Consequences of Dietary Manganese Deficiency or Mn2O3 Nanoparticles Supplementation on Rat Manganese Biodistribution and Femur Morphology
Abstract
1. Introduction
2. Materials and Methods
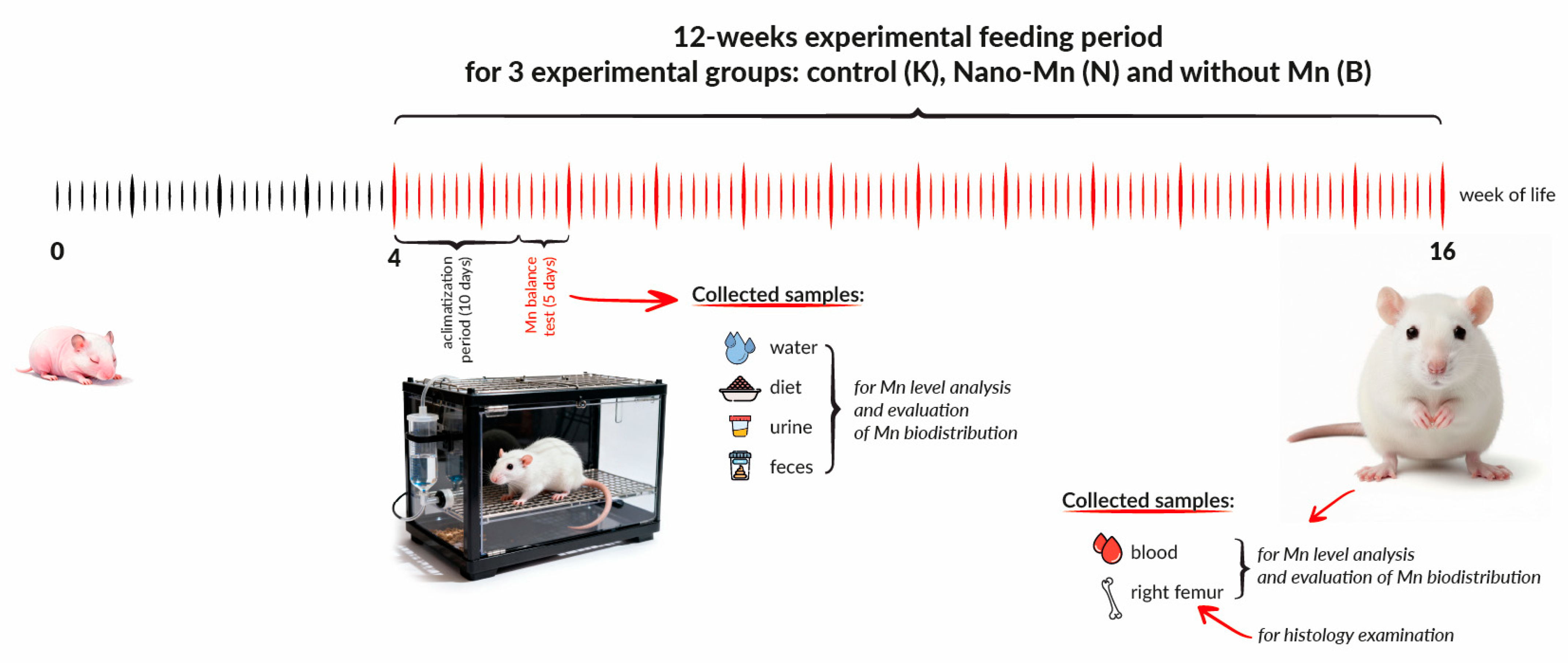
2.1. Experimental Animals and Housing
2.2. Dietary Treatments
2.3. Sample Collection and Monitoring
2.4. Sample Preparation and Manganese Quantification
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
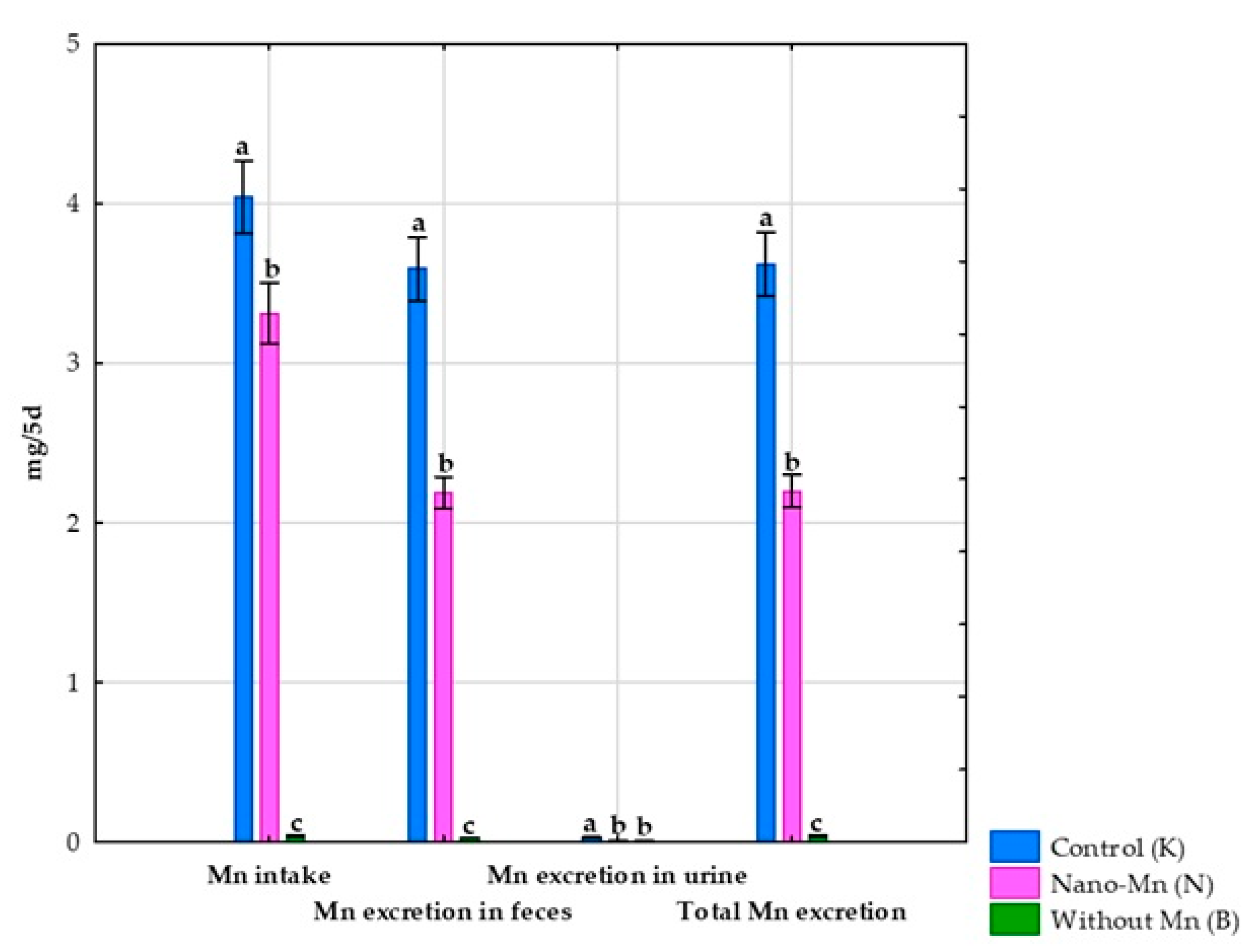
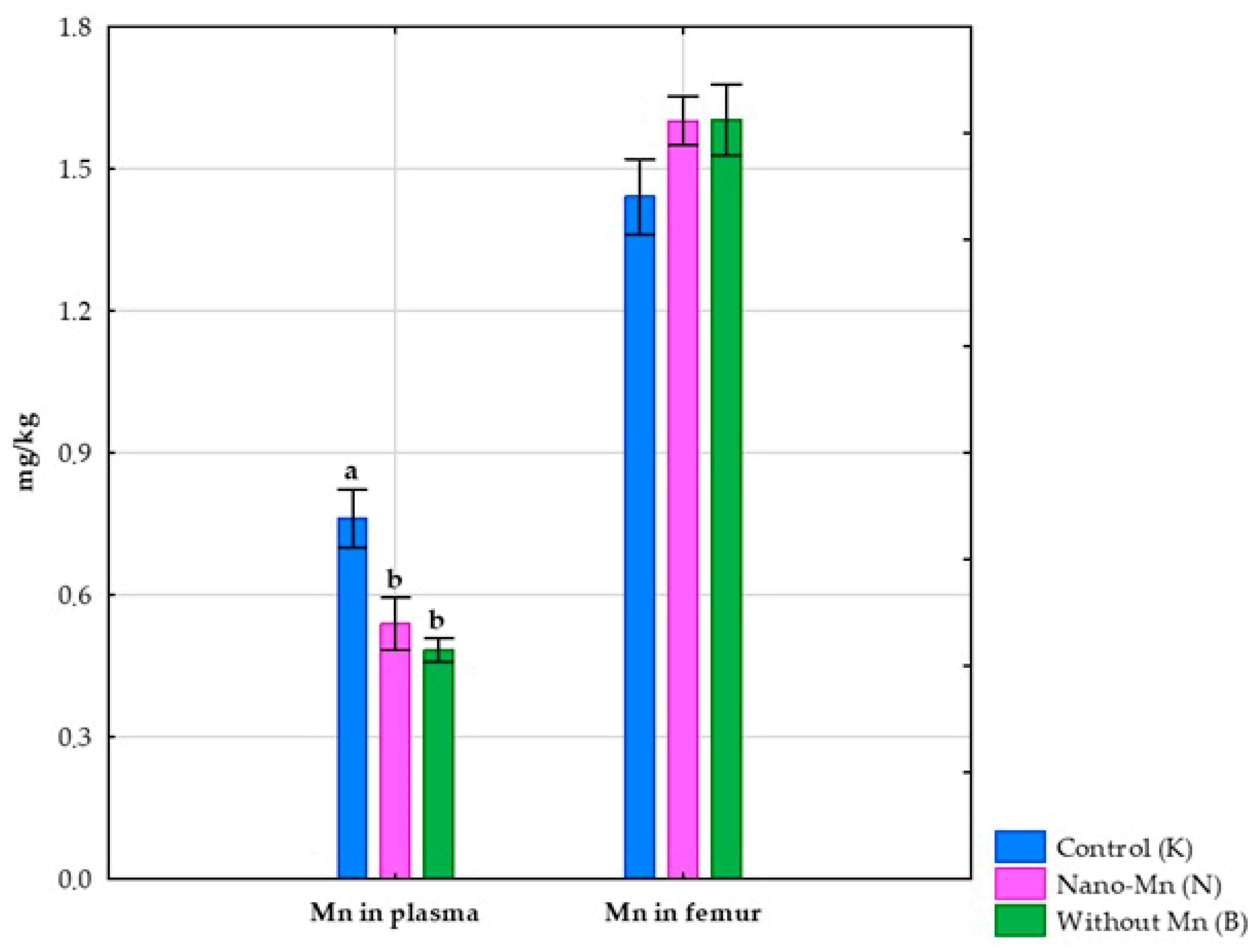
3.1. Effects of Mn Exclusion from the Mineral Mixture in the Rats’ Diet
3.2. Effect of Replacing Standard MnCO3 with Mn2O3 Nanoparticles (Mn2O3NPs) in the Rats’ Diet
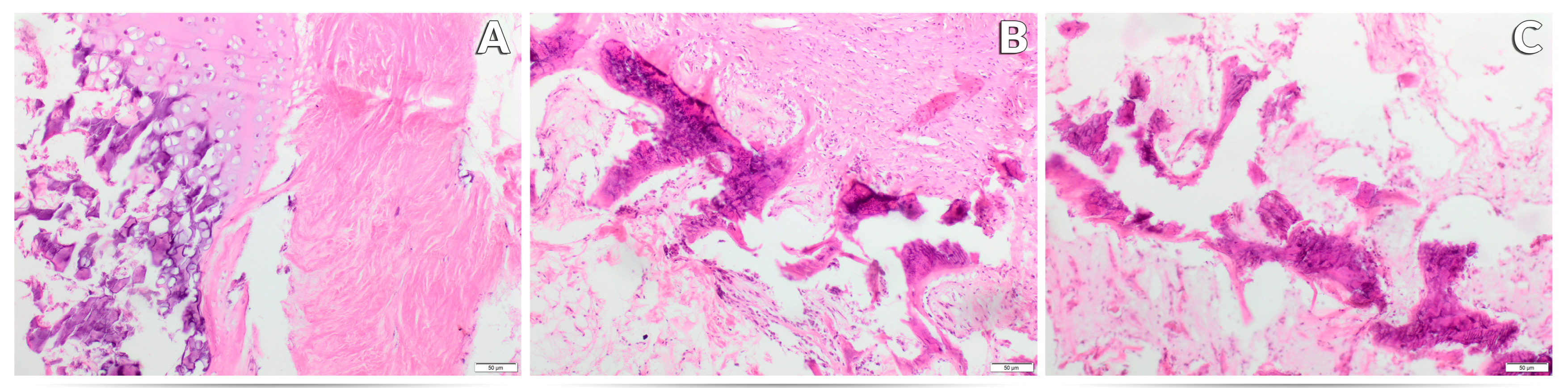
3.3. Effect of Experimental Factors on the Histological Image of the Femur
4. Discussion
4.1. Manganese Biodistribution and Bone Tissue Alterations in Response to Dietary Manganese Deficiency
4.2. Manganese Biodistribution and Bone Tissue Alterations in Response to Dietary Mn2O3 Nanoparticles Supplementation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Mn2O3NPs | Mn2O3 nanoparticles |
References
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Devi, S.; Jatav, R.S.; Dimri, U. Role of trace elements in animals: A review. Vet. World 2013, 6, 963–967. [Google Scholar] [CrossRef]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met. Ions Life Sci. 2019, 19, 253–266. [Google Scholar]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Essentiality of manganese for bone health: An overview and update. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Taskozhina, G.; Batyrova, G.; Umarova, G.; Issanguzhina, Z.; Kereyeva, N. The manganese–bone connection: Investigating the role of manganese in bone health. J. Clin. Med. 2024, 13, 4679. [Google Scholar] [CrossRef]
