Abstract
Background: Sufficient choline supply is essential for tissue functions via phosphatidylcholine and sphingomyelin within membranes and secretions like bile, lipoproteins and surfactant, and in one-carbon metabolism via betaine. Choline requirements are linked to age and genetics, folate and cobalamin via betaine, and arachidonic (ARA) and docosahexaenoic (DHA) acid transport via the phosphatidylcholine moiety of lipoproteins. Groups at risk of choline deficiency include preterm infants, children with cystic fibrosis (CF) and patients dependent on parenteral nutrition. Fortifiers, formula and supplements may differently impact their choline supply. Objective: To evaluate added amounts of choline, folate, cobalamin, ARA and DHA in fortifiers, supplements and formula used in pediatric care from product files. Methods: Nutrient contents from commonly used products, categorized by age and patient groups, were obtained from public sources. Data are shown as medians and interquartile ranges. Results: 105 nutritional products including fortifiers, formula and products for special indications were analyzed. Choline concentrations were comparable in preterm and term infant formulas (≤6 months) (31.9 [27.6–33.3] vs. 33.3 [30.8–35.2] mg/100 kcal). Products for toddlers, and patients with CF, kidney or Crohn’s disease showed Choline levels from 0 to 39 mg/100 kcal. Several products contain milk components and lecithin-based emulsifiers potentially increasing choline content beyond indicated amounts. Conclusions: Choline addition is standardized in formula for term and preterm infants up to 6 months, but not in other products. Choline content may be higher in several products due to non-declared sources. The potential impact of insufficient choline supply in patients at risk for choline deficiency suggests the need for biochemical analysis of products.
1. Introduction
1.1. Essential Nature of Choline
Choline has been acknowledged as an essential nutrient by the Institute of Medicine (IoM) and the European Food Safety Authority (EFSA) since 1998 and 2016, respectively, because endogenous synthesis via the phosphatidylethanolamine-N-methyltransferase (PEMT) pathway is not sufficient to meet requirements [1,2]. Moreover, any endogenous choline synthesis by PEMT is partly fed by betaine which is derived from exogenous choline [3,4,5], and the release of free choline from PC synthesized by the PEMT pathway is low [6,7], irrespective of PEMT single nucleotide polymorphisms (SNPs, see below). In addition, choline has been approved by EFSA for the promotion of liver development and function, and clinical data highlight its role in human development and patient care [8,9,10]. Although EFSA provides health claim data on the choline metabolite betaine as well, betaine is not added to human formula and supplements [11]. We will therefore focus on choline administration in infants and children.
Choline is a critical nutrient in the overall population, particularly in several vulnerable groups at risk for deficiency, such as preterm infancy (PI), cystic fibrosis (CF) with exocrine pancreatic insufficiency, long-term total parenteral nutrition (TPN), small intestine bacterial overgrowth (SIBO) resulting in intestinal choline degradation prior to absorption, and frequent SNPs of the PEMT gene [7]. Such conditions and SNPs corrupting the estrogen-dependent expression of the PEMT pathway, result in a further increase in choline requirements and susceptibility to develop liver steatosis. In all these patients, plasma choline levels are frequently below their age-adjusted levels, impairing cellular choline uptake and potentially also organ function, particularly of the liver [12,13,14].
1.2. Choline Concentrations and Metabolism
The plasma levels of choline and its metabolite betaine indicate choline status, which depends on choline supply by regular nutrition, formula and nutritional supplements ([6], supplement) and [13,15,16]. Reference values of choline are age-dependent, being 41 (32–51) µmol/L in the fetus, 14 (10–17) µmol/L in pregnant women, and 9 (6–11) µmol/L in non-pregnant adults. Values display an untimely drop to 22 (16–28) µmol/L in preterm infants after birth during NICU stay and are only 6 (5–7) mmol/L in CF with exocrine pancreatic insufficiency and TPN patients. Values for betaine are 26 (18–39)µmol/L for the fetus and adult, but low in pregnant women (11 (10–14) µmol/L) and CF patients (19 (15–25) µmol/L) [6,17,18,19].
The PEMT pathway for endogenous choline synthesis requires betaine and further downstream metabolites of choline. It, therefore, depends on exogenous choline. Betaine is synthesized from choline in the kidneys for osmolytic functions, and in the liver to convert homocysteine to methionine, for the formation of S-adenosylmethionine (SAM). Notably, 40% of choline is metabolized to betaine, facilitating methyl group transfer rather than membrane phospholipid homeostasis. SAM is a substrate for the PEMT reaction, generating phosphatidyletanolamine-derived PC for very-low density lipoprotein (VLDL) formation, creatine synthesis, and epigenetic regulation of DNA and histones [9,20,21].
The functional impact of choline is that the concentrations of its major metabolites, phosphatidylcholine (PC) and sphingomyelin (SPH), are high in parenchyma as well as in bile, lipoproteins and other secretions, such as surfactant and gastric juice. PC concentrations in membranes and secretions are tightly regulated with characteristic fatty acid profiles [22,23,24]. PC and SPH synthesis essentially depend on exogenous choline supply [1,2,8] that, in addition to the consequences of PEMT SNPs, is further increased in CF patients due to choline losses and low PEMT expression, while PEMT expression is virtually absent in (preterm) infants [17,21,25].
