Abstract
Background/Objectives: Ketone bodies are increasingly studied for their potential therapeutic effects, particularly through exogenous ketosis, in a variety of diseases. This systematic review aimed to rigorously assess the clinical efficacy of exogenous ketosis in adults with medical conditions. Methods: Following PRISMA guidelines, we systematically searched MEDLINE and Scopus databases. Our inclusion criteria were defined according to the PICOS framework, focusing on studies involving exogenous ketosis in adult patients with specific diseases. The study is registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42023492846). Results: After a stringent selection process, fifty-one studies were analyzed. Twenty-two studies focused on neurological disorders, one on psychiatric disorders, twenty-two on metabolic disorders, five on cardiovascular disorders, and one on an inflammatory disorder. Exogenous ketosis demonstrated potential benefits across multiple conditions, including Alzheimer’s disease, mild cognitive impairment, McArdle’s disease, various forms of heart failure, cardiogenic shock, pulmonary hypertension, and COVID-19-related acute respiratory distress syndrome, although evidence is mostly limited to surrogate endpoints with insufficient hard outcome data. Subtherapeutic ketone concentrations induced by medium-chain triglycerides and limited follow-up periods often precluded firm conclusions regarding clinically meaningful outcomes. Conclusions: Exogenous ketosis shows potential in neurological, metabolic, and cardiovascular disorders, while evidence in psychiatric and inflammatory conditions remains scarce and preliminary. Ketone esters appear preferable for effective and tolerable ketosis. Future research should focus on identifying responsive patient populations, optimizing treatment regimens, and conducting long-term clinical trials with hard endpoints to validate these findings.
1. Introduction
Ketone bodies are fundamental metabolites that are best known for their function as an alternative fuel source to glucose during energy restriction with reduced carbohydrate intake [1]. In this physiological state, ketogenesis in the liver converts fatty acids into β-hydroxybutyrate (βHB), acetoacetate (AcAc), and acetone. The main characteristics of ketone bodies and the different ways to induce ketosis are described in Figure 1. βHB and AcAc can then serve as an energy source for extrahepatic tissues. βHB is the most abundant and stable ketone body [1]. In a normal fed state, circulating βHB levels are typically less than 0.5 mmol/L but this can rise to 6–7.5 mmol/L during extended fasting [2]. Ketosis is defined as a blood βHB level greater than 0.5 mmol/L [2].
Ketosis can be achieved endogenously or exogenously. Endogenous ketosis occurs when the liver produces ketone bodies from free fatty acids, typically triggered by a ketogenic diet (low carbohydrate, moderate protein, high fat) or intermittent fasting. Both methods can effectively induce ketosis [3,4]. However, depending on its composition and the individual’s metabolic profile, a ketogenic diet may be associated with elevations in low-density lipoprotein cholesterol (LDL-c) and Apolipoprotein B [5]-biomarkers linked with atherosclerosis risk- and can also lead to nutritional deficiencies [6] or nephrolithiasis [7]. Moreover, compliance rates with these regimens are poor, reaching only 38% to 56% in some cases [8], with systematic reviews showing short-term adherence around 66–80% but declining to about 38% after three years [9]. Adherence to intermittent fasting is also poor, with a drop-out rate of 38% in a major study [10].
Exogenous ketosis can be achieved alternatively through the use of exogenous ketone supplements. Three main categories of exogenous ketone supplements are available: ketone salts, ketone esters, and medium-chain triglyceride (MCT). Ketone salts are composed of βHB bound to a mineral (potassium, sodium, or magnesium) [11,12]. Ketone esters often consist of a ketone body bound to a precursor molecule such as 1,3-butanediol, that is typically also metabolized to a ketone body. Ketone esters are more effective in raising blood ketone levels and induce fewer adverse events compared to ketone salts [11,13]. MCTs are highly ketogenic fats that are metabolized to ketone bodies in the liver with direct intestine-to-liver portal absorption [14]. MCTs are not exogenous ketone supplements per se, they act as exogenous ketogenic substrates and are pragmatically regarded as exogenous ketogenic supplements in clinical practice. More recently, additional exogenous ketones have emerged, such as pure ketone diol (containing exclusively 1,3-butanediol) or free bioidentical βHB [15]. While daily administration of diluted free βHB (10 g) once a day for 28 days in healthy participants was well tolerated and safe [15], these molecules have accumulated less clinical experience.
In therapeutic studies using exogenous ketosis, βHB concentrations typically range between 1.0 and 3.0 mmol/L with ketone esters, and often remain below 1.0 mmol/L when using MCTs [11,12]. Reported adverse events associated with the use of exogenous ketone supplements are generally mild and include gastrointestinal symptoms such as nausea, bloating, and diarrhea, particularly with higher doses or with MCT-based approaches [14].
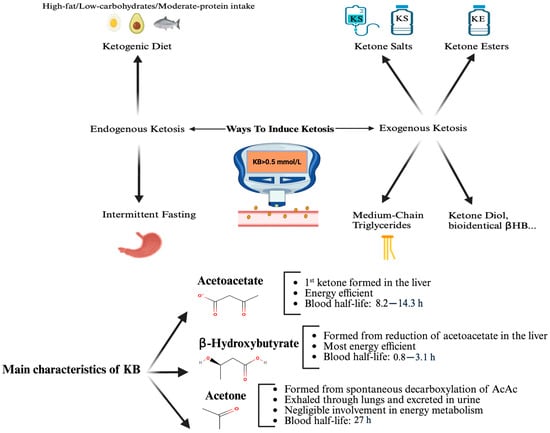
Figure 1.
The main characteristics of ketone bodies and the different ways to induce ketosis. AcAc, acetoacetate; KB, ketone bodies; βHB, β-hydroxybutyrate; KS, ketone salt; KE, ketone ester. Adapted from [16,17].
Beyond serving as an alternative energy source, ketone bodies also serve as signaling molecules. βHB has been shown to act as an effector via G-protein coupled receptors, such as the hydrocarboxylic acid receptor 2 [18], modulating cellular homeostasis of many cell subtypes. By inhibiting the pro-inflammatory transcription factor nuclear factor-kappa B (NF-κB) and enhancing autophagy, βHB can provide anti-inflammatory effects, leading to decreased levels of proinflammatory mediators like interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α)) [19]. Furthermore, βHB has been shown to decrease oxidative stress by increasing the expression of antioxidant genes [20] and improving mitochondrial function [21]. βHB also functions as an epigenetic gene regulator by inhibiting class I histone deacetylases. This leads to an increase in histone acetylation, influencing the expression of genes that reduce oxidative stress and enhance mitochondrial homeostasis [22]. Finally, βHB can influence appetite and satiety [23], inhibit sympathetic nervous system activity, and reduce heart rate (HR) and total energy expenditure [24].
These pleiotropic effects hold therapeutic potential in several diseases, explaining an increased interest in ketosis among researchers, clinicians, and the general public. Initially, interest in ketosis—induced by a ketogenic diet or intermittent fasting—emerged several decades ago as a treatment for epilepsy and diabetes mellitus [25,26]. More recently, the widespread availability of exogenous ketone supplements has spurred claims of diverse health benefits, underscoring the need for a thorough evaluation of the scientific evidence.
Although several systematic reviews have examined exogenous ketosis, most have either focused on healthy populations or restricted their scope to a single disease domain [27,28]. To our knowledge, this is the first systematic review to focus exclusively on adults with established medical conditions, deliberately excluding healthy individuals. By synthesizing evidence from various disorders, our review provides a comprehensive, clinically oriented overview. With rigorous methodology, PROSPERO registration, and an updated search through February 2025, our review not only assesses therapeutic potential but also the methodological limitations and knowledge gaps for future research.
