Associations Between Dietary Iron, SNP rs2794720, and Metabolic Syndrome Risk in Chinese Males and Females: A Community-Based Study in a Chinese Metropolis
Abstract
1. Introduction
2. Materials and Methods
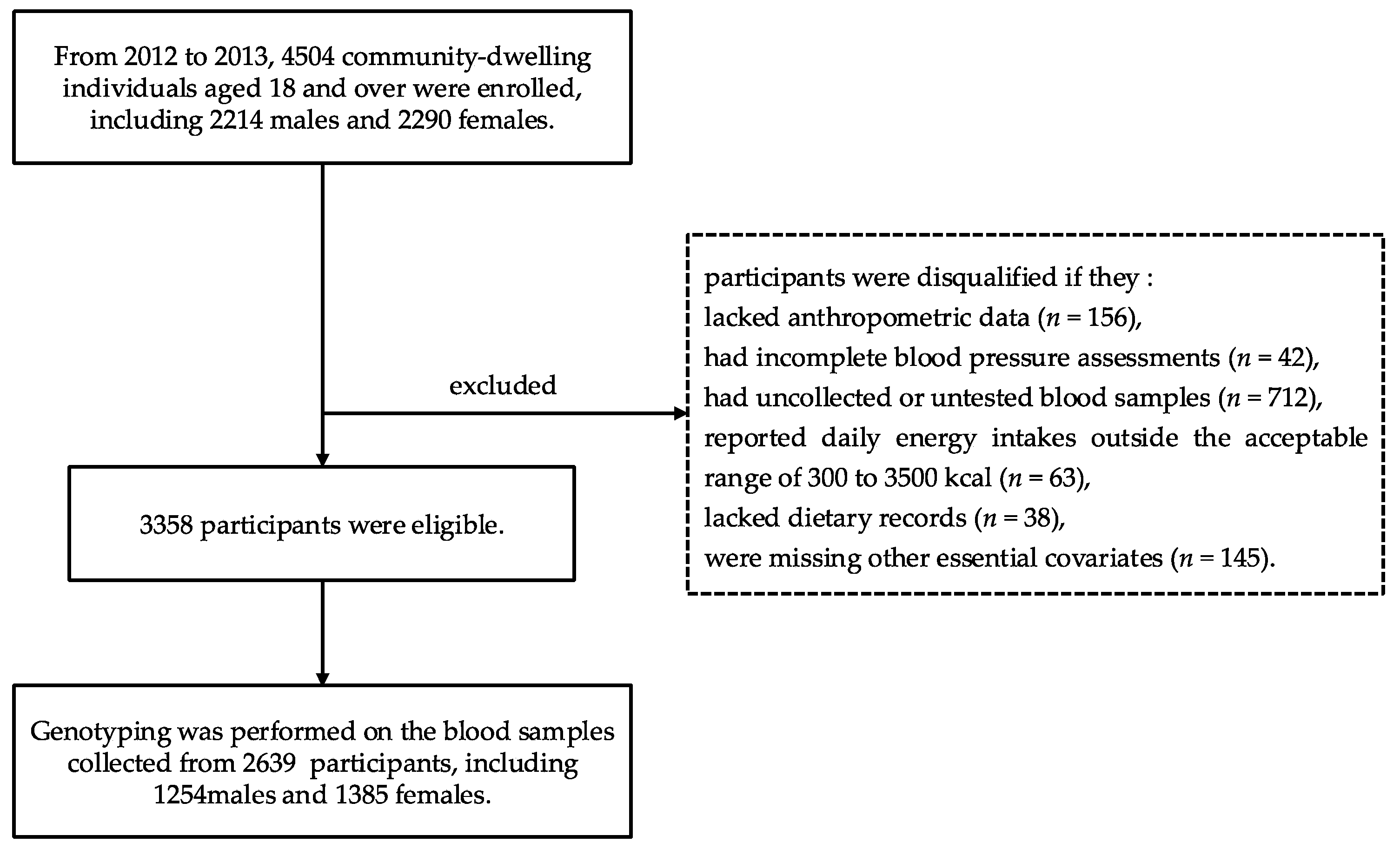
2.1. Study Population
2.2. Dietary Assessment
2.3. Anthropometric and Laboratory Measurements and Genotyping
2.4. Metabolic Syndrome Definition
2.5. Potential Confounders
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Genotypes of the rs2794720
3.3. Associations of MetS Risk Stratified with Dietary Iron and the rs2794720
3.4. Associations Between Dietary Iron and Risk of MetS Stratified by rs2794720
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MetS | Metabolic Syndrome |
| IRS-1 | Insulin Receptor Substrate-1 |
| HFE | Homeostatic Iron Regulator |
| TFR2 | Transferrin Receptor 2 |
| HH | Hereditary Hemochromatosis |
| SDHS | Shanghai Diet and Health Survey |
| FFQ | Food Frequency Questionnaires |
| MSG | Monosodium Glutamate |
| SNP | Single Nucleotide Polymorphism |
| NCEP-ATP III | National Cholesterol Education Program Adult Treatment Panel III |
| HDL-C | High-Density Lipoprotein Cholesterol |
| SD | Standard Deviation |
| ORs | Odds Ratios |
| CIs | Confidence Intervals |
| RERI | Relative Excess Risk Due to Interaction |
| RMB | Renminbi, the currency of P.R. China |
| MAF | Minor Allele Frequency |
| IO | Iron Overload |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Lu, J.; Wang, L.; Li, M.; Xu, Y.; Jiang, Y.; Wang, W.; Li, J.; Mi, S.; Zhang, M.; Li, Y.; et al. Metabolic Syndrome Among Adults in China: The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2017, 102, 507–515. [Google Scholar] [CrossRef]
- Yao, F.; Bo, Y.; Zhao, L.; Li, Y.; Ju, L.; Fang, H.; Piao, W.; Yu, D.; Lao, X. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. Nutrients 2021, 13, 4475. [Google Scholar] [CrossRef]
