Abstract
(1) Background: Metabolic-dysfunction-associated steatotic liver disease (MASLD) is now the most prevalent chronic liver disease worldwide, posing a growing public health concern. While dietary improvements are key to prevention, the impact of different vegetable types remains unclear. This study focuses on the association between vegetable consumption and the risk of MASLD in a cohort of Southern Italy. (2) Methods: This research involved 1297 participants from the NUTRIHEP study, examining overall vegetable intake and classifying them into color subgroups to determine optimal quantity and variety for risk reduction. (3) Results: Daily consumption of approximately 325 g (two servings) of total vegetables significantly reduces the risk of MASLD (OR: 0.521; 95% CI: 0.317; 0.858). Among the subgroups, green vegetables were most protective at 35 g/day, while red and orange vegetables offered protection at 130 g/day. A higher intake of the other vegetable category, specifically onions, was associated with a reduced probability of MASLD (OR = 0.995; 95%CI: 0.989; 0.999). (4) Conclusions: These findings suggest a threshold effect, where moderate but regular consumption of specific vegetables offers maximal protection. Consuming excessive amounts may not enhance this benefit within this cohort. Cultural and regional dietary patterns should be considered when designing targeted nutritional interventions.
1. Introduction
Metabolic-dysfunction-associated steatotic liver disease (MASLD), previously named non-alcoholic fatty liver disease (NAFLD), is defined as the presence of excess triglyceride storage in the liver in the existence of one or more cardiometabolic risk factor such as obesity, type 2 diabetes, hypertension, or dyslipidemia and the absence of harmful alcohol intake [1].
MASLD is currently the most common chronic liver disease, with an estimated prevalence of 38% of the global adult population and around 13% of children and adolescents, becoming a major threat to public health due to its very high prevalence and related morbidity and mortality [2].
The pathogenesis of MASLD is complex and influenced by various factors, including metabolic, genetic, environmental, and lifestyle elements [3]. A key factor in this condition is dysregulated lipid metabolism, which leads to excessive lipid accumulation in the liver and hepatocyte damage. Furthermore, insulin resistance and hyperinsulinemia seem to have a huge relevance in amplifying lipogenesis and reducing lipid oxidation, which promote steatosis and metabolic stress [4]. Insulin resistance is often associated with obesity and type 2 diabetes, which represent the metabolic diseases with the strongest impact on the natural history and progression of MASLD [5].
Chronic inflammation and oxidative stress, often stemming from mitochondrial dysfunction and lipid peroxidation, promote cellular injury and fibrogenesis, driving progression to metabolic-dysfunction-associated steatohepatitis (MASH), characterized by hepatic inflammation and fibrosis, which may further lead to cirrhosis and hepatocellular carcinoma [4,6].
Genetic predispositions and epigenetic modifications also play significant roles in MASLD susceptibility and progression [7,8]. Environmental and lifestyle factors, including dietary habits and physical inactivity, significantly impact the development and progression of MASLD, underscoring the importance of holistic approaches to prevention and treatment [3].
The management of MASLD is multifaceted, involving lifestyle modifications, pharmacotherapy, and, in some cases, surgical interventions. Dietary changes, weight loss, physical exercise, and discouraging alcohol consumption are considered the most effective recommendations to prevent the development of MASLD and its complications [5].
A Mediterranean diet, the Dietary Approaches to Stop Hypertension (DASH) diet, low-carbohydrate and ketogenic diets, and intermittent fasting have shown benefits in reducing liver fat, improving insulin sensitivity, and mitigating inflammation [9,10,11,12]. All these dietary patterns emphasize the importance of incorporating a generous and regular intake of raw and cooked vegetables, highlighting their essential role in promoting overall health and well-being. On the contrary, diets rich in red and processed meat as well as ultra-processed foods (UPFs) are associated with a higher risk of developing MASLD and MASLD-related conditions due to high energy intake and saturated fat [13,14].
Diets high in red meat are contrasted with those rich in vegetables, which seem to have the most beneficial effect on hepatic steatosis due to their antioxidant and anti-inflammatory effects [11,15]. However, it is unclear whether all types of vegetables benefit MAFLD. Vegetables rich in flavonoids, carotenoids, and α-tocopherol have been shown to reduce the risk of MASLD and possibly effectively prevent liver fibrosis [16,17,18,19].
Recent studies have investigated the association between different types of vegetables and MASLD in other populations [18,20]. Consumption of dark green vegetables has been associated with reduced odds of MASLD, particularly among females and non-Hispanic white individuals [20]. Frequent consumption of soy products was significantly and negatively associated with the onset of MASLD in Japanese women [21]. However, there are not enough studies investigating this specific topic in the Mediterranean region, particularly in the southern Italian areas.
This study aims to assess how various types of vegetables influence the risk of MASLD in a Southern Italian cohort, and to determine the optimal quantity and variety of vegetable consumption necessary to reduce that risk.
2. Materials and Methods
2.1. Study Population
The NUTRIHEP study is a cohort initiated in 2005–2006, using a systematic random sample of attendees over 18 years from Putignano Primary Care Physicians’ list procedure [22].
We used data from General Practitioners’ (GP) registers instead of census records, as there were no notable differences in the age and gender distribution between the general population of Putignano and the data recorded in the GP registers. Between 2015 and 2018, all Nutrihep participants were invited for the initial follow-up. A total of 1426 participants responded, and they followed the same protocol as during the initial enrollment. All participants signed informed consent forms after receiving detailed information about the medical data to be studied. To address this, this study was designed as cross-sectional, focusing solely on the follow-up measurement. This study was approved by the Ethical Committee of the Minister of Health (DDG-CE-792/2014, on 14 February 2014).
