In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GFPs
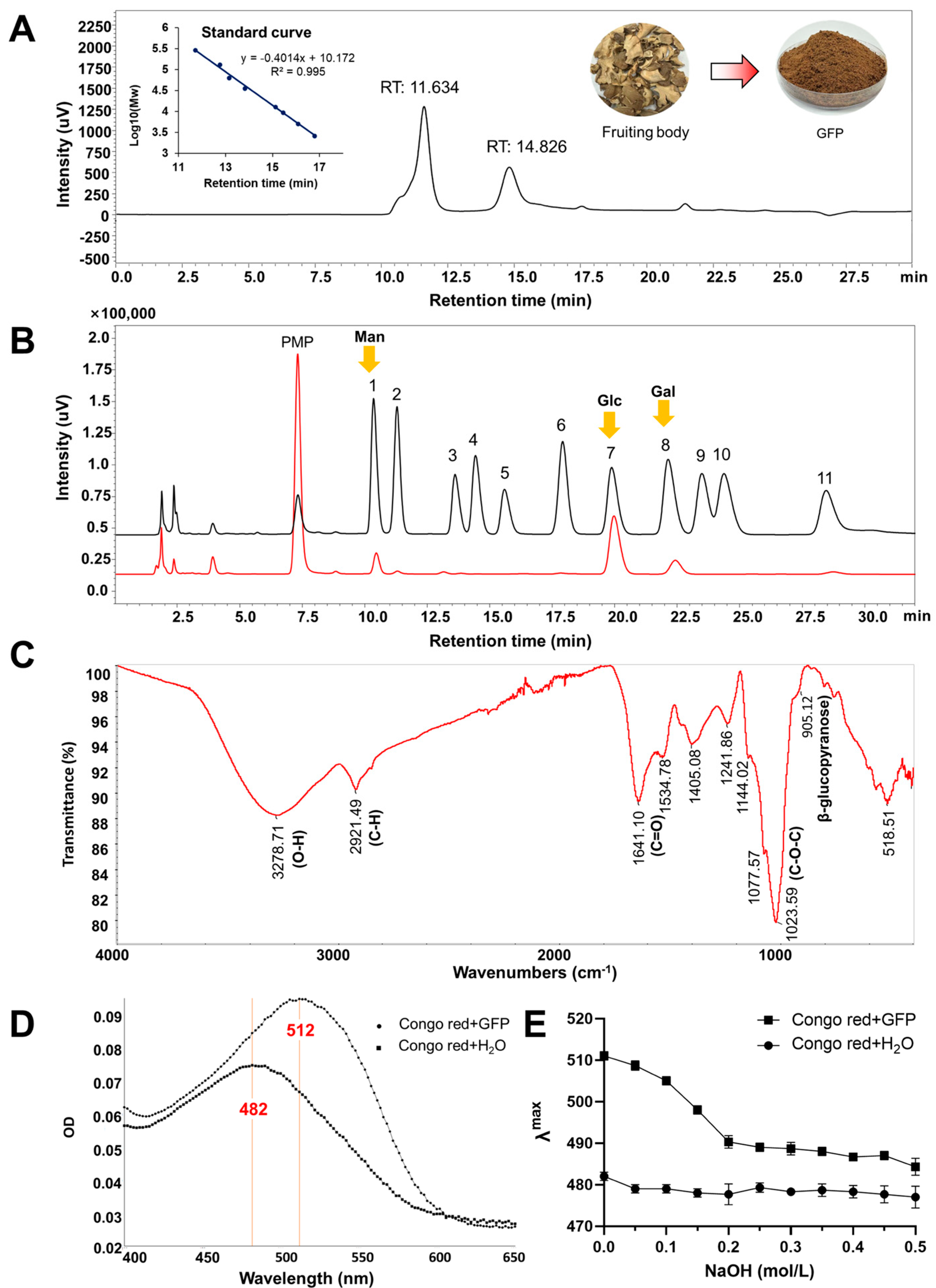
2.3. Analysis of Physicochemical Properties of GFPs
2.3.1. Basic Composition Determination
2.3.2. Molecular Weight Distribution Detection
2.3.3. Monosaccharide Composition Analysis
2.3.4. Fourier Transform Infrared Spectrum Analysis
2.3.5. Congo Red Assay
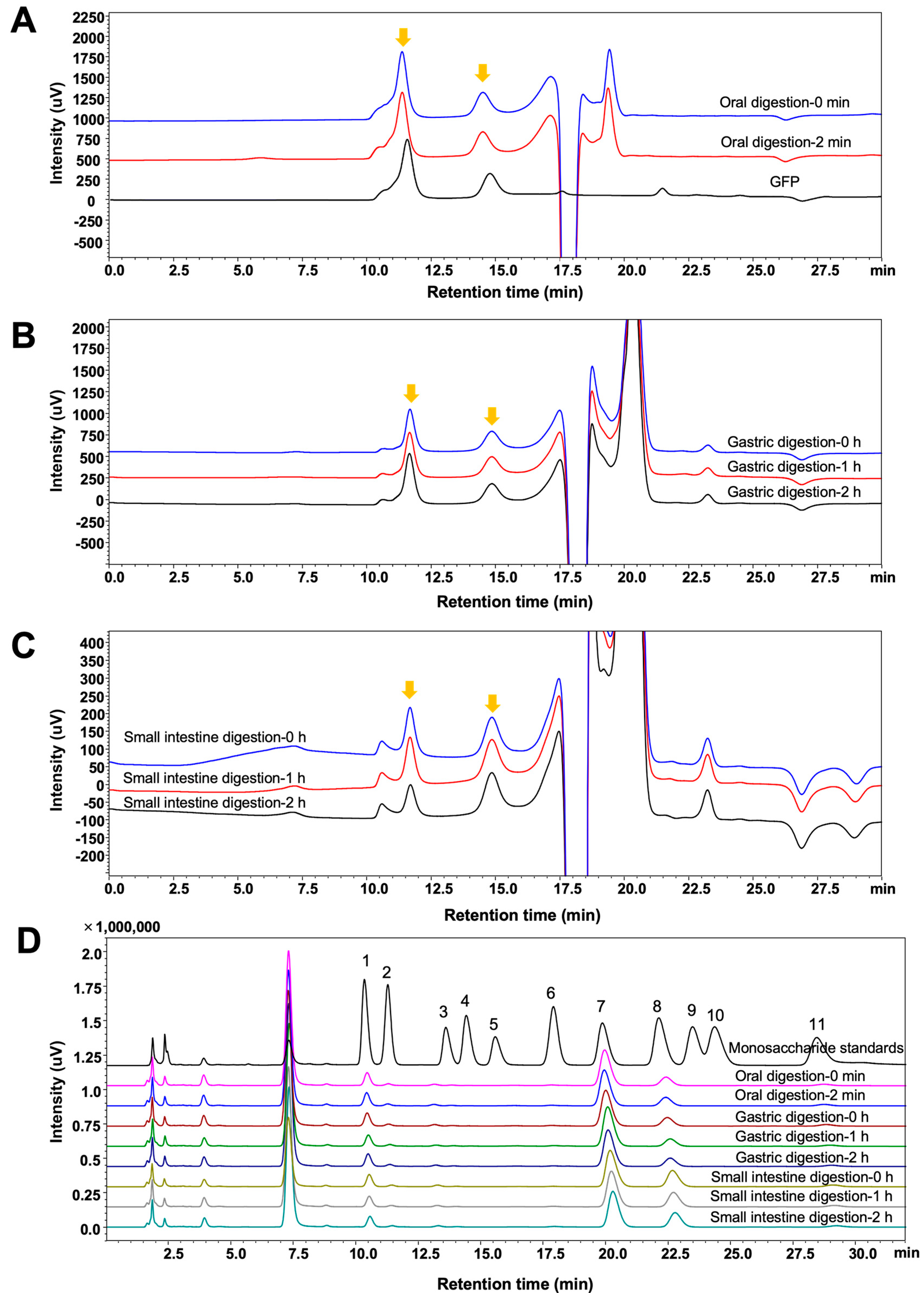
2.4. In Vitro Simulated Digestion
2.4.1. Oral Digestion
2.4.2. Gastric Digestion
2.4.3. Small Intestine Digestion
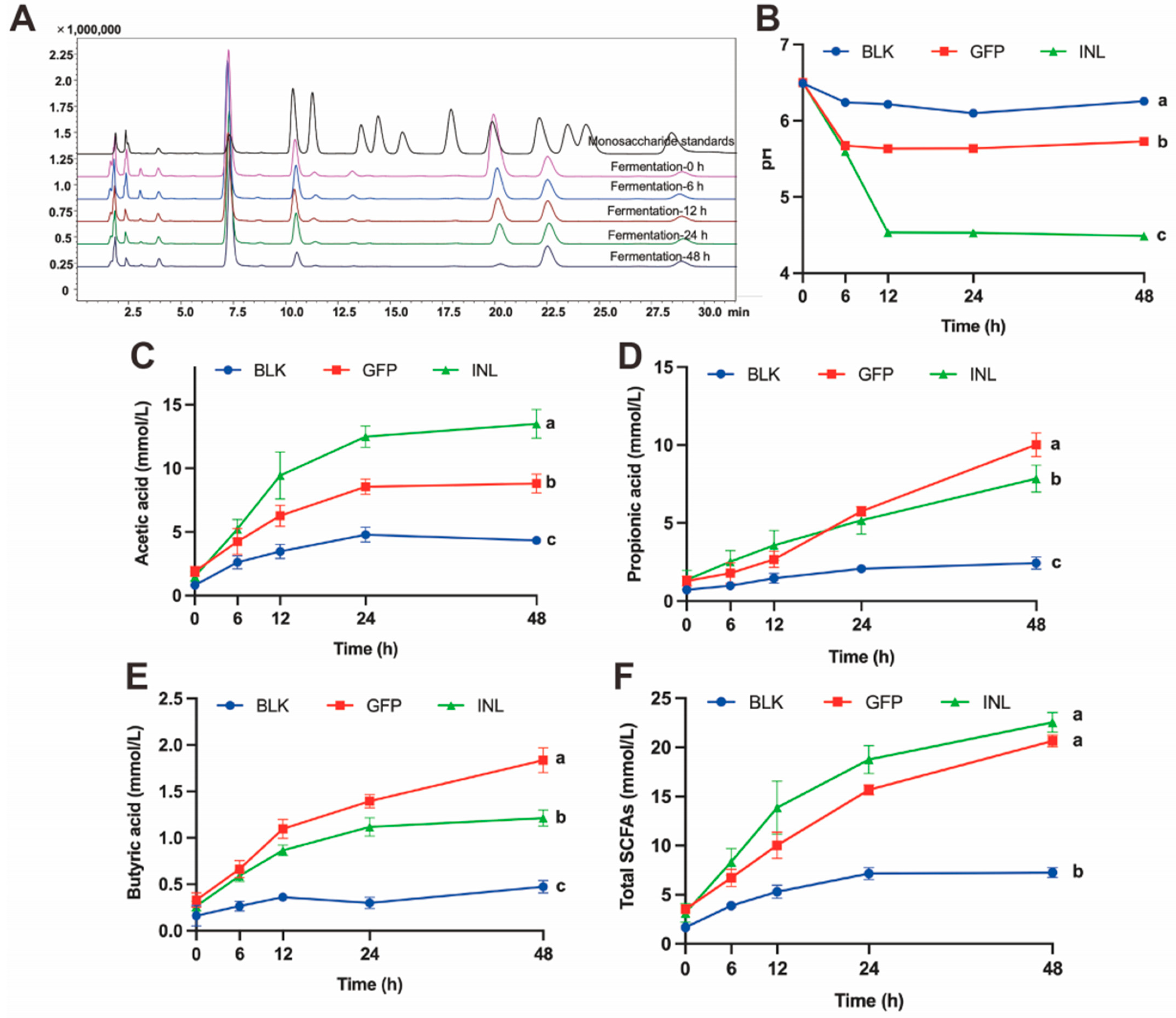
2.5. In Vitro Simulated Fermentation of GFPs
2.6. Dynamic Monitoring of GFPs During Digestion and Fermentation
2.7. Determination of SCFAs in Fermentation Broth
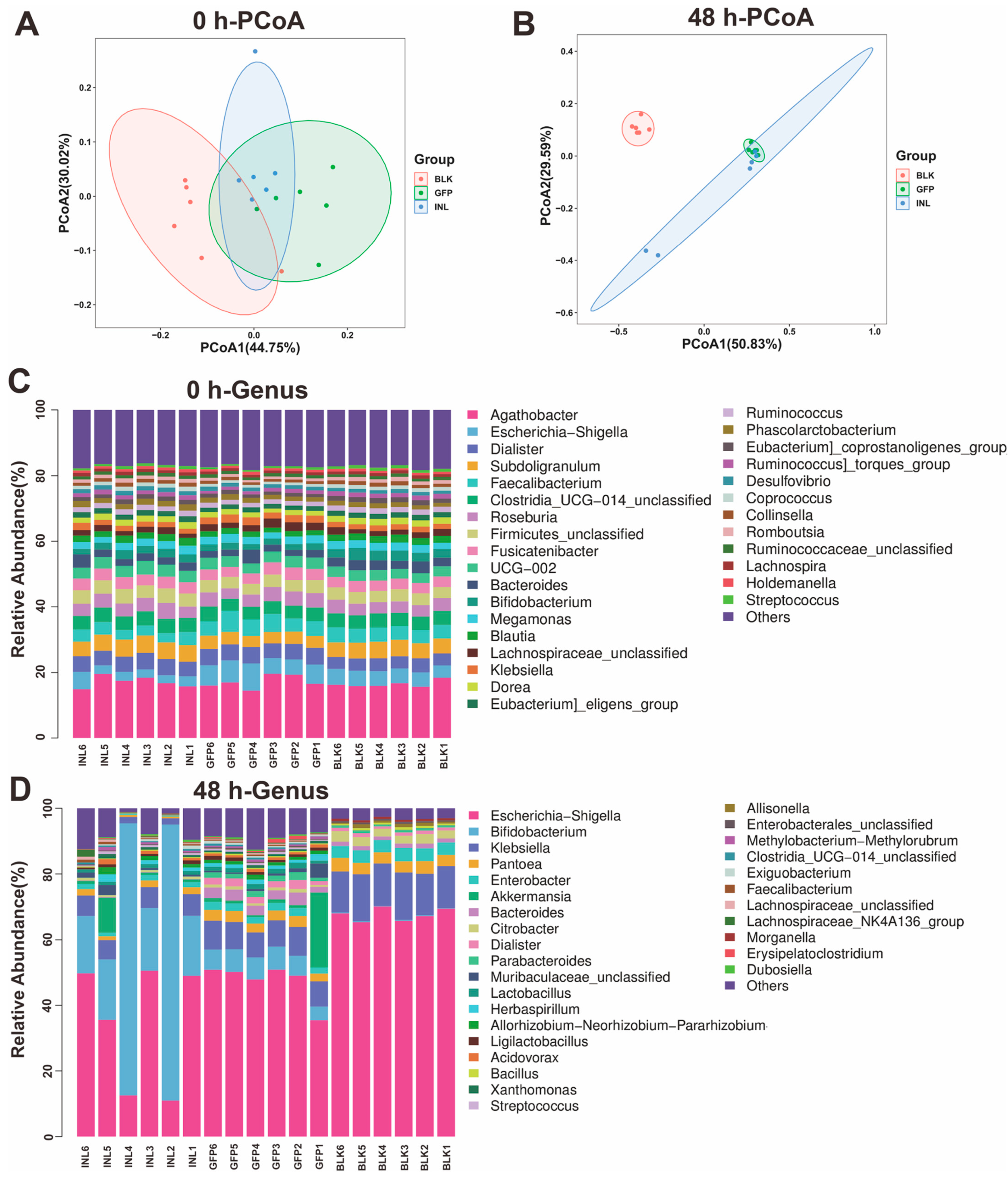
2.8. 16S rDNA Sequencing of Gut Microbiota
2.9. Non-Targeted Metabolites Analysis of Fermentation Fluid
2.10. Single Bacterial Fermentation
2.11. Determination of GABA in the Fermentation Broth by LC-MS/MS
2.12. Statistical Analysis
3. Results
3.1. Prepared and Physicochemical Properties Analysis of GFPs
3.2. Characteristics of GFPs During In Vitro Simulated Digestion
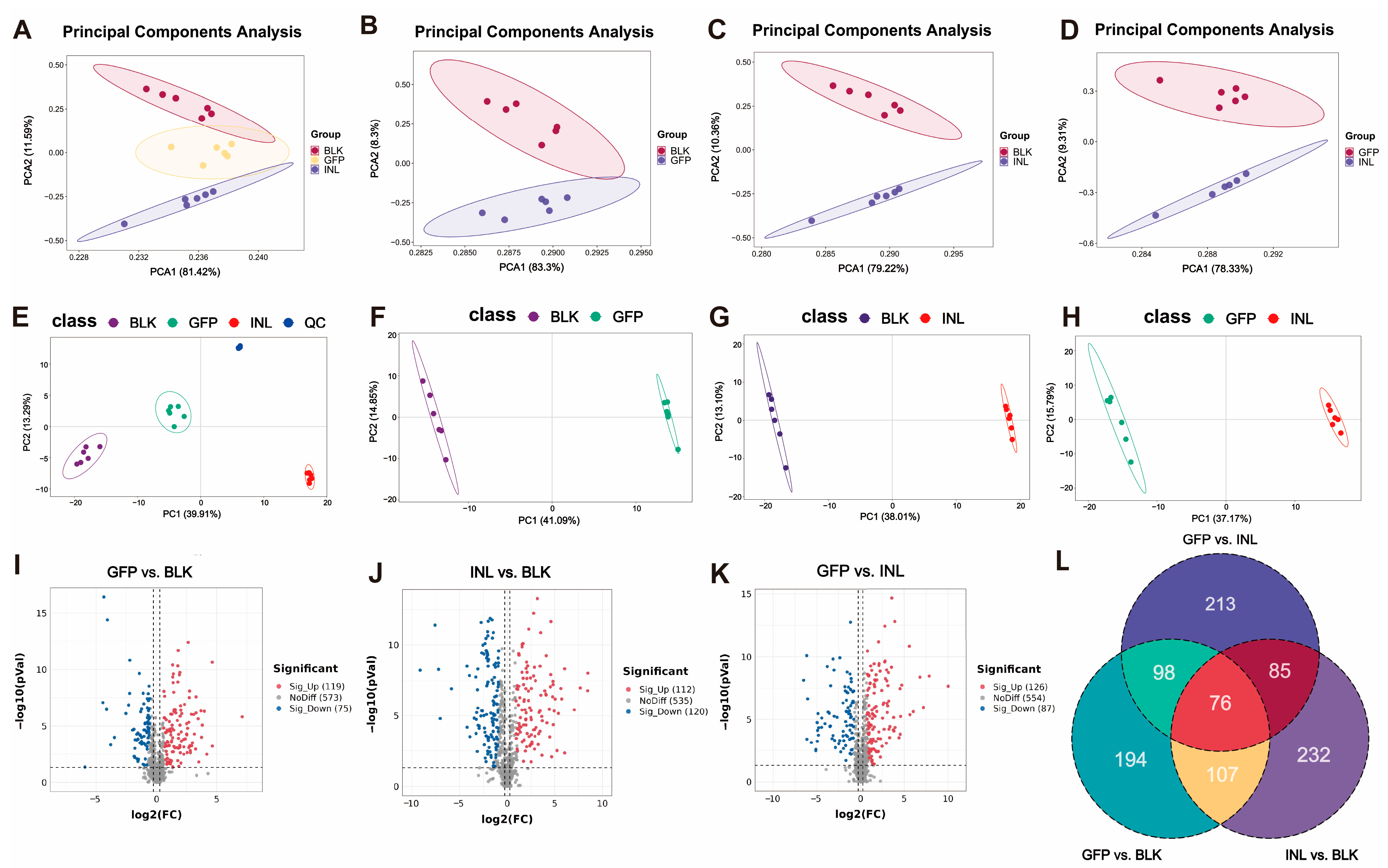
3.3. Interaction Between GFPs and Gut Microbiota During In Vitro Fermentation
3.3.1. Dynamic Changes During GFPs Fermentation
3.3.2. Regulatory Role of GFPs in Modulating Gut Microbiota
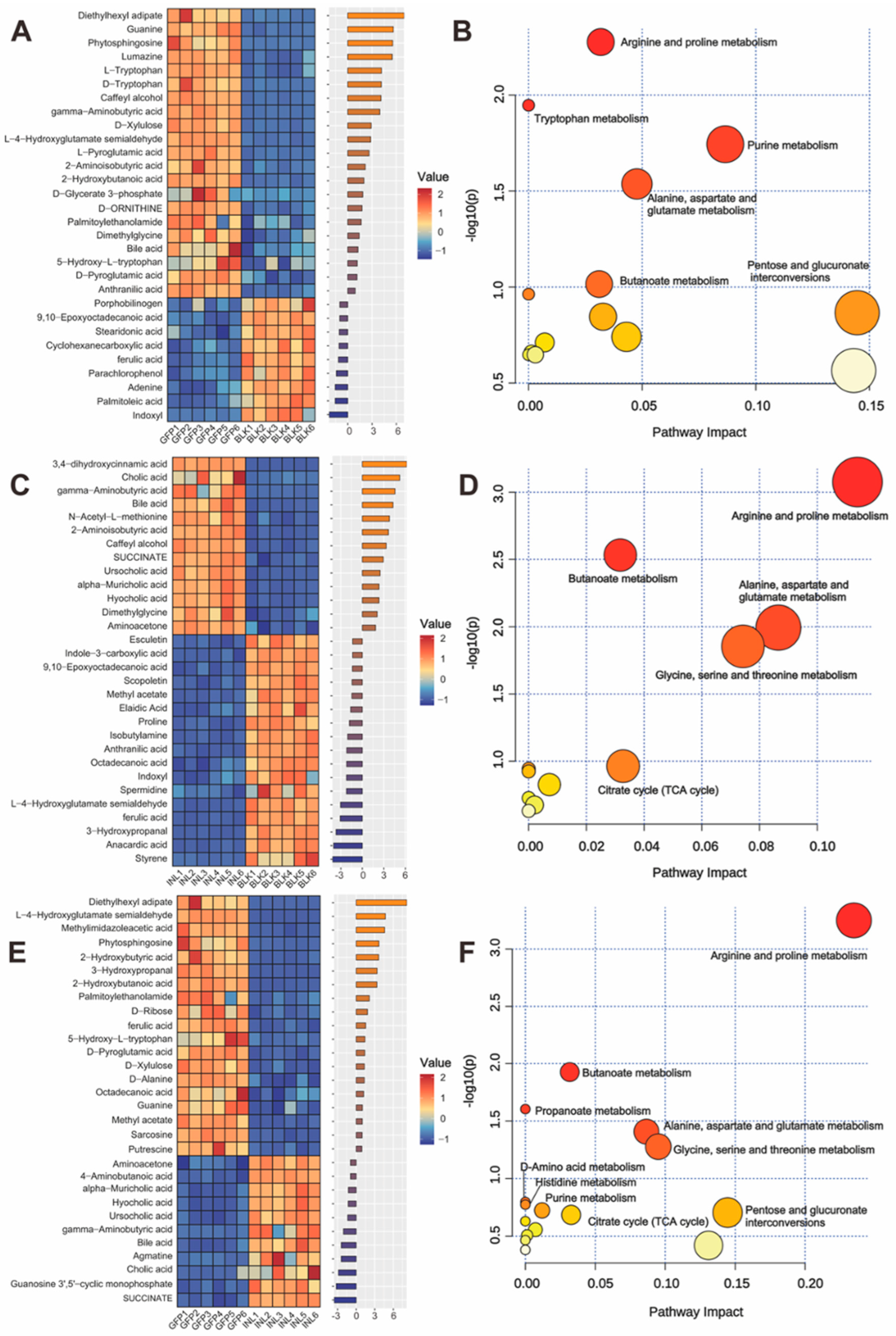
3.3.3. Effects of GFP on Metabolite Production
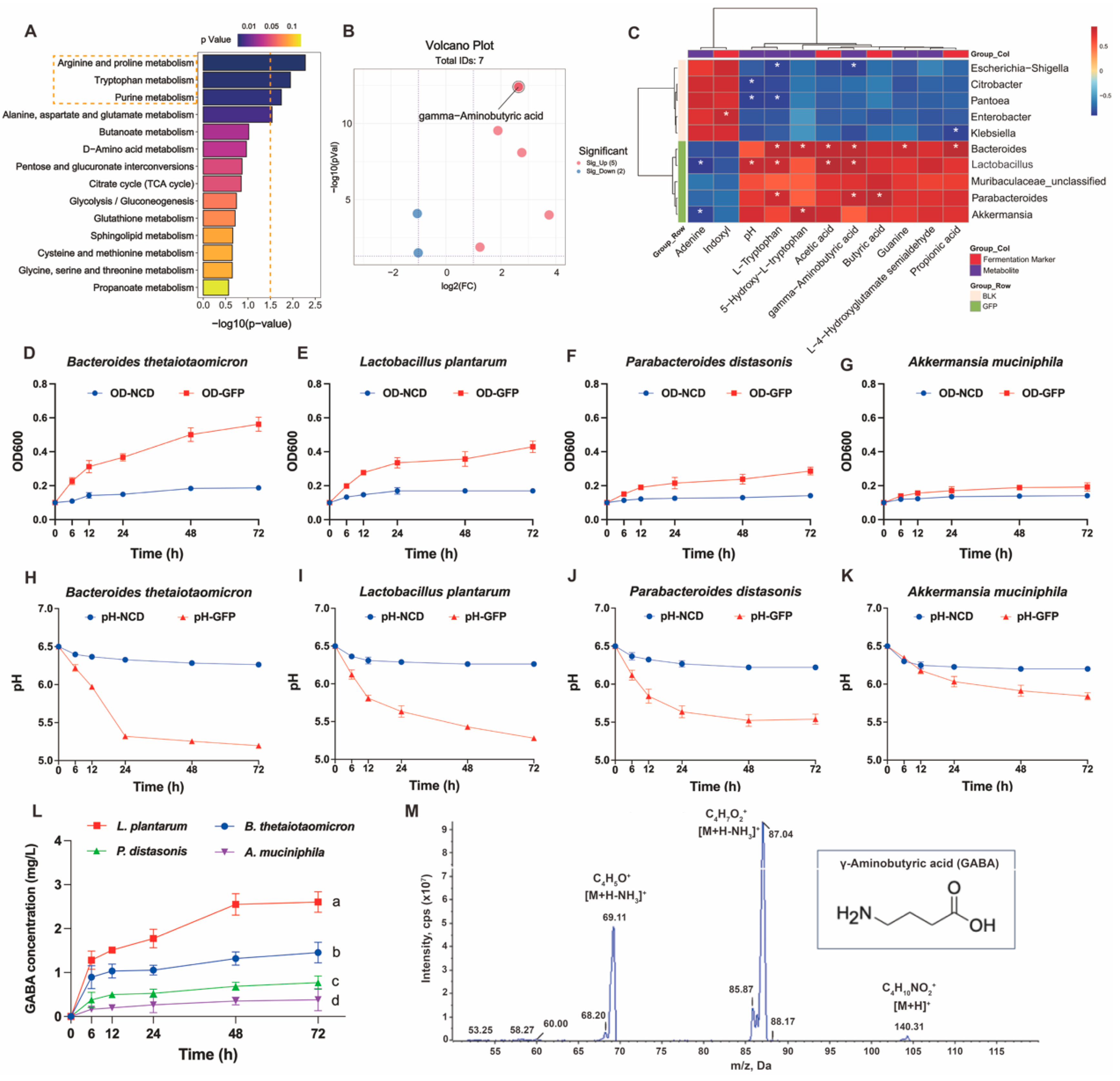
