Effects of Citrulline or Watermelon Supplementation on Body Composition: A Systematic Review and Dose–Response Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Selection Criteria
2.2. Data Extraction
2.3. Statistical Analysis
2.4. Quality Assessment
2.5. GARDE
3. Results
3.1. Study Selection
3.2. Study Characteristics
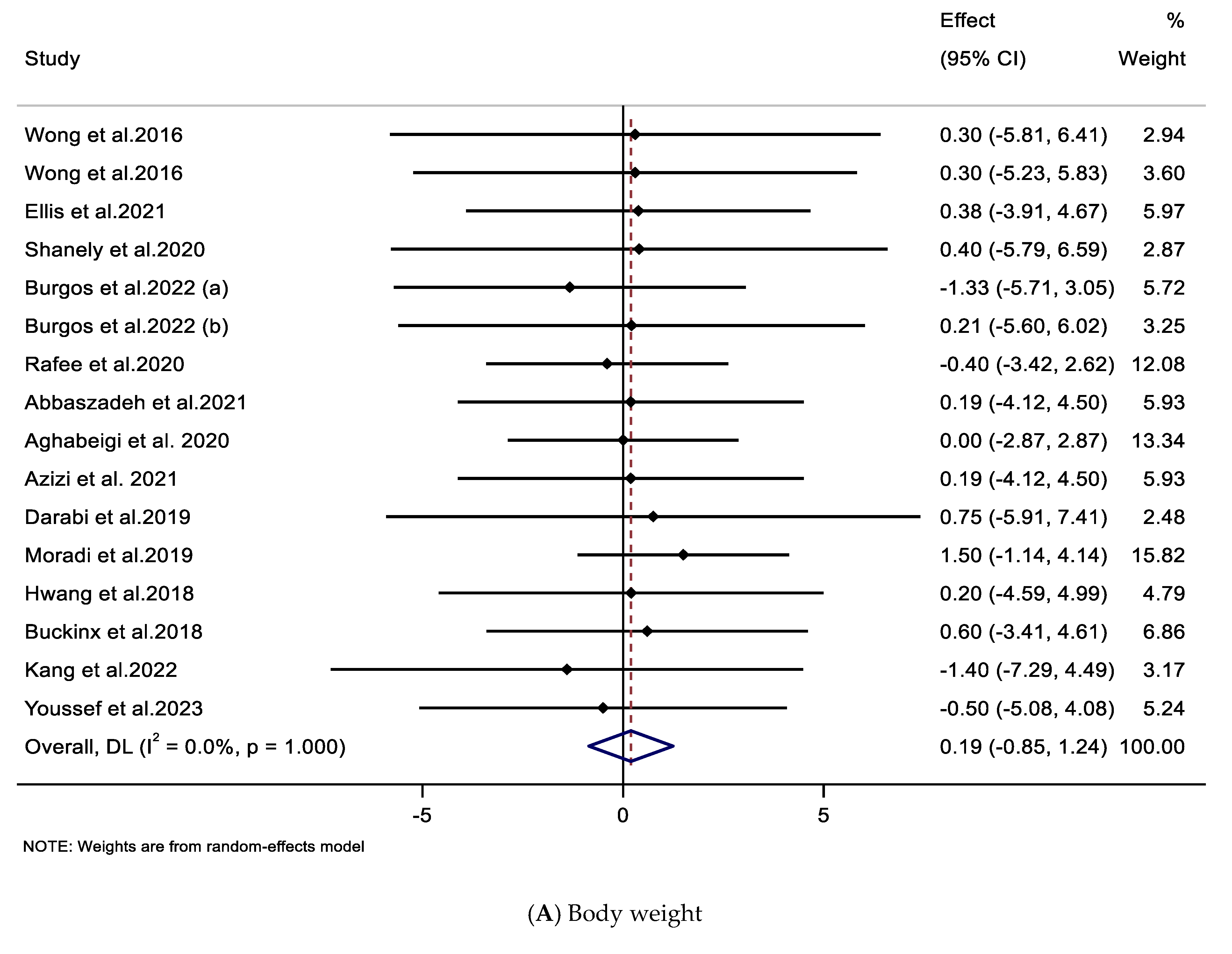
3.3. Effect of Supplementation with CIT on Body Weight and BMI
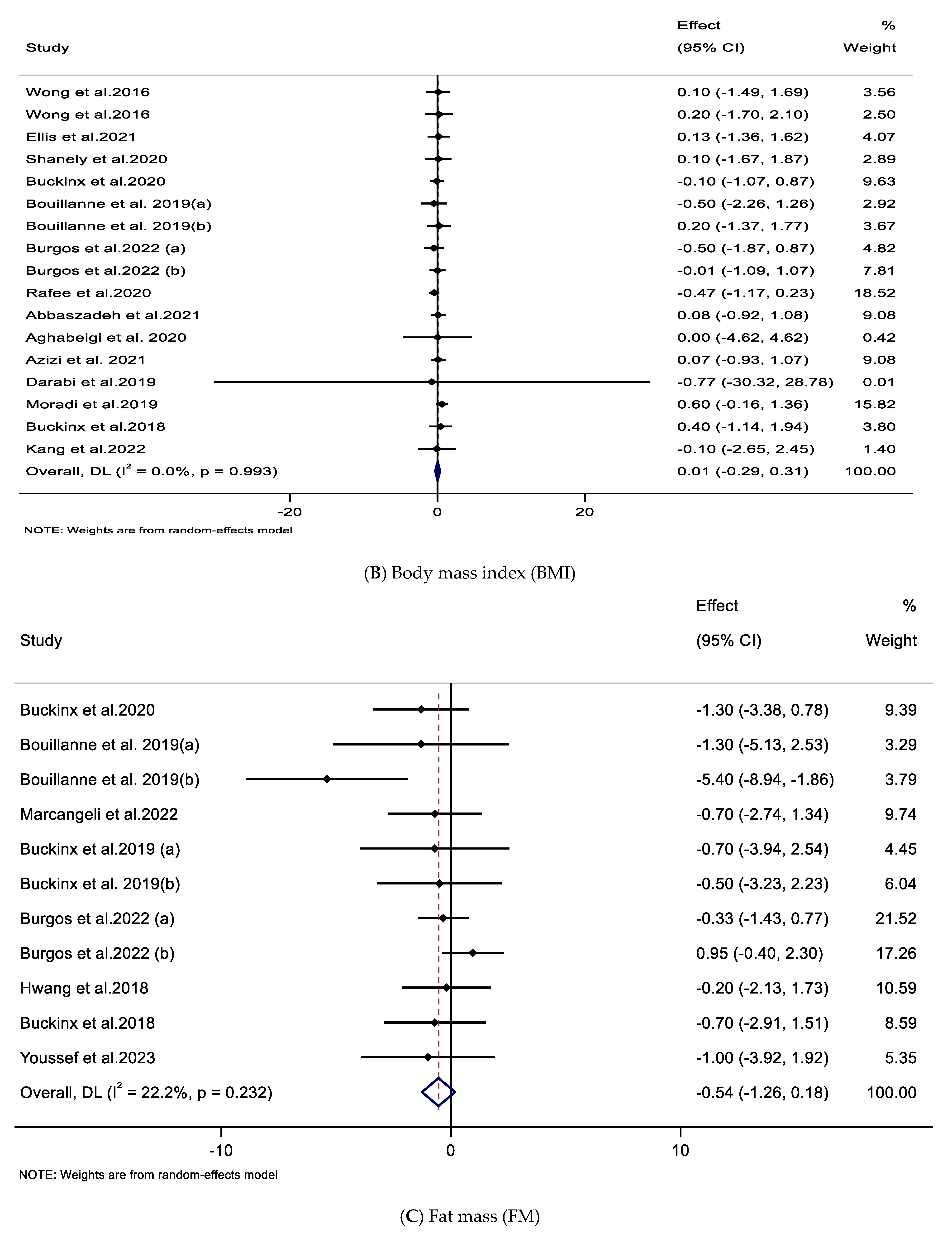
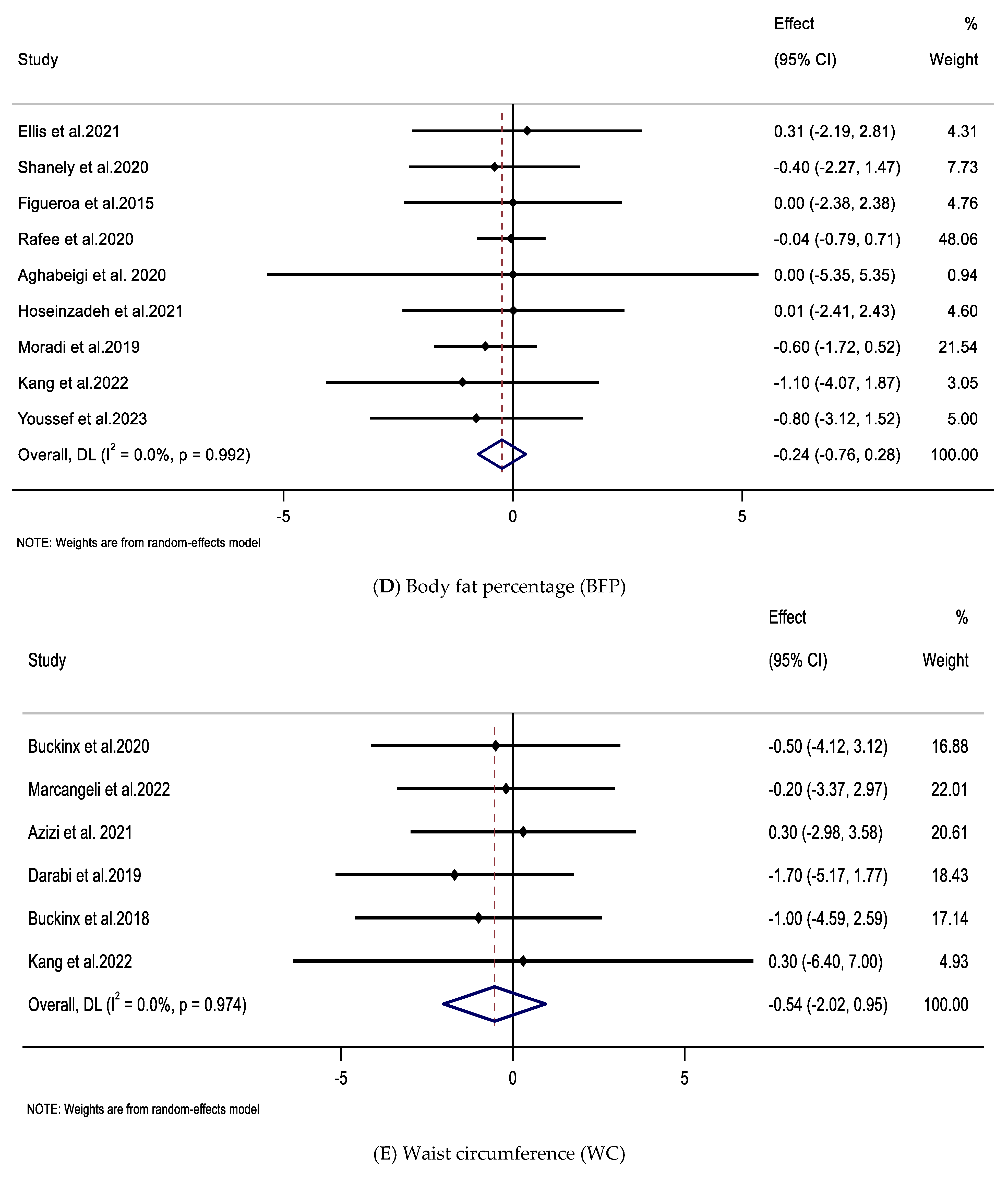
3.4. Impacts of Supplementation with CIT on FM, BFP, and WC
3.5. Impact of Supplementation with CIT on FFM
3.6. Risk of Bias
3.7. Publication Bias
3.8. Certainty of Evidence
3.9. Linear and Non-Linear Dose–Response
3.10. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CIT | L-citrulline |
| WAT | Watermelon |
| BMI | Body mass index |
| FM | Fat mass |
| BFP | Body fat percentage |
| WC | Waist circumference |
| FFM | Fat-free mass |
| RCT | Randomized controlled trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | The International Prospective Register of Systematic Reviews |
| WMD | Weighted mean differences |
| CI | Confidence intervals |
| SD | Standard deviation |
| RT | Resistance training |
| HIIT | High-intensity interval training |
| CT | Combined training |
| MA | Martial art training |
| RoB | Risk of Bias |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| NAFLD | Non-alcoholic fatty liver disease |
| NO | Nitric oxide |
| HTN | Hypertension |
| T2DM | Type 2 diabetes |
| ATP | Adenosine triphosphate |
References
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Aparecida Silveira, E.; Vaseghi, G.; de Carvalho Santos, A.S.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; de Oliveira, C. Visceral obesity and its shared role in cancer and cardiovascular disease: A scoping review of the pathophysiology and pharmacological treatments. Int. J. Mol. Sci. 2020, 21, 9042. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Aune, D.; Freisling, H.; Hardikar, S.; Jaafar, R.; Rinaldi, S.; Gunter, M.J.; Dossus, L. Association of metabolic obesity phenotypes with risk of overall and site-specific cancers: A systematic review and meta-analysis of cohort studies. Br. J. Cancer 2024, 131, 1480–1495. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G. Obesity and cardiovascular disease: An executive document on pathophysiological and clinical links promoted by the Italian Society of Cardiovascular Prevention (SIPREC). Front. Cardiovasc. Med. 2023, 10, 1136340. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.A.; Cini, K.I.; Francis, K.L.; Sawyer, S.M.; Azzopardi, P.S.; Patton, G.C.; Dhungel, B.; Jebasingh, F.K.; Abate, Y.H.; Abbas, N. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Mathews, E.M.; Wagner, D.R. Prevalence of overweight and obesity in collegiate American football players, by position. J. Am. Coll. Health 2008, 57, 33–38. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Ghanavati, M.; Lamuchi-Deli, N.; Payami, S.A.; Alavi-Rad, S.; Boustaninejad, M.; Afrisham, R.; Abbasnezhad, A.; Alipour, M. Rapid weight loss vs. slow weight loss: Which is more effective on body composition and metabolic risk factors? Int. J. Endocrinol. Metab. 2017, 15, e13249. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Daneghian, S.; Alipour, M.; Rafiei, H.; Ghanavati, M.; Mohammadpour, R.; Kooti, W.; Ashtary-Larky, P.; Afrisham, R. Waist circumference to height ratio: Better correlation with fat mass than other anthropometric indices during dietary weight loss in different rates. Int. J. Endocrinol. Metab. 2018, 16, e55023. [Google Scholar] [CrossRef]
- Lukaski, H.; Raymond-Pope, C.J. New Frontiers of Body Composition in Sport. Int. J. Sports Med. 2021, 42, 588–601. [Google Scholar] [CrossRef]