- Bai, S.-P.; Lu, L.; Wang, R.-L.; Xi, L.; Zhang, L.-Y.; Luo, X.-G. Manganese source affects manganese transport and gene expression of divalent metal transporter 1 in the small intestine of broilers. Br. J. Nutr. 2012, 108, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Ponzoni, S.; Aschner, M. Manganese homeostasis and transport. Met. Ions Life Sci. 2013, 12, 169–201. [Google Scholar] [CrossRef]
- Katz, N.; Rader, D.J. Manganese homeostasis: From rare single-gene disorders to complex phenotypes and diseases. J. Clin. Invest. 2019, 129, 5082–5085. [Google Scholar] [CrossRef]
- Freeland-Graves, J.H.; Mousa, T.Y.; Kim, S. International variability in diet and requirements of manganese: Causes and consequences. J. Trace Elem. Med. Biol. 2016, 38, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Anagianni, S.; Tuschl, K. Genetic disorders of manganese metabolism. Curr. Neurol. Neurosci. Rep. 2019, 19, 33. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K. Manganese. Adv. Nutr. 2017, 8, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. (Landmark Ed.) 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Liu, Q.; Barker, S.; Knutson, M.D. Iron and manganese transport in mammalian systems. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118890. [Google Scholar] [CrossRef]
- Ye, Q.; Park, J.E.; Gugnani, K.; Betharia, S.; Pino-Figueroa, A.; Kim, J. Influence of iron metabolism on manganese transport and toxicity. Metallomics 2017, 9, 1028–1046. [Google Scholar] [CrossRef]
- Peres, T.V.; Schettinger, M.R.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef]
