Clinical Benefits of Exogenous Ketosis in Adults with Disease: A Systematic Review
Abstract
1. Introduction
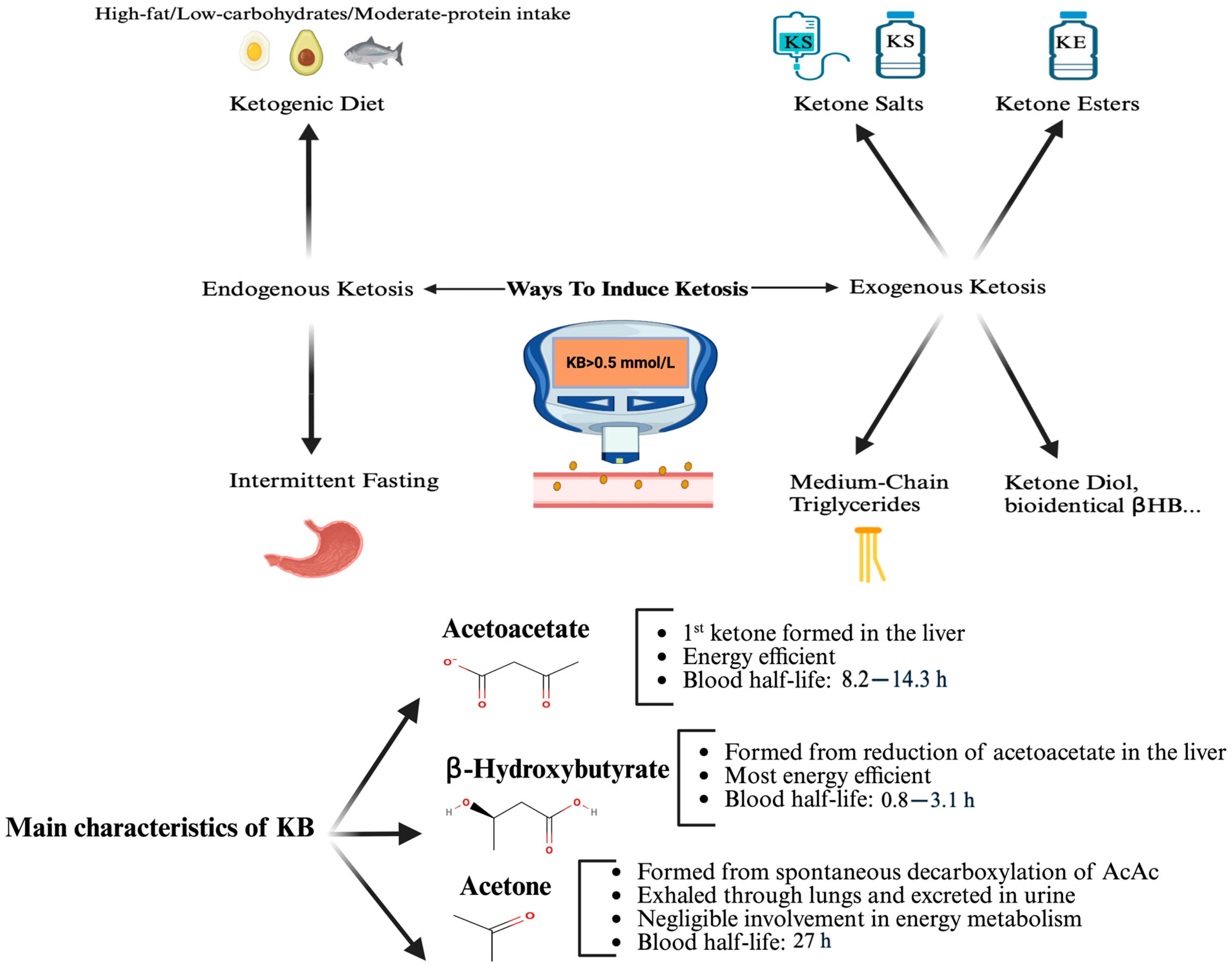
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Data Extraction, Risk of Bias Assessment and Analysis
3. Results
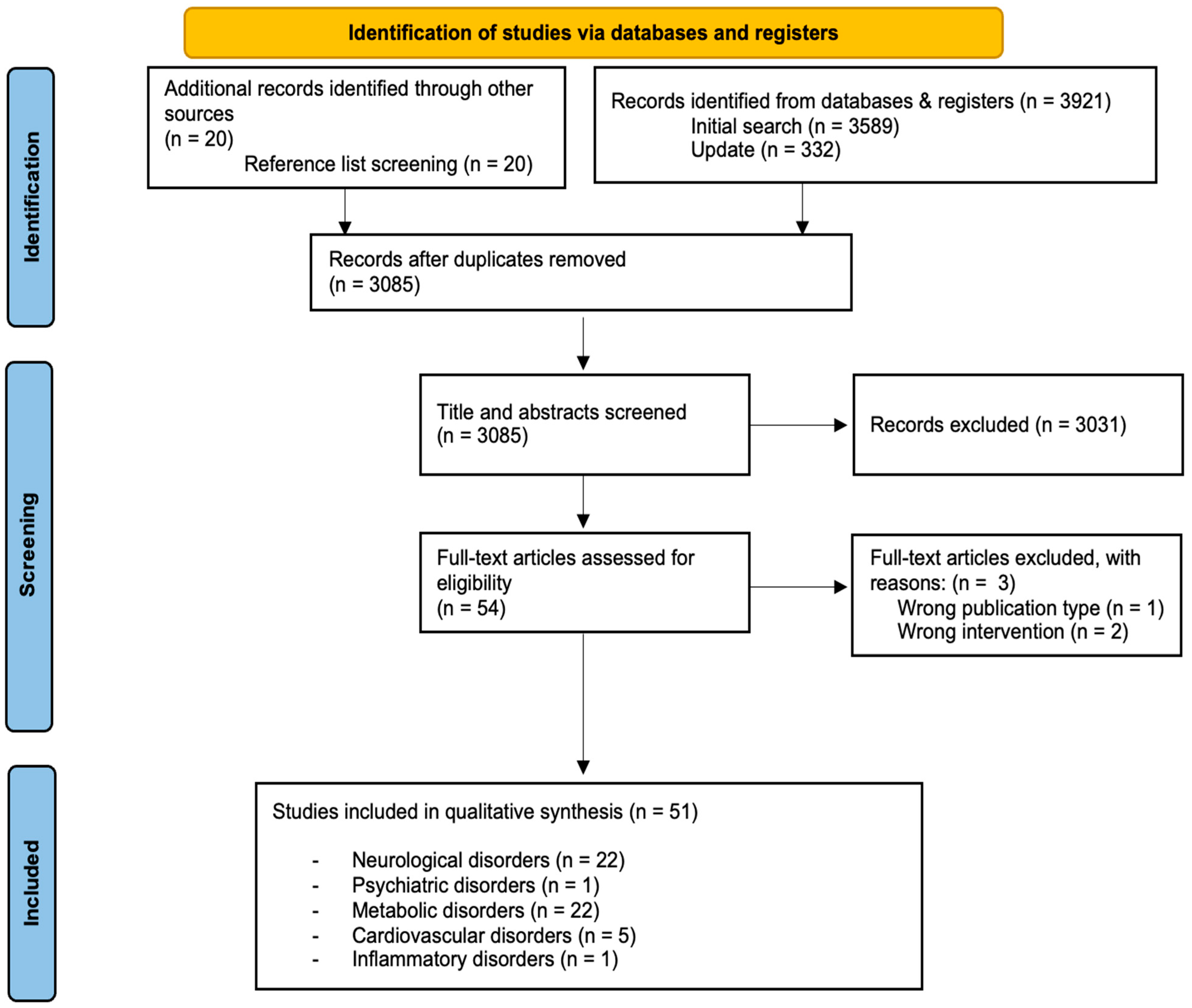
3.1. Study Selection and Characteristics
3.2. Risk of Bias in Studies
3.2.1. Randomized Controlled Trials
3.2.2. Non-Randomized and Open Label Studies
3.2.3. Systematic Patterns
3.3. Results of Individual Studies
3.3.1. Neurological Disorders
3.3.2. Psychiatric Disorders
3.3.3. Metabolic Disorders
3.3.4. Cardiovascular Disorders
3.3.5. Inflammatory Disorders
3.4. Synthesis Across Studies
4. Discussion
4.1. Summary of Main Findings
4.1.1. Neurological Disorders
4.1.2. Psychiatric Disorders
4.1.3. Metabolic Disorders
4.1.4. Cardiovascular Disorders
4.1.5. Inflammatory Disorders
4.2. Strengths and Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A signaling metabolite. Ann. Rev. Nutr. 2017, 37, 51–76. [Google Scholar]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Rad. Biol. Med. 2016, 95, 268–277. [Google Scholar]
- Cahill, G.F.; George, F.C. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of ketogenic diet on health outcomes: An umbrella review of meta-analyses of randomized clinical trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Acharya, C.; Thongprayoon, C.; Hansrivijit, P.; Kanduri, S.R.; Kovvuru, K.; Medaura, J.; Vaitla, P.; Anton, D.F.G.; Mekraksakit, P.; et al. Incidence and Characteristics of Kidney Stones in Patients on Ketogenic Diet: A Systematic Review and Meta-Analysis. Diseases 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.J.; Jiang, W.L.; Sun, H.-B.; Liu, J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J. Clin. Neurol. 2015, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lopes Neri, L.C.; Guglielmetti, M.; Fiorini, S.; Pasca, L.; Zanaboni, M.P.; de Giorgis, V.; Tagliabue, A.; Ferraris, C. Adherence to ketogenic dietary therapies in epilepsy: A systematic review of literature. Nutr. Res. 2024, 126, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Falkenhain, K.; Daraei, A.; Forbes, S.C.; Little, J.P. Effects of Exogenous Ketone Supplementation on Blood Glucose: A Systematic Review and Meta-analysis. Adv. Nutr. 2022, 13, 1697–1714. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Kirk, T.; Evans, R.D.; Clarke, K. Gastrointestinal Effects of Exogenous Ketone Drinks are Infrequent, Mild, and Vary According to Ketone Compound and Dose. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 596–603. [Google Scholar] [CrossRef]
- Harvey, C.J.; Schofield GMWilliden, M.; McQuillan, J.A. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef]
- Pimentel-Suarez, L.I.; Soto-Mota, A. Evaluation of the safety and tolerability of exogenous ketosis induced by orally administered free beta-hydroxybutyrate in healthy adult subjects. BMJ Nutr. Prev. Health 2023, 6, 122–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; King, M.T.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; VanItallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Jones, A.W. Elimination half-life of acetone in humans: Case reports and review of the literature. J. Anal. Toxicol. 2000, 24, 8–10. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.-Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.-J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Laeger, T.; Metges, C.C.; Kuhla, B. Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 2010, 54, 450–455. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Newburgh, L.H.; Marsh, P.L. The use of a high fat diet in the treatment of diabetes mellitus: First paper. Arch. Intern. Med. (Chic.) 1920, 26, 647–662. [Google Scholar] [CrossRef][Green Version]
- Wilder, R.J. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar][Green Version]
- Bohnen, J.L.B.; Albin, R.L.; Bohnen, N.I. Ketogenic interventions in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease: A systematic review and critical appraisal. Front. Neurol. 2023, 14, 1123290. [Google Scholar] [CrossRef]
- Marcotte-Chénard, A.; Tremblay, R.; Falkenhain, K.; Little, J.P.; Riesco, E. Effect of Acute and Chronic Ingestion of Exogenous Ketone Supplements on Blood Pressure: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2024, 21, 408–426. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; the PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. (Lond.) 2009, 6, 31. [Google Scholar] [CrossRef]
- Henderson, S.T.; Morimoto, B.H.; Cummings, J.L.; Farlow, M.R.; Walker, J. A Placebo-Controlled, Parallel-Group, Randomized Clinical Trial of AC-1204 in Mild-to-Moderate Alzheimer’s Disease. J. Alzheimers Dis. 2020, 75, 547–557. [Google Scholar] [CrossRef]
- Ohnuma, T.; Toda, A.; Kimoto, A.; Takebayashi, Y.; Higashiyama, R.; Tagata, Y.; Ito, M.; Ota, T.; Shibata, N.; Arai, H. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: A prospective, open-label pilot study. Clin. Interv. Aging 2016, 11, 29–36. [Google Scholar] [CrossRef]