- Samadpour Masouleh, S.; Bagheri, R.; Ashtary-Larky, D.; Cheraghloo, N.; Wong, A.; Yousefi Bilesvar, O.; Suzuki, K.; Siahkouhian, M. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients 2021, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.; Hooshmand Moghadam, B.; Bagheri, R.; Ashtary-Larky, D.; Eskandari, E.; Nordvall, M.; Dutheil, F.; Wong, A. Effects of Interval Jump Rope Exercise Combined with Dark Chocolate Supplementation on Inflammatory Adipokine, Cytokine Concentrations, and Body Composition in Obese Adolescent Boys. Nutrients 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Rezaei Kelishadi, M.; Larky, D.A.; Bagheri, R.; Amirani, N.; Goudarzi, K.; Kargar, F.; Ghanavati, M.; Zamani, M. The effects of green tea extract supplementation on body composition, obesity-related hormones and oxidative stress markers: A grade-assessed systematic review and dose-response meta-analysis of randomised controlled trials. Br. J. Nutr. 2024, 131, 1125–1157. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A non-essential amino acid with important roles in human health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Karimi, E.; Abaj, F.; Gholizadeh, M.; Asbaghi, O.; Amini, M.R.; Ghaedi, E.; Hadi, A. Watermelon consumption decreases risk factors of cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2023, 202, 110801. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W., Jr.; Moyen, N.E. Acute citrulline malate supplementation improves upper- and lower-body submaximal weightlifting exercise performance in resistance-trained females. Eur. J. Nutr. 2017, 56, 775–784. [Google Scholar] [CrossRef]
- Perez-Guisado, J.; Jakeman, P.M. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J. Strength Cond. Res. 2010, 24, 1215–1222. [Google Scholar] [CrossRef]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Pashayee-Khamene, F.; Heidari, Z.; Asbaghi, O.; Ashtary-Larky, D.; Goudarzi, K.; Forbes, S.C.; Candow, D.G.; Bagheri, R.; Ghanavati, M.; Dutheil, F. Creatine supplementation protocols with or without training interventions on body composition: A GRADE-assessed systematic review and dose-response meta-analysis. J. Int. Soc. Sports Nutr. 2024, 21, 2380058. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Candow, D.G.; Forbes, S.C.; Hajizadeh, L.; Antonio, J.; Suzuki, K. Effects of Creatine and beta-Alanine Co-Supplementation on Exercise Performance and Body Composition: A Systematic Review. Nutrients 2025, 17, 2074. [Google Scholar] [CrossRef]
- Grgic, J.; Trexler, E.T.; Lazinica, B.; Pedisic, Z. Effects of caffeine intake on muscle strength and power: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2018, 15, 11. [Google Scholar] [CrossRef]
- Tabrizi, R.; Saneei, P.; Lankarani, K.B.; Akbari, M.; Kolahdooz, F.; Esmaillzadeh, A.; Nadi-Ravandi, S.; Mazoochi, M.; Asemi, Z. The effects of caffeine intake on weight loss: A systematic review and dos-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2688–2696. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Tinsley, G.M.; Asbaghi, O.; Salehpour, S.; Kashkooli, S.; Kooti, W.; Wong, A. Betaine supplementation fails to improve body composition: A systematic review and meta-analysis. Br. J. Nutr. 2022, 128, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Afrisham, R.; Farrokhi, V.; Ghanavati, M.; Asbaghi, O.; Mohammadi, S.; Mohammadian, M.; Taghvaei-Yazdeli, T.; Safaei-Kooyshahi, S.; Jadidi, Y.; Ashtary-Larky, D. The effects of beetroot and nitrate supplementation on body composition: A GRADE-assessed systematic review and meta-analysis. Br. J. Nutr. 2023, 130, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Ghanavati, M.; Asbaghi, O.; Wong, A.; Stout, J.R.; Suzuki, K. Effects of beta-alanine supplementation on body composition: A GRADE-assessed systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2022, 19, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Melchior, J.C.; Faure, C.; Paul, M.; Canoui-Poitrine, F.; Boirie, Y.; Chevenne, D.; Forasassi, C.; Guery, E.; Herbaud, S.; et al. Impact of 3-week citrulline supplementation on postprandial protein metabolism in malnourished older patients: The Ciproage randomized controlled trial. Clin. Nutr. 2019, 38, 564–574. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2022, 15, 74. [Google Scholar] [CrossRef]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.P.; Marcangeli, V.; El Hajj Boutros, G.; Dulac, M.; Noirez, P.; Morais, J.A.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of High-Intensity Interval Training Combined with L-Citrulline Supplementation on Functional Capacities and Muscle Function in Dynapenic-Obese Older Adults. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef]
