Impact of a Transition Clinic on Long-Term Care and Nutritional Management in Patients with Inborn Errors of Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Transition Consultation Protocol
2.4. Clinical Assessment and Data Collection
2.5. Nutritional Adherence
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics and Diagnostic Pathways
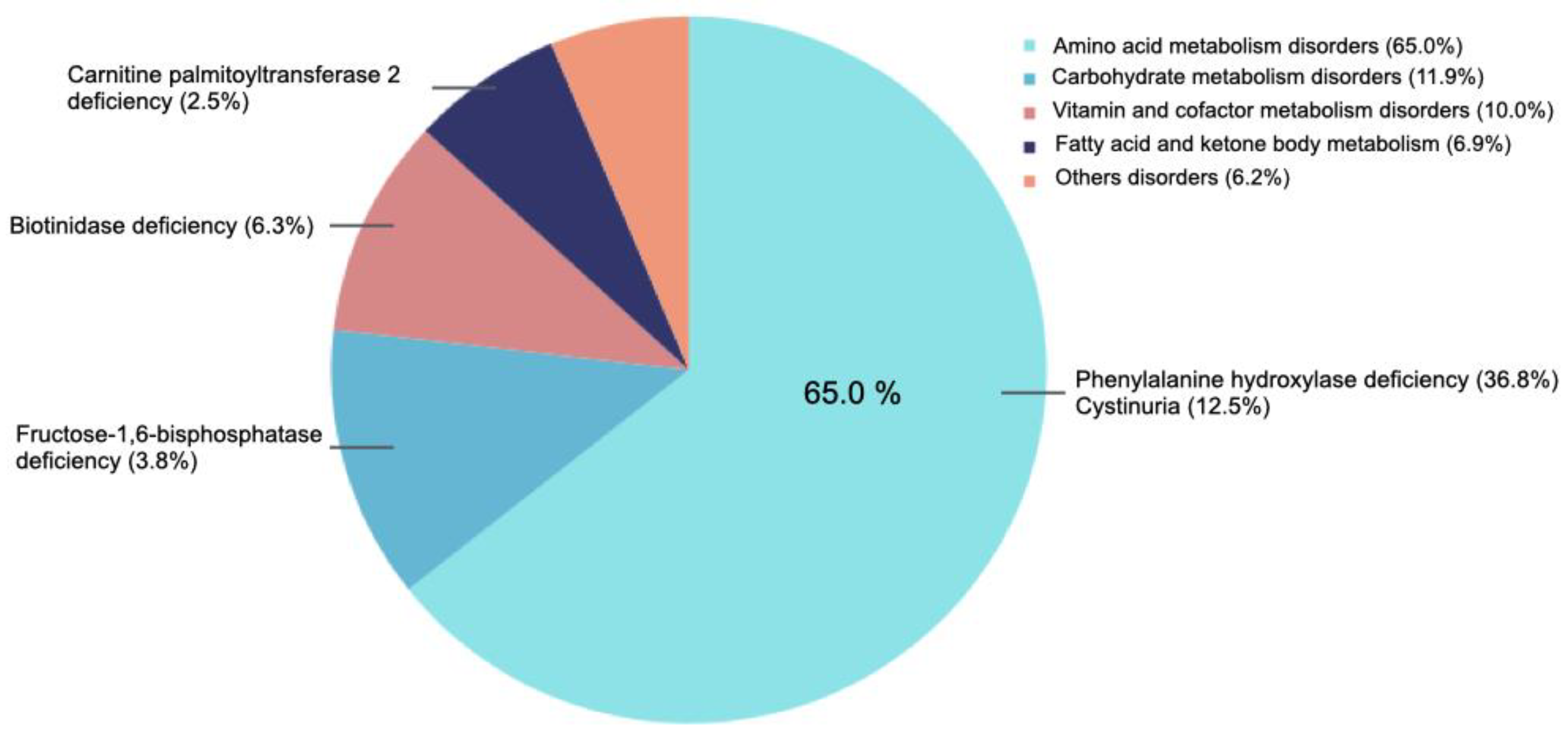
3.2. Inborn Errors of Metabolism Distribution
3.3. Demographic Characteristics of Transitioned Vs. Non-Transitioned Patients
3.4. Diagnostic Pathways by Group
3.5. Treatment Modalities and Dietary Adherence
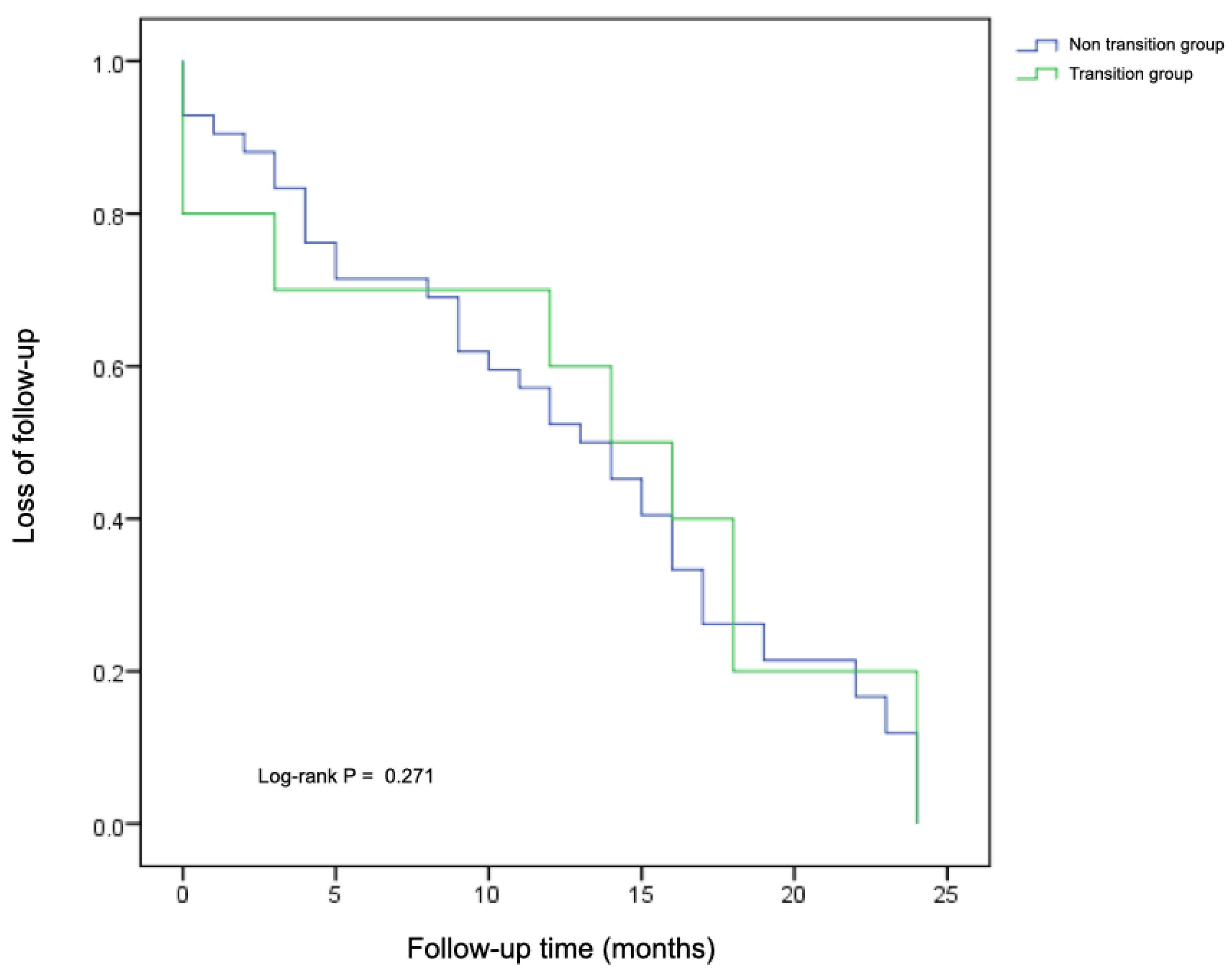
3.6. Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-López, J.; Ceberio-Hualde, L.; Morillo, J.S.G.; Grau-Junyent, J.M.; Ameijeiras, Á.H.; López-Rodríguez, M.; Morales-Conejo, M.; Mateos, J.J.N.; Azuara, L.J.A.E.; Campistol, J.; et al. Transition process from paediatric to adult care in patients with inborn errors of metabolism. Consensus statement. Med. Clin. 2016, 147, 506.e1–506.e7. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Rahman, S.; Keller, M.; Zschocke, J.; ICIMD Advisory Group. An international classification of inherited metabolic disorders (ICIMD). J. Inherit. Metab. Dis. 2021, 44, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, M. Clinical approach to the diagnoses of inborn errors of metabolism. Pediatr. Clin. N. Am. 2008, 55, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Brodosi, L.; Bettocchi, I.; Noto, D.; Pochiero, F.; Urban, M.L.; Burlina, A. The management of transitional care of patients affected by phenylketonuria in Italy: Review and expert opinion. Mol. Genet. Metab. 2022, 136, 94–100. [Google Scholar] [CrossRef]
- Stolwijk, N.N.; Bosch, A.M.; Bouwhuis, N.; Häberle, J.; van Karnebeek, C.; van Spronsen, F.J.; Langeveld, M.; Hollak, C.E.M. Food or medicine? A European regulatory perspective on nutritional therapy products to treat inborn errors of metabolism. J. Inherit. Metab. Dis. 2023, 46, 1017–1028. [Google Scholar] [CrossRef]