1.3. Choline Function and Organotypic Metabolism
PC/choline concentrations have to be constant as any significant decrease, particularly of PC, results in organ damage [26,27]. Moreover, PC/choline is required for SPH synthase which converts ceramides to SPH via phosphocholine transfer, a process that is quantitatively important—particularly for lung metabolism and integrity [5,28,29]. An adequate choline supply is also required for acetylcholine synthesis, which is fundamental to synaptogenesis, the development and function of the brain and leucocytes [18,30,31]. Hence, choline exerts complex effects on organ integrity, immunology, and epigenetics. Notably, choline requirements are proportional to growth, with the highest defined postnatal adequate intake (AI) in term infants (TI) (17–18 mg/kg/d) [1]. Requirements may even be higher in fetuses and preterm infants (PI) due to their 3–4-times higher growth rate, as physiologic plasma levels are not achieved by current feeding strategies [17,19]. The same applies to CF and TPN patients, due to inadequate intake/administration or losses [6,12].
Turnover of lung surfactant PC is very low; only its PC secretion in contribution to systemic lipoprotein trafficking is high. However, the hepatic PC secretion rate is very high for both triglyceride emulsification and lipase activation via bile and for VLDL (20% PC) assembly to release triglycerides [28,32,33,34]. It surmounts 50% of the hepatic PC pool per day in adults and may be even higher at higher metabolic rates [35,36]. In essence, for choline, PC, SPH and betaine (see above) homeostasis, the organism requires adequate exogenous choline intake that is proportional to growth. It is further increased in case of choline losses, such as exocrine pancreatic insufficiency in CF [6]. Choline/betaine exhaustion rapidly results in liver disease like steatosis [37,38]. For survival during choline deprivation, systemic prioritization of the liver occurs at the expense of other organs such as the lungs [32,33,36].
1.4. Choline in Nutrition
Unprocessed food frequently contains high amounts of choline in the form of organic esters that are either water-soluble, such as phosphocholine and glycerophosphocholine (GPC) in milk, or lipid-soluble, such as PC, SPH and lyso-PC in unprocessed eggs, viscera, meat, fish and many cereals. Free choline is high in processed food and formula [39]. In formula, the physiological esters are not specifically added, but may originate from basic ingredients or emulsifiers rather than from specifically added components to meet regulatory requirements [40,41]. Notably, choline absorption and plasma kinetics as well as its intestinal degradation to trimethylamine (TMA) by the microbiota prior to absorption, increasing TMA oxide (TMAO) in plasma, differ among these compounds. PC, phosphocholine and glycerophosphocholine show no or low TMAO formation, whereas the frequently used choline bitartrate shows the highest rate of TMAO formation [42]. This may be particularly important for CF patients with exocrine pancreatic insufficiency frequently showing SIBO and high TMAO levels from infancy onwards [6].
For vulnerable groups, the addition of choline is under official regulation by European Union (EU) enactments, defining the choline content of formula to be 25–50 mg/100 kcal for term born infant formula from 0 to 6 months, whereas no supplementation is advised beyond 6 months of age [41]. However, guidelines and recommendations from IoM, EFSA and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), as well as scientific data, are not congruent here. The statement of the EU committee that beyond the age of 6 months, other foods and endogenous synthesis are sufficient, opposes both the recommendations of the IoM and clinical data, which indicate high requirements and low endogenous synthesis [41]. Furthermore, ESPGHAN statements on the minimal choline requirements of preterm infants are not in line with the IoM recommendations either [43]. Here, the minimum ESPGHAN recommendations for choline supply in preterm infants are only ~45% of the IoM recommendations for term infants, despite their 3–4 fold higher physiologic growth rate [1,44]. For other pediatric diseases, where large amounts of choline are lost via feces, as in cystic fibrosis (CF) with exocrine pancreatic insufficiency [6,37], official guidelines do not address choline as a critical nutrient [45]. This is despite the fact that in CF patients, low plasma choline and betaine levels are pathognomonic and associated with hepatosteatosis [9].
1.5. Aim of Study
As choline is a potentially critical nutrient, the aim of this study was to investigate the choline content of frequently used commercial pediatric nutritional products in Germany. Furthermore, as individual nutrient requirements change during development and in response to diseases, this was assessed in relation to total energy and nutrients closely linked to choline metabolism, such as folate, cobalamin, DHA, and ARA. We assessed their content in fortifiers and formula for preterm and term infants, in liquid food for toddlers, in supplements and in formulations designed for children with specific indications, such as cow milk allergy, food allergies, other gastrointestinal conditions including malabsorption and inflammation (e.g., Crohn’s disease), increased nutrient requirements or kidney disease. We focused on the product information concerning added choline together with macronutrients, folate, cobalamin (vitamin B12), ARA and DHA. We further evaluated whether additional, unquantified food components that contain choline may increase choline intake over the producers’ information.