- Burgos, J.; Viribay, A.; Calleja-Gonzalez, J.; Fernandez-Lazaro, D.; Olasagasti-Ibargoien, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Long-Term Combined Effects of Citrulline and Nitrate-Rich Beetroot Extract Supplementation on Recovery Status in Trained Male Triathletes: A Randomized, Double-Blind, Placebo-Controlled Trial. Biology 2022, 11, 75. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg 2021, 88, 105906. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Berlin, J.A. Publication bias: A problem in interpreting medical data. J. R. Stat. Soc. Ser. A Stat. Soc. 1988, 151, 419–445. [Google Scholar] [CrossRef]
- Mitchell, M.N. Interpreting and Visualizing Regression Models Using Stata; Stata Press: College Station, TX, USA, 2012; Volume 558. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Aghabeigi, A.P.; Azizi, M.; Tahmasebi, W.; Bashiri, P. The Effects of Six Weeks Ingestion of Watermelon Juice on Nitric Oxide in Elite Female Taekwondo. J. Neyshabur Univ. Med. Sci. 2020, 8, 100–188. [Google Scholar]
- Azizi, S.; Mahdavi, R.; Mobasseri, M.; Aliasgharzadeh, S.; Abbaszadeh, F.; Ebrahimi-Mameghani, M.J.P.R. The impact of L-citrulline supplementation on glucose homeostasis, lipid profile, and some inflammatory factors in overweight and obese patients with type 2 diabetes: A double-blind randomized placebo-controlled trial. Phytother. Res. 2021, 35, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Marcangeli, V.; Pinheiro Carvalho, L.; Dulac, M.; Hajj Boutros, G.; Gouspillou, G.; Gaudreau, P.; Morais, J.; Noirez, P.; Aubertin-Leheudre, M. Initial Dietary Protein Intake Influence Muscle Function Adaptations in Older Men and Women Following High-Intensity Interval Training Combined with Citrulline. Nutrients 2019, 11, 1685. [Google Scholar] [CrossRef]
- Buckinx, F.; Carvalho, L.; Marcangeli, V.; Dulac, M.; Hajj Boutros, G.; Gouspillou, G.; Gaudreau, P.; Noirez, P.; Aubertin-Leheudre, M. High intensity interval training combined with L-citrulline supplementation: Effects on physical performance in healthy older adults. Exp. Gerontol. 2020, 140, 111036. [Google Scholar] [CrossRef] [PubMed]
- Darabi, Z.; Darand, M.; Yari, Z.; Agah, S. Effects of Citrulline on Non-alcoholic Fatty Liver Disease: A Randomized-controlled Clinical Trial. Iran. J. Nutr. Sci. Food Technol 2019, 14, 1–9. [Google Scholar]
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% watermelon juice consumption and vascular function among postmenopausal women: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2959–2968. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Ormsbee, M.J.; Madzima, T.A.; Campbell, J.C.; Wong, A. Impact of L-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp. Gerontol. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Hosein Zade, F.; Farzaneh, A. Effects of low-frequency High-Intensity Interval training combined with L-Citrulline supplementation on myostatin and some physiological parameters in inactive elderly men. J. Sport Biosci. 2021, 12, 493–506. [Google Scholar]
- Hwang, P.; Morales Marroquín, F.E.; Gann, J.; Andre, T.; McKinley-Barnard, S.; Kim, C.; Morita, M.; Willoughby, D.S. Eight weeks of resistance training in conjunction with glutathione and L-Citrulline supplementation increases lean mass and has no adverse effects on blood clinical safety markers in resistance-trained males. J. Int. Soc. Sports Nutr. 2018, 15, 30. [Google Scholar] [CrossRef]