- Kelishadi, R. Metabolic Syndrome Burden in Children and Adolescents. Lancet Child. Adolesc. Health 2022, 6, 138–139. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, X.; Fang, Y.; Zhang, J.; Yang, Z.; Wang, Z.; Liu, A.; He, L.; Sun, J.; Lian, Y.; et al. Trends and Disparities of Energy Intake and Macronutrient Composition in China: A Series of National Surveys, 1982–2012. Nutrients 2020, 12, 2168. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, H.; Luo, B.; Wu, C.; Guo, C.; Wang, Z.; Zang, J.; Wu, F.; Zhu, Z. The Association Between Dietary Iron, the SNP of the JAZF1 rs864745, and Glucose Metabolism in a Chinese Population. Nutrients 2024, 16, 3831. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, W.; Wang, Y.; Yi, C.; Yu, B.; Pang, X.; Li, K.; Li, H.; Dai, Y. Crosstalk between intestinal flora and Human Iron Metabolism: The Role in Metabolic Syndrome-Related Comorbidities and Its Potential Clinical Application. Microbiol. Res. 2024, 282, 127667. [Google Scholar] [CrossRef]
- Chen, P.; Wu, S.; He, J.; Sui, Y.; Li, K.; Fang, A. Long-Term Dietary Iron Intake and Risk of non-Fatal Cardiovascular Diseases in the China Health and Nutrition Survey. Eur. J. Prev. Cardiol. 2023, 30, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of Ferroptosis in Murine Models of Hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Zang, J.; Lu, Y.; Xiao, Z.; Zheng, G.; Wu, F. The SNP rs516946 Interacted in the Association of MetS with Dietary Iron among Chinese Males but Not Females. Nutrients 2022, 14, 2024. [Google Scholar] [CrossRef]
- Fathi Dizaji, B. The Investigations of Genetic Determinants of the Metabolic Syndrome. Diabetes Metab. Syndr. 2018, 12, 783–789. [Google Scholar] [CrossRef]
- Bridle, K.R.; Frazer, D.M.; Wilkins, S.J.; Dixon, J.L.; Purdie, D.M.; Crawford, D.H.; Subramaniam, V.N.; Powell, L.W.; Anderson, G.J.; Ramm, G.A. Disrupted Hepcidin Regulation in HFE-Associated Haemochromatosis and the Liver as a Regulator of Body Iron Homoeostasis. Lancet 2003, 361, 669–673. [Google Scholar] [CrossRef]
- Pantopoulos, K. TfR2 Links Iron Metabolism and Erythropoiesis. Blood 2015, 125, 1055–1056. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron Metabolism and Iron Disorders Revisited in the Hepcidin Era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary Hemochromatosis: Pathogenesis, Diagnosis, and Treatment. Gastroenterology 2010, 139, 393–408, 408.e391-392. [Google Scholar] [CrossRef]