2. Materials and Methods
2.1. Study Design
We collected information on fortifiers, formulas and other products that were either clinically used, prescribed, or available to clinical staff responsible for the respective patient groups from July 2024 to January 2025. This product selection encompassed insights from nurses, physicians and dietary staff members, and was based on their practical and potential applications according to clinical indications (neonatology, pediatric gastroenterology, CF) and common practices related to dietary acceptance (nurses, dieticians, CF, pediatric gastroenterology). All patient files from the CF outpatient clinic at the University Children’s Hospital Tübingen were reviewed for products used (Ethical approval by Ethics Committee, Medical Faculty, Tübingen University Clinic, No. 121/2020BO2 19 March 2020). All identified products and their components were compiled into an Excel sheet (Microsoft Office Professional Plus 2024, Microsoft Corporation, Redmond, WA, USA) for quantitative analysis.
2.2. Sample Collection
We collected the information on contents in energy, protein, carbohydrate, choline, folate, cobalamin (vitamin B12), ARA and DHA in the above-mentioned products used as formula, fortifiers, substitutes and supplements in our Neonatal Intensive Care Unit (NICU), CF outpatient clinic, department of gastroenterology, and nutritional support unit of the Children’s Hospital Tübingen.
Data were systematically grouped according to indications (Table 1) so that a total of 75 products, i.e., 3 fortifiers for preterm infants, 11 preterm infant formulas, 39 formulas for term infants from 0 to 6 months and 14 for infants from >6–12 months and 8 products for toddlers (12–36 months) were selected (Appendix A Table A1). For special indications, like galactosemia, lactose intolerance, increased energy requirements, tube feeding or kidney disease, an additional 30 products were identified (Appendix A Table A2).

Table 1.
Product groups assessed in study.
2.3. Sample Characterization
Products were characterized according to their contents in macronutrients (energy; protein, carbohydrates, fat), micronutrients (choline, folate, cobalamin) and their content in long-chain polyunsaturated fatty acids (LC-PUFA), namely ARA and DHA acid (Table 2) according to the producers’ information, being available online. We additionally screened all selected products for components that essentially contain choline, either on the basis of skimmed milk or whey (mainly phosphocholine, GPC, or on the basis of emulsifiers containing phospholipids (PC, SPH and lyso-PC), such as soya lecithin and egg lipids. While this information was available via online links (see Appendix A Table A1 and Table A2), no information was provided on the amount of additional choline from such components.

Table 2.
Compounds assessed in study.
2.4. Statistics
For descriptive statistics, data are expressed as median and interquartile range (IQR), as data were frequently not normally distributed. Group comparisons were performed of the indicated numbers of sample (N) with non-parametric testing, and correction for multiple testing according to Dunn, using GraphPad Instat®, version 3.0 (San Diego, CA, USA). Statistical significance was accepted at p < 0.05.
3. Results
An overview of evaluated products is shown in the online supplement (Table A1 and Table A2), showing their use for age and patient groups, and special indications as well as the amount of choline added, the component used (choline chloride or bitartrate), as well as the use of choline-containing emulsifiers. To none of the analyzed products, choline compounds prevalent in breast milk or other foods, like GPC, phosphocholine, PC or SPH [40] were added.
3.1. Energy
Energy density of products for term infants and toddlers was 65 to 68 kcal/100 mL of final product. It was marginally higher in products for preterm infants 73.5 (67.3–80.0) kcal/100 mL (p < 0.05) but partly was significantly higher in products for special indications like older patients suffering from CF, Crohn’s or kidney disease and others (Figure 1A; Appendix A Table A2). Notably, for preterm infant fortifiers, energy density was provided as the incremental energy per unit breast milk or formula, if fortified according to the manufacturers’ recommendations.
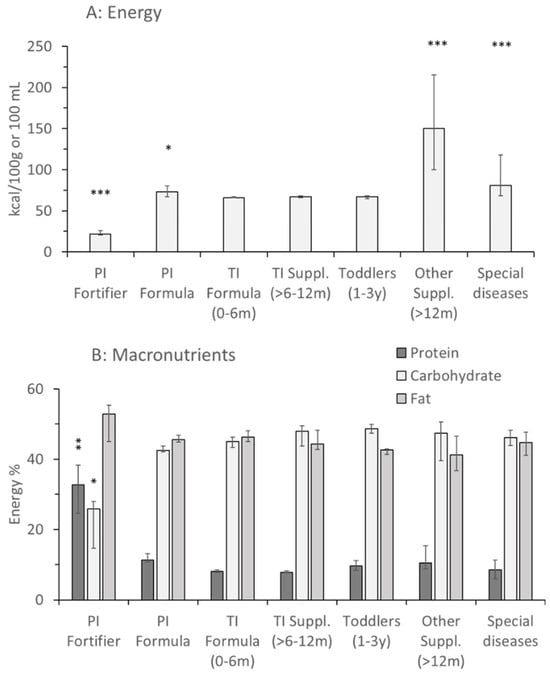
Figure 1.
Energy concentration and macronutrient distribution of fortifiers, formulas and supplements for healthy children and pediatric patients. Data are median values and interquartile ranges of preterm infant (PI) fortifiers (n = 3), PI formula (n = 14), term infant (TI) formula (0–6 months [m]; n = 38), TI supplements (6–12 m; n = 14), toddlers (1–3 years [y]; n = 9), other supplements for higher energy demands, e.g., in cystic fibrosis (M = 19) and special indications like kidney disease, Crohn’s disease, etc., (n = 12). Values are median and interquartile range. Abbreviations: *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. TI formula 0–6 m.
