca) at 5788 µg/g and magnesium (Mg) at 1369 µg/g. Sodium (Na) and selenium (Se) were also present in significant amounts, at 291.7 µg/g and 292.9 µg/g, respectively, both with very low variability (CVs of 0.16% and 0.09%). Zinc (Zn) was detected at a lower concentration of 33.12 µg/g, with a higher CV of 3.15%, indicating slightly greater variability in measurements. Iron (Fe), copper (Cu), and manganese (Mn) were not detected (nd) under the applied analytical conditions, suggesting their concentrations were below the detection limits of the method. Example of a spectral window for the analysis of potassium, magnesium, and zinc using inductively coupled plasma optical emission spectrometry (ICP-OES) is presented in Supplementary Material (Figure S6).

Table 5.
Elemental composition of the E. senticosus fruit extract determined by ICP-OES.
Trace elements are fundamental for liver function and protection [46]. For example, zinc is a cofactor for over 300 enzymes. It stabilizes cell membranes, regulates immune responses, and supports liver regeneration and detoxification. Zinc deficiency is common in liver diseases like cirrhosis and fatty liver and is linked to impaired immune function and slower hepatocyte repair. Magnesium is as a cofactor in ATP synthesis and metabolic regulation. It also has anti-inflammatory effects and aids in detox processes. Disruptions in magnesium homeostasis are observed in cirrhosis and have been associated with worse liver outcomes. Selenium is integral to selenoproteins like glutathione peroxidase, which combats lipid peroxidation and oxidative stress. Patients with chronic liver conditions often exhibit low selenium levels, while supplementation has shown benefits in non-alcoholic fatty liver disease (NAFLD) by reducing oxidative damage and inflammation. However, it should be noted that the roles of some elements, such as iron and copper, are dual. On one hand, they are essential for metabolic activity; on the other, when present in excess, they may accumulate in hepatocytes and catalyze oxidative damage.
3. Materials and Methods
3.1. Plant Material
The extract was prepared from the fruits of E. senticosus (Rupr. et Maxim.) Maxim., following the procedures described in our previous work [47]. The fruits were collected from the Medicinal and Cosmetic Plant Garden in Bydgoszcz, Poland, in September 2024 (53°07′36.55″ N; 18°01′51.64″ E). Plant samples were deposited at the Department of Pharmaceutical Botany and Pharmacognosy of the Collegium Medicum in Bydgoszcz (Cat. Nr. ES/9/2024). The authenticity of the plant material was verified with morphological examination and confirmed by HPLC-DAD analysis, comparing the results with reference standards. Briefly, the process of extraction involved macerating fresh fruits (20 g) in 100 mL of 40% (v/v) ethanol (Cat. No.: E9508, Merck KGaA, Darmstadt, Germany) for 30 days at room temperature in the absence of light (this type of preparation is referred to as intractum). After maceration, the mixture was filtered through Whatman No. 4 filter paper. The solvent was then removed under reduced pressure at 45 °C, and the residue was frozen at −20 °C and subsequently lyophilized. The resulting lyophilized extract was stored at 4 °C until further use.
3.2. Evaluation of the Hepatoprotective Effect of the Eleutherococcus senticosus Fruit Extract in a Model of Liver Damage with Paracetamol
A detailed study protocol, including the research question, key design features, and analysis plan, was prepared prior to the experiment in accordance with local ethical committee requirements, but it was not registered in a public database.
The female BALB/c mice (n = 25, 8 weeks old) were used in the study. Animals were housed under specific pathogen-free (SPF) conditions in individually ventilated cages (5 animals per cage) at 21–23 °C, with 50% relative humidity and a 12 h light/dark cycle. Standard laboratory chow and water were provided ad libitum. All experimental procedures were approved by the Local Ethics Committee (approval number: 055/2024/P1). After a quarantine period, mice were randomized into five groups (n = 5 per group) based on body weight:
- Group 1 (Control): Water (per os (PO), 0.1 mL/10 g) for 7 days + vehicle (EtOH/PEG/NaCl) intraperitoneal (IP) on day 8,
- Group 2: Paracetamol (300 mg/kg, IP, day 8),
- Group 3: Eleutherococcus senticosus fruit extract (750 mg/kg, PO, twice daily for 7 days and once on day 8) + paracetamol (300 mg/kg, IP, day 8),
- Group 4: Eleutherococcus senticosus fruit extract (1500 mg/kg, PO, twice daily for 7 days and once on day 8) + paracetamol (300 mg/kg, IP, day 8),
- Group 5: Silymarin (50 mg/kg, PO, twice daily for 7 days and once on day 8) + paracetamol (300 mg/kg, IP, day 8).