2.2. Data Collection
During follow-up visits, the participants completed all assessments outlined in the protocol. Trained physicians and/or nutritionists interviewed them to collect data on sociodemographic details, health status, personal history, and lifestyle factors. This included a history of tobacco use, food intake, educational level [23], work profile [24], and marital status.
The participants’ weight and height were measured while wearing only underclothing and no shoes. We used an electronic balance (SECA©, Hamburg, Germany) to record weight to the nearest 1 kg, and a wall-mounted stadiometer (SECA©) to measure height to the nearest 1 cm. Blood pressure (BP) was measured following international guidelines [25,26], and the average of three measurements was calculated. The participants completed the EPIC food questionnaire independently to gather information about their eating habits [27,28]. The following blood measurements were taken: fasting serum glucose (FSG), fasting insulin, HbA1c, triglycerides, total cholesterol, LDL-C, HDL-C, AST, ALT, GGT, ferritin, and high-sensitivity C-reactive protein. Analyses were performed using the COBAS 8000 autoanalyzer (ROCHE Diagnostics SPA, Monza, Italy).
Insulin resistance was estimated using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) [29], calculated by the following formula:
HOMA-IR = FSG (mg/dL) × fasting Insulin (μIU/mL)/405
All subjects underwent standardized ultrasound exams using a Hitachi H21 Vision (Hitachi Medical Corporation, Tokyo, Japan). The liver parenchyma was examined with a 3.5 MHz transducer. A scoring system was used to semi-quantitatively assess hepatic fat content.
The degree of hepatic fat infiltration was determined based on liver echotexture, hepatic echo penetration, hepatic blood vessel clarity, and hepatic diaphragm differentiation in echo amplitude [30]. Figure S1 illustrates the ultrasound board employed in this study to determine the steatosis grade.
The European Prospective Investigation into Cancer and Nutrition (EPIC) Food Frequency Questionnaire (FFQ) [31] was used to document habitual food intake at baseline. The participants self-completed the EPIC questionnaire and returned it after one week. Nutritionists validated all responses and uploaded the questionnaire into a custom online tool. Afterwards, the entered nutritional data was transformed into micro- and macro-nutrients.
2.3. Outcome Assessment
MASLD is defined by the presence of hepatic steatosis combined with at least one of the following cardiometabolic risk factors: (1) BMI over 25 kg/m2 or waist circumference exceeding 94 cm in men and 80 cm in women; (2) fasting serum glucose of 100 mg/dL or higher, 2 h post-load glucose of 140 mg/dL or higher, HbA1c of 5.7% or higher, or being on specific medication; (3) blood pressure of 130/85 mmHg or higher, or on specific medication; (4) plasma triglycerides at or above 150 mg/dL, or on specific medication; (5) plasma HDL cholesterol below 40 mg/dL in men and below 50 mg/dL in women, or on specific medication. Additionally, the MASLD definition continues to restrict alcohol intake (similar to NAFLD) in those with steatosis to an average daily intake of 20–50 g for women and 30–60 g for men [1].
Finally, other forms of liver disease coexisting with MASLD, such as MASLD + HCV, HBV, were ruled out to avoid altering the natural history of the disease [32] (Figure 1).
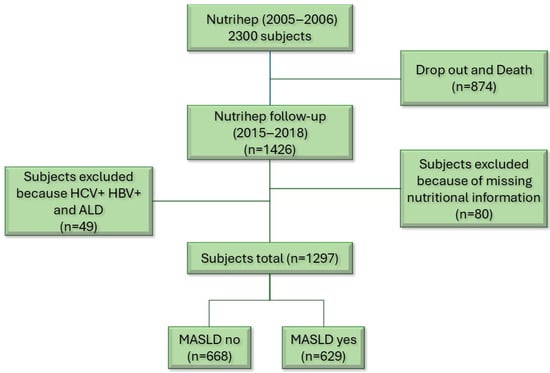
Figure 1.
Flow chart.
2.4. Exposure Variable
The EPIC questionnaire provided data on the frequencies and amounts of 30 vegetables, which we later categorized by color, as displayed in Table 1. Neither the type of cooking nor whether the vegetables were consumed raw or cooked was considered in this study.

Table 1.
Vegetables categorized by color.
2.5. Confounding Variables
Covariates were chosen based on previous research and both clinical and statistical judgment of their potential link to MASLD. After evaluating collinearity risks, we included demographic variables such as Age, Gender, Education, and Personal assessment of family income; behavioral factors like smoking, red wine consumption, daily energy intake, total vegetable intake regardless of exposure category, and food groups (fruits, legumes, cereals, fresh fish, olive oil, total meat, dairy products) excluding vegetables; and laboratory measures including AST/ALT, HOMA, and ɣgt.
To prevent collinearity issues, variables defining MASLD- such as BMI, waist circumference, fasting glucose, triglycerides, blood pressure, HDL cholesterol, and HbA1c- were excluded.
2.6. Statistical Analysis
The individual characteristics are reported as means and standard deviations (M ± SD) or medians and interquartile ranges for continuous variables and as frequencies and percentages (%) for categorical variables. We applied the Wilcoxon rank-sum test for comparing continuous variables between two groups. For categorical variables, we used the χ2 test to assess differences. Additionally, a logistic regression model was fitted to estimate odds ratios (ORs) and 95% confidence intervals (CIs), with MASLD as the outcome variable and vegetable intake (both continuous and categorical) as predictors.
Odds ratio (OR) is a measure of association between an exposure and an outcome.
An odds ratio of 1 indicates no association between the exposure and outcome. An odds ratio greater than 1 suggests that the exposure is associated with an increased likelihood of the outcome (a risk factor). An odds ratio of less than 1 suggests that the exposure is associated with a decreased likelihood of the outcome (a protective factor [33]).