- Croteau, E.; Castellano, C.A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; Turcotte, É.E.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 64, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Y.; Zhang, X.; Liu, L.; Zhou, B.; Mo, R.; Li, Y.; Li, H.; Li, F.; Tao, Y.; et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4-/-: A double-blind, randomized, placebo-controlled crossover trial. Clin. Nutr. 2020, 39, 2092–2105. [Google Scholar] [CrossRef]
- Chan, S.C.; Esther, G.E.; Yip, H.L.; Sugathan, S.; Chin, P.S. Effect of cold pressed coconut oil on cognition and behavior among patients with Alzheimer’s disease—A pilot intervention study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1432–1435. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef]
- Juby, A.G.; Blackburn, T.E.; Mager, D.R. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: A randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimers Dement. (N. Y.) 2022, 8, e12259. [Google Scholar] [CrossRef]
- Torosyan, N.; Sethanandha, C.; Grill, J.D.; Dilley, M.L.; Lee, J.; Cummings, J.L.; Ossinalde, C.; Silverman, D.H. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp. Gerontol. 2018, 111, 118–121. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef]
- Fernando, M.G.; Silva, R.; Fernando, W.M.A.D.B.; de Silva, H.A.; Wickremasinghe, A.R.; Dissanayake, A.S.; Sohrabi, H.R.; Martins, R.N.; Williams, S.S. Effect of Virgin Coconut Oil Supplementation on Cognition of Individuals with Mild-to-Moderate Alzheimer’s Disease in Sri Lanka (VCO-AD Study): A Randomized Placebo-Controlled Trial. J. Alzheimer’s Dis. 2023, 96, 1195–1206. [Google Scholar] [CrossRef]
- Rebello, C.J.; Keller, J.N.; Liu, A.G.; Johnson, W.D.; Greenway, F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015, 3, 123–125. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; St-Pierre, V.; Myette-Côté, É.; Langlois, F.; Roy, M.; Morin, M.; Bocti, C.; Fulop, T.; Godin, J.; et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef]
- Fortier, M.; Castellano, C.A.; Croteau, E.; Langlois, F.; Bocti, C.; St-Pierre, V.; Vandenberghe, C.; Bernier, M.; Roy, M.; Descoteaux, M.; et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019, 15, 625–634. [Google Scholar] [CrossRef]
- Roy, M.; Fortier, M.; Rheault, F.; Edde, M.; Croteau, E.; Castellano, C.; Langlois, F.; St-Pierre, V.; Cuenoud, B.; Bocti, C.; et al. A ketogenic supplement improves white matter energy supply and processing speed in mild cognitive impairment. Alzheimers Dement. (N. Y.) 2021, 7, e12217. [Google Scholar] [CrossRef]
- Myette-Côté, É.; St-Pierre, V.; Beaulieu, S.; Castellano, C.-A.; Fortier, M.; Plourde, M.; Bocti, C.; Fulop, T.; Cunnane, S.C. The effect of a 6-month ketogenic medium-chain triglyceride supplement on plasma cardiometabolic and inflammatory markers in mild cognitive impairment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102236. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Dearlove, D.J.; Lu, M.; Clarke, K.; Dawes, H.; Hu, M.T. A Ketone Ester Drink Enhances Endurance Exercise Performance in Parkinson’s Disease. Front. Neurosci. 2020, 14, 584130. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; Ortí, J.E.d.l.R. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Sancho-Cantus, D.; Benlloch, M.; Ceron, J.J.; Peres Rubio, C.; García-Pardo, M.P.; López-Rodríguez, M.M.; de la Rubia Ortí, J.E. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life 2021, 11, 353. [Google Scholar] [CrossRef]
- Putananickal, N.; Gross, E.C.; Orsini, A.L.; Schmidt, S.; Hafner, P.; Gocheva, V.; Nagy, S.; Henzi, B.C.; Rubino, D.; Vogt, D.R.; et al. Efficacy and safety of exogenous beta-hydroxybutyrate for preventive treatment in episodic migraine: A single-centred, randomised, placebo-controlled, double-blind crossover trial. Cephalalgia 2022, 42, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.; Patel, V.; Tideman, S.; Frech, R.; Frigerio, R.; Narayanan, J. Efficacy of supplemental MCT oil on seizure reduction of adult drug-resistant epilepsy—A single-center open-label pilot study. Nutr. Neurosci. 2023, 26, 535–539. [Google Scholar] [CrossRef]
- Youssef, N.A.; Holland-Winkler, A.M.; Phung, P.; Waller, J.L.; Ponkshe, S. A randomized, double-blind, clinical pilot trial of adjunct ketone supplement compared to placebo for treating posttraumatic stress disorder. Ann. Clin. Psychiatry 2022, 34, 240–244. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Cho, J.; Alarcon Ramos, G.C.; Ko, J.; Cameron-Smith, D.; Petrov, M.S. Acute Nutritional Ketosis and Its Implications for Plasma Glucose and Glucoregulatory Peptides in Adults with Prediabetes: A Crossover Placebo-Controlled Randomized Trial. J. Nutr. 2021, 151, 921–929. [Google Scholar] [CrossRef]
- Bharmal, S.H.; Alarcon Ramos, G.C.; Ko, J.; Petrov, M.S. Abdominal fat distribution modulates the metabolic effects of exogenous ketones in individuals with new-onset prediabetes after acute pancreatitis: Results from a randomized placebo-controlled trial. Clin. Nutr. ESPEN 2021, 43, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kimita, W.; Bharmal, S.H.; Ko, J.; Cho, J.; Petrov, M.S. Effect of β-hydroxybutyrate monoester on markers of iron metabolism in new-onset prediabetes: Findings from a randomised placebo-controlled trial. Food Funct. 2021, 12, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of acute ketosis on lipid profile in prediabetes: Findings from a cross-over randomized controlled trial. Cardiovasc. Diabetol. 2022, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of d-β-Hydroxybutyrate-(R)-1,3 Butanediol on Appetite Regulation in People with Prediabetes. Mol. Nutr. Food Res. 2023, 67, e2200615. [Google Scholar] [CrossRef]