- Gurol, K.C.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. Role of excretion in manganese homeostasis and neurotoxicity: A historical perspective. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G79–G92. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Yenice, E.; Mızrak, C.; Gültekin, M.; Atik, Z.; Tunca, M. Effects of organic and inorganic forms of manganese, zinc, copper, and chromium on bioavailability of these minerals and calcium in late-phase laying hens. Biol. Trace Elem. Res. 2015, 167, 300–307. [Google Scholar] [CrossRef]
- Jankowski, J.; Ognik, K.; Stępniowska, A.; Zduńczyk, Z.; Kozłowski, K. The effect of manganese nanoparticles on apoptosis and on redox and immune status in the tissues of young turkeys. PLoS ONE 2018, 13, e0201487. [Google Scholar] [CrossRef]
- Mattar, G.; Haddarah, A.; Haddad, J.; Pujola, M.; Sepulcre, F. New approaches, bioavailability and the use of chelates as a promising method for food fortification. Food Chem. 2022, 373 Pt A, 131394. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Singh, A.K.; Kochewad, S.A.; Reddy, K.S. Manganese nanoparticles control the gene regulations against multiple stresses in Pangasianodon hypophthalmus. Sci. Rep. 2023, 13, 15900. [Google Scholar] [CrossRef]
- Gopi, M.; Pearlin, B.; Kumar, R.D.; Shanmathy, M.; Prabakar, G. Role of nanoparticles in animal and poultry nutrition: Modes of action and applications in formulating feed additives and food processing. Int. J. Pharm. 2017, 13, 724–731. [Google Scholar] [CrossRef]
- Sołek, P.; Różaniecka, K.; Juśkiewicz, J.; Fotschki, B.; Stępniowska, A.; Ognik, K. Consequences of dietary manganese-based nanoparticles supplementation or deficiency on systemic health and gut metabolic dynamics in rats. Nanotechnol. Sci. Appl. 2025, 18, 19–34. [Google Scholar] [CrossRef]