The models were adjusted for the following variables: age, gender, smoking, education, daily kcal, γGT, AST/ALT, HOMA, intake of total vegetables devoid of exposure category, food groups (fruits, legumes, cereals, fresh fish, olive oil, total meat, dairy products) without vegetables, personal assessment of family income and red wine intake (g/day), and the estimated coefficients were transformed into odds ratio (OR).
Models in which the exposure variable was total daily vegetable consumption were adjusted for age, gender, smoking, education, daily kcal, γGT, AST/ALT, HOMA, Food groups (fruits, legumes, cereals, fresh fish, olive oil, total meat, dairy products) without vegetables, personal assessment of family income, and red wine intake (g/day).
Initially, confounding variables were chosen based on the existing literature. Then, the minimum absolute reduction and selection (LASSO) method was used to reduce the number of candidate predictors and highlight the most valuable ones for building the model [34]. When selecting variables as confounders for the model, those already included in the definition of MASLD, such as BMI, waist circumference, HDL cholesterol, triglycerides, fasting glucose, HbA1c, and blood pressure, were excluded. Additionally, the Variance Inflation Factor (VIF) was assessed to detect multicollinearity, and confounders with VIF > 5 were removed (Table S1) [35].
We conducted a detailed sensitivity analysis to identify the optimal exposure threshold with the most significant protective effect on outcomes, where total vegetable intake was significantly related to an increased likelihood of MASLD. We calculated intake increments between the different vegetable groups to identify the daily intake category with the lowest odds ratio (OR) and statistical significance, based on daily intake.
Total vegetable intake was ranked in 25 g/day intervals from 150 (lower) to 400 (higher) g/day. The intake of green vegetables was ranked in 5 g/day intervals from 30 (lower) to 55 (upper) g/day. The intake of red and orange vegetables (g/day) was classified into 10 g/day intervals, ranging from 70 g/day (lower) to 170 g/day (upper).
A forest plot was created to compare the OR values from the model fitting, using Total Vegetable intake as a categorical variable.
The two-tailed probability level was set at 0.05 to test the null hypothesis of non-association.
The analyses were conducted with StataCorp 2025 Stata Statistical Software: Release 19 (College Station, TX, USA: StataCorp LLC.), while the forest plots were created using RStudio (“Mariposa Orchid” Release (ab7c1bc7, 2025-06-01)) and its packages “forestplot”.
3. Results
3.1. Participants’ Characteristics
Table 2 presents the main characteristics of the 1297 participants, classified according to the presence or absence of MASLD. The sample consisted of 48.50% with MASLD, with most of them being male (54.6%).

Table 2.
Characteristics of participants by MASLD Nutrihep Study, Putignano (BA), Italy 2015–2018.
As shown in Table 2, the participants with MAFLD were older and mainly female. They also had a higher prevalence of hypertension and hyperlipidemia, along with greater BMI and weight: 30.28 (4.97) and 79.58 (14.73), respectively. Those with MASLD had a lower educational level compared to non-MASLD participants; 423 individuals had primary or secondary education as their highest level, while 214 had no MASLD. Among MASLD subjects, 59 were college graduates versus 119 in the non-MASLD group. Blood parameters were higher in MASLD patients, with statistically significant differences between groups. No significant differences in vegetable intake were observed; however, MASLD subjects consumed fewer green vegetables and more red/orange and other vegetables. There were no differences in Mediterranean Diet scores between groups, indicating similar adherence regardless of disease status. Likewise, macronutrient and micronutrient intake showed no significant variation between MASLD and non-MASLD groups (Table S2).
In Table 3, we have broken down the vegetables by MASLD, demonstrating no statistically significant differences between those with MASLD and those without, except for the consumption of onions.

Table 3.
Intake of different types of vegetables broken down by MASLD.
3.2. The Associations Between Different Kinds of Vegetable Intake and the Occurrence of MASLD
Table 4 presents the results of logistic regression models that examine the association between MASLD and total vegetable intake, presented as either a continuous or a categorical variable.

Table 4.
Logistic regression analysis of the association between total vegetable intake and MASLD. Daily vegetable consumption was expressed both continuously and categorically.
The results of the logistic regression model shown in Table 4 indicate that the category with the lowest OR was total vegetable consumption ≤325 (g/day) [OR 0.521 (95% CI 0.317; 0.858)] versus consumption >325 (g/day), after adjustment for covariates. This suggests that individuals with a maximum daily vegetable intake of 325 g had a 47.9% probability of not having MASLD, considering a model adjusted for age, gender, daily calories, ɣGT, AST/ALT, food groups without vegetables, red wine intake, and personal income assessment.
The odds ratio (OR) slightly above 1 (OR = 1.002; 95% CI: 1.001; 1.004, p-value = 0.007) indicates a positive association between daily vegetable intake and the likelihood of having MASLD. In simpler terms, this implies that individuals who consume more vegetables may be slightly more likely to develop MASLD.
The forest diagram (Figure 2) illustrates the OR values for each category of total vegetables, aiming to identify the one with the lowest risk value.
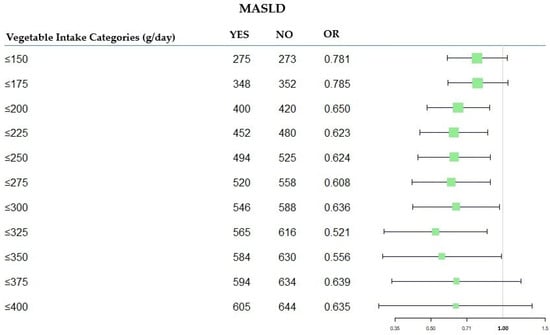
Figure 2.