- Marcangeli, V.; Youssef, L.; Dulac, M.; Carvalho, L.P.; Hajj-Boutros, G.; Reynaud, O.; Guegan, B.; Buckinx, F.; Gaudreau, P.; Morais, J.A.; et al. Impact of high-intensity interval training with or without l-citrulline on physical performance, skeletal muscle, and adipose tissue in obese older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 1526–1540. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic l-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. 2016, 198, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.; Durand, S.; Aprahamian, F.; Lefevre, D.; Bourgin, M.; Maiuri, M.C.; Dulac, M.; Hajj-Boutros, G.; Marcangeli, V.; Buckinx, F.; et al. Serum metabolomic adaptations following a 12-week high-intensity interval training combined to citrulline supplementation in obese older adults. Eur. J. Sport Sci. 2023, 23, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Tahmasebi, W.; Azizi, M. The Simultaneous Effect of Citrulline Malate Supplementation and HIIT Exercise Training on Nitric Oxide Serum Levels and Performance in Elite Male Wrestlers. Appl. Health Stud. Sport Physiol. 2019, 6, 7–14. [Google Scholar]
- Abbaszadeh, F.; Azizi, S.; Mobasseri, M.; Ebrahimi-Mameghani, M. The effects of citrulline supplementation on meta-inflammation and insulin sensitivity in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2021, 13, 52. [Google Scholar] [CrossRef]
- Rafee, M.; Azizi, M.; Tahmasebi, W.; Hoseini, R. The effect of 6 weeks of watermelon juice supplementation on the activity of CK and LDH enzymes and exercise performance in elite female taekwondo athletes. Stud. Med. Sci. 2020, 30, 856–866. [Google Scholar]
- Xie, S.; Li, S.; Shaharudin, S. The Effects of Combined Exercise with Citrulline Supplementation on Body Composition and Lower Limb Function of Overweight Older Adults: A Systematic Review and Meta-Analysis. J. Sports Sci. Med. 2023, 22, 541–548. [Google Scholar] [CrossRef]
- Toomey, C.M.; McCormack, W.G.; Jakeman, P. The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry. Eur. J. Appl. Physiol. 2017, 117, 567–574. [Google Scholar] [CrossRef]
- Bone, J.L.; Ross, M.L.; Tomcik, K.A.; Jeacocke, N.A.; Hopkins, W.G.; Burke, L.M. Manipulation of muscle creatine and glycogen changes DXA estimates of body composition. Med. Sci. Sports Exerc. 2016, 49, 1029–1035. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients 2023, 15, 660. [Google Scholar] [CrossRef]
- Suzuki, K. Recent Progress in Applicability of Exercise Immunology and Inflammation Research to Sports Nutrition. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef]
- Gough, L.A.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F.; Newbury, J.W.; Trexler, E.; Faghy, M.A.; Bridge, C.A. A critical review of citrulline malate supplementation and exercise performance. Eur. J. Appl. Physiol. 2021, 121, 3283–3295. [Google Scholar] [CrossRef]
- Schierbauer, J.; Francis, L.; Greco, F.; Zimmermann, P.; Moser, O. Ergogenic Effects of a 10-Day L-Citrulline Supplementation on Time to Exhaustion and Cardiorespiratory and Metabolic Responses in Healthy Individuals. A Double-Blind, Randomized, Placebo-Controlled Crossover Trial. Front. Sports Act. Living 2025, 7, 1627743. [Google Scholar] [CrossRef]
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes. 2022, 12, 35. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Bagheripour, F.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Metabolic effects of L-citrulline in type 2 diabetes. Acta Physiol. 2023, 237, e13937. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Samadian, L.; Saedmocheshi, S.; Prieto-Gonzalez, P.; MacDonald, C. Overview of mechanisms related to citrulline malate supplementation and different methods of high-intensity interval training on sports performance: A narrative review. Heliyon 2025, 11, e42649. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Garcia, A.; Pascual-Fernandez, J.; Noriega-Gonzalez, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Cordova-Martinez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef]