2. Methods
A protocol was drafted for this systematic review in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) checklist [29]. This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) on 12 December 2023, with the following number: CRD42023492846. The study was conducted according to the PRISMA statement [30].
2.1. Search Strategy
The MEDLINE and Scopus databases were explored. Although the original protocol specified four databases, including the Cochrane Database of Systematic Reviews and PROSPERO, these databases were not searched because systematic reviews were listed as an exclusion criterion. The search terms and key concepts used were as follows: (Exogenous ketogenic supplements) OR (exogenous ketones supplements) OR (ketone supplementation) OR (ketone supplements). Articles published from the inception of each database until 5 December 2023 were retrieved. We subsequently updated our systematic review with a new database search on 13 February 2025. There were no geographical restrictions but languages other than English were excluded.
In addition, the reference lists of retrieved papers were screened to identify other articles of interest. The grey literature was not searched.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria applied in this systematic review (Table 1) were proposed according to the Participants, Interventions, Comparators, Outcomes, and Study design (PICOS) framework.

Table 1.
Inclusion and exclusion criteria based on PICOS algorithm.
Studies were eligible if they assessed at least one clinical, biological, or radiological outcome, or adverse event, regardless of whether the results demonstrated improvement, harm, or no effect.
2.3. Selection Process
Two reviewers (OM, SB) conducted independent searches for relevant studies and determined which ones should be included (using Rayyan). The records were managed using Rayyan, a free web tool (Rayyan.ai). Rayyan detects duplicates by considering various factors such as the title, authors, journal, and year. A second check for duplicate articles was carried out by two reviewers (OM, SB).
After removing duplicates, titles and abstracts were screened. The full text of a study was reviewed, and the study was considered to be potentially relevant when it could not clearly be excluded based on its title and abstract following discussion between the two independent reviewers. In the event of disagreement or inadequate information in an abstract, the full text was sought. When a study was determined by both reviewers to meet the inclusion criteria from the complete text, it was included. After discussion, if there was a disagreement, a third reviewer (PJ) mediated.
2.4. Data Extraction, Risk of Bias Assessment and Analysis
Two reviewers extracted the data independently via a standardized extraction form in Excel including the following summary data: reference (year), condition, study design, population (characteristics and size), type and dose of exogenous ketone supplement, ketone blood levels, primary outcome measures, other outcome measures, results, and study limitations.
Risk of bias was assessed using the Risk Of Bias In Non-randomized Studies-of Interventions (ROBINS-I) in non-randomized controlled trials and the Cochrane Risk of Bias tool in randomized controlled trials [31]. The same initial reviewers (OM, SB) independently evaluated the risk of bias for each trial included.
Due to the heterogeneity of study populations, interventions, and outcomes, a quantitative synthesis was not feasible. Instead, we conducted a structured narrative synthesis. Studies were grouped by disease domain (neurological, psychiatric, metabolic, cardiovascular, inflammatory), and findings were tabulated to allow comparison of study characteristics, type and dose of exogenous ketone, achieved βHB levels, and primary outcomes. Within each disease domain, results were summarized narratively to highlight consistencies, divergences, and limitations.
3. Results
3.1. Study Selection and Characteristics
The initial literature search retrieved 3941 articles. After removing duplicates, 3085 records remained. Next, a screening of the titles and abstracts was conducted. A total of 3031 records were excluded and 54 articles remained. The full texts of the remaining 54 articles were then carefully reviewed and assessed for eligibility. Finally, 51 articles [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] were chosen that met the inclusion criteria. A PRISMA statement flow diagram illustrating the study selection process is shown in Figure 2.
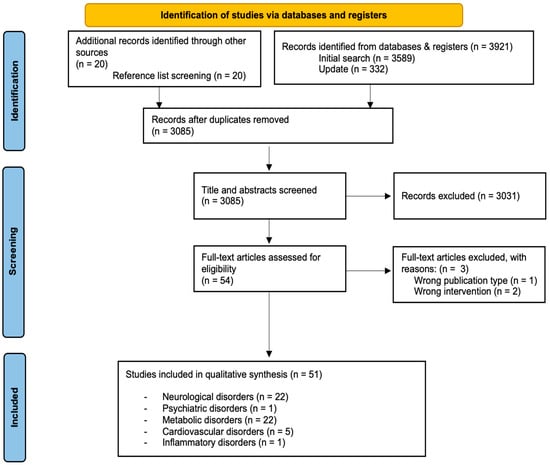
Figure 2.
PRISMA flow diagram of the study selection process.
The distribution of included studies by disorder type is detailed in Table 2.

Table 2.
Distribution of included studies by disorder type.
Among these, 51 included articles and half (25/51) involved ketone esters [49,55,56,57,58,59,60,61,62,63,64,65,66,68,69,70,71,73,74,75,76,77,79,80,82]. In 21 studies [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,51,53,72], MCTs were used and, in the remaining 5 studies [52,54,67,78,81], ketone salts were administered.
The main characteristics of studies on neurological, psychiatric, metabolic, cardiovascular, and inflammatory disorders are presented in Table 3, Table 4, Table 5, Table 6, and Table 7, respectively. Within these tables, it has been decided to report blood βHB levels expressed as mean (+/− standard deviation), with some exceptions which will be mentioned. The limitations reported correspond to those mentioned by the authors of each article.

Table 3.
Overview of studies on neurological disorders.

Table 4.
Overview of studies on psychiatric disorders.

Table 5.
Overview of studies on metabolic disorders.

Table 6.
Overview of studies on cardiovascular disorders.

Table 7.
Overview of studies on inflammatory disorders.
3.2. Risk of Bias in Studies
The risk of bias graph for randomized controlled trials, using the Cochrane Risk of Bias tool, is available as Supplementary Material S1, and the results of the ROBINS-I (traffic light plot) used to assess the risk of bias in non-randomized controlled trials or uncontrolled trials are available as Supplementary Material S2.
3.2.1. Randomized Controlled Trials
Most RCTs were judged at low-to-moderate risk of bias. Randomization and allocation concealment were often insufficiently described, and blinding was unclear in several trials, particularly older MCT studies. Attrition bias due to high dropout rates (notably in Alzheimer’s trials using MCTs) was common.
3.2.2. Non-Randomized and Open Label Studies
Across five non-randomized studies [35,36,53,63,77], overall risk of bias was mostly moderate, but confounding was serious in four studies [35,53,63,77], reflecting the absence of comparators and lack of adjustment. Selection bias was serious in two small, highly selected cohorts [36,53]. In contrast, classification of interventions was uniformly low risk, and deviations, missing data, measurement, and reporting ranged from low to moderate, with the lowest risks in studies using objective physiological outcomes [36,77].
3.2.3. Systematic Patterns
Blinding and allocation concealment were the most frequent unclear domains in RCTs. Confounding was the most problematic domain in non-RCTs.
3.3. Results of Individual Studies
3.3.1. Neurological Disorders
Twenty-two studies [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] investigated neurological disorders, focusing primarily on Alzheimer’s disease (AD) and mild cognitive impairment (MCI). Across these studies, supplementation with MCTs was generally associated with modest improvements in cognitive domains such as memory, executive function, and language, particularly among Apoε4-negative individuals. However, the extent of ketosis achieved was typically low (<0.6 mmol/L), which limited the magnitude of clinical benefit. Dropout rates were high and biomarker confirmation was lacking, thereby reducing confidence in the findings. Other neurological conditions showed more heterogeneous outcomes.