- Różaniecka-Zwolińska, K.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Ognik, K. Manganese deficiency or dietary manganese(III) oxide nanoparticle supplementation: Consequences for hematology, and intestinal and brain immunity in rats. Front. Immunol. 2025, 16, 1528770. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, L276, 33–79.
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Richardson, C.; Roberts, E.K.; Nelms, S.; Roberts, N.B. Optimisation of whole blood and plasma manganese assay by ICP-MS without use of a collision cell. Clin. Chem. Lab. Med. 2011, 50, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Aring, L.; Das, N.K.; Solanki, S.; Inohara, N.; Iwase, S.; Samuelson, L.C.; Shah, Y.M.; Seo, Y.A. Impact of dietary manganese on experimental colitis in mice. FASEB J. 2020, 34, 2929–2943. [Google Scholar] [CrossRef]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.W.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.L.; Begun, D.L.; Westendorf, J.J.; McGee-Lawrence, M.E. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mo, Y.; Sun, Y.; Li, J.; An, Y.; Feng, N.; Liu, Y. Intestinal nanoparticle delivery and cellular response: A review of the bidirectional nanoparticle–cell interplay in mucosa based on physiochemical properties. J. Nanobiotechnol. 2024, 22, 669. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, M.; Kumari, S.I.; Rahman, M.F.; Mahboo, M.; Grover, P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. J. Appl. Toxicol. 2013, 33, 1165–1179. [Google Scholar] [CrossRef]
- Sobańska, Z.; Roszak, J.; Kowalczyk, K.; Stępnik, M. Applications and biological activity of nanoparticles of manganese and manganese oxides in in vitro and in vivo models. Nanomaterials 2021, 11, 1084. [Google Scholar] [CrossRef]