- Charles, S.; Liu, Y.; Kimita, W.; Ko, J.; Bharmal, S.H.; Petrov, M.S. Effect of D-β-hydroxybutyrate-(R)-1,3 butanediol on plasma levels of asprosin and leptin: Results from a randomised controlled trial. Food Funct. 2023, 14, 759–768. [Google Scholar] [CrossRef]
- Charles, S.; Liu, Y.; Bharmal, S.H.; Kimita, W.; Petrov, M.S. Effect of Acute Nutritional Ketosis on Circulating Levels of Growth Differentiation Factor 15: Findings from a Cross-Over Randomised Controlled Trial. Biomolecules 2024, 14, 665. [Google Scholar] [CrossRef]
- Nakagata, T.; Tamura, Y.; Kaga, H.; Sato, M.; Yamasaki, N.; Someya, Y.; Kadowaki, S.; Sugimoto, D.; Satoh, H.; Kawamori, R.; et al. Ingestion of an exogenous ketone monoester improves the glycemic response during oral glucose tolerance test in individuals with impaired glucose tolerance: A cross-over randomized trial. J. Diabetes Investig. 2021, 12, 756–762. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.; Clarke, K.; Barber, T.M. Exogenous ketosis in patients with type 2 diabetes: Safety, tolerability and effect on glycaemic control. Endocrinol. Diabetes Metab. 2021, 4, e00264. [Google Scholar] [CrossRef]
- Falkenhain, K.; Oliveira, B.F.; Islam, H.; Neudorf, H.; Cen, H.H.; Johnson, J.D.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. The effect of acute and 14-day exogenous ketone supplementation on glycemic control in adults with type 2 diabetes: Two randomized controlled trials. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E61–E72. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Falkenhain, K.; Davy, B.; Davy, K.; Little, J. Acute effect of an exogenous ketone monoester supplement on appetite and food intake in adults with type 2 diabetes. Appl. Physiol. Nutr. Metab. 2024, 49, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.J.; Falkenhain, K.; Whelehan, G.; Neudorf, H.; Abdelrahman, D.R.; Murton, A.J.; Wall, B.T.; Stephens, F.B.; Little, J.P. A ketone monoester drink reduces postprandial blood glucose concentrations in adults with type 2 diabetes: A randomised controlled trial. Diabetologia 2024, 67, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.J.; Nilsson, M.; Ingerslev, J.S.; Olsen, D.A.; Fenger, M.; Svart, M.; Møller, N.; Zander, M.; Miskowiak, K.W.; Rungby, J. Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur. J. Endocrinol. 2020, 182, 233–242. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Caldwell, H.G.; Ainslie, P.N.; Clarke, K.; Little, J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 1491–1501. [Google Scholar] [CrossRef]
- Neudorf, H.; Myette-Côté, É.; P. Little, J. The Impact of Acute Ingestion of a Ketone Monoester Drink on LPS-Stimulated NLRP3 Activation in Humans with Obesity. Nutrients 2020, 12, 854. [Google Scholar] [CrossRef]
- Walsh, J.J.; Caldwell, H.G.; Neudorf, H.; Ainslie, P.N.; Little, J.P. Short-term ketone monoester supplementation improves cerebral blood flow and cognition in obesity: A randomized cross-over trial. J. Physiol. 2021, 599, 4763–4778. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef]
- Kanta, J.M.; Lundsgaard, A.M.; Havelund, J.F.; Armour, S.L.; Bæk, O.; Nguyen, D.N.; Richter, E.A.; Knudsen, J.G.; Kleinert, M.; Færgeman, N.J.; et al. Metabolic effects of medium-chain triacylglycerol consumption are preserved in obesity. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E1–E20. [Google Scholar] [CrossRef]
- Yu, Q.; Wong, K.K.; Lei, O.K.; Armada-Da-Silva, P.A.; Wu, Z.; Nie, J.; Shi, Q.; Kong, Z. Acute ketone monoester supplementation in young adults: Modulating metabolic and neurocognitive functions across body weights. Appl. Physiol. Nutr. Metab. 2025, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, J.C.; Visser, G.; Clarke, K.; Ferdinandusse, S.; de Haan, F.H.; Houtkooper, R.H.; Ijlst, L.; Kok, I.L.; Langeveld, M.; van der Pol, W.L.; et al. Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2020, 43, 787–799. [Google Scholar] [CrossRef]
- Løkken, N.; Storgaard, J.H.; Revsbech, K.L.; Voermans, N.C.; Van Hall, G.; Vissing, J.; Ørngreen, M.C. No effect of oral ketone ester supplementation on exercise capacity in patients with McArdle disease and healthy controls: A randomized placebo-controlled cross-over study. J. Inherit. Metab. Dis. 2022, 45, 502–516. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Santalla, A.; Alejo, L.B.; Bustos, A.; Ozcoidi, L.M.; Castellote-Bellés, L.; Ferrer-Costa, R.; Villarreal-Salazar, M.; Morán, M.; Barranco-Gil, D.; et al. Acute ketone supplementation in the absence of muscle glycogen utilization: Insights from McArdle disease. Clin. Nutr. 2024, 43, 692–700. [Google Scholar] [CrossRef]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment with the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Berg-Hansen, K.; Christensen, K.H.; Ladefoged, B.T.; Poulsen, S.H.; Andersen, M.J.; Borlaug, B.A.; Nielsen, R.; Møller, N.; Wiggers, H. Randomized Crossover Trial of 2-Week Ketone Ester Treatment in Patients with Type 2 Diabetes and Heart Failure with Preserved Ejection Fraction. Circulation 2024, 150, 1570–1583. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Christensen, K.H.; Gopalasingam, N.; Nielsen, R.; Eiskjær, H.; Møller, N.; Birkelund, T.; Christensen, S.; Wiggers, H. Beneficial Effects of Ketone Ester in Patients with Cardiogenic Shock: A Randomized, Controlled, Double-Blind Trial. JACC Heart Fail. 2023, 11, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Christensen, K.H.; Gopalasingam, N.; Berg-Hansen, K.; Seefeldt, J.; Homilius, C.; Boedtkjer, E.; Andersen, M.J.; Wiggers, H.; Møller, N.; et al. Hemodynamic Effects of Ketone Bodies in Patients with Pulmonary Hypertension. J. Am. Heart Assoc. 2023, 12, e028232. [Google Scholar] [CrossRef]
- Shahtaghi, N.R.; Bigdelitabar, S.; Thakur, S.; Kaur, M.; Singh, H.; Saini, M.; Singh, M.; Singh, K.; Jain, S.K. Oral beta-hydroxybutyrate alleviates COVID-19 related acute respiratory distress syndrome: A randomized, single-blind, placebo-controlled trial. Res. Soc. Adm. Pharm. 2024, 20, 760–767. [Google Scholar] [CrossRef]
- McCall, A.L. The impact of diabetes on the CNS. Diabetes 1992, 41, 557–570. [Google Scholar] [CrossRef]
- Hoyer, S. Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol. Chem. Neuropathol. 1992, 16, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004, 292, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.J.; Santana, I.; Brás, J.M.; Santiago, B.; Paiva, A.; Oliveira, C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener. Dis. 2007, 4, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Fenoglio, C.; Lovati, C.; Venturelli, E.; Guidi, I.; Corrà, B.; Scalabrini, D.; Clerici, F.; Mariani, C.; Bresolin, N.; et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1763–1768. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonça, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef]
- Johnston, G.A. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef]
- Pflanz, N.C.; Daszkowski, A.W.; James, K.A.; Mihic, S.J. Ketone body modulation of ligand-gated ion channels. Neuropharmacology 2019, 148, 21–30. [Google Scholar] [CrossRef]
- Hartman, A.L.; Gasior, M.; Vining, E.P.; Rogawski, M.A. The neuropharmacology of the ketogenic diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.D.; Chen, P.E.; Walker, M.C.; Williams, R.S.B. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139 Pt 2, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef]
- Balasse, E.O.; Ooms, H.A.; Lambilliotte, J.P. Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm. Metab. Res. 1970, 2, 371–372. [Google Scholar] [CrossRef]
- Svart, M.; Rittig, N.; Pedersen, S.B.; Jessen, N.; Møller, N. Oral 3-hydroxybutyrate ingestion decreases endogenous glucose production, lipolysis, and hormone-sensitive lipase phosphorylation in adipose tissue in men: A human randomized, controlled, crossover trial. Diabet. Med. 2021, 38, e14385. [Google Scholar] [CrossRef]
- Yamanashi, T.; Iwata, M.; Kamiya, N.; Tsunetomi, K.; Kajitani, N.; Wada, N.; Iitsuka, T.; Yamauchi, T.; Miura, A.; Pu, S.; et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017, 7, 7677. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Alosco, M.L.; Gunstad, J. The negative effects of obesity and poor glycemic control on cognitive function: A proposed model for possible mechanisms. Curr. Diabetes Rep. 2014, 14, 495. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Seifert, T.; Secher, N.H.; Grøndal, T.; van Hall, G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015, 100, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Trevisan, R.; Velussi, M.; Cernigoi, A.; DE Riva, C.; Bressan, M.; Doria, A.; Pauletto, N.; Angeli, P.; DE Doná, C.; et al. Glomerular filtration rate is increased in man by the infusion of both D,L-3-hydroxybutyric acid and sodium D,L-3-hydroxybutyrate. J. Clin. Endocrinol. Metab. 1987, 65, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gadegbeku, C.A.; Dhandayuthapani, A.; Shrayyef, M.Z.; Egan, B.M. Hemodynamic effects of nicotinic acid infusion in normotensive and hypertensive subjects. Am. J. Hypertens. 2003, 16, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Izawa, H.; Sobue, T.; Ishihara, H.; Somura, F.; Nishizawa, T.; Nagata, K.; Iwase, M.; Yokota, M. Prognostic value of mechanical efficiency in ambulatory patients with idiopathic dilated cardiomyopathy in sinus rhythm. J. Am. Coll. Cardiol. 2002, 39, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Galiè, N.; Ussia, G.; Passarelli, P.; Parlangeli, R.; Branzi, A.; Magnani, B. Role of pharmacologic tests in the treatment of primary pulmonary hypertension. Am. J. Cardiol. 1995, 75, 55A–62A. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D.; Orfanos, S.E.; Giovinazzo, M.; Schlesinger, R.D.; Naeije, R.; Fox, B.D.; Abualsaud, A.O.; Blenkhorn, F.; Rudski, L.G.; Catravas, J.D. Pulmonary capillary surface area in supine exercising humans: Demonstration of vascular recruitment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L361–L368. [Google Scholar] [CrossRef]