For multiple sclerosis (MS), two trials [50,51] reported reductions in anxiety, depressive symptoms, and abdominal fat distribution, but interpretation was hampered by concurrent dietary interventions.
A trial in migraine patients [52] using ketone salts failed to demonstrate reductions in migraine frequency or severity, most likely because of insufficient ketosis.
In Parkinson’s disease [49], a small exploratory study with ketone esters demonstrated improved endurance performance.
An uncontrolled epilepsy trial [53] suggested seizure reduction with MCTs but was at very high risk of bias.
Overall, neurological evidence is promising but inconsistent, and methodological limitations prevent firm conclusions.
3.3.2. Psychiatric Disorders
In psychiatric disorders, evidence is currently limited to a single small randomized controlled trial in post-traumatic stress disorder (PTSD) [54]. This study suggested potential symptom improvement with ketone salt supplementation, but no between-group differences were detected, and blood ketone levels were not measured. Thus, evidence in psychiatric conditions remains very preliminary.
3.3.3. Metabolic Disorders
Metabolic disorders were investigated in twenty-two studies [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76].
Acute ketone ester supplementation in pre-diabetes and type 2 diabetes consistently improved glycemia (only in fasting conditions in type 2 diabetes), insulin response, and lipid parameters. Longer-term exogenous ketosis (2–4 weeks) showed a beneficial effect on HbA1c [63], but conflicting results on fructosamine [63,64], though limited by small sample sizes.
In obesity, ketone esters reduced post-prandial glucose, improved vascular function, and enhanced some aspects of cognitive performance.
Rare metabolic diseases such as very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD) and McArdle disease were also studied. In VLCADD [74], ketone ester supplementation improved muscle bioenergetics, while in McArdle disease, results were inconsistent: one ketone ester trial [75] showed no improvement in exercise tolerance, while another [76] reported worsened performance compared to carbohydrate supplementation.
3.3.4. Cardiovascular Disorders
Five studies addressed cardiovascular disorders [77,78,79,80,81].
In heart failure with reduced ejection fraction (HFrEF) [77,78], both ketone esters and salts acutely improved cardiac output (CO), stroke volume, and ventricular function, with consistent reductions in systemic vascular resistance (SVR).
In heart failure with preserved ejection fraction (HFpEF) [79], short-term ketone ester supplementation increased cardiac output, improved diastolic function, and reduced pulmonary capillary wedge pressure both at rest and during exercise, although potential carry-over effects could not be excluded.
In cardiogenic shock [80], ketone esters improved CO, cardiac power output, and systemic oxygen delivery without adverse safety signals.
In pulmonary hypertension [81], ketone salts increased CO and right ventricular contractility, though effects were largely acute and may have been influenced by hemodynamic changes induced by sodium load.
Overall, cardiovascular studies consistently demonstrated improvements in hemodynamic parameters and myocardial energetics, though evidence remains limited to short-term interventions in small samples.
3.3.5. Inflammatory Disorders
Finally, evidence in inflammatory disorders derives from a single randomized controlled trial in patients with COVID-19-related acute respiratory distress syndrome [82]. In this study, supplementation with a βHB formulation reduced pro-inflammatory cytokines, increased anti-inflammatory mediators, improved oxygenation, and shortened hospital stay. These results suggest potential immunomodulatory effects, but the evidence remains preliminary and requires replication in larger, longer-term studies.
3.4. Synthesis Across Studies
Across the diverse disease domains studied, several broad patterns can be discerned. The most consistent benefits of exogenous ketosis were observed in metabolic regulation and cardiovascular performance, where ketone esters in particular improved glycemic control, lipid metabolism, and hemodynamic function.
Neurological outcomes were more variable: while some improvements in cognition were reported in AD and MCI, especially in Apoε4-negative individuals, effects were generally modest and limited by low levels of ketosis in MCT-based studies.
Non-randomized and open-label studies tended to report more favorable outcomes than randomized trials, underscoring the importance of rigorous methodology. Across all domains, however, clinically meaningful endpoints such as long-term disease progression, quality of life, and survival remain underreported. The evidence base as a whole is characterized by small sample sizes, heterogeneous designs, and reliance on surrogate outcomes. Taken together, the current findings indicate promising therapeutic potential of exogenous ketosis, but confirmatory large-scale trials with long-term follow-up are needed to establish clinical efficacy and safety across different patient populations.
4. Discussion
4.1. Summary of Main Findings
This systematic review evaluated the clinical impact of exogenous ketosis in various diseases. Fifty-one clinical studies were included, mostly focusing on neurological, metabolic, and cardiovascular disorders. Exogenous ketosis demonstrated potential benefits on cognitive function, metabolic regulation, cardiac performance, and inflammatory processes. However, therapeutic responses were heterogenous and depended on factors such as ketone type, dosage, and patient characteristics. The majority of evidence is based on surrogate markers rather than hard clinical outcomes, and the duration of follow-up was typically limited.
4.1.1. Neurological Disorders
Therapeutic insights and mechanisms of action: Exogenous ketosis shows particular promise in AD and MCI, conditions associated with impaired cerebral glucose metabolism [83,84]. Ketone bodies may partially compensate by serving as alternative cerebral energy substrates, while also exerting anti-inflammatory and antioxidant properties via inhibition of NF-kB signaling and enhancing mitochondrial function [85,86,87,88].
In the studies reviewed, ketone supplementation was associated with modest improvements in variable outcome measures for episodic memory, executive function, and language skills, particularly in Apoε4-negative individuals who appear to respond more favorably to ketone supplementation. Six-month administration of MCTs had some effects on various circulating cardiometabolic and inflammatory markers.
In the context of MS [50,51], exogenous ketosis may reduce inflammation, anxiety, depression, and abdominal fat in MS patients. The benefits appear linked to ketone body–mediated neuroprotection, gamma-aminobutyric acid (GABA)-ergic [89] and N-methyl-D-aspartate modulation [90], and improved metabolic regulation.
Ketone bodies are considered [91] to stabilize brain excitability by enhancing GABAergic tone, reducing glutamatergic activity, and limiting cortical spreading depression, a key process in migraines. Ketones may also improve mitochondrial efficiency and reduce oxidative stress, potentially lowering susceptibility to migraine triggers. However, the relatively low systemic ketone levels achieved [52] suggest that therapeutic benefit depends on attaining higher and more sustained ketosis.
Finally, MCTs support ketosis and generate decanoic acid, which exerts direct antiseizure effects through inhibition of excitatory AMPA receptors [92]. Beyond seizure control, these mechanisms highlight the broader potential of MCT-induced ketosis for stabilizing neuronal networks. By combining ketone body–mediated neuroprotection, anti-excitatory receptor modulation, and improved cerebral energy metabolism, MCTs may represent a targeted metabolic strategy relevant to epilepsy.
Taken together, these findings suggest that the effects of ketone bodies in neurological disorders are not limited to their role as an alternative fuel source. Rather, they appear to act through multiple converging mechanisms, including improved mitochondrial bioenergetics, reduction of oxidative stress, modulation of excitatory–inhibitory neurotransmission, and attenuation of neuroinflammation. Importantly, these mechanisms are shared across distinct disorders such as AD, MS, migraine, and epilepsy, suggesting that exogenous ketosis may represent a unifying metabolic strategy for stabilizing neuronal function and resilience.