3.2. Macronutrients
Protein contents of formula (0–0.5 y) and add-on products for older term infants (0.5–1 y) were 8.1 (7.8–8.5)% and 7.8 (7.7–8.2)% of energy, respectively, but were higher for preterm compared to term infant formula (11.3 [11.8–13.8]%; p < 0.01). In products for older patients and for special diseases, values were more variable, according to indications. In preterm infant fortifiers, protein was 32.8 (24.5–38.3)% relative to energy (p < 0.01), as it was only added in 5–20% amounts to breastmilk or preterm infant formula. Except for preterm infant fortifiers, where fat surmounted carbohydrates by far (52.8 [45.1–55.3]% vs. 25.9 [14.6–27.9]%, respectively; p < 0.05), there was a balanced contribution of 41–46% fat and 42–49% carbohydrates in all products (Figure 1B).
3.3. Choline
Figure 2A–C displays the information on choline, folate and vitamin B12 (cobalamin) added to the product groups shown in Figure 1. Contents of choline added per 100 mL and per 100 kcal were almost identical in preterm and term infant (0–6 months) formula, with only marginal differences (Figure 2A). Added choline per 100 kcal was slightly lower in products for preterm compared to term infants (31.9 [27.6–33.3] vs. 33.3 [30.8–35.2] mg/100 kcal; p > 0.05), with minimum values of 20.5 vs. 23.0 mg/100 kcal. However, maximum value was highest (46.8 mg/100 kcal; O-HP-2, Humana) for PI formula.
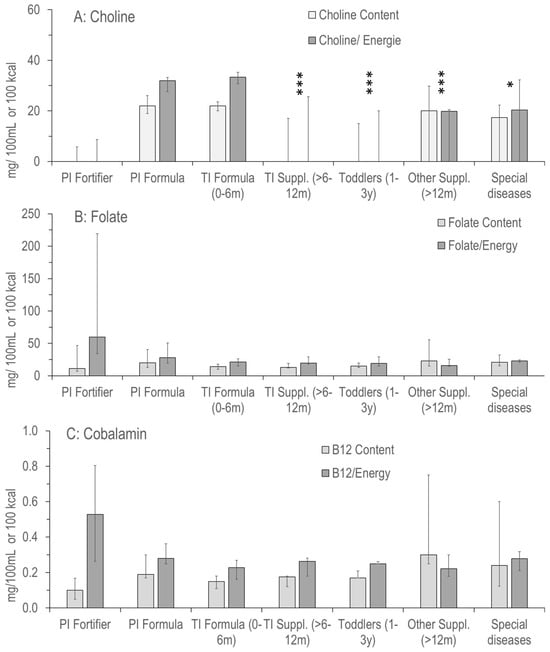
Figure 2.
Concentration and density of choline (A) and its metabolically related vitamins folate (B) and vitamin B12 (C) in the products as indicated in Figure 1. Abbreviations: PI, preterm infant; TI, term infant; ***, p < 0.001; *, p < 0.05 vs. PI and TI Formula.
Other products frequently did not contain added choline, with median zero values per 100 mg or per 100 kcal (PI fortifiers, PI and TI formula, products for toddlers, other supplements and products for special diseases [see above]) (Figure 1A). Maximum values were 17.3, 37.3, 22.1, 36.7 and 37.9 mg/100 kcal, respectively. In a total of 105, no choline was added to 27, choline chloride was added to 29 and choline bitartrate was added to 34 products, whereas no specification of added choline was provided in 15 cases.
However, in many products, soya lecithin or egg lipids were used as emulsifiers (Table 3) or whole and skimmed milk, as well as demineralized whey were added without exact quantification in the summary of product characteristics, prohibiting any precise estimation of total choline content, but providing information on the minimum choline supply by the respective products (Table 3 compared to Appendix A, Table A1 and Table A2): three out of 3 preterm infant fortifiers comprised milk lipids, lecithin and/or whole milk compounds. Five out of 11 preterm infant formulas contained such compounds, whereas among term infant formula (0–6 Months) 30 out of 39 products contained additional choline-containing ingredients, and in formula for 6–12 month olds it was 13 out of 14. For toddlers, it was 4 out of 8, and for special indications it was 16 out of 24 products.

Table 3.
Products with choline-containing emulsifiers and milk components.
There was a significant overlap of the products’ content or absence of both added choline and ingredients containing choline components, so that there are products containing precisely the indicated choline value from zero to 39 mg/100 kcal or more than indicated without any value given.
3.4. Choline Related Nutrients: Folate, Cobalamin and Long-Chain Polyunsaturated Fatty Acids
The low variability of folate (Figure 2B) and cobalamin (vitamin B12; Figure 2C) values was in sharp contrast to added choline, showing no median zero values and much narrower IQRs for both. Only exceptions were high maximum values in preterm infant fortifiers, and supplements for older children. No significant differences were observed between groups (p > 0.05). Notably, although bioavailability differs for different components, such as cyano-, hydroxy- or methylcobalamin, no specific information was provided here. For folate, it was recorded that Bio Combiotic PRE, HA Combiotic PRE-HA, Bio Combiotic 1+2 and HiPP Comfort (all HiPP GmbH & Co, Pfaffenhofen, Germany) contained 5-methyltetrahydrofolate rather than folate.