- Jurecki, E.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.; Sheinin, M.; Cohen-Pfeffer, J. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef]
- Walter, J.; White, F.; Hall, S.; MacDonald, A.; Rylance, G.; Boneh, A.; Francis, D.; Shortland, G.; Schmidt, M.; Vail, A. How practical are recommendations for dietary control in phenylketonuria? Lancet 2002, 360, 55–57. [Google Scholar] [CrossRef]
- Borghi, L.; Moreschi, C.; Toscano, A.; Comber, P.; Vegni, E. The PKU & ME study: A qualitative exploration, through co-creative sessions, of attitudes and experience of the disease among adults with phenylketonuria in Italy. Mol. Genet. Metab. Rep. 2020, 23, 100585. [Google Scholar]
- Enns, G.M.; Koch, R.; Brumm, V.; Blakely, E.; Suter, R.; Jurecki, E. Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence. Mol. Genet. Metab. 2010, 101, 99–109. [Google Scholar] [CrossRef]
- Bulut, F.D.; Seydaoğlu, G.; Kor, D.; Kılavuz, S.; Boz, A.; Önenli Mungan, N. Perspectives of adult patients with lysosomal storage diseases on the transition from pediatric to adult healthcare in Turkey. Arch. Pediatr. 2023, 30, 450–454. [Google Scholar] [CrossRef]
- Cooley, W.C.; Sagerman, P.J.; American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group. Supporting the Health Care Transition from Adolescence to Adulthood in the Medical Home. Pediatrics 2011, 128, 182–200, Erratum in Pediatrics 2018, 142, e20182587. [Google Scholar] [CrossRef]
- Chabrol, B.; Jacquin, P.; Francois, L.; Broué, P.; Dobbelaere, D.; Douillard, C.; Dubois, S.; Feillet, F.; Perrier, A.; Fouilhoux, A.; et al. Transition from pediatric to adult care in adolescents with hereditary metabolic diseases: Specific guidelines from the French network for rare inherited metabolic diseases (G2M). Arch. Pediatr. 