Limitations in existing studies and future directions: Clinical results are modest at best. Most clinical trials utilized MCTs, achieving relatively low βHB levels (often <0.5 mmol/L), which may be insufficient to elicit robust clinical results. Additionally, studies with MCTs often faced high dropout rates (up to 25% of participants in the interventional group in one trial [33]) due to gastrointestinal side effects, potentially compromising adherence and outcome reliability. A gradual increase in MCT dose [35] could improve tolerance and compliance.
A notable finding in MCI and AD contexts is the differential response based on Apoε4 status [32,33,37,44], with Apoε4-negative participants often consistently showing greater cognitive benefits, indicating the need for genetic stratification in future studies.
More broadly, when assessing the relevance of exogenous ketosis for a particular neurological disease, inter-individual variability in disease severity needs to be considered. Many disorders (e.g., migraine, AD) are heterogeneous conditions, potentially limiting efficacy to subgroups of patients.
Further research is warranted to explore the long-term efficacy of ketone esters in neurological disorders, as these formulations induce potent sustained ketosis with fewer side effects. Given the heterogeneous nature of AD and MCI, future studies should aim to identify biomarkers that predict response to exogenous ketosis, with a focus on distinguishing responders from non-responders based on genetic, metabolic, and inflammatory profiles.
4.1.2. Psychiatric Disorders
Therapeutic Insights and Mechanisms of Action: Exogenous ketosis in PTSD subjects [54] may enhance standard pharmacological treatment by modulating neurotransmitter systems and reducing neuroinflammation [93]. These mechanisms suggest potential benefits in symptom reduction, faster response, and improved overall outcomes.
Limitations in Existing Studies and Future Directions: As a pilot trial [54], this study is limited by small sample size, short duration, and heterogeneity among participants, restricting generalizability. Future research should include larger, longer trials with biomarker assessments to clarify mechanisms, identify responsive subgroups, and establish long-term safety and efficacy.
4.1.3. Metabolic Disorders
Therapeutic insights and mechanisms of action: Exogenous ketosis may be associated with improved glycemic control, insulin sensitivity, and lipid metabolism, particularly in pre-diabetic and obese individuals. Ketone esters and MCT lowered blood glucose and triglycerides while modestly increasing insulin levels. However, studies were performed in selected populations and results are not uniform. Ketone bodies may modulate glucose metabolism through multiple pathways, including increased insulin secretion [94], suppression of lipolysis [18], and/or a reduction of gluconeogenesis [95].
One cohort [55,56,57,58,59,60,61] evaluated the acute impact of exogenous ketosis in a specific cohort of new-onset prediabetes after pancreatitis. As in healthy and obese populations [68,96], acute exogenous ketosis may lead to a decrease in blood glucose levels, modulated by abdominal fat distribution [56].
In type II diabetic participants, both acute and chronic administration of ketone ester in one study [64] did not lead to a detectable drop in blood fasting glucose or fructosamine levels, but significantly and slightly increased insulin without a significant variation in C-peptide. It was hypothesized that the increase in circulating insulin may not have been sufficient to affect glucose clearance or hepatic gluconeogenesis, in the context of insulin resistance. Also, a decreased insulin-secreting capacity of β cells alongside insulin resistance minimize any insulin-mediated reductions in blood glucose. However, in contrast with this clinical trial [64], in which a single dose of ketone ester was administered under fasting conditions and away from a meal, Monteyne et al. [66] proved that ketone ester administration (vs. placebo) with a liquid mixed-meal tolerance test using a dual-glucose tracer approach leads to a significant reduction in post-prandial glucose concentrations, mainly as a result of a decrease in the 2 h rate of glucose appearance following meal ingestion. As no effect on post-prandial endogenous glucose production nor plasma insulin were demonstrated, the authors concluded that reduced post-prandial glucose concentration within this type II diabetes population may be induced by a delayed glucose absorption. A prior systematic review with meta-analysis had already demonstrated the blood glucose–lowering effect of exogenous ketosis. However, the majority of included studies were conducted in healthy participants, with only 6 of the 51 studies involving individuals with type II diabetes, prediabetes, or obesity [96].
Next, while βHB demonstrated anti-inflammatory effects via the NLRP3 inflammasome pathway in preclinical studies [97,98], exogenous ketosis did not inhibit NLRP3 inflammasome activation in a clinical study [69], likely due to an exposition of monocytes to weak levels of βHB and LPS.
Finally, since obesity is also associated with neurocognitive dysfunction [99], the effects of ketone ester supplementation were studied and early data suggest potential cognitive benefits with an improvement in cerebral blood flow [70,73].
Bleeker et al. [74] induced exogenous ketosis prior to exercise in patients with VLCADD. The uptake of βHB by skeletal muscles was significant and blood glucose levels remained normal, suggesting utilization of ketone bodies as an energy substrate. A lower Pi/PCr ratio in leg muscle during exercise suggested an improved intramuscular energy balance.
Patients with McArdle disease have blocked glycogen breakdown and ketone bodies could serve as an alternative energy fuel. However, exercise capacity was not significantly improved after induction of exogenous ketosis [75]. This was explained by the fact that ketone bodies induce inhibition of lipolysis [11] and liver glucose output [100], leading to reduced free fatty acid (FFA) and glucose availability in the muscle. A recent study [76] confirmed these results, demonstrating an important impairment in exercise capacity following acute ketone ester supplementation in McArdle disease.
Limitations in Existing Studies and Future Directions: Most studies in metabolic disorders assessed only the acute effects of ketosis and often lacked long-term follow-up, limiting the conclusions on sustained benefits. Additionally, a variability in ketone sources, dosages, and the metabolic profiles of participants introduces significant heterogeneity. The induction of exogenous ketosis might be of benefit in various diseases resulting from inborn errors of metabolism, including multiple acyl-CoA dehydrogenation deficiency. Future trials should evaluate the long-term effects of ketone esters in larger, well-characterized cohorts, focusing on outcomes such as insulin sensitivity, body composition, and cardiovascular risk markers.
4.1.4. Cardiovascular Disorders
Therapeutic Insights and Mechanisms of Action: In the setting of HFrEF, exogenous ketosis was associated with improved CO and left ventricular function, by increasing myocardial efficiency and lowering SVR [78,101]. βHB may enhance cardiac energy efficiency by serving as an oxygen-sparing fuel source, thus improving mechano-energetic coupling in heart failure. Ketone bodies may also act as agonists for cellular receptors involved in HR regulation [102], explaining the observed increase in HR. It is very interesting to note that despite the increase in cardiac work, βHB did not deteriorate mechano-energetic coupling in terms of myocardial external efficiency (MEE), as βHB similarly increased cardiac work and MVO2. Given that a decrease in MEE is associated with a worse prognosis [103], the maintenance of an invariable MEE is a strong argument for the administration of βHB in these patients. Moreover, myocardial βHB fractional extraction increased linearly in proportion to ketone delivery with no upper threshold and correlated positively with hallmarks of LV remodeling [77]. Finally, the increased βHB fractional extraction was associated with an enhanced uptake of lactate, without any variation of extraction of glucose or FFA, indicating improved myocardial metabolic flexibility.
Even in the situation of cardiogenic shock and ongoing vasoactive medication, enteral treatment with ketone ester may be associated with an improved CO and an increase in mixed venous oxygen saturation (SVO2), without any significant variation of mean arterial pressure [80]. Moreover, as suggested by the lower blood glucose levels and circulating FFAs during ketone ester supplementation while insulin levels remained similar between treatments, ketone ester administration may help to minimize the vicious metabolic cycle of cardiogenic shock by enhancing insulin sensitivity and limiting excessive stress-induced lipolysis.