- Figueroa, A.; Dillon, K.N.; Levitt, D.E.; Kang, Y. Citrulline Supplementation Improves Microvascular Function and Muscle Strength in Middle-Aged and Older Adults with Type 2 Diabetes. Nutrients 2025, 17, 2790. [Google Scholar] [CrossRef]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef]
- Ashtary-Larky, D. Are plant-based and omnivorous diets the same for muscle hypertrophy? A narrative review of possible challenges of plant-based diets in resistance-trained athletes. Nutrition 2025, 135, 112742. [Google Scholar] [CrossRef]
- Baz-Valle, E.; Balsalobre-Fernandez, C.; Alix-Fages, C.; Santos-Concejero, J. A Systematic Review of The Effects of Different Resistance Training Volumes on Muscle Hypertrophy. J. Hum. Kinet. 2022, 81, 199–210. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of resistance training combined with a ketogenic diet on body composition: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5717–5732. [Google Scholar] [CrossRef]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef]
- Varvik, F.T.; Bjornsen, T.; Gonzalez, A.M. Acute Effect of Citrulline Malate on Repetition Performance During Strength Training: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 350–358. [Google Scholar] [CrossRef]


| Component | Description | |
|---|---|---|
| Population | Adults (≥18 years), including healthy individuals, older adults, obese/overweight participants, trained athletes, and patients with metabolic disorders; excluding pregnant women | |
| Intervention | Citrulline supplementation as a standalone supplement (including pure L-citrulline or watermelon-derived citrulline) | |
| Comparison | Placebo or control group | |
| Outcome | Body composition parameters, including BMI, BFP, FM, body weight, WC, and FFM | |
| Study Design | RCTs, including both parallel and crossover designs, with an intervention duration of ≥2 weeks | |
| Studies | Country | Study Design | Participants | Sex | Sample Size | Trial Duration (Weeks) | Mean Age | Mean BMI | Intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | Intervention Type | Supplement Dose (g/day) | CG | ||||||
| Wong et al. 2016 [53] | USA | P, R, PC | Obese postmenopausal women | ♀ (23) | 12 | 11 | 8 | 58 ± 4 | 58 ± 3 | 32.2 ± 2.4 | 32.9 ± 3.6 | CIT | 6 | MD |
| Wong et al. 2016 [52] | USA | P, R, PC | Obese postmenopausal women | ♀ (27) | 13 | 14 | 8 | 58 ± 3 | 58 ± 4 | 33.8 ± 3.9 | 35 ± 3.4 | CIT | 6 | MD |
| Ellis et al. 2021 [46] | USA | C, R, DB, PC | Healthy postmenopausal women | ♀ (17) | 17 | 17 | 4 | 60 ± 4 | 60 ± 4 | 25.0 ± 3.5 | 25.0 ± 3.5 | WAT | 720 mL/day (1.63 g) | Isocaloric placebo |
| Shanely et al. 2020 [51] | USA | P, R, CO | Obese postmenopausal women | ♀ (45) | 26 | 19 | 6 | 60 ± 5 | 60 ± 7 | 30.9 ± 4.6 | 30.3 ± 4.8 | WAT | 710 mL/day (1.61 g) | No intervention |
| Buckinx et al. 2020 [44] | Canada | P, R, DB, PC | Healthy older adults | ♀/♂ (24/20) | 23 | 21 | 12 | 68 ± 5 | 68 ± 3 | 26.1± 2.8 | 26.1 ± 2.2 | CIT | 10 | MD |
| Bouillanne et al. 2019 (a) [28] | France | P, R, DB, PC | Malnourished older women | ♀ (18) | 8 | 10 | 3 | 89 ± 6 | 88 ± 4 | 19.9 ± 2.4 | 20.7 ± 3.7 | CIT | 10 | Mixture of six NEAAs |
| Bouillanne et al. 2019 (b) [28] | France | P, R, DB, PC | Malnourished older patients | ♀/♂ (18/6) | 11 | 13 | 3 | 89 ± 6 | 88 ± 4 | 19.7 ± 2.5 | 21.6 ± 3.8 | CIT | 10 | Mixture of six NEAAs |
| Marcangeli et al. 2022 [50] | France | P, R, DB, PC | Obese older adults | ♀/♂ (43/38) | 45 | 36 | 12 | 67 ± 5 | 68 ± 4 | 29.1 ± 4.3 | 29.3 ± 5.1 | CIT | 10 | MD |
| Figueroa et al. 2015 [47] | USA | P, R, PC | Postmenopausal women with HTN | ♀ (27) | 13 | 14 | 8 | 58 ± 4 | 58 ± 4 | 33.8 ± 4 | 35 ± 3.4 | CIT | 6 | MD |
| Rafee et al. 2020 [57] | Iran | P, R, SB, PC | Elite taekwondo athletes | ♀ (25) | 15 | 10 | 6 | 22 ± 2 | 21 ± 2 | 20.7 ± 1.9 | 20.9 ± 0.9 | WAT | 500 mL/day (1.17 g) | Placebo |
| Buckinx et al. 2019 (a) [43] | Canada | P, R, DB, PC | Sedentary obese older adults | ♀/♂ (17/16) | 19 | 14 | 12 | 68 ± 5 | 68 ± 4 | 30.4 ± 4 | 31.9 ± 6 | CIT | 10 | MD + Protein |