Forest plot of OR values in the MASLD by total vegetable categories (g/day). MASLD: Metabolic-Dysfunction-Associated Steatotic Liver Disease; OR: Odds Ratio.
Multivariate logistic regression models were subsequently employed to verify the associations between MASLD and various types of vegetable intake (see Table 5).

Table 5.
Logistic regression analysis of the association between different kinds of vegetable intake and MASLD. Daily vegetable consumption was expressed both continuously and categorically.
We performed a detailed analysis to determine the optimal exposure cutoff with the most significant protective effect on the outcome (Table 5).
Green, red/orange vegetables were included in the models as both continuous and categorical variables, while other vegetables were considered as total continuous intake. The total intake and categories of vegetable consumption (g/day) with the lowest statistically significant odds ratios are presented in Table 5.
After adjustment for covariates, it was observed that the intake of the other vegetables group was negatively associated with MASLD (OR = 0.995, 95%CI: 0.989; 0.999, p-value = 0.047), while a higher intake of green vegetables and red/orange vegetables was significantly associated with increased odds of MASLD (OR = 1.005; 95% CI: 1.001; 1.009, p-value = 0.009, OR = 1.004; 95% CI: 1.001; 1.007, p-value = 0.004, respectively). In simpler terms, this implies that individuals who consume more green vegetables and red/orange vegetables may be slightly more likely to develop MASLD (see Table 5).
Table 5 shows the consumption categories for each group of vegetables studied. In the category “green vegetables”, the most protective intake was 35 g/day (OR = 0.616; 95% CI: 0.446; 0.851; p-value = 0.003). In other words, individuals consuming a daily intake of 35 g of “green vegetables” had a 39% reduced probability of not having MASLD compared to those with the highest consumption. However, eating up to 45 g of green vegetables daily can help protect against MASL, as this effect remains statistically significant.
In the “orange-red vegetables” category, it was an intake of 130 g per day that provided the greatest protection against MASLD (OR = 0.457; 95% CI: 0.274; 0.762; p-value 0.003). Thus, subjects consuming up to 130 g/day of red and/or orange vegetables had a 54.3% chance of not having MASLD compared to those with the highest consumption. Nevertheless, consumption of 150 g/day of red/orange vegetables still provides a statistically significant protective effect against MASLD.
4. Discussion
This research explored the impact of various vegetable types on the risk of developing MASLD among a cohort of 1297 Southern Italians from the NUTRIHEP study.
Our findings indicate that the greatest benefit was observed at an intake of approximately 325–350 g per day of total vegetables, equivalent to about two servings. This ideal daily amount would align with the national food-based dietary guidelines and the Mediterranean Diet, which suggests consuming at least two servings of vegetables per day [36,37]. Interestingly, in this cohort, consuming more than this amount did not provide any additional protection.
This indicates that, despite current scientific evidence consistently indicating that increased vegetable consumption is associated with a reduced risk of MASLD [11,15,18,20], certain factors may mitigate this protective effect. Along with following healthy dietary patterns and maintaining high overall diet quality, genetic variations may influence individual responses to dietary components [38]. A further study conducted on our cohort showed that a balanced diet, based on the principles of the Mediterranean Diet and rich in vegetables, combined with physical activity, can reduce the risk of MASLD and improve liver health in subjects with a specific genetic profile, such as FOXO3 TT [7]. This suggests that genetic variants could contribute to the development of MASLD, independent of vegetable intake. Additionally, various lifestyle factors, such as a lack of physical activity, may reduce the protective effects of vegetables on the liver [39]. Socioeconomic factors, medication use, and the presence of additional comorbidities could also have influenced the results of this study [3].
It is also worth noting that the population group investigated in this study moderately adheres to a Mediterranean Diet pattern, specifically using extra virgin olive oil as the primary seasoning, recognized for its antioxidant and anti-inflammatory effects. These dietary habits already serve as a protective factor against MASLD, which may explain why increasing vegetable consumption in this population might not necessarily reduce the odds of developing MASLD [40]. On the other hand, in different populations, including Chinese, Korean, and American adults with varying dietary habits, the increased consumption of vegetables seems to decrease the risk of NAFLD or MASLD [15,18,20]. These findings support the hypothesis that results may vary between populations, as previously hypothesized by Wang et al. [41].
When analyzing vegetables by color group, a similar trend emerged. Vegetables were grouped based on their pigments and bioactive compounds. For instance, the green group includes all vegetables rich in chlorophyll, which gives them their characteristic color, as well as compounds like β-Carotene, which confer antioxidant properties. For each color group, we identified a specific intake threshold at which the protective effect against MASLD was strongest. Red/orange vegetables, primarily represented by tomatoes and carrots, are most effective when eaten at a daily intake of 130 g, followed by green vegetables, especially leafy greens, lettuce, and cruciferous, at 35 g per day. In this cohort, exceeding these thresholds did not provide any additional protection. Conversely, for other vegetables, such as purple and white varieties—particularly onions, fennel, and celery—we did not identify a threshold.
Red/orange vegetables are rich in carotenoids, especially lycopene, which is abundant in tomatoes, a key ingredient in southern Italian cuisine. Lycopene has shown significant benefits for liver health and plays a crucial role in preventing and managing liver disease. It helps improve liver enzyme levels, reduces oxidative stress, lowers inflammation, and modulates gut microbiota—all of which are essential for preventing liver damage [16,42,43,44]. Lycopene supplementation has been shown to suppress hepatic inflammation by inhibiting the NF-κB/NLRP3 inflammasome pathway, leading to decreased expression of pro-inflammatory cytokines such as TNF-α and IL-6 [43]. Lycopene stands out as a promising agent in preventing MASLD due to its comprehensive benefits on liver health and metabolic regulation.