In HFpEF, ketone esters were associated with an increased CO and also reduced cardiac filling pressures and myocardial stiffness at rest and during exercise [79]. Since inflammation has been shown to play a major role in cardiac stiffness in HFpEF [104], the authors hypothesized that the anti-inflammatory activity of ketone bodies would explain the reduction in cardiac stiffness.
Finally, an increase in CO and a decrease in pulmonary vascular resistance (PVR) following the induction of exogenous ketosis were also observed in patients with PH [81]. However, despite this decrease in PVR, a small increase in mean pulmonary arterial pressure (mPAP) was reported. The authors hypothesized that the decrease in PVR was due to the recruitment of pulmonary vessels following the increased in CO [105], as seen during exercise [106].
Limitations in Existing Studies and Future Directions: Small sample sizes and short intervention durations hinder the generalizability of findings. The hemodynamic benefits observed may also be contingent on the high βHB levels achieved with ketone esters, underscoring the need for dosage standardization in future research. Larger clinical trials with longer follow-up periods could help establish the role of exogenous ketosis as an adjunct therapy in cardiovascular diseases.
4.1.5. Inflammatory Disorders
Therapeutic Insights and Mechanisms of Action: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can trigger ARDS. Activation of the NF-κB signaling pathway represents a major contribution in SARS-CoV-2-induced cytokine storm [106]. In the setting of mild COVID-19-related ARDS [82], exogenous ketosis may be associated with a decreased inflammation, increased peripheral oxygen saturation, and reduced duration of hospitalization and muscle fatigue.
Limitations in Existing Studies and Future Directions: The efficacy of exogenous ketosis in a population with severe COVID-19-related ARDS has yet to be demonstrated. Future studies should assess the impact of exogenous ketosis on other inflammatory processes.
4.2. Strengths and Limitations of the Review
This comprehensive overview of clinically relevant outcomes of exogenous ketosis in adults with various diseases offers an accessible summary for clinicians and researchers. The results highlight multiple challenges in interpreting the efficacy of exogenous ketosis across diverse populations. The heterogeneity in study designs, patient populations, types of ketone supplements, and outcome measures, as well as the short duration of most studies, limits the comparability of findings and restricts conclusions on long-term safety and effectiveness on clinically relevent endpoints. Risk of bias was also variable, which further reduces certainty of the evidence. Compared with previous systematic reviews, which often included healthy participants or focused on single disease areas or ketogenic diets, our review uniquely synthesizes evidence on exogenous ketosis in adults with established medical conditions. Taken together, this provides a complementary perspective while underscoring the need for larger, well-controlled, long-term clinical trials. Finally, the database search was restricted to MEDLINE and Scopus; while these databases provide broad coverage and considerable overlap with Embase and Cochrane CENTRAL, some relevant studies may not have been captured.
5. Conclusions
This systematic review highlights that exogenous ketosis may hold therapeutic potential across a spectrum of diseases, including neurological, psychiatric, metabolic, cardiovascular, and inflammatory conditions. However, the current body of evidence is insufficient to establish clear clinical efficacy. Most available studies are limited by small sample sizes, short follow-up periods, high dropout rates, reliance on surrogate endpoints, and methodological constraints, which preclude firm conclusions regarding long-term or clinically meaningful benefits. While ketone esters appear more effective than other formulations in achieving sustained ketosis with acceptable tolerability, the overall results remain heterogeneous and modest. Future high-quality, adequately powered randomized controlled trials with longer follow-up and clinically relevant hard outcomes are needed before exogenous ketosis can be considered a robust therapeutic strategy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17193125/s1, Figure S1: The risk of bias graph for randomized controlled trials using the Cochrane Risk of Bias tool. Figure S2: ROBINS-I traffic light matrix for the assessing the risk of bias in non-randomized controlled trials or uncontrolled trials. N/A: Not Applicable.
Author Contributions
O.M.: Drafting of the manuscript, elaboration of tables and figures, elaboration of the systematic review protocol, role of first reviewer, conducted the literature search and collected data; S.B.: role of second reviewer, elaboration of the systematic review protocol, conducted the literature search and collected data; B.J.G.: elaboration of the systematic review protocol; L.L.: elaboration of the systematic review protocol; E.D.W.: elaboration of the systematic review protocol, work supervision; D.B.: elaboration of the systematic review protocol, work supervision; C.L.: elaboration of the systematic review protocol, work supervision; P.J.: drafting of the manuscript, elaboration of the systematic review protocol, work supervision, role of third reviewer. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this work was provided by Fonds Wetenschappelijk Onderzoek (FWO) grant number G075423N and Wetenschappelijk Fonds Willy Gepts (WFWG).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to warmly thank the researchers and authors of the studies included in our systematic review who shared their manuscripts with us. The authors acknowledge the contribution of a medical writer, Sandy Field, for English language editing of this manuscript.
Conflicts of Interest
No conflicts of interest are declared for the authors of this manuscript.
Abbreviations
3-OHB: 3-hydroxybutyrate; 11C: carbon-11; 18F-FDG: [18F]-fluorodeoxyglucose; 31P-MR: phosphorus-31 magnetic resonance; AcAc: acetoacetate; ACTH: adrenocorticotropic hormone; AD: Alzheimer’s disease; ADAS-Cog: Alzheimer’s Disease Assessment Scale Alzheimer’s-cognitive; ADAS-Cog-C: Alzheimer’s Disease Assessment Scale–Cognitive Subscale Chinese version; ADAS-Jcog: Alzheimer’s Disease Assessment Scale (Japanese version) cognitive subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change; ADL: activities of daily living; AF: atrial fibrillation; ANOVA: analysis of variance; Apoε4: apolipoprotein ε4; ARDS: acute respiratory distress syndrome; ATP: adenosine triphosphate; AUC: area under the curve; BDNF: brain-derived neurotrophic factor; BMI: body mass index; BNP: brain natriuretic peptide; CBF: cerebral blood flow; CCA: common carotid artery; CDIS: clock drawing interpretation scale; CMR: cerebral metabolic rate; CO: cardiac output; COVID-19: coronavirus disease 2019; CPO: cardiac power output; CRP: C-reactive protein; CrSO2: cerebral regional tissue saturation; CS: coronary sinus; CTPEH: chronic thromboembolic pulmonary hypertension; CVP: central venous pressure; DSM-5: Diagnostic and Statistical Manual of Mental Disorders-5; DSST: Digit Symbol Substitution Task; EDPVR: end-diastolic pressure-volume relationship; EDSS: Expanded Disability Status Scale; EGCG: epigallocatechin gallate; FE%: fractional extraction; FFA: free fatty acid; FMD: flow mediated dilation; GDF-15: Growth Differentiation Factor-15; GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like peptide-1; GLS: global longitudinal strain; HbA1c: hemoglobin A1c; HDL-c: high-density lipoprotein cholesterol; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HIT-6: Headache Impact Test-6; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; HR: heart rate; IL: interleukin; LCT: long-chain triglycerides; LDL-c: low-density lipoprotein cholesterol; LPS: lipopolysaccharides; LV: left ventricular; LVEF: left ventricular ejection fraction; LVOT VTI: left ventricular outflow tract velocity Time Integral; MAP: mean arterial pressure; MBF: myocardial blood flow; MCI: mild cognitive impairment; MCT: medium-chain triglyceride; MEE: myocardial external efficiency; MIDAS: Migraine Disability Test; MMSE: Mini-Mental State Examination; MOCA: Montreal Cognitive Assessment; mPAP: mean pulmonary arterial pressure; MRI: magnetic resonance imaging; MVO2: myocardial oxygen consumption; Na-3-OHB: sodium 3-hydroxybutyrate; NEFA: non-esterified fatty acid; NF-κB: nuclear factor-kappa B; NLRP3: NOD-like receptor pyrin-domain-containing 3; NPI-Q: Neuropsychiatric Inventory Questionnaire; OGTT: Oral Glucose Tolerance Test; P/F ratio: arterial oxygen partial pressure to fractional inspired oxygen ratio; PBMC: peripheral blood mononuclear cell; PCL-5: PTSD Checklist for DSM-5; PCWP: pulmonary capillary wedged pressure; PD: Parkinson’s disease; PET: positron emission tomography; PFC: prefrontal cortex; PH: pulmonary hypertension; PICOS: Participants, Interventions, Comparators, Outcomes, and Study design; PPO: peak power output; PRISMA-P: Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols; PROMs: Patient-Reported Outcome Measures; PrSO2: peripheral regional tissue oxygenation; PTSD: post-traumatic stress disorder; PVR: pulmonary vascular resistance; QOL-AD: quality of life–Alzheimer’s disease; RAP: right atrial pressure; RCT: randomized controlled trial; ROBINS-I: Risk Of Bias In Non-randomized Studies-of Interventions; RPM: revolutions per minute; RUD-Lite: resource utilization in dementia-lite; RV: right ventricle; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SNAP: suspected non-Alzheimer’s disease pathophysiology; STAI: State-Trait Anxiety Inventory; SV: stroke volume; SVO2: mixed venous oxygen saturation; SVR: systemic vascular resistance; TAPSE: tricuspid annular peak systolic excursion; TG: triglyceride; TNF-α: tumor necrosis factor α; TST: Task Switching Task; VIS: Vasoactive Inotropic Score; VLCADD: very long-chain acyl-CoA dehydrogenase deficiency; VT: ventilatory threshold; WAIS-LNS: Wechsler Adult Intelligence Scale–Letter–Number Sequencing; WM: white matter; βHB: β-hydroxybutyrate.