For the (conditionally) essential LC-PUFA, ARA and DHA, values were similar in all formulas for preterm and term infants up to 6 months of age. However, for ARA from 6 months onwards, and for DHA from 1 year onwards, the values showed extreme ranges, including even zero-values for some products (e.g., high energy) (Figure 3). In toddler products and special health issues, ARA and DHA values were frequently zero, but could also be high (30.8 mg/100 kcal ARA and DHA, Beba Expert HA; 29.9 mg/100 kcal ARA and DHA, Beba AR-anti reflux; both Nestlé). Consequently, although being metabolically intimately connected with choline, the ratio of added choline vs. folate, cobalamin, ARA and DHA was highly variable throughout, with the lowest variability in formulas for preterm and term infants up to 6 months. However, ratios between added choline and folate, cobalamin, ARA and DHA only partly reflected the real situation as information on additional choline-containing compounds was not provided (Appendix A Figure A1, Appendix B).
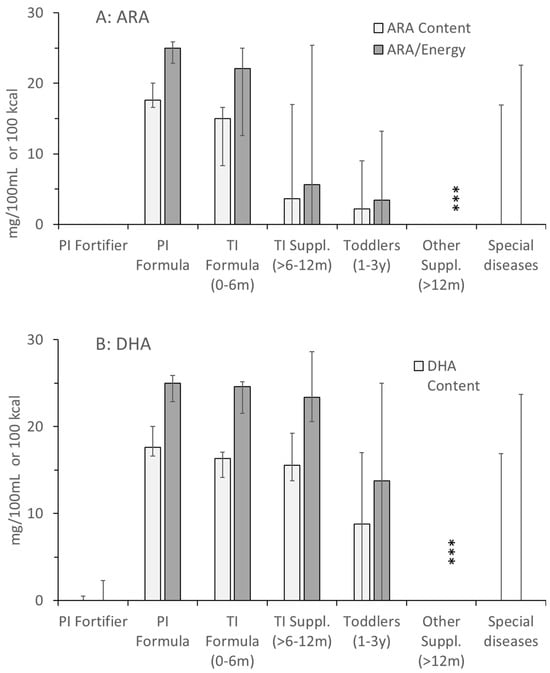
Figure 3.
Concentrations and densities of arachidonic (ARA; (A)) and docosahexaenoic acid (DHA, (B)) in the products as indicated in Figure 1. Abbreviations: PI, preterm infant; TI, term infant; ***, p < 0.001.
4. Discussion
In this study, we investigated preterm and term infant fortifiers and formulas, as well as products for older children and for children with additional nutrient requirements due to an underlying disease. Our main focus was on the supply of choline, as an essential nutrient per se, and as a critical nutrient under special conditions. Such conditions principally are an increased requirement to achieve and maintain concentrations of choline and its primary water-soluble metabolite betaine in plasma that match age-adjusted reference levels. We evaluated this in relation to other macronutrients, such as energy and fractions of protein, carbohydrate and fat. Moreover, we investigated the other main micronutrients that are related to choline metabolism, namely folate and cobalamin which are involved in one-carbon metabolism via betaine as a choline metabolite, as well as ARA and DHA that are linked to choline via the PC moiety of very low-density lipoproteins (VLDL) as the main plasma carrier of these fatty acids (for details see Figure 4 and [19,40]).
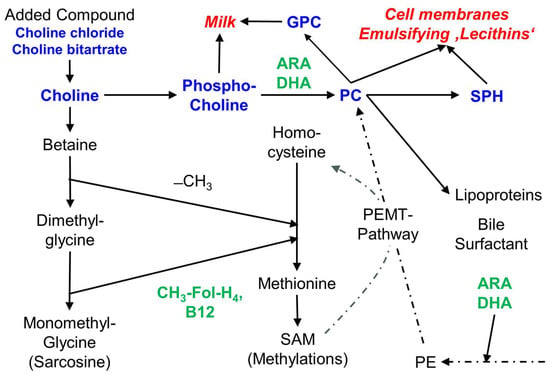
Figure 4.
Scheme of choline components and metabolism, and the relation of choline to folate, vitamin B12, ARA and DHA. Choline and choline-containing components are bold in blue, choline-related micronutrients are bold in green. Abbreviations: ARA, arachidonic acid; B12, vitamin B12 (cobalamin); −CH3, methyl group; CH3-FolH4, methyl tetrahydrofolate; DHA, docosahexaenoic acid; GPC, glycerophosphocholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PEMT, PE-N-methyl transferase; SPH, sphingomyelin.
4.1. Balance and Imbalance of Choline Compared to Other Nutrients
All these latter components were present in balanced concentrations and fractions, with only few exceptions: there were lower concentrations of energy in final dilutions of preterm infant fortifiers, being an add-on to formula or breast milk, and a higher fraction of protein and fat in such products. Moreover, ARA and DHA were partly absent from formula beyond 6 months of age, and in ‘other products’ compensating increased energy expenditure or those for characteristic intestinal (such as food allergies, malabsorption or Crohn’s disease) or kidney diseases. For folate and cobalamin, whose metabolism is tightly linked to that of choline due to their function as methyl group carriers for betaine and other downstream products, concentrations were similar and balanced throughout (see Section 3, Figure 2B,C).