PO administrations were performed using a flexible gastric gavage tube. Paracetamol was dissolved in a mixture of 96% EtOH, PEG, and 0.9% NaCl (5:10:85, v/v/v) (Pol-Aura, Warsaw, Poland; EtOH cat. no.: PA-06-396420113; PEG cat. no.: PA-04-F1243; NaCl cat no.: PA-31-HI7037L) due to low water solubility. Silymarin (Silimax, Filofarm, Bydgoszcz, Poland) was prepared as a water suspension. The experimenter, who was responsible for conducting the study, was aware of the group allocation throughout the experimental procedure. All animals were included in the experiment and analysis, and no exclusion criteria were applied; all data points were taken into account according to generally accepted standards.
Twenty-four hours after paracetamol administration (day 9), mice were euthanized, following analgesia with buprenorphine and anesthesia with isoflurane; blood and organs (liver, kidneys, spleen, lungs, brain) were collected. Blood was used for hematological and biochemical analyses (ALT, AST, urea, creatinine, total protein, albumin) and serum isolation. Organs were weighed and fixed in 10% neutral-buffered formalin for histopathological evaluation.
3.3. Histopathological Studies
The brains and kidneys of animals from all experimental groups were examined to assess cell morphology and arrangement of individual structures. Histological marking was performed using hematoxylin and eosin (H&E) staining. Fixed biological material from each experimental group was cryoprotected by immersion in 10, 20 and 30% (w/v) sucrose solutions in PBS (Merck KGaA, Darmstadt, Germany), cat. no.: P3813 (for 24 h, 3 days and 6 days, respectively). Next, the brains and kidneys were frozen on dry ice, sectioned into 20-μm thick slices using a CM 1850 UV cryostat (Leica, Wetzlar, Germany). The sections were processed for histological/morphological analysis using routine hematoxylin and eosin staining (hematoxylin No. 1.50174 and eosin No. 1.09844, Merck, Darmstadt, Germany). Finally, the stained sections were mounted with DPX mounting medium (Sigma-Aldrich, Schnelldorf, Germany), cat. no.: 06522. Images were examined and captured using a Nikon light microscope (Nikon, Tokyo, Japan) equipped with a CCD camera and image analysis system.
3.4. Ultra-High Performance Liquid Chromatography (UHPLC-DAD-MS)
Ultra-high-performance liquid chromatograph (UHPLC) Infinity Series II with a DAD detector and an Agilent 6224 ESI/TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) coupled with a Kinetex C18 column 1.7 µm, 150 mm × 2.1 mm (Phenomenex, Torrance, CA, USA) was used in the investigation. The column temperature was 30 °C. Water with 0.05% formic acid (LiChropur®, LC–MS grade; Merck KGaA, Darmstadt, Germany), cat. no. 113228 (solvent A) and acetonitrile LC–MS, LiChrosolv® hypergrade (Merck KGaA, Darmstadt, Germany), cat. no. 100029 with 0.05% formic acid (solvent B) at the flow rate 0.2 mL/min was used as a mobile phase. Phenolic compounds were separated according to following elution gradient: 0–2 min from 98% A, 2–35 min from 98% A to 80% A, 35–50 min from 80% A to 75% A, 50–60 min from 75% A to 60% A, 60–85 min from 60% A to 35% A. Triterpenic acids were analyzed using 25% solvent A and 75% solvent B. Amino acids and low molecular weight organic acids were analyzed according to following elution gradient 0–5 min 100% A and 5–20 min from 100% A to 75% A. LC–MS conditions: the drying gas temperature was set at 325 °C, with a gas flow rate of 8 L/min. The nebulizer pressure was maintained at 30 psi, while the capillary voltage was set to 3500 V. The skimmer voltage was 65 V, and the fragmentor voltage was 140 V and 240 V in negative mode and 120 V in positive mode.