| Buckinx et al. 2019 (b) [43] | Canada | P, R, DB, PC | Sedentary obese older adults | ♀/♂ (23/17) | 21 | 19 | 12 | 67 ± 5 | 68 ± 4 | 27.7 ± 5 | 27.6 ± 3.8 | CIT | 10 | MD+ Protein |
| Burgos et al. 2022 (a) [31] | Spain | P, R, DB, PC | Trained male triathletes | ♂ (16) | 8 | 8 | 9 | 33 ± 7 | 34 ± 7 | 24.5 ± 2.5 | 24.0 ± 1.8 | CIT | 3 | Cellulose |
| Burgos et al. 2022 (b) [31] | Spain | P, R, DB, PC | Trained male triathletes | ♂ (16) | 8 | 8 | 9 | 34 ± 8 | 33 ± 7 | 22.5 ± 1.6 | 23.2 ± 1.8 | CIT | 3 | Nitrate-rich beetroot extract |
| Abbaszadeh et al. 2021 [56] | Iran | P, R, DB, PC | Patients with T2DM | ♀/♂ (16/29) | 23 | 22 | 8 | 48 ± 6 | 50 ± 5 | 29.7± 3.2 | 28.2 ± 2.1 | CIT | 3 | Microcrystalline cellulose |
| Aghabeigi et al. 2020 [41] | Iran | P, R, SB, PC | Elite taekwondo players | ♀ (25) | 15 | 10 | 6 | 22 ± 2 | 22 ± 2 | 20.7 ± 1.9 | 20.9 ± 0.9 | WAT | 214 mL/day (0.5 g) | Placebo |
| Azizi et al. 2021 [42] | Iran | P, R, DB, PC | Patients with T2DM | ♀/♂ (16/29) | 23 | 22 | 8 | 48 ± 6 | 50 ± 5 | 29.7± 3.2 | 28.2 ± 2.1 | CIT | 3 | Microcrystalline cellulose |
| Hosein Zade et al. 2021 [48] | Iran | P, R, DB, PC | Inactive elderly men | ♂ (18) | 9 | 9 | 8 | 64 ± 5 | 64 ± 5 | 28.6 | 28.6 | CIT | 6 | Dextrose |
| Darabi et al. 2019 [45] | Iran | P, R, DB, PC | Patients with NAFLD | ♀/♂ (21/23) | 22 | 22 | 12 | 46 ± 12 | 45± 11 | 32.5 ± 6.6 | 33.5 ± 5.8 | CIT | 2 | Starch |
| Moradi et al. 2019 [55] | Iran | P, R, SB, PC | Elite male wrestlers | ♂ (19) | 10 | 9 | 6 | 22 ± 2 | 22 ± 2 | 24.6 ± 0.5 | 23.8 ± 1.2 | CIT | 2 | Cellulose |
| Hwang et al. 2018 [49] | USA | P, R, DB, PC | Resistance-trained males | ♂ (50) | 25 | 25 | 8 | 20 ± 2 | 20 ± 2 | 24.9 | 25.8 | CIT | 2 | Cellulose |
| Buckinx et al. 2018 [30] | Canada | P, R, DB, PC | Dynapenic-obese older adults | ♀/♂ (28/28) | 26 | 30 | 12 | 66 ± 4 | 68 ± 4 | 30.5 ± 4.1 | 30.5 ± 4.9 | CIT | 10 | MD |
| Kang et al. 2022 [29] | USA | P, R, DB, PC | Postmenopausal women with HTN | ♀ (24) | 13 | 11 | 8 | 62 ± 7 | 63 ± 3 | 29.6 ± 4 | 29.2 ± 5.6 | CIT | 10 | MD |
| Youssef et al. 2023 [54] | Canada | P, R, DB, PC | Obese older adults | ♀/♂ (59) | 33 | 26 | 12 | ≥65 | ≥65 | 25 | 25.7 | CIT | 10 | Placebo |
| Sub-Groups | Number of Effect Sizes | WMD (95%CI) | p-Value | Heterogeneity | ||
|---|---|---|---|---|---|---|
| p-Value Heterogeneity | I2 (%) | p-Value Between Sub-Groups | ||||
| Impacts of CIT supplementation on body weight (kg) | ||||||
| Overall effect | 16 | 0.19 (−0.85, 1.24) | 0.716 | 1.000 | 0 | |
| Trial duration (weeks) | ||||||
| ≤8 | 11 | 0.30 (−0.89, 1.50) | 0.622 | 0.999 | 0 | 0.719 |
| >8 | 5 | −0.15 (−2.31, 2.00) | 0.891 | 0.971 | 0 | |
| Type of CIT | ||||||
| CIT | 12 | 0.31 (−0.97, 1.61) | 0.630 | 0.999 | 0 | 0.750 |
| WAT | 4 | −0.04 (−1.83, 1.75) | 0.964 | 0.990 | 0 | |
| Supplement dose (g/day) | ||||||
| ≤6 | 13 | 0.26 (−0.87, 1.40) | 0.649 | 1.00 | 0 | 0.758 |
| >6 | 3 | −0.19 (−2.87, 2.49) | 0.888 | 0.848 | 0 | |
| Baseline BMI | ||||||
| Normal | 6 | 0.23 (−1.17, 1.64) | 0.745 | 0.905 | 0 | 0.991 |
| OW | 2 | 0.28 (−2.75, 3.32) | 0.854 | 0.951 | 0 | |
| OB | 8 | 0.09 (−1.72, 1.91) | 0.918 | 1.000 | 0 | |
| Sex | ||||||
| Female | 7 | −0.08 (−1.67, 1.49) | 0.913 | 0.999 | 0 | 0.864 |
| Male | 4 | 0.60 (−1.32, 2.52) | 0.542 | 0.744 | 0 | |
| Both | 5 | 0.21 (−1.82, 2.25) | 0.839 | 0.997 | 0 | |
| Age | ||||||
| ≤40 | 6 | 0.23 (−1.17, 1.64) | 0.745 | 0.905 | 0 | 0.934 |
| >40 | 10 | 0.14 (−1.41, 1.70) | 0.855 | 1.000 | 0 | |
| Training | ||||||
| Yes | 10 | 0.15 (−1.07, 1.37) | 0.810 | 0.992 | 0 | 0.887 |
| No | 6 | 0.32 (−1.73, 2.37) | 0.758 | 1.000 | 0 | |
| Training type | ||||||
| RT | 3 | −0.20 (−3.29, 2.87) | 0.895 | 0.897 | 0 | 0.911 |
| HIIT | 3 | 0.90 (−1.08, 2.88) | 0.372 | 0.749 | 0 | |
| CT | 2 | −0.77 (−4.27, 2.72) | 0.666 | 0.679 | 0 | |
| MA | 2 | −0.19 (−2.27, 1.89) | 0.858 | 0.851 | 0 | |
| Impacts of CIT supplementation on BMI (kg/m2) | ||||||
| Overall effect | 17 | 0.01 (−0.29.0.31) | 0.947 | 0.993 | 0 | |
| Trial duration (weeks) | ||||||
| ≤8 | 12 | 0.04 (−0.31, 0.39) | 0.823 | 0.948 | 0 | 0.744 |