However, this is in contrast to choline, both for the amounts of added choline per 100 mL volume and per 100 kcal supplied with a product. Some products for infants older than 6 months comprised no added choline, whereas others did. While infants beyond 6 months are frequently fed with complementary solid food already, their choline supply from formula may be insufficient, as their requirement per kg body weight is nearly identical to that of younger infants [1].
The only constancy in choline added to products, as officially regulated [41], is found in formula for PI and TI up to 6 months of age, comprising 30–35 mg added choline per 100 kcal. However, the results of our own research indicate that the choline amounts added to TI formula are not sufficient for PI. Under current feeding conditions, with both breast milk and formula, PI do not show plasma choline and betaine corresponding to fetal levels (postnatal PI plasma: ~20 µmol/L vs. cord blood: ~40 µmol/L). As the uptake of choline into cells is proportional to concentration in a range of ~2–100 µmol/L [27], PI formula may be inadequate for optimal growth. Notably, this applies similarly to unfortified breast milk that supplies the same median amount of choline per 100 kcal as PI formula, however with a much larger intra- and inter-individual variability [46].
A similar objection may be raised for products used for special clinical conditions such as CF and other diseases. We recently demonstrated that in CF patients with exocrine pancreatic insufficiency, plasma choline levels are significantly decreased and they further decrease with age and such low plasma choline values are associated with liver disease [6,9,38]. Products frequently used in these patients to compensate for increased energy expenditure have a substantial impact on plasma choline concentrations, if they contain additional choline ([6], Appendix A, Table A1 and Table A2). In essence, the choline content of such products is important to normalize plasma concentrations, and using an adequate product is a matter of knowledge and choice. From a clinical point of view, this is very important as there are strong correlations between plasma choline and its supply, and liver and lung function [9,21]. This may similarly apply for products used in other patient groups, e.g., d those with pediatric Crohn’s (e.g., Modulen IBD®, Nestlé), cachexia (Duocal®, Nutricia), liver (Heparon Junior®, Nutricia) or kidney disease (Nephea Infant®, Nephea Kid®, MetaX; restoric® nephro prae, Vitasyn medical) (see Appendix A, Table A2).
4.2. The Indicated Choline Content May Not Match the Actual Amount in the Products
However, there is a discrepancy between the amounts of ‘intentionally added choline’ and that ‘actually present’ in some products (i.e., that choline form lecithin emulsifiers or milk components), which applies to all product groups from preterm infant fortifiers to special needs products. If added, mostly choline bitartrate, followed by choline chloride, is a quantified product ingredient. It is noteworthy that choline bitartrate, followed by choline chloride, is the least favourable compound as its conversion to TMA and TMAO surmounts that of other compounds, such as GPC, phosphocholine and PC [20,42]. However, 71 out of 105 products comprised ingredients that essentially contain choline esters, and potentially free choline mainly due to processing. These are (1) milk and whey, primarily containing GPC and phosphocholine, and not found in non-milk-based products for allergy reasons, and (2) phospholipid-based emulsifiers, like soya lecithin and egg lipids. Whereas egg lipids mainly contain PC as a phospholipid, soya lecithin is a mixture mainly of PC, phosphatidylethanolamine and phosphatidylinositol [47]. All these phospholipids do not increase TMAO formation [20,42].
4.3. Clinical Suggestions for Product Choice
While the amounts of choline added by milk components and lecithin-based emulsifiers are not indicated in the respective products (Appendix A, Table A1 and Table A2), they may substantially improve the choline supply to patients. Hence, for all patients at risk of choline undersupply or deficiency, that is preterm infants, CF patients, and patients of any age with malabsorption or dependence on parenteral nutrition, the choice of products comprising additional choline from milk or PC-containing emulsifiers may be useful. For example, products that do not contain choline chloride or bitartrate, may nevertheless be useful for CF patients with exocrine pancreatic insufficiency, as they may contain choline via lecithin (Table 3).
This holds true even though their quantitative contribution to choline supply is yet unknown, because of the very high upper tolerable limit of intake according to IoM and EFSA, and the virtual absence of choline toxicity (if not given intravenously as a high-dosage bolus injection) [1,2,8].
While choline supply is frequently higher than estimated from the provided information, when using such products, it may vary. In a previous analysis, two batches of a preterm infant formula, PC was the major choline component, and choline levels varied by 50%, reaching the upper limit of the ESPGHAN recommendation in one batch [40,43,44]. To the best of our knowledge, no choline toxicity has been reported for any of these products providing additional choline. Nevertheless, a declaration of choline from compounds like emulsifiers, and a straightforward biochemical analysis of formulas, fortifiers and add-on formulations would be helpful for clinicians and dieticians to enable informed choices. Compared to the value of a single batch, such costs seem negligible. Furthermore, replacing phospholipid emulsifiers with choline-free fatty acid methyl esters and similar compounds by companies is discouraged by these authors.
4.4. Limitations
While we used the most recent information on products, the market is fluctuating, and product characteristics change rapidly. Moreover, we have not evaluated the global market but focused on products being prescribed to or used by patients visiting our local outpatient clinic or during clinical treatment. However, the declared choline content, and its relation to folate, cobalamin, and long-chain polyunsaturated acids may be incorrect for those fortifiers, formula, liquid food and special nutrition products containing milk or lecithin compounds, adding natural choline components which are not quantified in the product information.