3.3.4. GFPs Enhance GABA Production by Upregulating Beneficial Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, B.; Wei, S.; Huang, H. Recent advances in Grifola frondosa polysaccharides: Production, properties, and bioactivities. Curr. Opin. Food Sci. 2023, 49, 100946. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Wen, C.; Liu, J.; Xu, X.; Liu, G.; Kan, J.; Qian, C.; Jin, C. New light on Grifola frondosa polysaccharides as biological response modifiers. Trends Food Sci. Technol. 2022, 119, 565–578. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Wang, H.; Geng, F.; Nie, S. Effect of β-glucan on metabolic diseases: A review from the gut microbiota perspective. Curr. Opin. Food Sci. 2022, 47, 100907. [Google Scholar] [CrossRef]
- Gao, X.; Liu, D.; Gao, L.; Ouyang, Y.; Wen, Y.; Ai, C.; Chen, Y.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Wellness 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Chen, S.; Liu, H.; Ma, H.; Hu, T.; Luo, P.; Wei, S. Grifola frondosa polysaccharide’s therapeutic potential in oxazolone-induced ulcerative colitis. Carbohydr. Polym. 2024, 344, 122517. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, J.; Zuo, S.; Zhang, S.; Li, M.; Nie, S. In vitro gastrointestinal digestion and fermentation models and their applications in food carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 62, 5349–5371. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Liu, C.; Hua, H.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Comprehensive analysis of Sparassis crispa polysaccharide characteristics during the in vitro digestion and fermentation model. Food Res. Int. 2022, 154, 111005. [Google Scholar] [CrossRef]