2018, 25, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.H.; White, F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health 2004, 16, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sirrs, S.; Hollak, C.; Merkel, M.; Sechi, A.; Glamuzina, E.; Janssen, M.C.; Lachmann, R.; Langendonk, J.; Scarpelli, M.; Ben Omran, T.; et al. The frequencies of different inborn errors of metabolism in adult metabolic centres: Report from the SSIEM Adult Metabolic Physicians Group. JIMD Rep. 2016, 27, 85–91. [Google Scholar] [PubMed]
- Stepien, K.M.; Kieć-Wilk, B.; Lampe, C.; Tangeraas, T.; Cefalo, G.; Belmatoug, N.; Francisco, R.; del Toro, M.; Wagner, L.; Lauridsen, A.-G.; et al. Challenges in transition from childhood to adulthood care in rare metabolic diseases: Results from the first multi-center European survey. Front. Med. 2021, 8, 652358. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; BéLanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Genevaz, D.; Arnoux, A.; Marcel, C.; Brassier, A.; Pichard, S.; Feillet, F.; Labarthe, F.; Chabrol, B.; Berger, M.; Lapointe, A.-S.; et al. Transition from child to adult health care for patients with lysosomal storage diseases in France: Current status and priorities—The TENALYS study, a patient perspective survey. Orphanet J. Rare Dis. 2022, 17, 68. [Google Scholar] [CrossRef]
- Somanadhan, S.; Brinkley, A.; Larkin, P.J. Living through liminality? Situating the transitional experience of parents of children with mucopolysaccharidoses. Scand. J. Caring Sci. 2022, 36, 614–624. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, España. Relación de Centros, Servicios y Unidades de Referencia (CSUR) del Sistema Nacional de Salud Designados para la Atención o Realización de las Patologías o Procedimientos que se Indican. Available online: https://www.sanidad.gob.es/areas/csur/centros/patologias/docs/Listado_PTTPs.pdf (accessed on 14 October 2025).