3.5. Elemental Analysis
Samples of the extract were digested using a mixture of nitric acid (HNO3) Suprapur® (Merck KGaA, Darmstadt, Germany), cat. no. 100441 and distilled water in a volume ratio of 2:8 (v/v). The digestion was carried out with a DigiPREP MS heating block (SCP Science, Canada), following standard procedures to ensure complete decomposition of organic matter. Elemental concentrations were determined using inductively coupled plasma optical emission spectrometry (ICP-OES) with a PlasmaQuant PQ 9000 Elite instrument (Analytik Jena AG, Jena, Germany), as previously described [48]. The following operating conditions were used for the analysis: RF Power: 1300 W; Plasma Gas Flow Rate: 12.0 L/min, Auxiliary Gas Flow Rate: 0.50 L/min, Nebulizer Gas Flow Rate: 0.60 L/min, Observation Mode: Axial direction (with attenuated axial mode used for Na and K), Readout Time: 3 s (6 s for selenium). Outliers in the dataset were identified using Grubbs’ test at a 95.4% confidence level (corresponding to 2 sigma). Calibration was carried out using single-element standards PlasmaCAL, ISO 17034/ISO 17294 (SCP Science, Baie-D’Urfé, QC, Canada) series (1000 ppm), appropriately diluted. Calibration verification was performed using a multi-element standard PlasmaCAL, ISO 17034/ISO 17294 (SCP Science, Baie-D’Urfé, QC, Canada) at two concentration levels: 200 ppb and 400 ppb, with recovery rates within the range of 95–105% [49,50].
3.6. Statistical Analysis
Data were analyzed using GraphPad Prism 7. Non-parametric Kruskal–Wallis test was used to compare treatment groups with the control group. A p-value < 0.05 was considered statistically significant. To investigate the complex relationships between the experimental groups and the measured parameters, multivariate data analyses were performed. Principal Component Analysis (PCA) was conducted to visualize the interrelationships between samples and variables. Three separate PCA models were generated:
- on the complete dataset, including all biochemical and hematological parameters,
- on a subset of data containing only injury markers (ALT, AST, creatinine, urea),
- on a subset of data comprising hematological and immune response parameters.
The results were presented as biplots, displaying the scores for individual samples and the vectors for each variable. Principal Component Analyses were performed using Python 3.13. The Hierarchical Cluster Analysis was conducted and the dendrogram was generated using Statistica 13.3 software. The stability of the clusters identified by HCA was assessed using bootstrap validation with 1000 iterations.
4. Conclusions
Although Eleutherococcus senticosus fruits are growing in popularity as food ingredients, rigorous evidence-based safety is still needed. The present study provides new evidence supporting the hepatoprotective potential of Eleutherococcus senticosus fruit extract in a mouse model of paracetamol-induced liver injury. Pre-treatment with the extract, particularly at a dose of 750 mg/kg, helped maintain liver enzyme activity (ALT, AST) and renal function markers closer to physiological levels compared to the paracetamol-only group, while modulating immune parameters and platelet indices. No histopathological changes were observed in the kidneys or brains of treated animals, confirming the safety of the extract at the tested doses. However, the higher dose (1500 mg/kg) demonstrated reduced benefits of usage, in some biochemical parameters, suggesting a possible paradoxical effect associated with excessive intake of plant-based preparations. Further studies are needed to clarify dose-dependent mechanisms and establish safe and effective dosing regimens for adaptogens in hepatoprotection.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17213456/s1, Table S1: Chromatographic and mass data of phenolic constituents identified in Eleutherococcus senticosus fruit extract. Figure S1: Mass spectrum of the main amino acids found in E. senticosus fruit extract including tyrosine (Rt = 4.60), phenylalanine (Rt = 9.60), leucine (Rt = 4.17), and tryptophan (Rt = 14.19); Figure S2: Example of extracted ion chromatograms showing spectral window characteristic for proline, valine/norvaline, methionine, glutamine, and glutamic acid; Figure S3: Base peak chromatogram in negative mode showing analysis of low molecular organic acids including gluconic acid (RT = 1.77 min), malic acid (RT = 2.29 min), and citric acid (RT = 3.39); Figure S4: UV-Vis spectrum and mass spectrum of the anthocyanin found in E. senticosus fruit extract; Figure S5: Extracted ion chromatogram from E. senticosus fruit extract (blue line) and standard (red line), showing a extraction window with mass range corresponding to quinic acid (C7H12O6, a mass error from −5.76 to 4.63 ppm), Eleutheroside E (C34H46O18, a mass error from −4.87 to 4.56), and ursolic acid (C30H48O3, a mass error from −6.72 to 4.23); Figure S6: Example of a spectral window for the analysis of potassium, magnesium, and zinc using inductively coupled plasma optical emission spectrometry (ICP-OES).