- Kesika, P.; Thangaleela, S.; Sisubalan, N.; Radha, A.; Sivamaruthi, B.S.; Chaiyasut, C. The Role of the Nuclear Factor-Kappa B (NF-κB) Pathway in SARS-CoV-2 Infection. Pathogens 2024, 13, 164. [Google Scholar] [CrossRef]
| Inclusion Criteria | Patient (P) | Adults with Disease |
| Intervention (I) | Induction of exogenous ketosis via:
| |
| Comparison (C) |
| |
| Outcome (O) | Assessment of disease-related outcomes, including clinical, biological, or radiological parameters, or adverse events | |
| Study design (S) |
| |
| Exclusion Criteria | Patient (P) |
|
| Intervention (I) | Induction of endogenous ketosis via:
| |
| Comparison (C) | / | |
| Outcome (O) | / | |
| Study design (S) | Animal studies, in vitro studies, observational studies, reviews, opinion articles, guidelines, letters, editorials, comments, case reports/series, abstracts, dissertations, theses, and articles published in any other language than English |
| Type of Disorder | Disease | N° Studies Included with References |
|---|---|---|
| Neurological | MCI | 5 [44,45,46,47,48] |
| AD | 11 [33,34,35,36,37,38,39,40,41,42,43] | |
| MCI and AD | 1 [32] | |
| PD | 1 [49] | |
| Multiple sclerosis | 2 [50,51] | |
| Episodic migraine | 1 [52] | |
| Drug-resistant epilepsy | 1 [53] | |
| Psychiatric | PTSD | 1 [54] |
| Metabolic | Pre-diabetes | 8 [55,56,57,58,59,60,61,62] |
| Type II diabetes mellitus | 5 [63,64,65,66,67] | |
| Obesity | 6 [68,69,70,71,72,73] | |
| VLCADD | 1 [74] | |
| McArdle disease | 2 [75,76] | |
| Cardiovascular | HFrEF | 2 [77,78] |
| HFpEF | 1 [79] | |
| Cardiogenic shock | 1 [80] | |
| PH | 1 [81] | |
| Inflammatory | COVID-19-related ARDS | 1 [82] |
| Condition | Reference (Year) | Study Design | Participants (N) | Type of Exogenous Ketone | Dose of Exogenous Ketone | Blood βHB Level | Primary Outcomes | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| MCI | Myette-Côté et al. (2021) [48] | Double-blinded RCT (vs. placebo) | 39 (intervention: 19; placebo: 20) | MCT (60% caprylic + 40% capric acid) | 30 g/day (250 mL) for 6 months | Significant increase in total ketones post-intervention (mean + 0.416 mmol/L) in MCT group, 2 h after last administration of MCT | Cardiometabolic markers and peripheral inflammation |
|
|
| Fortier et al. (2021) [45] | Double-blinded RCT (vs. placebo) | 122 | MCT (60% caprylic + 40% capric acid) | 30 g/day (250 mL) for 6 months | Mean βHB increased to 0.4 (+/− 0.3) mmol/L 30 min after administration of MCT | Cognition Plasma ketone Metabolic profile |
|
| |
| Fortier et al. (2019) [46] | Double-blinded RCT (vs. placebo) | 52 | MCT (60% caprylic + 40% capric acid) | 30 g/day (250 mL) for 6 months | Mean βHB significantly increased to 0.543 (+/− 0.32) mmol/L | Metabolic rate of AcAc and glucose in the brain measured by PET with the 11C-AcAc and 18F-FDG tracers |
| Inadequately powered to assess the effect of Apoε4 status on cognitive outcomes | |
| Roy et al. (2021) [47] | Double-blinded RCT (vs. placebo) | 33 (intervention: 17; placebo: 16) | MCT (60% caprylic + 40% capric acid) | 30 g/day (250 mL) for 6 months | Tested +2 h post-prandial. Mean βHB increased significantly to 0.572 (+/− 0.32) mmol/L | Ketone metabolism by WM fascicles assessed by PET and diffusion imaging |
|
| |
| Rebello et al. (2015) [44] | Double-blinded RCT (vs. placebo) | 6 (intervention: 3; placebo: 3) | MCT | 56 g/day for 6 months | Tested +90 min post-prandial. βHB increased in Apoε4-, but was progressively less with measures repetition (0.15 mmol/L at week 24). βHB showed consistent increase in Apoε4+ status (0.54 mmol/L at week 24) | Blood βHB levels Cognition (ADAS-Cog/Trail Making Test/DSST) |
|
| |
| AD | Henderson et al. (2009) [33] | Double-blinded RCT (vs. placebo) | 152 (intervention: 86; placebo: 66) | MCT (AC-1202, glycerin + caprylic acid) | 20 g/day for 90 days | Tested +2 h post-prandial. Post-dose βHB levels significantly elevated to mean of 0.36–0.39 mmol/L for MCT group | Cognition (ADAS-Cog/ADCS-CGIC) |
|
|
| Henderson et al. (2020) [34] | Double-blinded RCT (vs. placebo) | 413 (intervention: 208; placebo: 205) | MCT (AC-1204, 50% caprylic acid) | 40 g/day for 6 months | Tested +1 h post prandial. Post-dose βHB levels significantly elevated to mean of 0.25 mmol/L for MCT group | ADAS-Cog11 score in Apoε4 non carriers |
|
| |
| Croteau et al. (2018) [36] | Cross-over unrandomized controlled trial (C8C10 or C8) | 15 | MCT (60% caprylic + 40% capric acid or tricaprylin) | 30 g/day (250 mL), each MCT formulation for 1 month | Tested +2 h post-prandial. Mean βHB significantly increased to 0.57 (+/− 0.27) mmol/L post-C8 -mean βHB significantly increased to 0.46 (+/− 0.19) mmol/L post-C8C10 | Brain ketone and glucose uptake by PET |
|
| |
| Ohnuma et al. (2016) [35] | Uncontrolled open-label trial | 24 | MCT (Axona, caprylic acid) | 20 g/day for 3 months | Timing of testing post-intervention undisclosed. Mean βHB: 0.25 (+/− 0.20) mmol/L at M1 (then stable at M2 and M3) |
|
| Low levels of blood βHB achieved | |
| Xu et al. (2020) [37] | Cross-over double-blinded RCT (vs. placebo) | 53 | MCT (caprylic + capric acid) | 17.3 g/day for 30 days | Timing of testing post-intervention undisclosed. Very weak mean βHB: 0.09 (+/− 0.07) mmol/L at M1 | Cognition (ADAS-Cog-C) |
|
| |