- Ye, K.; Fu, C.; Ma, S.; Du, H.; Chen, S.; Liu, D.; Ma, G.; Xiao, H. Comprehensive assessment of Hypsizygus marmoreus polysaccharides through simulated digestion and gut microbiota fermentation in vitro. Food Hydrocoll. 2023, 144, 108989. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, Y.; Ye, X.; Song, Z.; Yuan, J.; Xiong, B.; Jing, Y.; Shi, Y.; Xu, T.; Wu, J.; et al. Extraction, purification and characterization of sulphated polysaccharide from Bellamya quadrata and its stabilization roles on atherosclerotic plaque. Int. J. Biol. Macromol. 2020, 152, 314–326. [Google Scholar] [CrossRef]
- Wang, W.; Chen, F.; Zheng, F.; Russell, B.T. Optimization of synthesis of carbohydrates and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology (RSM) for improved carbohydrate detection. Food Chem. 2020, 309, 125686. [Google Scholar] [CrossRef]
- Hao, R.; Zhou, X.; Zhao, X.; Lv, X.; Zhu, X.; Gao, N.; Jiang, Y.; Wu, M.; Sun-Waterhouse, D.; Li, D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total Environ. 2023, 877, 162910. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, D.; Zhao, R.; Gao, J.; Kimatu, B.M.; Hu, Q.; Chen, G.; Zhao, L. Effects of ultrasound-assisted extraction on antioxidant activity and bidirectional immunomodulatory activity of Flammulina velutipes polysaccharide. Int. J. Biol. Macromol. 2019, 140, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Dai, W.; Shang, Q.; Yu, G. Bacteroides salyersiae is a potent chondroitin sulfate-degrading species in the human gut microbiota. Microbiome 2024, 12, 41. [Google Scholar] [CrossRef]