Author Contributions
F.G. conceived and supervised the study, provided the plant materials, prepared the samples, formal analyses, and drafted the manuscript. K.K. drafting, correction of the manuscript. D.G.-K. made a correction, and statistical analysis. M.W. and I.S.—chromatographic analyses. D.S. histopathological analyses. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The study was supported by an internal research grant under the “DEBIUTY” program within the Excellence Initiative—Research University (IDUB) from Collegium Medicum, Nicolaus Copernicus University in Toruń.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee Hirszfeld Institute of Immunology and Experimental Therapy (protocol code: 055/2024/P1; date of approval: 20 November 2024).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| COX-2 | cyclooxygenase-2 |
| CV | coefficient of variation |
| ES | Eleutherococcus senticosus |
| EtOH/PEG/NaCl | ethanol/polyethylene glycol/sodium chloride |
| GFAP | glial fibrillary acidic protein |
| H&E | hematoxylin and eosin |
| HGB | hemoglobin |
| HPLC-DAD | high-performance liquid chromatography with diode-array detection |
| ICP-OES | inductively coupled plasma optical emission spectrometry |
| IP | intraperitoneal |
| MVP | mean platelet volume |
| NAFLD | non-alcoholic fatty liver disease |
| NAPQI | N-acetyl-p-benzoquinoneimine |
| PCT | plateletcrit |
| PDW | platelet distribution width (platelet anisocytosis) |
| PO | per os (by mouth) |
| RBC | red blood cells |
| TCA | tricarboxylic acid |
| UHPLC | ultra-high-performance liquid chromatography |
References
- Schmidt, M.; Thomsen, M.; Kelber, O.; Kraft, K. Myths and Facts in Herbal Medicines: Eleutherococcus senticosus (Siberian Ginseng) and Its Contraindication in Hypertensive Patients. hypertensive patients. Bot. Targets Ther. 2014, 27, 27–32. [Google Scholar] [CrossRef]
- Li, W.; Luo, Q.; Jin, L.H. Acanthopanax senticosus Extracts Have a Protective Effect on Drosophila Gut Immunity. J. Ethnopharmacol. 2013, 146, 257–263. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Verpoorte, R.; Khan, I.; Kuźniewski, R.; Nowak, R. Phytoconstituents and Nutritional Properties of the Fruits of Eleutherococcus divaricatus and Eleutherococcus sessiliflorus: A Study of Non-European Species Cultivated in Poland. Oxid. Med. Cell. Longev. 2017, 2017, 8374295. [Google Scholar] [CrossRef]
- Chang, Y.; Jiang, Y.; Chen, J.; Li, S.; Wang, Y.; Chai, L.; Ma, J.; Wang, Z. Comprehensive Analysis of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. Fruits Based on UPLC–MS/MS and GC–MS: A Rapid Qualitative Analysis. Food Sci. Nutr. 2024, 12, 1911–1927. [Google Scholar] [CrossRef]
- Gębalski, J.; Małkowska, M.; Kiełkowska, E.; Graczyk, F.; Wnorowska, S.; Hołyńska-Iwan, I.; Strzemski, M.; Wójciak, M.; Załuski, D. Chemical Composition and In Vitro Biological Activity of the Polar and Non-Polar Fractions Obtained from the Roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Int. J. Mol. Sci. 2025, 26, 5619. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Meng, Y.; Zhai, C.; Wang, M.; Avula, B.; Yuk, J.; Smith, K.M.; Isaac, G.; Khan, I.A. The Chemical Characterization of Eleutherococcus senticosus and Ci-Wu-Jia Tea Using UHPLC-UV-QTOF/MS. Int. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cho, M.L.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Lee, J.S.; Park, S.O.; Lee, S.J.; Shin, H.M.; Lee, O.H. The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef]