References
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A signaling metabolite. Ann. Rev. Nutr. 2017, 37, 51–76. [Google Scholar]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Rad. Biol. Med. 2016, 95, 268–277. [Google Scholar]
- Cahill, G.F.; George, F.C. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of ketogenic diet on health outcomes: An umbrella review of meta-analyses of randomized clinical trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Acharya, C.; Thongprayoon, C.; Hansrivijit, P.; Kanduri, S.R.; Kovvuru, K.; Medaura, J.; Vaitla, P.; Anton, D.F.G.; Mekraksakit, P.; et al. Incidence and Characteristics of Kidney Stones in Patients on Ketogenic Diet: A Systematic Review and Meta-Analysis. Diseases 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.J.; Jiang, W.L.; Sun, H.-B.; Liu, J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J. Clin. Neurol. 2015, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lopes Neri, L.C.; Guglielmetti, M.; Fiorini, S.; Pasca, L.; Zanaboni, M.P.; de Giorgis, V.; Tagliabue, A.; Ferraris, C. Adherence to ketogenic dietary therapies in epilepsy: A systematic review of literature. Nutr. Res. 2024, 126, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Falkenhain, K.; Daraei, A.; Forbes, S.C.; Little, J.P. Effects of Exogenous Ketone Supplementation on Blood Glucose: A Systematic Review and Meta-analysis. Adv. Nutr. 2022, 13, 1697–1714. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Kirk, T.; Evans, R.D.; Clarke, K. Gastrointestinal Effects of Exogenous Ketone Drinks are Infrequent, Mild, and Vary According to Ketone Compound and Dose. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 596–603. [Google Scholar] [CrossRef]
- Harvey, C.J.; Schofield GMWilliden, M.; McQuillan, J.A. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef]
- Pimentel-Suarez, L.I.; Soto-Mota, A. Evaluation of the safety and tolerability of exogenous ketosis induced by orally administered free beta-hydroxybutyrate in healthy adult subjects. BMJ Nutr. Prev. Health 2023, 6, 122–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; King, M.T.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Jones, A.W. Elimination half-life of acetone in humans: Case reports and review of the literature. J. Anal. Toxicol. 2000, 24, 8–10. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.-Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.-J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Laeger, T.; Metges, C.C.; Kuhla, B. Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 2010, 54, 450–455. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Newburgh, L.H.; Marsh, P.L. The use of a high fat diet in the treatment of diabetes mellitus: First paper. Arch. Intern. Med. (Chic.) 1920, 26, 647–662. [Google Scholar] [CrossRef][Green Version]
- Wilder, R.J. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar][Green Version]
- Bohnen, J.L.B.; Albin, R.L.; Bohnen, N.I. Ketogenic interventions in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease: A systematic review and critical appraisal. Front. Neurol. 2023, 14, 1123290. [Google Scholar] [CrossRef]
- Marcotte-Chénard, A.; Tremblay, R.; Falkenhain, K.; Little, J.P.; Riesco, E. Effect of Acute and Chronic Ingestion of Exogenous Ketone Supplements on Blood Pressure: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2024, 21, 408–426. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. (Lond.) 2009, 6, 31. [Google Scholar] [CrossRef]
- Henderson, S.T.; Morimoto, B.H.; Cummings, J.L.; Farlow, M.R.; Walker, J. A Placebo-Controlled, Parallel-Group, Randomized Clinical Trial of AC-1204 in Mild-to-Moderate Alzheimer’s Disease. J. Alzheimers Dis. 2020, 75, 547–557. [Google Scholar] [CrossRef]
- Ohnuma, T.; Toda, A.; Kimoto, A.; Takebayashi, Y.; Higashiyama, R.; Tagata, Y.; Ito, M.; Ota, T.; Shibata, N.; Arai, H. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: A prospective, open-label pilot study. Clin. Interv. Aging 2016, 11, 29–36. [Google Scholar] [CrossRef]
- Croteau, E.; Castellano, C.A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; Turcotte, É.E.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 64, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Y.; Zhang, X.; Liu, L.; Zhou, B.; Mo, R.; Li, Y.; Li, H.; Li, F.; Tao, Y.; et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4-/-: A double-blind, randomized, placebo-controlled crossover trial. Clin. Nutr. 2020, 39, 2092–2105. [Google Scholar] [CrossRef]
- Chan, S.C.; Esther, G.E.; Yip, H.L.; Sugathan, S.; Chin, P.S. Effect of cold pressed coconut oil on cognition and behavior among patients with Alzheimer’s disease—A pilot intervention study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1432–1435. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef]
- Juby, A.G.; Blackburn, T.E.; Mager, D.R. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: A randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimers Dement. (N. Y.) 2022, 8, e12259. [Google Scholar] [CrossRef]
- Torosyan, N.; Sethanandha, C.; Grill, J.D.; Dilley, M.L.; Lee, J.; Cummings, J.L.; Ossinalde, C.; Silverman, D.H. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp. Gerontol. 2018, 111, 118–121. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef]
- Fernando, M.G.; Silva, R.; Fernando, W.M.A.D.B.; de Silva, H.A.; Wickremasinghe, A.R.; Dissanayake, A.S.; Sohrabi, H.R.; Martins, R.N.; Williams, S.S. Effect of Virgin Coconut Oil Supplementation on Cognition of Individuals with Mild-to-Moderate Alzheimer’s Disease in Sri Lanka (VCO-AD Study): A Randomized Placebo-Controlled Trial. J. Alzheimer’s Dis. 2023, 96, 1195–1206. [Google Scholar] [CrossRef]
- Rebello, C.J.; Keller, J.N.; Liu, A.G.; Johnson, W.D.; Greenway, F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015, 3, 123–125. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; St-Pierre, V.; Myette-Côté, É.; Langlois, F.; Roy, M.; Morin, M.; Bocti, C.; Fulop, T.; Godin, J.; et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; Croteau, E.; Langlois, F.; Bocti, C.; St-Pierre, V.; Vandenberghe, C.; Bernier, M.; Roy, M.; Descoteaux, M.; et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019, 15, 625–634. [Google Scholar] [CrossRef]
- Roy, M.; Fortier, M.; Rheault, F.; Edde, M.; Croteau, E.; Castellano, C.; Langlois, F.; St-Pierre, V.; Cuenoud, B.; Bocti, C.; et al. A ketogenic supplement improves white matter energy supply and processing speed in mild cognitive impairment. Alzheimers Dement. (N. Y.) 2021, 7, e12217. [Google Scholar] [CrossRef]