- Powell, L.W.; Seckington, R.C.; Deugnier, Y. Haemochromatosis. Lancet 2016, 388, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, C.; Luo, B.; Zang, J.; Wang, Z.; Guo, C.; Jia, X.; Wang, W.; Shen, X.; Lu, Y.; et al. The Dietary Intake and Its Features across Four Seasons in the Metropolis of China. J. Nutr. Sci. Vitaminol. 2019, 65, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Liu, A.; Yao, Y.; Ma, Y.; Ding, C.; Song, C.; Yuan, F.; Zhang, Y.; Feng, G.; Chen, Z.; et al. Nutrient Supplement Use among the Chinese Population: A Cross-Sectional Study of the 2010–2012 China Nutrition and Health Surveillance. Nutrients 2018, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). dbSNP: rs2794720. 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2794720 (accessed on 31 December 2024).
- Sala, A.; Shoaib, M.; Anufrieva, O.; Mutharasu, G.; Yli-Harja, O.; Kandhavelu, M. Origins of Transcriptional Transition: Balance between Upstream and Downstream Regulatory Gene Sequences. mBio 2015, 6, e02182-14. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, H.; An, P.; Tao, Y.; Deng, J.; Zhang, Z.; Shen, Y.; Chen, C.; Min, J.; Wang, F. HJV and HFE Play Distinct Roles in Regulating Hepcidin. Antioxid. Redox Signal 2015, 22, 1325–1336. [Google Scholar] [CrossRef]
- Feder, J.N. The hereditary hemochromatosis gene (HFE). Immunol. Res. 1999, 20, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Distante, S.; Berg, J.P.; Lande, K.; Haug, E.; Bell, H. HFE Gene Mutation (C282Y) and Phenotypic Expression Among a Hospitalised Population in a High Prevalence Area of Haemochromatosis. Gut 2000, 47, 575–579. [Google Scholar] [CrossRef]
- Sánchez, M.; Bruguera, M.; Bosch, J.; Rodés, J.; Ballesta, F.; Oliva, R. Prevalence of the Cys282tyr and His63asp Hfe Gene Mutations in Spanish Patients with Hereditary Hemochromatosis and in Controls. J. Hepatol. 1998, 29, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Dixon, J.L.; Ramm, G.A.; Hewett, D.G.; Lincoln, D.J.; Anderson, G.J.; Subramaniam, V.N.; Dodemaide, J.; Cavanaugh, J.A.; Bassett, M.L.; et al. The Clinical Relevance of Compound Heterozygosity for the C282Y and H63D Substitutions in Hemochromatosis. Clin. Gastroenterol. Hepatol. 