- Graczyk, F.; Orzechowska, B.; Franz, D.; Strzemski, M.; Verpoorte, R.; Załuski, D. The Intractum from the Eleutherococcus senticosus Fruits Affects the Innate Immunity in Human Leukocytes: From the Ethnomedicinal Use to Contemporary Evidence-Based Research. J. Ethnopharmacol. 2021, 268, 113636. [Google Scholar] [CrossRef]
- Załuski, D.; Janeczko, Z. Variation in Phytochemicals and Bioactivity of the Fruits of Eleutherococcus Species Cultivated in Poland. Nat. Prod. Res. 2015, 29, 2207–2211. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Sulejczak, D.; Małkowska, M.; Wójciak, M.; Gawenda-Kempczyńska, D.; Piskorska, E.; Krolik, K.; Markiewicz, M.; Kondrzycka-Dąda, A.; et al. UHPLC-DAD/ESI-TOF-MS Phytochemical Characterization and Evaluation of the Impact of Eleutherococcus senticosus Fruit Intractum on Biochemical, Hepatological, and Blood Parameters in Balb/c Mice. Int. J. Mol. Sci. 2024, 25, 9295. [Google Scholar] [CrossRef]
- Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Shiokawa, Y.; Yamada, S.; Shinozuka, K. Acanthopanax senticosus Root Extract Exerts Dual Action on Mouse Ileal Smooth Muscle Function, Leading to Modulation of Gastrointestinal Motility. Biol. Pharm. Bull. 2020, 43, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Van Crombruggen, K.; Holtappels, G.; Bachert, C. A Herbal Composition of Scutellaria Baicalensis and Eleutherococcus senticosus Shows Potent Anti-Inflammatory Effects in an Ex Vivo Human Mucosal Tissue Model. Evid.-Based Complement. Altern. Med. 2012, 2012, 673145. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Harbi, M.S. Amelioration of Paracetamol Hepatotoxicity and Oxidative Stress on Mice Liver with Silymarin and Nigella Sativa Extract Supplements. Asian Pac. J. Trop. Biomed. 2015, 5, 521–531. [Google Scholar] [CrossRef]
- Papackova, Z.; Heczkova, M.; Dankova, H.; Sticova, E.; Lodererova, A.; Bartonova, L.; Poruba, M.; Cahova, M. Silymarin Prevents Acetaminophen-Induced Hepatotoxicity in Mice. PLoS ONE 2018, 13, e0191353. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, Á.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna y González-Rubio, M.G.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective Effect of Silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and Antioxidant Effects of Gallic Acid in Paracetamol-Induced Liver Damage in Mice. J. Pharm. Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Makuch-Kocka, A.; Gawenda-Kempczyńska, D.; Ptaszyńska, A.A.; Grzyb, S.; Bogucka-Kocka, A.; Załuski, D. Phenolic Profile, Antioxidant, Anti-Enzymatic and Cytotoxic Activity of the Fruits and Roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Molecules 2022, 27, 5579. [Google Scholar] [CrossRef]
- Facchinetti, F.; Neri, I.; Tarabusi, M. Eleutherococcus senticosus Reduces Cardiovascular Stress Response in Healthy Subjects: A Randomized, Placebo-Controlled Trial. Stress. Health 2002, 18, 11–17. [Google Scholar] [CrossRef]
- Lim, S.H.; Park, Y.H.; Kwon, C.J.; Ham, H.J.; Jeong, H.N.; Kim, K.H.; Ahn, Y.S. Anti-Diabetic and Hypoglycemic Effect of Eleutherococcus Spp. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1761–1768. [Google Scholar] [CrossRef]