- Myette-Côté, É.; St-Pierre, V.; Beaulieu, S.; Castellano, C.-A.; Fortier, M.; Plourde, M.; Bocti, C.; Fulop, T.; Cunnane, S.C. The effect of a 6-month ketogenic medium-chain triglyceride supplement on plasma cardiometabolic and inflammatory markers in mild cognitive impairment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102236. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Dearlove, D.J.; Lu, M.; Clarke, K.; Dawes, H.; Hu, M.T. A Ketone Ester Drink Enhances Endurance Exercise Performance in Parkinson’s Disease. Front. Neurosci. 2020, 14, 584130. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; Ortí, J.E.d.l.R. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Sancho-Cantus, D.; Benlloch, M.; Ceron, J.J.; Peres Rubio, C.; García-Pardo, M.P.; López-Rodríguez, M.M.; de la Rubia Ortí, J.E. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life 2021, 11, 353. [Google Scholar] [CrossRef]
- Putananickal, N.; Gross, E.C.; Orsini, A.L.; Schmidt, S.; Hafner, P.; Gocheva, V.; Nagy, S.; Henzi, B.C.; Rubino, D.; Vogt, D.R.; et al. Efficacy and safety of exogenous beta-hydroxybutyrate for preventive treatment in episodic migraine: A single-centred, randomised, placebo-controlled, double-blind crossover trial. Cephalalgia 2022, 42, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.; Patel, V.; Tideman, S.; Frech, R.; Frigerio, R.; Narayanan, J. Efficacy of supplemental MCT oil on seizure reduction of adult drug-resistant epilepsy—A single-center open-label pilot study. Nutr. Neurosci. 2023, 26, 535–539. [Google Scholar] [CrossRef]
- Youssef, N.A.; Holland-Winkler, A.M.; Phung, P.; Waller, J.L.; Ponkshe, S. A randomized, double-blind, clinical pilot trial of adjunct ketone supplement compared to placebo for treating posttraumatic stress disorder. Ann. Clin. Psychiatry 2022, 34, 240–244. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; Alarcon Ramos, G.C.; Ko, J.; Cameron-Smith, D.; Petrov, M.S. Acute Nutritional Ketosis and Its Implications for Plasma Glucose and Glucoregulatory Peptides in Adults with Prediabetes: A Crossover Placebo-Controlled Randomized Trial. J. Nutr. 2021, 151, 921–929. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Alarcon Ramos, G.C.; Ko, J.; Petrov, M.S. Abdominal fat distribution modulates the metabolic effects of exogenous ketones in individuals with new-onset prediabetes after acute pancreatitis: Results from a randomized placebo-controlled trial. Clin. Nutr. ESPEN 2021, 43, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kimita, W.; Bharmal, S.H.; Ko, J.; Cho, J.; Petrov, M.S. Effect of β-hydroxybutyrate monoester on markers of iron metabolism in new-onset prediabetes: Findings from a randomised placebo-controlled trial. Food Funct. 2021, 12, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of acute ketosis on lipid profile in prediabetes: Findings from a cross-over randomized controlled trial. Cardiovasc. Diabetol. 2022, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of d-β-Hydroxybutyrate-(R)-1,3 Butanediol on Appetite Regulation in People with Prediabetes. Mol. Nutr. Food Res. 2023, 67, e2200615. [Google Scholar] [CrossRef]
- Charles, S.; Liu, Y.; Kimita, W.; Ko, J.; Bharmal, S.H.; Petrov, M.S. Effect of D-β-hydroxybutyrate-(R)-1,3 butanediol on plasma levels of asprosin and leptin: Results from a randomised controlled trial. Food Funct. 2023, 14, 759–768. [Google Scholar] [CrossRef]
- Charles, S.; Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial. Biomolecules 2024, 14, 665. [Google Scholar] [CrossRef]
- Nakagata, T.; Tamura, Y.; Kaga, H.; Sato, M.; Yamasaki, N.; Someya, Y.; Kadowaki, S.; Sugimoto, D.; Satoh, H.; Kawamori, R.; et al. Ingestion of an exogenous ketone monoester improves the glycemic response during oral glucose tolerance test in individuals with impaired glucose tolerance: A cross-over randomized trial. J. Diabetes Investig. 2021, 12, 756–762. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.; Clarke, K.; Barber, T.M. Exogenous ketosis in patients with type 2 diabetes: Safety, tolerability and effect on glycaemic control. Endocrinol. Diabetes Metab. 2021, 4, e00264. [Google Scholar] [CrossRef]
- Falkenhain, K.; Oliveira, B.F.; Islam, H.; Neudorf, H.; Cen, H.H.; Johnson, J.D.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. The effect of acute and 14-day exogenous ketone supplementation on glycemic control in adults with type 2 diabetes: Two randomized controlled trials. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E61–E72. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Falkenhain, K.; Davy, B.; Davy, K.; Little, J. Acute effect of an exogenous ketone monoester supplement on appetite and food intake in adults with type 2 diabetes. Appl. Physiol. Nutr. Metab. 2024, 49, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Falkenhain, K.; Whelehan, G.; Neudorf, H.; Abdelrahman, D.R.; Murton, A.J.; Wall, B.T.; Stephens, F.B.; Little, J.P. A ketone monoester drink reduces postprandial blood glucose concentrations in adults with type 2 diabetes: A randomised controlled trial. Diabetologia 2024, 67, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.J.; Nilsson, M.; Ingerslev, J.S.; Olsen, D.A.; Fenger, M.; Svart, M.; Møller, N.; Zander, M.; Miskowiak, K.W.; Rungby, J. Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur. J. Endocrinol. 2020, 182, 233–242. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Caldwell, H.G.; Ainslie, P.N.; Clarke, K.; Little, J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 1491–1501. [Google Scholar] [CrossRef]
- Neudorf, H.; Myette-Côté, É.; P. Little, J. The Impact of Acute Ingestion of a Ketone Monoester Drink on LPS-Stimulated NLRP3 Activation in Humans with Obesity. Nutrients 2020, 12, 854. [Google Scholar] [CrossRef]
- Walsh, J.J.; Caldwell, H.G.; Neudorf, H.; Ainslie, P.N.; Little, J.P. Short-term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: A randomized cross-over trial. J. Physiol. 2021, 599, 4763–4778. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef]
- Kanta, J.M.; Lundsgaard, A.M.; Havelund, J.F.; Armour, S.L.; Bæk, O.; Nguyen, D.N.; Richter, E.A.; Knudsen, J.G.; Kleinert, M.; Færgeman, N.J.; et al. Metabolic effects of medium-chain triacylglycerol consumption are preserved in obesity. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E1–E20. [Google Scholar] [CrossRef]