2006, 4, 1403–1410. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Gao, H.; Yang, J.; Pan, W.; Yang, M. Iron Overload and the Risk of Diabetes in the General Population: Results of the Chinese Health and Nutrition Survey Cohort Study. Diabetes Metab. J. 2022, 46, 307–318. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Yang, X.; Wu, Q.; An, P.; Jin, X.; Liu, W.; Huang, X.; Li, Y.; Yan, S.; et al. Auranofin Mitigates Systemic Iron Overload and Induces Ferroptosis via Distinct Mechanisms. Signal Transduct. Target. Ther. 2020, 5, 138. [Google Scholar] [CrossRef]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Wiegerinck, E.; Yeap, B.B.; Trinder, D.; Peeling, P. Interleukin-6 and Hepcidin Levels during Hormone-Deplete and Hormone-Replete Phases of an Oral Contraceptive Cycle: A Pilot Study. Ann. Nutr. Metab. 2017, 70, 100–105. [Google Scholar] [CrossRef]
- Kesharwani, P.; Dash, D.; Koiri, R.K. Deciphering the Role of Hepcidin In Iron Metabolism and Anemia Management. J. Trace Elem. Med. Biol. 2025, 87, 127591. [Google Scholar] [CrossRef]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Zhang, Q.; Lin, H.; Zhu, L.; Liu, Q.; Zhao, C. Association between Serum Ferritin and Blood Lipids: Influence of Diabetes and hs-CRP Levels. J. Diabetes Res. 2020, 2020, 4138696. [Google Scholar] [CrossRef]
- Jahng, J.W.S.; Alsaadi, R.M.; Palanivel, R.; Song, E.; Hipolito, V.E.B.; Sung, H.K.; Botelho, R.J.; Russell, R.C.; Sweeney, G. Iron Overload Inhibits Late Stage Autophagic Flux Leading to Insulin Resistance. EMBO Rep. 2019, 20, e47911. [Google Scholar] [CrossRef]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron Regulation of Pancreatic Beta-Cell Functions and Oxidative Stress. Annu. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary Intakes of Zinc and Heme Iron from Red Meat, but not from Other Sources, are Associated with Greater Risk of Metabolic Syndrome and Cardiovascular Disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Diabetes, E. Precision Nutrition: A Step too Far? Lancet Diabetes Endocrinol. 