- Oszlánczi, G.; Vezér, T.; Sárközi, L.; Horváth, E.; Kónya, Z.; Papp, A. Functional neurotoxicity of Mn-containing nanoparticles in rats. Ecotoxicol. Environ. Saf. 2010, 73, 2004–2009. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Z.; Sun, Y.; Nie, Y.; Zhang, Y.; Li, Z.; Luo, Z.; Zhang, W.; Wang, X.; Du, Y.; et al. 3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment. Bioact. Mater. 2025, 44, 354–370. [Google Scholar] [CrossRef]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef] [PubMed]
- HaMai, D.; Bondy, S.C. Pro- or anti-oxidant manganese: A suggested mechanism for reconciliation. Neurochem. Int. 2004, 44, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative stress–induced DNA damage by manganese dioxide nanoparticles in human neuronal cells. Biomed. Res. Int. 2017, 2017, 5478790. [Google Scholar] [CrossRef] [PubMed]
- Frick, R.; Müller-Edenborn, B.; Schlicker, A.; Rothen-Rutishauser, B.; Raemy, D.O.; Günther, D.; Hattendorf, B.; Stark, W.; Beck-Schimmer, B. Comparison of manganese oxide nanoparticles and manganese sulfate with regard to oxidative stress, uptake and apoptosis in alveolar epithelial cells. Toxicol. Lett. 2011, 205, 163–172. [Google Scholar] [CrossRef]
- Alhadlaq, H.A.; Akhtar, M.J.; Ahamed, M. Different cytotoxic and apoptotic responses of MCF-7 and HT1080 cells to MnO2 nanoparticles are based on similar mode of action. Toxicology 2019, 411, 71–80. [Google Scholar] [CrossRef]
- Jiang, X.; Gray, P.; Patel, M.; Zheng, J.; Yin, J.-J. Crossover between anti- and pro-oxidant activities of different manganese oxide nanoparticles and their biological implications. J. Mater. Chem. B 2020, 8, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Sadeghi, L.; Babadi, V.Y.; Tanwir, F. Manganese dioxide nanoparticle induces Parkinson like neurobehavioral abnormalities in rats. Bratisl. Lek. Listy 2018, 119, 379–384. [Google Scholar] [CrossRef]