5. Conclusions
The amount of the essential nutrient choline in commercial pediatric nutritional products is heterogenous, particularly for products for children > 6 months of age. This study provides a comprehensive analysis of 105 nutritional products used in children regarding their choline content. However, choline may frequently be higher in products containing milk constituents and/or lecithin. Their contribution to choline supply, however, is unclear. Such uncertainty may have implications on product choice for children at risk of choline deficiency, such as preterm infants, and patients suffering from CF, SIBO, malabsorption or Crohn’s disease, receiving exclusive diets or are TPN-dependent.
In perspective, for all products used in patients at risk for choline deficiency, we propose a mandatory characterization or biochemical analysis to assess their total choline content, based on all contributing components.
Author Contributions
W.B. conceptualized the study and wrote the manuscript draft. W.B. and A.S. collected the data. U.G.-M., J.H., C.W. and A.R.F. contributed to product collection. C.F.P. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Appendix A and Appendix B. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the staff of the cystic fibrosis outpatient clinic and nutritional support team of Children’s Hospital, Eberhard-Karls-University, Tübingen.
Conflicts of Interest
Financial Disclosure: W.B. and A.R.F. indicate that they received grants and honoraria from Hipp, manufacturing infant formula.
Abbreviations
AI, adequate intake; ARA, arachidonic acid; CF, cystic fibrosis; DHA, docosahexaenoic acid; EFSA, European Food Safety Authority; ESPGHAN, European Society for Paediatric Gastroenterology, Hepatology and Nutrition; EU, European Union; IoM, Institute of Medicine; LC-PUFA, long-chain polyunsaturated fatty acids; GPC, glycerophosphocholine; N, number; NICU, Neonatal Intensive Care Unit; PC, phosphatidylcholine; PEMT, phosphatidylethanolamine N-methyl transferase; PI, preterm infant; SAM, S-adenosylmethionine; SIBO, small intestinal bacterial overgrowth; SNP, single nucleotide polymorphism; SPH, sphingomyelin; TI, term infant; TMA, trimethylamine; TMAO, trimethylamine oxide; TPN, total parenteral nutrition; VLDL, very low-density lipoprotein.
Appendix A

Table A1.
Products for Preterm and Term Infants and for Toddlers.
Table A1.
Products for Preterm and Term Infants and for Toddlers.
| Product Name, Producer | Choline Concentration (mg/100 kcal) | Added Choline Compound |
|---|---|---|
| Preterm Infant Fortifier | ||
| BEBA FM 85, Nestlé * | 17.3 | Not specified |
| Humavant+4, Prolacta | 0 | ∅ |
| Aptamil FMS, Nutricia | 0 | ∅ |
| Preterm Infant Formula | ||
| 0-HP-1 Expert, Humana | 38.6 | Choline bitartrate |
| 0-HP-2, Humana | 46.8 | Choline bitartrate |
| Alphare HMO, Nestlé | 29.9 | Choline bitartrate |
| BEBA expert pro Comfort, Nestlé | 33.3 | Choline bitartrate |
| BEBA FG 1, Nestlé | 25.0 | Choline bitartrate |
| Nestlé | 20.5 | Choline bitartrate |
| Aptamil HA Pre, Nutricia | 33.3 | Choline chloride |
| Aptamil PDF, Nutricia | 31.9 | Choline chloride |
| Aptamil Prematil, Nutricia | 32.5 | Choline chloride |
| Aptamil Prematil HA, Nutricia | 32.5 | Choline chloride |
| Monogen, Nutricia | 23.0 | Choline chloride |
| Term Infant Formula 0–6 months | ||
| Bebivita-PRE HA, Bebivita | 37.9 | Not specified |
| Bebivita 1 Anfangsmilch, Bebivita | 38.8 | Not specified |
| Bio Combiotic PRE, Hipp | 37.9 | Not specified |
| HA Combiotic PRE-HA, Hipp | 37.9 | Not specified |
| Bio Combiotic 1, Hipp | 38.8 | Not specified |
| HiPP Comfort, Hipp | 37.9 | Not specified |
| Humana Anfangsmilch pre, Humana | 30.8 | Choline bitartrate |
| Humana Anfangsmilch 1, Humana | 37.3 | Choline bitartrate |
| kendamil first instant milk, Kendamil | 32.8 | Choline bitartrate |
| Kendamil first organic, Kendamil | 30.3 | Choline bitartrate |
| Kendamil first goat, Kendamil | 32.8 | Choline bitartrate |