2024, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Glenn, A.J.; Tessier, A.J.; Mei, Z.; Haslam, D.E.; Guasch-Ferré, M.; Tobias, D.K.; Eliassen, A.H.; Manson, J.E.; Clish, C.; et al. Integration of Epidemiological and Blood Biomarker Analysis Links Haem Iron Intake to Increased Type 2 Diabetes Risk. Nat. Metab. 2024, 6, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Yang, X.; Hemler, E.C.; Fang, Y.; Zhao, L.; Zhang, J.; Yang, Z.; Wang, Z.; He, L.; et al. The Dietary Transition and its Association with Cardiometabolic Mortality among Chinese Adults, 1982–2012: A Cross-Sectional Population-Based Study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef]
| Total | Male | Female | p | |
|---|---|---|---|---|
| n (%) | 2639 (100.0) | 1254 (47.5) | 1385 (52.4) | |
| Age (%) | ||||
| 15−44 years | 786 (29.8) | 363 (29.0) | 423 (30.6) | 0.560 |
| 45–59 years | 1019 (38.7) | 484 (38.6) | 535 (38.7) | |
| 60+ years | 831 (31.5) | 406 (32.4) | 425 (30.7) | |
| Annual Household Income (%) | ||||
| Above average level (RMB > 60,000) a | 120 (4.6) | 60 (4.8) | 60 (4.3) | 0.541 |
| Average level (RMB 30,000–59,999) | 1493 (56.6) | 703 (56.1) | 790 (57.1) | |
| Below average level (RMB < 30,000) | 812 (30.8) | 397 (31.7) | 415 (30.0) | |
| No answer | 212 (8.0) | 93 (7.4) | 119 (8.6) | |
| Years of Education, years (SD) b | 9.5 (4.5) | 10.1 (4.0) | 8.9 (4.8) | <0.001 |
| Intentional Physical Exercise (%) | ||||
| Yes | 1958 (74.4) | 930 (74.5) | 1028 (74.4) | 0.973 |
| no | 672 (25.6) | 318 (25.5) | 354 (25.6) | |
| Smoking Status (%) | ||||
| Never smoked | 1850 (70.2) | 485 (38.7) | 1365 (98.6) | <0.001 |
| Former smoker | 142 (5.4) | 137 (10.9) | 5 (0.4) | |
| Current smoker | 644 (24.4) | 630 (50.3) | 14 (1.0) | |
| Alcohol Use (%) | ||||
| Lifetime abstainers | 1980 (79.7) | 716 (62.2) | 1264 (94.8) | <0.001 |
| Nonheavy drinkers | 397 (16.0) | 336 (29.2) | 61 (4.6) | |
| Infrequent heavy drinkers | 32 (1.3) | 28 (2.4) | 4 (0.3) | |
| Frequent heavy drinkers | 75 (3.0) | 71 (6.2) | 4 (0.3) | |
| Dietary Intake | ||||
| Energy, kcal/day (SD) | 1763.3 (852.6) | 1941.6 (915.4) | 1602.0 (756.4) | <0.001 |
| Iron Intake | ||||
| Total iron, mg/day (SD) | 19.4 (16.4) | 21.8 (21.0) | 17.3 (10.4) | <0.001 |