| Chan et al. (2017) [38] | Double-blinded RCT (vs. placebo) | 40 (intervention: 20; placebo: 20) | MCT (cold pressed coconut oil) | 30 mL/day during 2 weeks then 60 mL/day during 22 weeks | Not measured |
|
|
| |
| De la Rubia Orti et al. (2018) [39] | Double-blinded RCT (vs. placebo) | 44 (intervention: 22; placebo: 22) | MCT (coconut oil) | 40 mL/day for 21 days | Not measured | Temporal orientation, visuospatial abilities, semantic and episodic memory |
|
| |
| Juby et al. (2022) [40] | Cross-over double-blinded RCT (vs. placebo) | 20 | MCT | 42 g/day or maximum tolerated for 4 months, then open label for 7 months | Tested in fasting condition. Baseline βHB: 0.19 mmol/L, then at study completion 0.22 mmol/L | Cognition (Cognigram tests/MMSE/MOCA) |
|
| |
| Torosyan et al. (2018) [41] | Double-blinded RCT (vs. placebo) | 16 (intervention: 14; placebo: 2) | MCT (caprylidene) | 40 g/day for 45 days | Not measured | Evaluation of acute and long-term effects of caprylidene on regional CBF |
|
| |
| Ota et al. (2019) [42] | Study 1: Cross-over double-blinded RCT (vs. placebo) Study 2: Open-label study | Study 1 and 2: 20 | MCT (Ketonformula®, 50 g of this formula contains 20 g MCT) | Study 1: single dose of 50 g Ketonformula Study 2: 50 g/day Ketonformula during 12 weeks | Study 1: Tested before consumption and then 2 h later with significant increase to 0.47 (+/− 0.29) mmol/L Study 2: Tested before consumption in weeks 4–8–12 with no significant increase | Effect of single (Study 1) and chronic (Study 2) administration of MCT on cognitive function | Study 1:
Study 2:
|
| |
| Fernando et al. (2023) [43] | Double-blinded RCT (vs. placebo) | 120 (intervention: 60; placebo: 60) | MCT (virgin coconut-oil) | 30 mL/day for 24 weeks | Not measured |
|
|
| |
| MCI and AD | Reger et al. (2004) [32] | Cross-over double-blinded RCT (vs. placebo) | 20 | MCT | 40 mL single dose | Tested at +90 min and +120 min post-prandial. Mean βHB increased to 0.54 (+/− 0.32) mmol/L at + 90 min and remained stable in Apoε4- participants. Mean βHB increased to 0.43 (+/− 0.16) mmol/L at + 90 min and again to 0.68 (+/− 0.36) mmol/L at +120 min in Apoε4+ participants | Neuropsychological testing (ADAS-Cog/Stroop Color Word Test/paragraph recall) |
|
|
| PD | Norwitz et al. (2020) [49] | Cross-over single-blinded RCT (vs. placebo) | 15 | Ketone ester (DeltaG) | 25 mL single dose | Mean βHB increased significantly to 3.5 (+/− 0.3) mmol/L within 30 min of ketone ester consumption | Length of time participants could sustain an 80 revolutions per minute (rpm) cycling cadence |
|
|
| Multiple Sclerosis | Platero et al. (2020) [50] | Double-blinded RCT (vs. placebo) | 51 (intervention: 27; placebo: 24) | MCT (extra-virgin coconut oil) | 60 mL/day for 4 months | Tested after an overnight fast. Median βHB: 0.05 (range 0.33) mmol/L |
|
| Impossible to distinguish the individual impact of each of the treatment components (EGCG, coconut oil, mediterranean diet) on the different outcomes evaluated |
| Platero et al. (2021) [51] | Double-blinded RCT (vs. placebo) | 51 (intervention: 27; placebo: 24) | MCT (extra-virgin coconut oil) | 60 mL/day for 4 months | Tested after an overnight fast. Median βHB: 0.05 (range 0.33) mmol/L | Cortisol activity related to fat loss and depression |
| No evaluation of variations in ACTH | |
| Episodic migraine | Putananickal et al. (2022) [52] | Cross-over double-blinded RCT (vs. placebo) | 41 | Ketone salt (Ergomax) (Ca-βHB (9 g)/Mg-βHB (9 g)) | 18 g/day for 3 months | Tested +40 min post-prandial. Median βHB: 0.40 (interquartile-range [0.30, 0.50]) | Number of migraine days in the last four weeks of treatment (adjusted for baseline) |
|
|
| Drug- resistant epilepsy | Rasmussen et al. (2023) [53] | Uncontrolled open-label trial | 9 | MCT (50% caprylic + 30% capric acid) | 112 g/day (target dose) for 3 months | Not measured. Patients were screened for urinary ketones (+ in 5/6 individuals at some point during the trial) | Reduction in number of seizures |
|
|
| PTSD | Youssef et al. (2022) [54] | Double-blinded RCT (vs. placebo) | 21 (intervention: 11; Placebo: 10) | Ketone salt | 14 g/day for 6 weeks | Not measured | PTSD symptoms (PCL-5) |
|
|
| Condition | Reference (Year) | Study Design | Participants (n) | Type of Exogenous Ketone | Dose of Exogenous Ketone | Blood βHB Level | Primary Outcomes | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Prediabetes | Bharmal et al. (2021) [55] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Blood glucose |
|
|
| Bharmal et al. (2021) [56] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Modulation of intra-abdominal fat distribution on the effect of exogenous ketones on glucoregulatory peptides |
|
| |
| Kimita et al. (2021) [57] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Plasma levels of markers of iron metabolism | No significant effect on circulating levels of markers of iron metabolism |
| |
| Liu et al. (2022) [58] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Plasma lipid profile |
|
| |
| Liu et al. (2023) [59] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Objective and subjective parameters of appetite regulation (acylated ghrelin, peptide YY, and hunger) |
|
| |