- Ministerio de Sanidad, España. Listado de Centros Españoles Participantes en las Redes Europeas de Referencia (ERN) Aprobadas por la Comisión Europea. Available online: https://www.sanidad.gob.es/areas/csur/redesEuropeas/docs/Centros_SNS_que_participan_en_las_ERN_.pdf (accessed on 14 October 2025).
- Cazzorla, C.; Bensi, G.; Biasucci, G.; Leuzzi, V.; Manti, F.; Musumeci, A.; Papadia, F.; Stoppioni, V.; Tummolo, A.; Vendemiale, M.; et al. Living with phenylketonuria in adulthood: The PKU ATTITUDE study. Mol. Genet. Metab. Rep. 2018, 16, 39–45. [Google Scholar] [CrossRef]
- Suddaby, J.S.; Sohaei, D.; Bell, H.; Tavares, S.; Lee, G.J.; Szybowska, M.; So, J. Adult patient perspectives on phenylketonuria care: Highlighting the need for dedicated adult management and services. Eur. J. Med. Genet. 2020, 63, 103818. [Google Scholar] [CrossRef]
- Mütze, U.; Thiele, A.G.; Baerwald, C.; Ceglarek, U.; Kiess, W.; Beblo, S. Ten years of specialized adult care for phenylketonuria—A single-centre experience. Orphanet J. Rare Dis. 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pintos, P.; Camba-Garea, M.J.; López-Pardo, B.M.; Couce, M.L. Odimet®: A Pioneering Tele-Health Tool to Empower Dietary Treatment and the Acute Management of Inborn Errors of Metabolism—An Assessment of Its Effectiveness during the COVID Pandemic. Nutrients 2024, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Enns, G.M.; Packman, W. The adolescent with an inborn error of metabolism: Medical issues and transition to adulthood. Adolesc. Med. 2002, 13, 315–329. [Google Scholar]
- Yeung, E.; Kay, J.; Roosevelt, G.E.; Brandon, M.; Yetman, A.T. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int. J. Cardiol. 2008, 125, 62–65. [Google Scholar] [CrossRef]
- Schwartz, L.A.; Tuchman, L.K.; Hobbie, W.L.; Ginsberg, J.P. A social-ecological model of readiness for transition to adult-oriented care for adolescents and young adults with chronic health conditions. Child Care Health Dev. 2011, 37, 883–895. [Google Scholar] [CrossRef]
- Peres, M.; Almeida, M.F.; Pinto, É.J.; Carmona, C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; MacDonald, A.; et al. Implementing a transition program from pediatric to adult services in phenylketonuria: Results after two years of follow-up with an adult team. Nutrients 2021, 13, 799. [Google Scholar] [CrossRef] [PubMed]
| Overall Cohort (n = 160) | Transition Group (n = 41) | Non-Transition Group (n = 119) | p Value | p Value Adjusted for Age | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sex (n, % women) | 95 (59.4) | 21 (51.2) | 74 (62.2) | 0.218 | 0.843 |
| Current age (years) | 36.2 ± 11.5 | 28.12 ± 9.9 | 38.9 ± 10.8 | <0.001 | - |
| Age at diagnosis (months) | 39 (344) | 1 (131) | 66 (359) | 0.008 | - |
| Age at first adult visit (years) | 30.4 ± 10.9 | 24.4 ± 9.5 | 32.3 ± 10.6 | <0.001 | - |