| Heme iron, mg/day (SD) | 1.6 (1.4) | 1.7 (1.5) | 1.5 (1.2) | <0.001 |
| Non-heme iron, mg/day (SD) | 17.9 (16.0) | 20.1 (20.5) | 15.9 (9.8) | <0.001 |
| Metabolic Syndrome | ||||
| Yes | 635 (24.1) | 273 (21.8) | 362 (26.1) | 0.010 |
| no | 2004 (75.9) | 981 (78.2) | 1023 (73.9) | |
| Frequency (%) | ||||
|---|---|---|---|---|
| Total | Male | Female | p Value f | |
| Genotype | ||||
| Major allele carriers a | 2380 (90.2) | 1130 (90.1) | 1250 (90.2) | 0.955 |
| CC b | 1427 (54.1) | 661 (52.7) | 766 (55.3) | |
| CG c | 953 (36.1) | 469 (37.4) | 484 (34.9) | |
| Minor allele homozygotes | ||||
| GG d | 259 (9.8) | 124 (9.9) | 135 (9.7) | |
| MAF e | ||||
| G | 27.9 | 28.6 | 27.3 | 1.000 |
| Model 1 b | Model 2 c | |||
|---|---|---|---|---|
| OR (95% CI) d | p Values | OR (95% CI) | p Values | |
| Total (Sex-Adjusted) | ||||
| Dietary iron | ||||
| Q1 (<12.69 mg/day) | Reference | Reference | ||
| Q2 (12.69–16.50 mg/day) | 1.25 (0.96, 1.63) | 0.101 | 1.28 (0.96, 1.70) | 0.090 |
| Q3 (16.50–19.90 mg/day) | 1.42 (1.09, 1.85) | 0.010 | 1.46 (1.09, 1.97) | 0.013 |
| Q4 (≥21.73 mg/day) | 1.39 (1.07, 1.82) | 0.015 | 1.58 (1.12, 2.24) | 0.010 |
| rs2794720 | ||||
| Minor allele homozygotes | Reference | Reference | ||
| Major allele carriers | 1.26 (0.92, 1.74) | 0.156 | 1.31 (0.95, 1.85) | 0.108 |
| Male | ||||
| Dietary iron | ||||
| Q1 (<14.13 mg/day) | Reference | Reference | ||
| Q2 (14.13–17.70 mg/day) | 1.24 (0.82, 1.89) | 0.313 | 1.29 (0.82, 2.05) | 0.273 |
| Q3 (17.70–23.49 mg/day) | 1.78 (1.21, 2.66) | 0.004 | 1.89 (1.19, 3.04) | 0.007 |
| Q4 (≥23.49 mg/day) | 1.83 (1.24, 2.72) | 0.003 | 2.10 (1.24, 3.58) | 0.006 |
| rs2794720 | ||||
| Minor allele homozygotes | Reference | Reference | ||
| Major allele carriers | 0.94 (0.61, 1.49) | 0.774 | 0.92 (0.57, 1.53) | 0.733 |
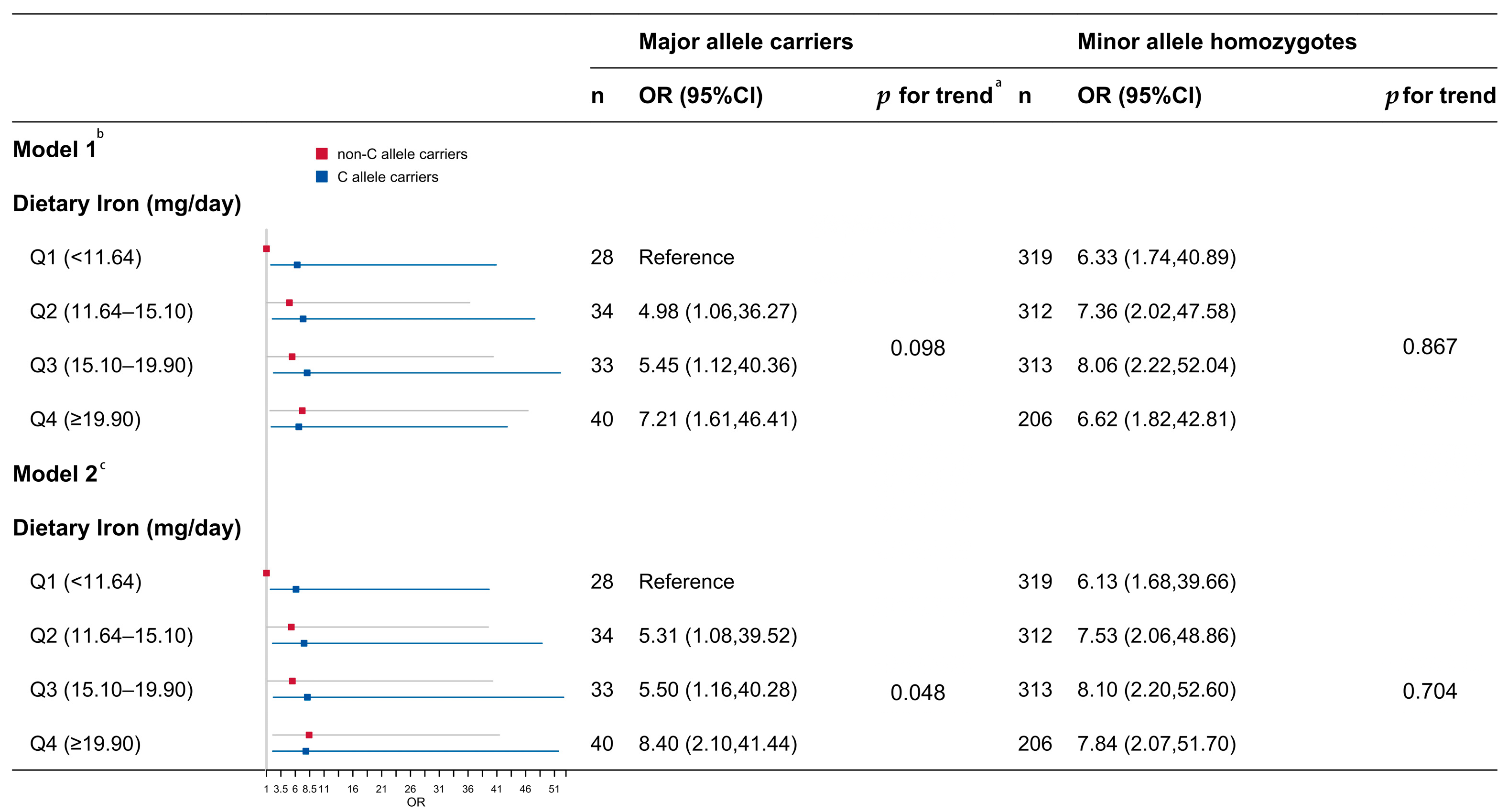
| Female | ||||
| Dietary iron | ||||
| Q1 (<11.64 mg/day) | Reference | Reference | ||
| Q2 (11.64–15.10 mg/day) | 1.26 (0.88, 1.81) | 0.211 | 1.33 (0.91, 1.96) | 0.140 |
| Q3 (15.10–19.90 mg/day) | 1.36 (0.95, 1.95) | 0.091 | 1.43 (0.97, 2.13) | 0.074 |
| Q4 (≥19.90 mg/day) | 1.18 (0.82, 1.70) | 0.362 | 1.46 (0.91, 2.35) | 0.120 |