The relationship between green leafy vegetables and metabolic-dysfunction-associated liver disease has been examined in several studies [18,20]. Additionally, it is observed that including a green leafy vegetable component in diets may mitigate the detrimental impacts of other foods. For instance, adding a leafy vegetable portion could decrease the mortality risks associated with red meat for MASLD patients, and substituting one serving of starchy carbohydrates with green leafy vegetables may enhance NAFLD biomarkers and lessen fibrosis [45,46]. The beneficial effects of these vegetables are attributed to their antioxidant properties and bioactive compounds, including α-tocopherol, flavonoids, and carotenoids, particularly β-carotene [17,42,47].
β-Carotene, a provitamin A carotenoid found abundantly in vegetables like spinach and kale, acts as a powerful scavenger of free radicals, protecting cellular components from oxidative damage. It also modulates immune responses by attenuating inflammatory signaling pathways, such as NF-κB and MAPK, thereby reducing the production of pro-inflammatory cytokines [42,48]. α-Tocopherol, the most active form of vitamin E in humans, is abundantly found in green leafy vegetables, although in lower concentrations compared to oils and nuts. It is a potent antioxidant that has shown potential therapeutic effects on liver enzyme levels and histological features in MASLD patients, particularly in reducing oxidative stress and inflammation [47,49]. Additionally, green leafy vegetables, including lettuce, are high in inorganic nitrate, which may aid in treating and preventing fatty liver disease [50]. Inorganic nitrate can be converted into nitric oxide (NO) through a process involving symbiotic host bacteria. Nitric oxide has the potential to reduce oxidative stress and improve cardiometabolic functions [51].
Cruciferous vegetables, largely because of their component sulforaphane (SFN), protect against NAFLD by enhancing the gut barrier, modulating gut microbiota, reducing harmful bacteria, inhibiting the production of lipopolysaccharides, and decreasing inflammation. Additionally, SFN appears to improve insulin resistance, one of the key factors in the physiopathology of MASLD [52,53].
Finally, among the “White and other color vegetables” group, which has demonstrated a consistent protective effect, the most consumed vegetables are fennel and onions. This is likely due to their availability throughout much of the year, especially onions, which are widely eaten in this region. Interestingly, these vegetables seem to lower the risk of MASLD in this cohort, regardless of quantity. Onion has been shown to possess numerous pharmacological properties, attributable to its rich content of bioactive compounds such as quercetin, thiosulphinate, and phenolic acids [54]. One of the main protective effects is on the gastrointestinal system, resulting in stimulation of beneficial microorganism growth, as well as normalization of liver enzymes activities. A study conducted by Emamat et al. [55] showed that onion consumption can help in NAFLD management when combined with a healthy dietary pattern, and regular consumption of onion powder can prevent the development of NAFLD, even in the presence of other risk factors such as obesity and high energy, fat, and sugar intakes [56].
The dietary habits and traditions of the studied group may help explain the findings. In this region of Southern Italy, it is common to find local fruits and vegetables that are available according to the seasons. For example, green leafy vegetables are typically in season only at specific times of the year, which means their daily intake varies throughout the year. In contrast, red/orange vegetables, particularly tomatoes and carrots, along with onions, are available year-round and are widely used in many recipes, leading to a consistently higher daily intake. Eating locally sourced and in-season vegetables and fruits is a trend commonly observed in Mediterranean regions, along with other cultural elements [57]. This emphasizes the importance of adapting dietary interventions to geographic and cultural contexts as well [58].
Another factor that could explain the results of this study is the various cooking methods participants used to prepare different vegetables. Cooking methods can significantly affect the nutritional value of vegetables, especially concerning MASLD. While vegetables are typically beneficial due to their fiber, antioxidants, and anti-inflammatory properties, how they are prepared can change these benefits. For example, boiling can lead to the loss of certain polyphenols, which diminishes their protective effects against liver inflammation and fat accumulation. Frying, especially deep-frying, can reduce the active compounds’ content, and it can introduce harmful trans fats and oxidative compounds that may worsen insulin resistance and contribute to fatty liver disease. On the other hand, steaming keeps most nutrients intact and may even enhance the absorption of some antioxidants [59,60].
Improving nutrition literacy could be a cost-effective way to prevent chronic conditions [61]. Although many people are aware of healthy eating, they often struggle to put that knowledge into practice due to limited nutrition literacy. This is especially concerning in populations at risk for MASLD, where low vegetable intake is common. Factors like socioeconomic status, food access, and education level can prevent understanding and using nutrition information effectively [62]. Increasing nutrition literacy may help these individuals make healthier food choices, such as including the right amount and type of vegetables in their diets, and understand the importance of these choices for liver health.
The findings from this study align with the recommendations of the Mediterranean dietary pattern, which has been shown to help prevent or delay hepatic steatosis [63]. However, while the classic Mediterranean Diet suggests at least two servings of a variety of raw and/or cooked vegetables, our study indicates that consuming more than two servings does not provide additional benefits against MASLD. Additionally, in this study, we aimed to identify specific types of vegetables within the Mediterranean dietary pattern to better adapt to the dietary habits, preferences, and availability specific to different regions of the Mediterranean. Our findings suggest that a moderate intake of green leafy vegetables and cruciferous vegetables, along with a good consumption of tomatoes, carrots, and regular inclusion of onions, is beneficial against MASLD.