- Hart, J.G.; Timbrell, J.A. The Effect of Age on Paracetamol Hepatotoxicity in Mice. Biochem. Pharmacol. 1979, 28, 3015–3017. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, P.C. Antioxidant and Hepatoprotective Effects of Acathopanax Senticosus. Phytother. Res. 2000, 14, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Elblehi, S.S.; Gad El-Karim, D.R.S. Potential Amelorative Effect of Panax Ginseng on Hepatorenal Damage Ocuured in Hydroxurea-Treated Rats. Slov. Vet. Res. 2019, 56, 595–606. [Google Scholar] [CrossRef]
- Hu, J.N.; Liu, Z.; Wang, Z.; Li, X.D.; Zhang, L.X.; Li, W.; Wang, Y.P. Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax Ginseng) on Acetaminophen-Mediated Liver Injury. Molecules 2017, 22, 664. [Google Scholar] [CrossRef]
- Jang, D.; Lee, J.; Eom, S.H.; Lee, S.M.; Gil, J.; Lim, H.B.; Hyun, T.K. Composition, Antioxidant and Antimicrobial Activities of Eleutherococcus Senticosus Fruit Extracts. J. Appl. Pharm. Sci. 2016, 6, 125–130. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Galanty, A.; Verpoorte, R.; Kuźniewski, R.; Nowak, R.; Bogucka-Kocka, A. Phytochemical Content and Pharma-Nutrition Study on Eleutherococcus senticosus Fruits Intractum. Oxid. Med. Cell. Longev. 2016, 2016, 9270691. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Kan, Q.; Chu, X. Antioxidant Activity of Acanthopanax senticosus Flavonoids in H2O2-Induced RAW 264.7 Cells and DSS-Induced Colitis in Mice. Molecules 2022, 27, 2872. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Liu, R.; Zhang, X.; Xue, J.; Li, Y.; Zhang, R.; Li, X.; Wang, X.; Wang, X.; et al. Antioxidant and Antiapoptotic Properties of N-Butanol Fraction the Acanthopanax senticosus Extracts in H2O2-RAW264.7 Cells and CCl4-Induced Liver Injury in Mice. Evid.-Based Complement. Altern. Med. 2023, 2023, 9190198. [Google Scholar] [CrossRef]
- Song, Z.; Jiao, C.; Chen, B.; Xu, W.; Wang, M.; Zou, J.; Xu, W.; Xu, Z.; Wang, Q. Dietary Acanthopanax senticosus Extracts Modulated the Inflammatory and Apoptotic Responses of Yellow Catfish to Protect against Edwardsiella ictaluri Infection. Aquac. Res. 2021, 52, 5078–5092. [Google Scholar] [CrossRef]
- Rostami, S.; Arefhosseini, S.; Tutunchi, H.; Khoshbaten, M.; Ebrahimi-Mameghani, M. Does Myo-Inositol Supplementation Influence Oxidative Stress Biomarkers in Patients with Non-Alcoholic Fatty Liver Disease? Food Sci. Nutr. 2024, 12, 1279–1289. [Google Scholar] [CrossRef]
- Pan, S.; Yan, X.; Dong, X.; Li, T.; Suo, X.; Tan, B.; Zhang, S.; Li, Z.; Yang, Y.; Zhang, H. The Positive Effects of Dietary Inositol on Juvenile Hybrid Grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu) Fed High-Lipid Diets: Growthperformance, Antioxidant Capacity and Immunity. Fish Shellfish Immunol. 2022, 126, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Li, N.R.; Zhao, W.L.; Liu, H.; Ge, M.X.; Zhang, Y.X.; Zhao, L.Y.; You, X.F.; He, H.W.; Shao, R.G. D-Chiro-Inositol Effectively Attenuates Cholestasis in Bile Duct Ligated Rats by Improving Bile Acid Secretion and Attenuating Oxidative Stress. Acta Pharmacol. Sin. 2018, 39, 213–221. [Google Scholar] [CrossRef]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol Consumption Improves Liver Health Status by Reducing Oxidative Stress and Fatty Acid Accumulation in Subjects with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and Prooxidant Activities of Phenolic Acids Commonly Existed in Vegetables and Their Relationship with Structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Kanane, A.; Rouaki, F.; Errahmani, M.B.; Laraba, A.; Mesbah, H.; Ammouche, A. Original Communication Pro-Oxidant Effects of a High α-Tocopherol Dose on Kidney Antioxidant Biomarkers and Histopathological Aspects. Int. J. Vitam. Nutr. Res. 2017, 87, 179–190. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The Role of Antioxidant versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef]