| rs2794720 | ||||
| Minor allele homozygotes | Reference | Reference | ||
| Major allele carriers | 1.59 (1.02, 2.55) | 0.047 | 1.57 (1.01, 2.54) | 0.048 |
| Model 1 b | Model 2 c | pLR e | |||
|---|---|---|---|---|---|
| OR (95% CI) d | p Values | OR (95% CI) | p Values | ||
| Total (Sex-Adjusted) | |||||
| Dietary iron | 1.16 (1.04, 1.30) | 0.007 | 1.29 (0.95, 1.76) | 0.099 | 0.465 |
| rs2794720 | 1.31 (0.95, 1.84) | 0.113 | 1.77 (0.75, 4.41) | 0.206 | |
| Interaction term | 0.89 (0.65, 1.21) | 0.466 | |||
| Male | |||||
| Dietary iron | 1.30 (1.10, 1.54) | 0.002 | 1.11 (0.70, 1.75) | 0.664 | 0.463 |
| rs2794720 | 0.92 (0.57, 1.53) | 0.731 | 0.60 (0.18, 2.16) | 0.411 | |
| Interaction term | 1.19 (0.75, 1.89) | 0.463 | |||
| Female | |||||
| Dietary iron | 1.13 (0.98, 1.31) | 0.104 | 1.70 (1.12, 2.67) | 0.016 | 0.042 |
| rs2794720 | 1.57 (1.00, 2.53) | 0.055 | 5.31 (1.49, 22.16) | 0.014 | |
| Interaction term | 0.64 (0.41, 0.98) | 0.047 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Liu, H.; Wang, Z.; Zang, J.; Wu, F.; Zhu, Z. Associations Between Dietary Iron, SNP rs2794720, and Metabolic Syndrome Risk in Chinese Males and Females: A Community-Based Study in a Chinese Metropolis. Nutrients 2025, 17, 3185. https://doi.org/10.3390/nu17203185
Hu Z, Liu H, Wang Z, Zang J, Wu F, Zhu Z. Associations Between Dietary Iron, SNP rs2794720, and Metabolic Syndrome Risk in Chinese Males and Females: A Community-Based Study in a Chinese Metropolis. Nutrients. 2025; 17(20):3185. https://doi.org/10.3390/nu17203185
Chicago/Turabian StyleHu, Zihan, Hongwei Liu, Zhengyuan Wang, Jiajie Zang, Fan Wu, and Zhenni Zhu. 2025. "Associations Between Dietary Iron, SNP rs2794720, and Metabolic Syndrome Risk in Chinese Males and Females: A Community-Based Study in a Chinese Metropolis" Nutrients 17, no. 20: 3185. https://doi.org/10.3390/nu17203185
APA StyleHu, Z., Liu, H., Wang, Z., Zang, J., Wu, F., & Zhu, Z. (2025). Associations Between Dietary Iron, SNP rs2794720, and Metabolic Syndrome Risk in Chinese Males and Females: A Community-Based Study in a Chinese Metropolis. Nutrients, 17(20), 3185. https://doi.org/10.3390/nu17203185