Strengths and Limitations
One of the key strengths of this study is its large sample size, which boosts the statistical power and enhances the reliability of the findings. Additionally, this research is novel as it is the first to investigate the effects of different types of vegetables on MASLD in a population from Southern Italy. This regional focus provides valuable insight into dietary patterns specific to the Mediterranean context, potentially informing culturally tailored nutritional recommendations. However, several limitations should be acknowledged, such as the observational design, which limits causal inference. In addition, another potential limitation is related to self-reporting of diet. Furthermore, the EPIC FFQ is an international questionnaire that does not take into account typical local foods, which results in several regionally consumed vegetables being omitted. This may lead to an underestimation or overestimation of some quantities. Finally, our study did not include a measure of physical activity, which represents a potentially serious limitation given the results of previous research linking physical activity with MASLD [64,65]. These limitations should be taken into account when interpreting the results. The residual confounding was consistent across all models analyzed, and the differing environmental impacts could partly explain the inconsistencies with other studies [66].
5. Conclusions
In conclusion, the results of this study suggest that consuming approximately two servings of vegetables per day may help reduce the risk of MASLD in a southern Italian population. Notably, white vegetables, such as onions, play a significant protective role against MASLD, regardless of the quantity consumed. In contrast, increasing the intake of other types of vegetables may not necessarily offer additional benefits in this population. Therefore, these findings indicate that an effective dietary intervention for individuals at risk of MASLD should focus on the specific types and portions of vegetables to be consumed while also taking geographic and cultural factors into account. Encouraging adequate vegetable consumption may be important in preventing liver disease in this small geographic population. Further research is needed to establish optimal intake levels and to understand the underlying mechanisms of action.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17152477/s1, Figure S1. Echografic sheet. Table S1. Collinearity diagnostics by VIF for the independent variables; Table S2. Micro- and macro-nutrients broken down by the presence or absence of MASLD.
Author Contributions
Conceptualization, C.B. and M.N.P.; methodology, R.D. and C.B.; software, R.D. and C.B.; validation, G.G.; formal analysis, C.B.; resources, G.G.; data curation, R.D. and C.B.; writing—original draft preparation, M.N.P. and R.T.; writing—review and editing, C.B., M.N.P., P.L.P. and R.T.; supervision, G.G.; project administration, G.G.; funding acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health with Ricerca Corrente 2025.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the National Institute of Gastroenterology and Research Hospital (DDG-CE-792/2014, dated 14 February 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.29390003.
Acknowledgments
The authors thank the NUTRIHEP Group and all volunteers who took part in this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NUTRIHEP | Nutrition and Hepatology |
| MASLD | Metabolic-Dysfunction-Associated Steatotic Liver Disease |
| OR | Odds Ratio |
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Proceedings of the 73 Rd Session of the WHO Regional Committee for Europe, Astana, Kazakhstan, 24–26 October 2023.
- Byrne, C.D.; Armandi, A.; Pellegrinelli, V.; Vidal-Puig, A.; Bugianesi, E. Μetabolic Dysfunction-Associated Steatotic Liver Disease: A Condition of Heterogeneous Metabolic Risk Factors, Mechanisms and Comorbidities Requiring Holistic Treatment. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 314–328. [Google Scholar] [CrossRef]
- Mejía-Guzmán, J.E.; Belmont-Hernández, R.A.; Chávez-Tapia, N.C.; Uribe, M.; Nuño-Lámbarri, N. Metabolic-Dysfunction-Associated Steatotic Liver Disease: Molecular Mechanisms, Clinical Implications, and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Huang, D.Q.; Wong, V.W.S.; Rinella, M.E.; Boursier, J.; Lazarus, J.V.; Yki-Järvinen, H.; Loomba, R. Metabolic Dysfunction-Associated Steatotic Liver Disease in Adults. Nat. Rev. Dis. Primers 2025, 11, 14. [Google Scholar] [CrossRef]
- Donghia, R.; Di Nicola, E.; Tatoli, R.; Forte, G.; Lepore Signorile, M.; Bonfiglio, C.; Latrofa, M.; De Marco, K.; Manghisi, A.; Disciglio, V.; et al. The Protective Effect of FOXO3 Rs2802292 G-Allele on Food Intake in a Southern Italian Cohort Affected by MASLD. Nutrients 2025, 17, 1315. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Tria, G.; Dongiovanni, P. Genetics Is of the Essence to Face Nafld. Biomedicines 2021, 9, 1359. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hosseinzadeh, M.; Parastouei, K. The Effect of Dietary Approaches to Stop Hypertension (DASH) Diet on Fatty Liver and Cardiovascular Risk Factors in Subjects with Metabolic Syndrome: A Randomized Controlled Trial. BMC Endocr. Disord. 2024, 24, 126. [Google Scholar] [CrossRef]