| Charles et al. (2023) [60] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | Plasma levels of asprosin and leptin | No variation in plasma levels of asprosin and leptin, even after stratification by abdominal fat phenotypes | Long-term effects of exogenous ketosis on plasma levels of asprosin and leptine not evaluated | |
| Charles et al. (2024) [61] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester (DeltaG) | 395 mg/kg single dose | Tested +30 min post-prandial. Mean βHB increased significantly to 3.47 (+/− 0.22) mmol/L | GDF-15 levels |
|
| |
| Nakagata et al. (2021) [62] | Cross-over double-blinded RCT (vs. placebo) | 9 | Ketone ester | 482 mg/kg single dose | Tested +90 min post-prandial. Significant increase in mean βHB: 2.4 (+/− 0.7) mmol/L | Blood glucose levels during the 75 g OGTT |
|
| |
| Type II Diabetes Mellitus | Jensen et al. (2020) [67] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone salt (Na-DL-3-hydroxybutyrate), intravenous | 0.22 g/kg/h during approximately 165 min | Significant increase in mean βHB levels throughout neurocognitive assessment (time: +120 to 165 min): 2.4 (+/− 0.6) mmol/L | Cognitive performance (global cognitive composite score, estimated by the average of 4 domains: verbal memory, working memory and executive functions, psychomotor speed, and sustained attention | No significant difference in global cognitive composite score, even if performance of WAIS-LNS (working memory) improved significantly with ketone infusion |
|
| Monteyne et al. (2024) [66] | Cross-over double-blinded RCT (vs. placebo) | 10 | Ketone ester | 500 mg/kg single dose | Tested +60 min post-prandial. Significant increase with peak βHB: 4.3 (+/− 1.2) mmol/L | Reduction in post-prandial blood glucose |
|
| |
| Oliveira et al. (2024) [65] | Cross-over double-blinded RCT (vs. placebo) | 18 | Ketone ester | 0.3 g/kg single dose | Tested +60 min post-prandial. Significant increase with peak βHB: 1.8 (+/− 0.6) mmol/L | Determine whether acute ingestion of ketone ester supplement influenced hunger, fullness and food intake |
|
| |
| Falkenhain et al. (2024) [64] | 2 cross-over double-blinded RCTs (vs. placebo) | Study 1: 18 Study 2: 15 | Ketone ester (DeltaG for study 1, KetoneAid for study 2) | 0.3 g/kg single dose for Study 1, 45 g/day (90 mL) for 14 days for Study 2 | Study 1: Tested at +60 min post-prandial, significant increase in mean βHB: 1.8 (+/− 0.6) mmol/L; Study 2: Tested at +30 min post-prandial, significant increase in mean βHB: 1.8 (+/− 0.7) mmol/L | Study 1: Plasma glucose Study 2: Serum fructosamine | Study 1:
| Study 1: Discontinuation of glucose-lowering medication only 1 day before each study visit Study 2: Continuation of glucose-lowering medications possibly inducing interference with study outcomes | |
| Soto-Mota et al. (2021) [63] | Uncontrolled open-label trial | 23 | Ketone ester | 75 g/day (75 mL) for 4 weeks | Tested +30 min post-prandial. Significant increase in βHB with range from 3.1 (+/− 0.5) to 3.8 (+/− 0.7) mmol/L | Safety, tolerability and effects on glycemic control |
|
| |
| Obesity | Myette-Côté et al. (2019) [68] | Cross-over double-blinded RCT (vs. placebo) | 15 | Ketone ester (DeltaG) | 482 mg/kg single dose | Peak βHB: 3.4 mmol/L. Tested +90 min post-prandial | Capacity to lower blood glucose concentration |
|
|
| Neudorf et al. (2020) [69] | Cross-over double-blinded RCT (vs. placebo) | 15 | Ketone ester (DeltaG) | 482 mg/kg single dose | Tested +60 min post-prandial. Mean βHB increased significantly to 2.96 (+/− 0.91) mmol/L | Effect on NLRP3 activation |
|
| |
| Kanta et al. (2025) [72] | Study 1: Cross-over single-blinded RCT (vs. LCT) Study 2: Cross-over double-blinded RCT (vs. LCT) but included only healthy participants (excluded from analysis) | 16 (with 8 healthy controls) | MCT (caprylic acid) | 35 g single dose during test days (pretest and posttest), then increasing doses from 10 g 2/day to 30 g 2/day during 7 days | Significant increase with Peak βHB: 0.7 (+/− 0.1) mmol/L. Tested +90 min post-prandial. | Post-prandial energy metabolism |
|
| |
| Yu et al. (2025) [73] | Cross-over double-blinded RCT (vs. placebo) | 40 (with 20 healthy controls) | Ketone ester (HVMN) | 395 mg/kg single dose | Significant increase with βHB level raised by 0.92 mmol/L compared to placebo group | Metabolic and neurocognitive indicators (i.e., PFC connectomes (causal density) and cognitive interference) | Linear mixed models analysis:
|
| |
| Walsh et al. (2021) [71] | Cross-over double-blinded RCT (vs. placebo) | 15 | Ketone ester (DeltaG) | 36 g/day for 2 weeks | Tested +15 min post-prandial. Significant increase in mean βHB to 1.8 (+/− 1.3) mmol/L | Post-prandial glycemia, vascular function and inflammatory markers |
|
| |
| Walsh et al. (2021) [70] | Cross-over double-blinded RCT (vs. placebo) | 15 | Ketone ester (DeltaG) | 36 g/day for 2 weeks | Tested +15 min post-prandial. Significant increase in mean βHB to 1.8 (+/−1.3) mmol/L | CBF, BDNF, and cognitive function assessed by DSST/Stroop task/TST |
|
| |
| VLCADD | Bleeker et al. (2020) [74] | Cross-over double-blinded RCT (vs. placebo) | 5 | Ketone ester | 395 mg/kg single dose | Tested +30 min post-prandial. Significant increase in βHB to 2.0 (+/− 0.2) mmol/L | Quadriceps phosphocreatine, inorganic phosphate and pH dynamics during exercise and recovery assayed by in vivo 31P-MR |