| >8 | 5 | −0.07 (−0.66, 0.51) | 0.805 | 0.945 | 0 | |
| Type of CIT | ||||||
| CIT | 13 | 0.12 (−0.22, 0.47) | 0.501 | 0.993 | 0 | 0.226 |
| WAT | 4 | −0.30 (−0.89, 0.28) | 0.313 | 0.859 | 0 | |
| Supplement dose (g/day) | ||||||
| ≤6 | 12 | 0.01 (−0.32, 0.35) | 0.923 | 0.940 | 0 | 0.933 |
| >6 | 5 | −0.01 (−0.66, 0.63) | 0.965 | 0.954 | 0 | |
| Baseline BMI | ||||||
| Normal | 7 | −0.04 (−0.45, 0.36) | 0.829 | 0.545 | 0 | 0.893 |
| OW | 3 | 0.01 (−0.61, 0.64) | 0.968 | 0.955 | 0 | |
| OB | 7 | 0.13 (−0.48, 0.76) | 0.670 | 1.000 | 0 | |
| Sex | ||||||
| Female | 8 | −0.23 (−0.73, 0.26) | 0.351 | 0.989 | 0 | 0.474 |
| Male | 3 | 0.22 (−0.37, 0.82) | 0.462 | 0.333 | 9 | |
| Both | 6 | 0.07 (−0.43, 0.58) | 0.772 | 0.997 | 0 | |
| Age | ||||||
| ≤40 | 5 | −0.03 (−0.53, 0.45) | 0.880 | 0.326 | 13.8 | 0.785 |
| >40 | 12 | 0.05 (−0.36, 0.46) | 0.807 | 1.000 | 0 | |
| Training | ||||||
| Yes | 9 | −0.01 (−0.38, 0.36) | 0.949 | 0.757 | 0 | 0.844 |
| No | 8 | 0.05 (−0.45, 0.55) | 0.843 | 1.000 | 0 | |
| Training type | ||||||
| RT | 2 | 0.09 (−1.43, 1.61) | 0.905 | 0.853 | 0 | 0.491 |
| HIIT | 3 | 0.34 (−0.21, 0.90) | 0.226 | 0.535 | 0 | |
| CT | 2 | −0.19 (−1.04, 0.65) | 0.648 | 0.582 | 0 | |
| MA | 2 | −0.45 (−1.15, 0.23) | 0.193 | 0.844 | 0 | |
| Impacts of CIT supplementation on WC (cm) | ||||||
| Overall effect | 6 | −0.54 (−2.02, 0.95) | 0.480 | 0.974 | 0 | |
| Trial duration (weeks) | ||||||
| ≤8 | 2 | 0.30 (−2.64, 3.24) | 0.842 | 1.000 | 0 | 0.519 |
| >8 | 4 | −0.82 (−2.54, 0.90) | 0.349 | 0.933 | 0 | |
| Type of CIT | ||||||
| CIT | 6 | −0.53 (−2.02, 0.95) | 0.480 | 0.974 | 0 | - |
| Supplement dose (g/day) | ||||||
| ≤6 | 2 | −0.64 (−3.02, 1.73) | 0.596 | 0.411 | 0 | 0.910 |
| >6 | 4 | −0.46 (−2.37, 1.43) | 0.631 | 0.983 | 0 | |
| Baseline BMI | ||||||
| OW | 2 | −0.33 (−2.71, 2.05) | 0.786 | 0.903 | 0 | 0.828 |
| OB | 4 | −0.66 (−2.57, 1.23) | 0.492 | 0.852 | 0 | |
| Sex | ||||||
| Female | 1 | 0.30 (−6.39, 6.99) | 0.930 | - | - | 0.802 |
| Both | 5 | −0.58 (−2.10, 0.94) | 0.456 | 0.940 | 0 | |
| Age | ||||||
| >40 | 6 | −0.53 (−2.02, 0.95) | 0.480 | 0.974 | 0 | - |
| Training | ||||||
| Yes | 4 | −0.46 (−2.37, 1.43) | 0.631 | 0.983 | 0 | 0.910 |
| No | 2 | −0.64 (−3.02, 1.73) | 0.596 | 0.411 | 0 | |
| Training type | ||||||
| RT | 1 | 0.30 (−6.39, 6.99) | 0.930 | - | - | 0.967 |
| HIIT | 3 | −0.53 (−2.52, 1.45) | 0.598 | 0.948 | 0 | |
| Impacts of CIT supplementation on FM (kg) | ||||||
| Overall effect | 11 | −0.54 (−1.26, 0.18) | 0.142 | 0.232 | 22.2 | 0.232 |
| Trial duration (weeks) | ||||||
| ≤8 | 3 | −2.08 (−5.23, 1.06) | 0.194 | 0.041 | 68.7 | 0.264 |
| >8 | 8 | −0.25 (−0.90, 0.39) | 0.440 | 0.691 | 0.0 | |
| Type of CIT | ||||||
| CIT | 11 | −0.54 (−1.26, 0.18) | 0.142 | 0.232 | 62.3 | - |
| Supplement dose (g/day) | ||||||
| ≤6 | 3 | 0.12 (−0.70, 0.95) | 0.760 | 0.333 | 9.2 | 0.039 |
| >6 | 8 | −1.18 (−2.10, −0.25) | 0.012 | 0.516 | 0 | |
| Baseline BMI | ||||||
| Normal | 5 | −0.64 (−2.11, 0.83) | 0.392 | 0.022 | 65.1 | 0.972 |
| OW | 3 | −0.88 (−2.16, 0.40) | 0.178 | 0.878 | 0 | |
| OB | 3 | −0.78 (−2.33, 0.76) | 0.321 | 0.986 | 0 | |
| Sex | ||||||
| Female | 1 | −1.30 (−5.12, 2.52) | 0.506 | - | - | 0.121 |
| Male | 3 | 0.12 (−0.70, 0.95) | 0.760 | 0.333 | 9.2 | |
| Both | 7 | −1.17 (−2.14, −0.20) | 0.017 | 0.401 | 3.2 | |
| Age | ||||||
| ≤40 | 3 | 0.12 (−0.70, 0.95) | 0.760 | 0.333 | 9.2 | 0.039 |
| >40 | 8 | −1.18 (−2.10, −0.25) | 0.012 | 0.516 | 0 | |
| Training | ||||||
| Yes | 9 | −0.24 (−0.86, 0.36) | 0.425 | 0.784 | 0 | 0.126 |
| No | 2 | −3.41 (−7.43, 0.59) | 0.095 | 0.123 | 57.9 | |
| Training type | ||||||
| RT | 1 | −0.20 (−2.12, 1.72) | 0.839 | - | - | |
| HIIT | 6 | −0.84 (−1.83, 0.14) | 0.095 | 0.998 | 0 | 0.265 |
| CT | 2 | 0.24 (−0.99, 1.49) | 0.696 | 0.150 | 51.8 | |
| Impacts of CIT supplementation on BFP (%) | ||||||
| Overall effect | 9 | −0.24 (−0.76, 0.28) | 0.367 | 0.992 | 0 | |
| Trial duration (weeks) | ||||||
| ≤8 | 8 | −0.21 (−0.74, 0.32) | 0.441 | 0.989 | 0 | 0.627 |
| >8 | 1 | −0.80 (−3.12, 1.52) | 0.500 | - | - | |