| Follow-up time (months) | 31 (24) | 36 (16) | 29 (34) | 0.364 | 0.440 |
| Diagnostic pathway | |||||
| Screening (n, % patients) | 72 (45.0) | 27 (65.9) | 45 (37.8) | 0.007 | 0.259 |
| Clinical presentation (n, % patients) | 45 (28.1) | 6 (14.6) | 39 (32.8) | ||
| Family history (n, % patients) | 43 (26.9) | 8 (19.5) | 35 (29.4) | ||
| Treatment and nutritional guidance | |||||
| Pharmacological treatment (n, % patients) | 74 (46.3) | 24 (58.5) | 50 (42.0) | 0.073 | 0.001 |
| Dietary recommendations (n, % patients) | 151 (94.4) | 41 (100) | 110 (92.4) | 0.064 | 0.442 |
| No dietary records (n, % patients) | 59 (36.9) | 7 (17.1) | 52 (43.7) | 0.007 | 0.064 |
| Patient home use of Odimet® (n, % patients) | 11 (6.9) | 2 (4.9) | 9 (7.6) | 0.730 | 0.563 |
| Overall adherence in Odimet® (n, % patients) | 83 (51.9) | 20 (48.8) | 63 (52.9) | 0.718 | 0.334 |
| Medical appointments | |||||
| Attended visits, median [IQR] | 7 [9] | 6 [6] | 8 [10] | 0.173 | 0.339 |
| Missed visits, median [IQR] | 1 [1] | 1 [1] | 1 [1] | 0.399 | 0.801 |
| Rescheduled visits, median [IQR] | 0 [1] | 0 [1] | 0 [1] | 0.852 | 0.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-López, E.J.; Fernández-Pombo, A.; Hermida-Ameijeiras, Á.; Gómez-Vázquez, E.; Rodríguez-Carnero, G.; Jiménez-López, N.; Villar-Taibo, R.; Cantón-Blanco, A.; Muñoz-Leira, V.; Sánchez-Pintos, P.; et al. Impact of a Transition Clinic on Long-Term Care and Nutritional Management in Patients with Inborn Errors of Metabolism. Nutrients 2025, 17, 3240. https://doi.org/10.3390/nu17203240
Díaz-López EJ, Fernández-Pombo A, Hermida-Ameijeiras Á, Gómez-Vázquez E, Rodríguez-Carnero G, Jiménez-López N, Villar-Taibo R, Cantón-Blanco A, Muñoz-Leira V, Sánchez-Pintos P, et al. Impact of a Transition Clinic on Long-Term Care and Nutritional Management in Patients with Inborn Errors of Metabolism. Nutrients. 2025; 17(20):3240. https://doi.org/10.3390/nu17203240
Chicago/Turabian StyleDíaz-López, Everardo Josué, Antia Fernández-Pombo, Álvaro Hermida-Ameijeiras, Eva Gómez-Vázquez, Gemma Rodríguez-Carnero, Noemí Jiménez-López, Rocío Villar-Taibo, Ana Cantón-Blanco, Virginia Muñoz-Leira, Paula Sánchez-Pintos, and et al. 2025. "Impact of a Transition Clinic on Long-Term Care and Nutritional Management in Patients with Inborn Errors of Metabolism" Nutrients 17, no. 20: 3240. https://doi.org/10.3390/nu17203240
APA StyleDíaz-López, E. J., Fernández-Pombo, A., Hermida-Ameijeiras, Á., Gómez-Vázquez, E., Rodríguez-Carnero, G., Jiménez-López, N., Villar-Taibo, R., Cantón-Blanco, A., Muñoz-Leira, V., Sánchez-Pintos, P., Couce, M.-L., & Olmos, M. A. M. (2025). Impact of a Transition Clinic on Long-Term Care and Nutritional Management in Patients with Inborn Errors of Metabolism. Nutrients, 17(20), 3240. https://doi.org/10.3390/nu17203240