|
|
| McArdle Disease | Løkken et al. (2022) [75] | Cross-over double-blinded RCT (vs. placebo) | 12 | Ketone ester (HVMN) | 395 mg/kg single dose | Tested +25 min post-prandial (immediately before exercise). Significant increase in mean βHB to 3.3 (+/− 1.3) mmol/L | Exercise capacity as indicated by heart rate response to exercise |
|
|
| Valenzuela et al. (2024) [76] | Cross-over double-blinded RCT (vs. placebo, vs. CHO drink) | 15 (with 7 healthy controls) | Ketone ester (KetoneAid) | 30 g single dose | Test +30 min post-prandial (immediately before exercise). Significant increase in mean to 3.7 (+/− 0.9) mmol/L | Exercise capacity (or performance) assessed by a constant-load then maximal ramp test |
Constant load test:
|
|
| Condition | Reference (Year) | Study Design | Participants (n) | Type of Exogenous Ketone | Dose of Exogenous Ketone | Blood βHB Level | Primary Outcomes | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Chronic HFrEF | Monzo et al. (2021) [77] | Uncontrolled open-label trial + observational study (excluded from analysis). 11 HFrEF participants and 6 non-HFrEF subjects | 17 | Ketone ester (HVMN) | 25 g single dose | Tested +80 min post-prandial. Mean βHB significantly increased by 12.9-fold (concentration not reported) | Myocardial substrate utilization |
|
|
| Nielsen et al. (2019) [78] | 3 cross-over single-blinded RCTs (vs. placebo) and 1 dose response study. Study 4 is performed in age-matched volunteers. Study 2 is a dose-response study | Study 1: 16 Study 2: 8 Study 3: 10 Study 4: 10 | Ketone salt (Na-βHB, racemic mixture 50/50 D/L), intravenous | Study 1: 0.18 g/kg/h for 3 h Study 2: 0.045 g/kg/h for 2 h then 0.09 g/kg/h for 2 h Study 3 and 4: 0.18 g/kg/h for 3 h | Study 1: Tested H + 3 in the interventional group, significant increase in mean βHB to 3.3 (+/− 0.4) mmol/L Study 2: Tested H + 2 for each intervention, mean βHB: 0.7 (+/− 0.1) mmol/L at an infusion rate of 0.045 g/kg/h and mean βHB: 1.6 (+/− 0.3) mmol/L at an infusion rate of 0.09 g/kg/h Study 3 and 4: Tested H + 3, mean βHB 3.4 (+/− 0.6) mmol/L in the interventional group with no difference between HFrEF (Study 3) and healthy individuals (Study 4) | Study 1 and 2: CO measured by thermodilution Study 3 and 4: MEE, MVO2 and MBF assessed by 11C-acetate PET | Study 1:
Study 3 and 4:
|
| |
| Chronic HFpEF | Gopalasingam et al. (2024) [79] | Cross-over double-blinded RCT (vs. placebo) | 28 (at randomization) | Ketone ester (KetoneAid) | 25 g 4/day for 2 weeks | Significant increase in mean βHB of the 4-h observation period at rest to approximately 1 mmol/L | CO measured by thermodilution at rest and cardiopulmonary exercise | During the 4 h rest period following ketone ester intake:
|
|
| Cardiogenic Shock | Berg-Hansen et al. (2023) [80] | Cross-over double-blinded RCT (vs. placebo) | 13 | Ketone ester | 500 mg/kg single dose | Significant rise in mean βHB to 2.9 (+/− 0.5) mmol/L during the 3-h treatment period | CO measured by thermodilution and expressed as AUC |
|
|
| PH | Nielsen et al. (2023) [81] | Cross-over double-blinded RCT (vs. placebo) | 20 | Ketone salt (Na-βHB, racemic mixture 50/50 D/L), intravenous | 0.18 g/kg/h for 2 h | Tested +30 min post-intervention. Significant rise in mean βHB to 3.4 mmol/L | CO measured by thermodilution |
|
|
| Condition | Reference (Year) | Study Design | Participants (n) | Type of Exogenous Ketone | Dose of Exogenous Ketone | Blood βHB Level | Primary Outcomes | Main Findings | Main Limitations |
|---|---|---|---|---|---|---|---|---|---|
| COVID-19-related ARDS | Shahtaghi el al. (2024) [82] | Single-blinded RCT (vs. placebo) | 75 (intervention: 38; placebo: 37) | βHB formulation (unspecified) | 25 g 2/day during 5 days | Tested +120 min post-prandial. Significant increase in mean βHB to 5.26 (+/− 0.2) mmol/L | Pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-18) and anti-inflammatory cytokines (IL-10) from baseline to day 5 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohib, O.; Bomans, S.; Jimenez Garcia, B.; Leemans, L.; Ligneel, C.; De Waele, E.; Beckwée, D.; Janssens, P. Clinical Benefits of Exogenous Ketosis in Adults with Disease: A Systematic Review. Nutrients 2025, 17, 3125. https://doi.org/10.3390/nu17193125
Mohib O, Bomans S, Jimenez Garcia B, Leemans L, Ligneel C, De Waele E, Beckwée D, Janssens P. Clinical Benefits of Exogenous Ketosis in Adults with Disease: A Systematic Review. Nutrients. 2025; 17(19):3125. https://doi.org/10.3390/nu17193125
Chicago/Turabian StyleMohib, Othmane, Sarah Bomans, Berenice Jimenez Garcia, Lynn Leemans, Claudine Ligneel, Elisabeth De Waele, David Beckwée, and Peter Janssens. 2025. "Clinical Benefits of Exogenous Ketosis in Adults with Disease: A Systematic Review" Nutrients 17, no. 19: 3125. https://doi.org/10.3390/nu17193125
APA StyleMohib, O., Bomans, S., Jimenez Garcia, B., Leemans, L., Ligneel, C., De Waele, E., Beckwée, D., & Janssens, P. (2025). Clinical Benefits of Exogenous Ketosis in Adults with Disease: A Systematic Review. Nutrients, 17(19), 3125. https://doi.org/10.3390/nu17193125