- Xu, B.; Song, S.; Yao, L.; Wang, H.; Sun, M.; Zhuang, H.; Zhang, X.; Liu, Q.; Yu, C.; Feng, T. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human gut microbiota of polysaccharides from Ficus carica Linn. Food Hydrocoll. 2024, 146, 109204. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Yao, A.; Cui, W.; Wei, Y.; Guo, S.; Duan, J.; Kang, H.; Zhou, X.; Su, S.; et al. In vitro fermentation characteristics and interaction of neutral and acidic polysaccharides from Lycii fructus on human gut microbiota. Food Hydrocoll. 2024, 152, 109940. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, L.; Zhai, Q.; Zhao, R.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. In vitro fermentation of heparin by the human gut microbiota: Changes in the microbiota community and metabolic functions. Food Chem. 2023, 406, 135010. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, J.; Narimatsu, Y.; de Haan, N.; Clausen, H.; de Vos, W.M.; Tytgat, H.L.P. Binding of Akkermansia muciniphila to mucin is O-glycan specific. Nat. Commun. 2024, 15, 4582. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; Kingsbury, D.D.; Velazquez, E.M.; Bäumler, A.J. Collateral damage: Microbiota-derived metabolites and immune function in the antibiotic era. Cell Host Microbe 2014, 16, 156–163. [Google Scholar] [CrossRef]
- Ezzine, C.; Loison, L.; Montbrion, N.; Bôle-Feysot, C.; Déchelotte, P.; Coëffier, M.; Ribet, D. Fatty acids produced by the gut microbiota dampen host inflammatory responses by modulating intestinal SUMOylation. Gut Microbes 2022, 14, 2108280. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Peng, X.; Zhu, Y.; Duan, W.; Ji, R.; Xiao, H.; Li, X.; Liu, G.; Yu, Y.; et al. Anti-inflammation mechanisms of a homogeneous polysaccharide from Phyllanthus emblica L. on DSS induced colitis mice via the gut microbiota and metabolites alteration. Food Chem. 2024, 459, 140346. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yu, J.; Shi, X.; Zhu, J.; Gao, X.; Liu, W. Polysaccharides catabolism by the human gut bacterium—Bacteroides thetaiotaomicron: Advances and perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 3569–3588. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- Pannerchelvan, S.; Rios-Solis, L.; Faizal Wong, F.W.; Zaidan, U.H.; Wasoh, H.; Mohamed, M.S.; Tan, J.S.; Mohamad, R.; Halim, M. Strategies for improvement of gamma-aminobutyric acid (GABA) biosynthesis via lactic acid bacteria (LAB) fermentation. Food Funct. 2023, 14, 3929–3948. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.X.; Goh, W.R.; Wu, R.N.; Yue, X.Q.; Luo, X.; Khine, W.W.T.; Wu, J.R.; Lee, Y.K. Revisit gut microbiota and its impact on human health and disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, J.; Fan, M.; Li, Y.; Huang, L.; Wang, L. In vitro fermentation reveals an interplay relationship between oat β-glucan and human gut Bacteroides and their potential role in regulating gut cytokines. Food Funct. 2024, 15, 7794–7811. [Google Scholar] [CrossRef]
- Kles, K.A.; Chang, E.B. Short-chain fatty acids impact on intestinal adaptation, inflammation, carcinoma, and failure. Gastroenterology 2006, 130, S100–S105. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Mu, G.; Tuo, Y. Exopolysaccharide secreted by Lactiplantibacillus plantarum Y12 showed inhibitory effect on the pathogenicity of Shigella flexneri in vitro and in vivo. Int. J. Biol. Macromol. 2024, 261, 129478. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Ning, X.; Qin, Y.; Wang, Y.; Yu, Z.; Dong, R.; Zhang, Y.; Sun, S. Expansion of Escherichia-Shigella in gut is associated with the onset and response to immunosuppressive therapy of IgA nephropathy. J. Am. Soc. Nephrol. 2022, 33, 2276–2292. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3987–4002. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Dong, S.; Wang, R.; Wang, S.; Yang, J.; Wang, X.; Lü, X. The role of a novel antibacterial substance, cyclic opine-producing Lacticaseibacillus rhamnosus LS8 in ameliorating ulcerative colitis: A fecal microbiota transplantation study. Food Sci. Hum. Wellness 2024, 13, 778–790. [Google Scholar] [CrossRef]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; Zhou, X.; Wang, X.; Liu, X.; Wang, Q.; Zhao, R.; Gao, M.; Li, Z.; Feng, Y.; et al. Effect of Lactobacillus fermentation on the structural feature, physicochemical property, and bioactivity of plant and fungal polysaccharides: A review. Trends Food Sci. Technol. 2024, 148, 104492. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Guan, J.; Chen, J.; Xu, X. Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front. Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xie, G.; Zhao, A.; Zhao, L.; Yao, C.; Chiu, N.H.L.; Zhou, Z.; Bao, Y.; Jia, W.; Nicholson, J.K.; et al. The footprints of gut microbial–mammalian co-metabolism. J. Proteome Res. 2011, 10, 5512–5522. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of gamma-aminobutyric acid in foods: From conventional methods to innovative technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef]