| Type of CIT | ||||||
| CIT | 5 | −0.51 (−1.35, 0.31) | 0.221 | 0.964 | 0 | 0.398 |
| WAT | 4 | −0.06 (−0.72, 0.60) | 0.859 | 0.975 | 0 | |
| Supplement dose (g/day) | ||||||
| ≤6 | 7 | −0.18 (−0.72, 0.36) | 0.515 | 0.988 | 0 | 0.451 |
| >6 | 2 | −0.91 (−2.74, 0.91) | 0.328 | 0.876 | 0 | |
| Baseline BMI | ||||||
| Normal | 3 | −0.21 (−0.82, 0.40) | 0.505 | 0.715 | 0 | 0.811 |
| OW | 2 | 0.15 (−1.58, 1.89) | 0.861 | 0.866 | 0 | |
| OB | 4 | −0.50 (−1.65, 0.63) | 0.384 | 0.940 | 0 | |
| Sex | ||||||
| Female | 6 | −0.10 (−0.72, 0.52) | 0.749 | 0.985 | 0 | 0.724 |
| Male | 2 | −0.49 (−1.50, 0.52) | 0.342 | 0.654 | 0 | |
| Both | 1 | −0.80 (−3.12, 1.52) | 0.500 | - | - | |
| Age | ||||||
| ≤40 | 3 | −0.21 (−0.82, 0.40) | 0.505 | 0.715 | 0 | 0.867 |
| >40 | 6 | −0.30 (−1.26, 0.64) | 0.529 | 0.976 | 0 | |
| Training | ||||||
| Yes | 7 | −0.25 (−0.80, 0.30) | 0.373 | 0.972 | 0 | 0.896 |
| No | 2 | −0.14 (−1.64, 1.35) | 0.849 | 0.656 | 0 | |
| Training type | ||||||
| RT | 2 | −0.43 (−2.28, 1.42) | 0.650 | 0.571 | 0 | 0.855 |
| HIIT | 2 | −0.54 (−1.47, 0.38) | 0.254 | 0.879 | 0 | |
| MA | 3 | −0.03 (−0.78, 0.70) | 0.917 | 0.988 | 0 | |
| Impacts of CIT supplementation on FFM (kg) | ||||||
| Overall effect | 11 | −0.22 (−1.69, 1.24) | 0.763 | 0.020 | 52.8 | |
| Trial duration (weeks) | ||||||
| ≤8 | 4 | 1.95 (0.43, 3.47) | 0.012 | 0.863 | 0 | 0.004 |
| >8 | 7 | −1.35 (−2.99, 0.27) | 0.103 | 0.119 | 40.9 | |
| Type of CIT | ||||||
| CIT | 11 | −0.22 (−1.68, 1.23) | 0.763 | 0.020 | 52.8 | |
| Supplement dose (g/day) | ||||||
| ≤6 | 4 | −1.29 (−6.73, 4.14) | 0.641 | 0.001 | 82.4 | 0.615 |
| >6 | 7 | 0.12 (−0.99, 1.25) | 0.823 | 0.669 | 0 | |
| Baseline BMI | ||||||
| Normal | 6 | −0.30 (−2.98, 2.92) | 0.984 | 0.002 | 73.9 | 0.942 |
| OW | 3 | −0.63 (−2.41, 1.14) | 0.483 | 0.734 | 0 | |
| OB | 2 | −0.41 (−2.52, 1.69) | 0.702 | 0.819 | 0 | |
| Sex | ||||||
| Female | 1 | 1.10 (−1.43, 3.63) | 0.396 | - | - | 0.621 |
| Male | 4 | −1.29 (−6.73, 4.14) | 0.641 | 0.001 | 82.4 | |
| Both | 6 | −0.10 (−1.36, 1.14) | 0.865 | 0.646 | 0 | |
| Age | ||||||
| ≤40 | 4 | −1.29 (−6.73, 4.14) | 0.641 | 0.001 | 82.4 | 0.615 |
| >40 | 7 | 0.12 (−0.99, 1.25) | 0.823 | 0.669 | 0 | |
| Training | ||||||
| Yes | 9 | −0.76 (−2.49, 0.96) | 0.385 | 0.023 | 55.0 | 0.081 |
| No | 2 | 1.59 (−0.41, 3.59) | 0.120 | 0.538 | 0 | |
| Training type | ||||||
| RT | 1 | 0.52 (−15.69, 16.73) | 0.950 | - | - | 0.325 |
| HIIT | 6 | 0.18 (−1.05, 1.42) | 0.772 | 0.358 | 9.1 | |
| CT | 2 | −3.81 (−9.66, 2.03) | 0.201 | 0.056 | 72.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashtary-Larky, D.; Mohammadi, S.; Mousavi, S.A.H.; Hajizadeh, L.; Candow, D.G.; Forbes, S.C.; Afrisham, R.; Farrokhi, V.; Antonio, J.; Suzuki, K. Effects of Citrulline or Watermelon Supplementation on Body Composition: A Systematic Review and Dose–Response Meta-Analysis. Nutrients 2025, 17, 3126. https://doi.org/10.3390/nu17193126
Ashtary-Larky D, Mohammadi S, Mousavi SAH, Hajizadeh L, Candow DG, Forbes SC, Afrisham R, Farrokhi V, Antonio J, Suzuki K. Effects of Citrulline or Watermelon Supplementation on Body Composition: A Systematic Review and Dose–Response Meta-Analysis. Nutrients. 2025; 17(19):3126. https://doi.org/10.3390/nu17193126
Chicago/Turabian StyleAshtary-Larky, Damoon, Shooka Mohammadi, Seyed Amir Hossein Mousavi, Leila Hajizadeh, Darren G. Candow, Scott C. Forbes, Reza Afrisham, Vida Farrokhi, Jose Antonio, and Katsuhiko Suzuki. 2025. "Effects of Citrulline or Watermelon Supplementation on Body Composition: A Systematic Review and Dose–Response Meta-Analysis" Nutrients 17, no. 19: 3126. https://doi.org/10.3390/nu17193126
APA StyleAshtary-Larky, D., Mohammadi, S., Mousavi, S. A. H., Hajizadeh, L., Candow, D. G., Forbes, S. C., Afrisham, R., Farrokhi, V., Antonio, J., & Suzuki, K. (2025). Effects of Citrulline or Watermelon Supplementation on Body Composition: A Systematic Review and Dose–Response Meta-Analysis. Nutrients, 17(19), 3126. https://doi.org/10.3390/nu17193126