- Huang, X.; Gan, D.; Fan, Y.; Fu, Q.; He, C.; Liu, W.; Li, F.; Ma, L.; Wang, M.; Zhang, W. The Associations between Healthy Eating Patterns and Risk of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Case-Control Study. Nutrients 2024, 16, 1956. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High Red and Processed Meat Consumption Is Associated with Non-Alcoholic Fatty Liver Disease and Insulin Resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- García, S.; Monserrat-Mesquida, M.; Ugarriza, L.; Casares, M.; Gómez, C.; Mateos, D.; Angullo-Martínez, E.; Tur, J.A.; Bouzas, C. Ultra-Processed Food Consumption and Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD): A Longitudinal and Sustainable Analysis. Nutrients 2025, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Shin, S. Fruit and Vegetable Consumption and Non-Alcoholic Fatty Liver Disease Among Korean Adults: A Prospective Cohort Study. J. Epidemiol. Community Health 2020, 74, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Donghia, R.; Campanella, A.; Bonfiglio, C.; Cuccaro, F.; Tatoli, R.; Giannelli, G. Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients 2024, 16, 562. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, C.; Tatoli, R.; Donghia, R.; Guido, D.; Giannelli, G. Exploratory Role of Flavonoids on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in a South Italian Cohort. Antioxidants 2024, 13, 1286. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ye, M.; Zhang, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Does a High Intake of Green Leafy Vegetables Protect from NAFLD? Evidence from a Large Population Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1691–1701. [Google Scholar] [CrossRef]
- Sasada, T.; Iino, C.; Sato, S.; Tateda, T.; Igarashi, G.; Yoshida, K.; Sawada, K.; Mikami, T.; Nakaji, S.; Sakuraba, H.; et al. The Impact of Japanese Dietary Patterns on Metabolic Dysfunction-Associated Steatotic Liver Disease and Liver Fibrosis. Nutrients 2024, 16, 2877. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Liu, J.; Liu, J.; Lu, T.; Yu, J.; Zhang, G.; Xu, K. Associations between Intake of Different Types of Vegetables and Metabolic Dysfunction-Associated Fatty Liver Disease: A Population-Based Study. BMC Public Health 2025, 25, 315. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ueda, M.; Kobayashi, Y.; Shima, T. BMI Gain and Dietary Characteristics Are Risk Factors of MASLD in Non-Obese Individuals. Sci. Rep. 2025, 15, 2606. [Google Scholar] [CrossRef]
- Cozzolongo, R.; Osella, A.R.; Elba, S.; Petruzzi, J.; Buongiorno, G.; Giannuzzi, V.; Leone, G.; Bonfiglio, C.; Lanzilotta, E.; Manghisi, O.G.; et al. Epidemiology of HCV Infection in the General Population: A Survey in a Southern Italian Town. Off. J. Am. Coll. Gastroenterol. ACG 2009, 104, 2740–2746. [Google Scholar] [CrossRef]
- International Standard Classification of Education (ISCED); The United Nations Educational, Scientific and Cultural Organization: Paris, France, 1997.
- International Standard Classification of Occupations (ISCO-08); International Labour Office: Geneva, Switzerland, 2012.
- Sever, P. New Hypertension Guidelines from the National Institute for Health Clinical Excellence the British Hypertension Society. J. Renin-Angiotensin-Aldosterone Syst. 2006, 7, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study Populations and Data Collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Riboli, E.; Kaaks, R. The EPIC Project: Rationale and Study Design. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26, S6. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Chiloiro, M.; Caruso, M.G.; Cisternino, A.M.; Inguaggiato, R.; Reddavide, R.; Bonfiglio, C.; Guerra, V.; Notarnicola, M.; De Michele, G.; Correale, M.; et al. Ultrasound Evaluation and Correlates of Fatty Liver Disease: A Population Study in a Mediterranean Area. Metab. Syndr. Relat. Disord. 2013, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P. Relative Validity and Reproducibility of a Food Frequency Dietary Questionnaire for Use in the Italian EPIC Centres. Int. J. Epidemiol. 1997, 26, S152–S160. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Simon, S.D. Understanding the Odds Ratio and the Relative Risk. J. Androl. 2001, 22, 533–536. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Society Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics; Wiley: Hoboken, NJ, USA, 1980; ISBN 9780471058564. [Google Scholar]
- Adinolfi, F.; Carcea, M.; De Gara, L. Linee Guida Definitive—CREA. 2019. Available online: https://sapermangiare.mobi/files/download/linee-guida/linee-guida-completo.pdf (accessed on 26 July 2025).
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Sato, S.; Iino, C.; Sasada, T.; Soma, G.; Furusawa, K.; Yoshida, K.; Sawada, K.; Mikami, T.; Nakaji, S.; Sakuraba, H.; et al. Epidemiological Study on the Interaction between the PNPLA3 (Rs738409) and Gut Microbiota in Metabolic Dysfunction-Associated Steatotic Liver Disease. Genes 2024, 15, 1172. [Google Scholar] [CrossRef]
- Us Altay, D.; Kaya, Y.; Mataraci Değirmenci, D.; Kocyiğit, E.; Üner, A.; Noyan, T. Non-Alcoholic Fatty Liver Disease: The Importance of Physical Activity and Nutrition Education—A Randomized Controlled Study. J. Gastroenterol. Hepatol. 2024, 39, 2723–2734. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yan, R.; Jiao, J.; Li, F.; Zhang, H.; Chang, Z.; Wei, H.; Yan, S.; Li, J. Fruit and Vegetable Intake and the Risk of Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Observational Studies. Front. Nutr. 2024, 11, 1398184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Jia, L.; Liu, J. Association between Carotenoid Intake and Metabolic Dysfunction-Associated Fatty Liver Disease among US Adults: A Cross-Sectional Study. Medicine 2023, 102, E36658. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Liu, M.; Zhao, H.; Sun, Y. Lycopene Prevents Non-Alcoholic Fatty Liver Disease through Regulating Hepatic NF-ΚB/NLRP3 Inflammasome Pathway and Intestinal Microbiota in Mice Fed with High-Fat and High-Fructose Diet. Front. Nutr. 2023, 10, 1120254. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Nagata, N.; Xu, L.; Yamamoto, S.; Fuke, N.; Ushida, Y.; Suganuma, H.; Kaneko, S.; et al. Lycopene Prevents the Progression of Lipotoxicity-Induced Nonalcoholic Steatohepatitis by Decreasing Oxidative Stress in Mice. Free Radic. Biol. Med. 2020, 152, 571–582. [Google Scholar] [CrossRef]