- de Bie, T.H.; Witkamp, R.F.; Balvers, M.G.J.; Jongsma, M.A. Effects of γ-aminobutyric acid supplementation on glucose control in adults with prediabetes: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2023, 118, 708–719. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Kook, M.C.; Seo, M.J.; Cheigh, C.I.; Lee, S.J.; Pyun, Y.R.; Park, H. Enhancement of γ-amminobutyric acid production by Lactobacillus sakei B2–16 expressing glutamate decarboxylase from Lactobacillus plantarum ATCC 14917. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 816–820. [Google Scholar] [CrossRef]
- Shan, Y.; Man, C.X.; Han, X.; Li, L.; Guo, Y.; Deng, Y.; Li, T.; Zhang, L.W.; Jiang, Y.J. Evaluation of improved γ-aminobutyric acid production in yogurt using Lactobacillus plantarum NDC75017. J. Dairy Sci. 2015, 98, 2138–2149. [Google Scholar] [CrossRef]
- Plaza-Vinuesa, L.; Hernandez-Hernandez, O.; Moreno, F.J.; de las Rivas, B.; Muñoz, R. Unravelling the diversity of glycoside hydrolase family 13 α-amylases from Lactobacillus plantarum WCFS1. Microb. Cell Factories 2019, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.T.; López, P. Functional analysis of lactic acid bacteria and Bifidobacteria and their effects on human health. Foods 2022, 11, 2293. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Suttipun, K. Unveiling the prebiotic properties of mushrooms in improving gut health and related diseases. Food Biosci. 2025, 70, 107040. [Google Scholar] [CrossRef]
- Willoughby, J.O.; Jervois, P.M.; Menadue, M.F.; Blessing, W.W. Activation of GABA receptors in the hypothalamus stimulates secretion of growth hormone and prolactin. Brain Res. 1986, 374, 119–125. [Google Scholar] [CrossRef]

| Time | Total Sugars (mg/mL) | Reducing Sugars (mg/mL) | |
|---|---|---|---|