- Yu, Q.; Wong, K.K.; Lei, O.K.; Armada-Da-Silva, P.A.; Wu, Z.; Nie, J.; Shi, Q.; Kong, Z. Acute ketone monoester supplementation in young adults: Modulating metabolic and neurocognitive functions across body weights. Appl. Physiol. Nutr. Metab. 2025, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, J.C.; Visser, G.; Clarke, K.; Ferdinandusse, S.; de Haan, F.H.; Houtkooper, R.H.; Ijlst, L.; Kok, I.L.; Langeveld, M.; van der Pol, W.L.; et al. Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2020, 43, 787–799. [Google Scholar] [CrossRef]
- Løkken, N.; Storgaard, J.H.; Revsbech, K.L.; Voermans, N.C.; Van Hall, G.; Vissing, J.; Ørngreen, M.C. No effect of oral ketone ester supplementation on exercise capacity in patients with McArdle disease and healthy controls: A randomized placebo-controlled cross-over study. J. Inherit. Metab. Dis. 2022, 45, 502–516. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Santalla, A.; Alejo, L.B.; Bustos, A.; Ozcoidi, L.M.; Castellote-Bellés, L.; Ferrer-Costa, R.; Villarreal-Salazar, M.; Morán, M.; Barranco-Gil, D.; et al. Acute ketone supplementation in the absence of muscle glycogen utilization: Insights from McArdle disease. Clin. Nutr. 2024, 43, 692–700. [Google Scholar] [CrossRef]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment with the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Berg-Hansen, K.; Christensen, K.H.; Ladefoged, B.T.; Poulsen, S.H.; Andersen, M.J.; Borlaug, B.A.; Nielsen, R.; Møller, N.; Wiggers, H. Randomized Crossover Trial of 2-Week Ketone Ester Treatment in Patients with Type 2 Diabetes and Heart Failure with Preserved Ejection Fraction. Circulation 2024, 150, 1570–1583. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Christensen, K.H.; Gopalasingam, N.; Nielsen, R.; Eiskjær, H.; Møller, N.; Birkelund, T.; Christensen, S.; Wiggers, H. Beneficial Effects of Ketone Ester in Patients with Cardiogenic Shock: A Randomized, Controlled, Double-Blind Trial. JACC Heart Fail. 2023, 11, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Christensen, K.H.; Gopalasingam, N.; Berg-Hansen, K.; Seefeldt, J.; Homilius, C.; Boedtkjer, E.; Andersen, M.J.; Wiggers, H.; Møller, N.; et al. Hemodynamic Effects of Ketone Bodies in Patients with Pulmonary Hypertension. J. Am. Heart Assoc. 2023, 12, e028232. [Google Scholar] [CrossRef]
- Shahtaghi, N.R.; Bigdelitabar, S.; Thakur, S.; Kaur, M.; Singh, H.; Saini, M.; Singh, M.; Singh, K.; Jain, S.K. Oral beta-hydroxybutyrate alleviates COVID-19 related acute respiratory distress syndrome: A randomized, single-blind, placebo-controlled trial. Res. Soc. Adm. Pharm. 2024, 20, 760–767. [Google Scholar] [CrossRef]
- McCall, A.L. The impact of diabetes on the CNS. Diabetes 1992, 41, 557–570. [Google Scholar] [CrossRef]
- Hoyer, S. Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol. Chem. Neuropathol. 1992, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004, 292, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.J.; Santana, I.; Brás, J.M.; Santiago, B.; Paiva, A.; Oliveira, C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener. Dis. 2007, 4, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Fenoglio, C.; Lovati, C.; Venturelli, E.; Guidi, I.; Corrà, B.; Scalabrini, D.; Clerici, F.; Mariani, C.; Bresolin, N.; et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1763–1768. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonça, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef]
- Johnston, G.A. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef]
- Pflanz, N.C.; Daszkowski, A.W.; James, K.A.; Mihic, S.J. Ketone body modulation of ligand-gated ion channels. Neuropharmacology 2019, 148, 21–30. [Google Scholar] [CrossRef]
- Hartman, A.L.; Gasior, M.; Vining, E.P.; Rogawski, M.A. The neuropharmacology of the ketogenic diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.D.; Chen, P.E.; Walker, M.C.; Williams, R.S.B. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139 Pt 2, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef]
- Balasse, E.O.; Ooms, H.A.; Lambilliotte, J.P. Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm. Metab. Res. 1970, 2, 371–372. [Google Scholar] [CrossRef]
- Svart, M.; Rittig, N.; Pedersen, S.B.; Jessen, N.; Møller, N. Oral 3-hydroxybutyrate ingestion decreases endogenous glucose production, lipolysis, and hormone-sensitive lipase phosphorylation in adipose tissue in men: A human randomized, controlled, crossover trial. Diabet. Med. 2021, 38, e14385. [Google Scholar] [CrossRef]
- Yamanashi, T.; Iwata, M.; Kamiya, N.; Tsunetomi, K.; Kajitani, N.; Wada, N.; Iitsuka, T.; Yamauchi, T.; Miura, A.; Pu, S.; et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017, 7, 7677. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Alosco, M.L.; Gunstad, J. The negative effects of obesity and poor glycemic control on cognitive function: A proposed model for possible mechanisms. Curr. Diabetes Rep. 2014, 14, 495. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Seifert, T.; Secher, N.H.; Grøndal, T.; van Hall, G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015, 100, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Trevisan, R.; Velussi, M.; Cernigoi, A.; DE Riva, C.; Bressan, M.; Doria, A.; Pauletto, N.; Angeli, P.; DE Doná, C.; et al. Glomerular filtration rate is increased in man by the infusion of both D,L-3-hydroxybutyric acid and sodium D,L-3-hydroxybutyrate. J. Clin. Endocrinol. Metab. 1987, 65, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gadegbeku, C.A.; Dhandayuthapani, A.; Shrayyef, M.Z.; Egan, B.M. Hemodynamic effects of nicotinic acid infusion in normotensive and hypertensive subjects. Am. J. Hypertens. 2003, 16, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Izawa, H.; Sobue, T.; Ishihara, H.; Somura, F.; Nishizawa, T.; Nagata, K.; Iwase, M.; Yokota, M. Prognostic value of mechanical efficiency in ambulatory patients with idiopathic dilated cardiomyopathy in sinus rhythm. J. Am. Coll. Cardiol. 2002, 39, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Galiè, N.; Ussia, G.; Passarelli, P.; Parlangeli, R.; Branzi, A.; Magnani, B. Role of pharmacologic tests in the treatment of primary pulmonary hypertension. Am. J. Cardiol. 1995, 75, 55A–62A. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D.; Orfanos, S.E.; Giovinazzo, M.; Schlesinger, R.D.; Naeije, R.; Fox, B.D.; Abualsaud, A.O.; Blenkhorn, F.; Rudski, L.G.; Catravas, J.D. Pulmonary capillary surface area in supine exercising humans: Demonstration of vascular recruitment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L361–L368. [Google Scholar] [CrossRef]
- Kesika, P.; Thangaleela, S.; Sisubalan, N.; Radha, A.; Sivamaruthi, B.S.; Chaiyasut, C. The Role of the Nuclear Factor-Kappa B (NF-κB) Pathway in SARS-CoV-2 Infection. Pathogens 2024, 13, 164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).