- Smalinskiene, A.; Lesauskaite, V.; Zitkevicius, V.; Savickiene, N.; Savickas, A.; Ryselis, S.; Sadauskiene, I.; Ivanov, L. Estimation of the Combined Effect of Eleutherococcus senticosus Extract and Cadmium on Liver Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 314–320. [Google Scholar] [CrossRef]
- Fu, J.; Fu, J.; Yuan, J.; Zhang, N.; Gao, B.; Fu, G.; Tu, Y.; Zhang, Y. Anti-Diabetic Activities of Acanthopanax senticosus Polysaccharide (ASP) in Combination with Metformin. Int. J. Biol. Macromol. 2012, 50, 619–623. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Yoon, J.; Kim, M.Y.; Choi, H.Y.; Kim, H. Neuroprotective Effects of Eleutherococcus senticosus Bark on Transient Global Cerebral Ischemia in Rats. J. Ethnopharmacol. 2012, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kim, S.W.; Seo, H.W.; Hyun, S.H.; Kyung, J.S.; Youn, S.H.; So, S.H.; In, G.; Park, C.K.; Yi, E.C.; et al. Long-Term Evaluation of Safety and Biological Effects of Korean Red Ginseng (Panax ginseng): A Long-Term in Vivo Study. BMC Complement. Med. Ther. 2022, 22, 284. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Matsuzawa, A.; Ogawa, H.; Hirata, Y.; Noguchi, T.; Kawashima, H. Adverse Roles of Citric Acid and L-Histidine in the Transition Metal-Dependent Generation of Hydroxyl Radical at Circumneutral PH. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Zhou, J.; Sun, P.; Zhao, L.; Zhou, F. Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods 2022, 11, 3014. [Google Scholar] [CrossRef]
- Kos, G.; Czarnek, K.; Sadok, I.; Krzyszczak-Turczyn, A.; Kubica, P.; Fila, K.; Emre, G.; Tatarczak-Michalewska, M.; Latalska, M.; Blicharska, E.; et al. Eleutherococcus senticosus (Acanthopanax senticosus): An Important Adaptogenic Plant. Molecules 2025, 30, 2512. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Current Trends of Essential Trace Elements in Patients with Chronic Liver Diseases. Nutrients 2020, 12, 2084. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, F.; Gębalski, J.; Piskorska, E.; Małkowska, M.; Słomka, A.; Gawenda-Kempczyńska, D.; Kondrzycka-Dąda, A.; Olszewska-Słonina, D.; Styczyński, J.; Taglialatela-Scafati, O.; et al. The Eleutherococcus senticosus Fruits’ Intractum Affects Changes in the Transepithelial Electric Potential in the Distal Section of the Rabbit’s Large Intestine and Inhibits Hyaluronidase. J. Ethnopharmacol. 2024, 325, 117847. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Wójciak, M.; Sowa, I.; Sawicki, J.; Strzemski, M.; Hawrylak-Nowak, B.; Hanaka, A. Accumulation Ability of Trace Metals by Silver Birch Leaves in Areas Contaminated by Zn–Pb Ore Processing: Effects of Excessive Trace Metal Accumulation on Specialized Metabolism. Chemosphere 2024, 362, 142719. [Google Scholar] [CrossRef]
- ISO 17294-1:2004; Water Quality—Applications of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidance. Available online: https://www.iso.org/standard/32957.html (accessed on 30 September 2020).
- ISO 17294-2:2016; Water Quality—Applications of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements, Including Isotopes of Uranium. Available online: https://www.iso.org/standard/62962.html (accessed on 30 September 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).