| Oral digestion | 0 min | 0.6565 ± 0.0059 a | 0.1198 ± 0.0121 a |
| 2 min | 0.6685 ± 0.0216 a | 0.1201 ± 0.0191 a | |
| Gastric digestion | 0 h | 0.3759 ± 0.0035 a | 0.1192 ± 0.0192 a |
| 1 h | 0.3711 ± 0.0129 a | 0.1181 ± 0.0301 a | |
| 2 h | 0.3754 ± 0.0078 a | 0.1212 ± 0.0212 a | |
| Small intestinal digestion | 0 h | 0.2476 ± 0.0021 a | 0.1109 ± 0.0141 a |
| 1 h | 0.2427 ± 0.0078 a | 0.1094 ± 0.0231 a | |
| 2 h | 0.2453 ± 0.0047 a | 0.1091 ± 0.0133 a | |
| Fecal fermentation | 0 h | 0.5594 ± 0.0336 a | 1.8533 ± 0.1704 a |
| 6 h | 0.1779 ± 0.0126 b | 0.4533 ± 0.0850 b | |
| 12 h | 0.0751 ± 0.0211 c | 0.2330 ± 0.0497 c | |
| 24 h | 0.0351 ± 0.0352 d | 0.0147 ± 0.0047 d | |
| 48 h | 0.0115 ± 0.0137 e | 0.0092 ± 0.0021 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Luo, Y.; Zhu, H.; Liu, X.; Xue, M.; Yang, G.; Chen, Y.; Chen, S.; Wen, Z. In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation. Nutrients 2025, 17, 3332. https://doi.org/10.3390/nu17213332
Wang Q, Luo Y, Zhu H, Liu X, Xue M, Yang G, Chen Y, Chen S, Wen Z. In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation. Nutrients. 2025; 17(21):3332. https://doi.org/10.3390/nu17213332
Chicago/Turabian StyleWang, Qingchi, Yuhang Luo, Huabo Zhu, Xiaoyang Liu, Mingyuan Xue, Guiling Yang, Yue Chen, Shiguo Chen, and Zhengshun Wen. 2025. "In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation" Nutrients 17, no. 21: 3332. https://doi.org/10.3390/nu17213332
APA StyleWang, Q., Luo, Y., Zhu, H., Liu, X., Xue, M., Yang, G., Chen, Y., Chen, S., & Wen, Z. (2025). In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation. Nutrients, 17(21), 3332. https://doi.org/10.3390/nu17213332