- Donghia, R.; Tatoli, R.; Campanella, A.; Cuccaro, F.; Bonfiglio, C.; Giannelli, G. Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort. Nutrients 2024, 16, 1207. [Google Scholar] [CrossRef]
- De Nucci, S.; Rinaldi, R.; Di Chito, M.; Donghia, R.; Giannuzzi, V.; Shahini, E.; Cozzolongo, R.; Pesole, P.L.; Coletta, S.; De Pergola, G.; et al. The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study. Nutrients 2023, 15, 2289. [Google Scholar] [CrossRef]
- Chee, N.M.Z.; Sinnanaidu, R.P.; Chan, W.K. Vitamin E Improves Serum Markers and Histology in Adults with Metabolic Dysfunction-Associated Steatotic Liver Disease: Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2024, 39, 2545–2554. [Google Scholar] [CrossRef]
- Wu, S.; Chen, R.; Chen, J.; Yang, N.; Li, K.; Zhang, Z.; Zhang, R. Study of the Anti-Inflammatory Mechanism of β-Carotene Based on Network Pharmacology. Molecules 2023, 28, 7540. [Google Scholar] [CrossRef] [PubMed]
- Abera, M.; Suresh, S.B.; Malireddi, A.; Boddeti, S.; Noor, K.; Ansar, M.; Malasevskaia, I. Vitamin E and Non-Alcoholic Fatty Liver Disease: Investigating the Evidence Through a Systematic Review. Cureus 2024, 16, e72596. [Google Scholar] [CrossRef]
- Mirmiran, P.; Teymoori, F.; Farhadnejad, H.; Mokhtari, E.; Salehi-Sahlabadi, A. Nitrate Containing Vegetables and Dietary Nitrate and Nonalcoholic Fatty Liver Disease: A Case Control Study. Nutr. J. 2023, 22, 3. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; Haworth, S.M.C.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; de Campos Cruz, J.; et al. AMP-Activated Protein Kinase Activation and NADPH Oxidase Inhibition by Inorganic Nitrate and Nitrite Prevent Liver Steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 217–226. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Huangfu, B.; Hu, Y.; Xu, J.; Gao, R.; Huang, K.; He, X. Sulforaphane Ameliorates Nonalcoholic Fatty Liver Disease Induced by High-Fat and High-Fructose Diet via LPS/TLR4 in the Gut–Liver Axis. Nutrients 2023, 15, 743. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Lei, Y.; Zhao, F.; Che, J.; Wu, Y.; Lei, P.; Kang, Y.E.; Shan, Y. Improving Insulin Resistance by Sulforaphane via Activating the Bacteroides and Lactobacillus SCFAs–GPR–GLP1 Signal Axis. Food Funct. 2024, 15, 8644–8660. [Google Scholar] [CrossRef] [PubMed]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium Cepa, Preclinical and Clinical Evidences: A Review. Iran. J. Pharm. Res. 2021, 20, 107–134. [Google Scholar] [CrossRef]
- Emamat, H.; Foroughi, F.; Eini–Zinab, H.; Taghizadeh, M.; Rismanchi, M.; Hekmatdoost, A. The Effects of Onion Consumption on Treatment of Metabolic, Histologic, and Inflammatory Features of Nonalcoholic Fatty Liver Disease. J. Diabetes Metab. Disord. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Emamat, H.; Foroughi, F.; Eini-Zinab, H.; Hekmatdoost, A. The Effects of Onion Consumption on Prevention of Nonalcoholic Fatty Liver Disease. Indian J. Clin. Biochem. 2018, 33, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Tormo, E.; Barat, J.M.; Fernández-Segovia, I. Importance of the Origin, Organic Production and Other Extrinsic Parameters in Fruit and Vegetable Choices. Food Sci. Technol. Int. 2023, 31, 275–286. [Google Scholar] [CrossRef]
- Nutrition Research Must Go Local. Nat. Med. 2025, 31, 1371. [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Schmiedeskamp, A.; Schreiner, M.; Baldermann, S. Impact of Cultivar Selection and Thermal Processing by Air Drying, Air Frying, and Deep Frying on the Carotenoid Content and Stability and Antioxidant Capacity in Carrots (Daucus Carota L.). J. Agric. Food Chem. 2022, 70, 1629–1639. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Ellerbeck, E.F.; Gajewski, B.J.; Gibbs, H.D. Nutrition Literacy Predicts Adherence to Healthy/Unhealthy Diet Patterns in Adults with a Nutrition-Related Chronic Condition. Public Health Nutr. 2019, 22, 2157–2169. [Google Scholar] [CrossRef]
- Donghia, R.; Bonfiglio, C.; Giannelli, G.; Tatoli, R. Impact of Education on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Southern Italy Cohort-Based Study. J. Clin. Med. 2025, 14, 1950. [Google Scholar] [CrossRef]
- Cueto-Galán, R.; Fontalba-Navas, A.; Gutiérrez-Bedmar, M.; Ruiz-Canela, M.; Martínez-González, M.A.; Alves, L.; Babio, N.; Fitó, M.; Ros, E.; Fiol, M.; et al. Adherence to the Mediterranean Diet to Prevent or Delay Hepatic Steatosis: A Longitudinal Analysis within the PREDIMED Study. Front. Nutr. 2025, 12, 1518082. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, C.B.; Bianco, A.; Bonfiglio, C.; Franco, I.; Verrelli, N.; Carella, N.; Shahini, E.; Zappimbulso, M.; Giannuzzi, V.; Pesole, P.L.; et al. Healthy Lifestyle Changes Improve Cortisol Levels and Liver Steatosis in MASLD Patients: Results from a Randomized Clinical Trial. Nutrients 2024, 16, 4225. [Google Scholar] [CrossRef] [PubMed]
- Fewell, Z.; Davey Smith, G.; Sterne, J.A.C. The Impact of Residual and Unmeasured Confounding in Epidemiologic Studies: A Simulation Study. Am. J. Epidemiol. 2007, 166, 646–655. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).