- Collins, N.E.; Moran, E.T. Influence of supplemental manganese and zinc on live performance and carcass quality of broilers. J. Appl. Poult. Res. 1999, 8, 222–227. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127 (Suppl. 5), 838S–841S. [Google Scholar] [CrossRef]
- National Research Council. Science and the Endangered Species Act; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, E.; Dworzański, W.; Juśkiewicz, J.; Listos, P.; Ognik, K. Consequences of Dietary Manganese Deficiency or Mn2O3 Nanoparticles Supplementation on Rat Manganese Biodistribution and Femur Morphology. Nutrients 2025, 17, 3184. https://doi.org/10.3390/nu17193184
Cholewińska E, Dworzański W, Juśkiewicz J, Listos P, Ognik K. Consequences of Dietary Manganese Deficiency or Mn2O3 Nanoparticles Supplementation on Rat Manganese Biodistribution and Femur Morphology. Nutrients. 2025; 17(19):3184. https://doi.org/10.3390/nu17193184
Chicago/Turabian StyleCholewińska, Ewelina, Wojciech Dworzański, Jerzy Juśkiewicz, Piotr Listos, and Katarzyna Ognik. 2025. "Consequences of Dietary Manganese Deficiency or Mn2O3 Nanoparticles Supplementation on Rat Manganese Biodistribution and Femur Morphology" Nutrients 17, no. 19: 3184. https://doi.org/10.3390/nu17193184
APA StyleCholewińska, E., Dworzański, W., Juśkiewicz, J., Listos, P., & Ognik, K. (2025). Consequences of Dietary Manganese Deficiency or Mn2O3 Nanoparticles Supplementation on Rat Manganese Biodistribution and Femur Morphology. Nutrients, 17(19), 3184. https://doi.org/10.3390/nu17193184