| Milumil Pre Anfangsmilch, Milupa | 36.4 | Choline chloride |
| Milumil 1 Anfangsmilch, Milupa | 32.4 | Choline chloride |
| Alfaré, Nestlé | 29.9 | Choline bitartrate |
| Alphare HMO, Nestlé | 29.9 | Choline bitartrate |
| BEBA EXPERT HA 1, Nestlé | 35.2 | Choline bitartrate |
| BEBA Optipro PRE, Nestlé | 30.8 | Choline bitartrate |
| BEBA Optipro 1, Nestlé | 30.7 | Choline bitartrate |
| BEBA Supreme Pre, Nestlé | 34.3 | Choline bitartrate |
| BEBA Supreme 1, Nestlé | 34.2 | Choline bitartrate |
| Aptamil Care Pre, Nutricia | 34.8 | Choline chloride |
| Aptamil Comfort, Nutricia | 33.3 | Choline chloride |
| Aptamil Organic PRE, Nutricia | 33.3 | Not specified |
| Aptamil Pronutra Pre, Nutricia | 33.3 | Choline chloride |
| Aptamil Pregomin, Nutricia | 28.8 | Choline chloride |
| Aptamil Pregomin AS, Nutricia | 28.0 | Choline bitartrate |
| Aptamil FMS, Nutricia | 0 | ∅ |
| Aptamil Anti Reflux, Nutricia | 33.3 | Choline chloride |
| Aptamil Care Pre, Nutricia | 34.8 | Choline chloride |
| Aptamil Bio PRE, Nutricia | 33.3 | Choline chloride |
| Aptamil Organic PRE, Nutricia | 33.3 | Not specified |
| Aptamil Organic 1, Nutricia | 33.3 | Choline chloride |
| Monogen, Nutricia | 23.0 | Choline chloride |
| Neocate Syneo, Nutricia | 27.9 | Not specified |
| Neocate Infant, Nutricia | 27.6 | Not specified |
| Holle Bio Pre, Holle | 33.3 | Choline bitartrate |
| Bio-Anfangsmilch, Holle | 33.8 | Choline bitartrate |
| Bio-Anfangsmilch 1, Holle | 33.3 | Choline bitartrate |
| Bio-Anfangsmilch 1 (from goat milk) | 32.4 | Choline bitartrate |
| Term Infants 6–12 months | ||
| Bebivita 2 Folgemilch, Bebivita | 0 | ∅ |
| Bio Combiotic 2, Hipp | 0 | ∅ |
| HiPP Bio Ziegenmilch 2, Hipp | 0 | ∅ |
| Humana Folgemilch 2, Humana | 37.3 | Choline bitartrate |
| Humana Folgemilch 3, Humana | 37.3 | Choline bitartrate |
| Follow-on milk, Kendamil | 32.8 | Choline bitartrate |
| kendamil goat, Kendamil | 24.2 | Choline bitartrate |
| Milumil 2 Folgemilch, Milupa | 25.0 | Choline chloride |
| Milumil 3 Folgemilch, Milupa | 25.0 | Choline chloride |
| BEBA Optipro 2, Nestlé | 0 | ∅ |
| BEBA Supreme 2, Nestlé | 0 | ∅ |
| BEBA Supreme 3, Nestlé | 0 | ∅ |
| BEBA EXPERT HA 2, Nestlé | 0 | ∅ |
| Aptamil Bio 2, Nutricia | 0 | ∅ |
| Humana Kindergetränk 1+, Humana | 0 | ∅ |
| Toddler milk, Kendamil | 0 | ∅ |
| Toddler milk Organic, Kendamil | 0 | ∅ |
| BEBA EXPERT HA 3, Nestlé | 0 | ∅ |
| BEBA Junior 12–36, Nestlé | 0 | ∅ |
| Aptamil Profutura DUOADVANCE 2, Nutricia | 22.1 | Choline chloride |
| Neocate Junior, Nutricia | 20.0 | Not specified |
| Nutrini Multi Fibre, Nutricia | 20.0 | Not specified |
*: for companies’ locations see legend to Table 3.

Table A2.
Products for special indications.
Table A2.
Products for special indications.
| Product Name, Producer | Choline Concentration (mg/100 kcal) | Added Choline Compound |
|---|---|---|
| RCF, Abbott * | 12 | Not specified |
| Calshake, Fresenius KABI | 0 | ∅ |
| Fresubin 5kcal Shot, Fresenius KABI | 0 | ∅ |
| Comfort Spezialnahrung (Opstipation, lactose intolerance of infants) | 37.9 | Not specified |
| Energea Pkid, MetaX | 0 | ∅ |
| Energea P, MetaX | 0 | ∅ |
| Maltocal 19, MetaX | 0 | ∅ |
| Nephea Infant (Kidney Disease), MetaX | 0 | ∅ |
| Nephea Kid (Kidney Disease), MetaX | 0 | ∅ |
| Alphamino, Nestlé | 34.3 | Choline bitartrate |
| Althera (Cow milk allergie), Nestlé | 28.8 | Choline bitartrate |
| Beba Anti Reflux, Nestlé | 31.3 | Choline bitartrate |
| Modulen IBD (Crohn’s Disease), Nestlé | 7.1 | Choline bitartrate |
| Peptamen (Tube feeding), Nestlé | 21 | Choline chloride |
| Peptamen Junior Advance (high energy tube feed), Nestlé | 16 | Choline chloride |
| Aptamil Eiweiß +, Nutricia | 0 | ∅ |
| Aptamil PDF, Nutricia | 31.9 | Choline chloride |
| Duocal, Nutricia (Cachexia) | 0 | ∅ |
| Heparon Junior, Nutricia (Liver disease) | 24.7 | Choline chloride |
| Fortimel Joghurt style, Nutricia | 36.7 | Choline chloride |
| Fortimel Pulver Neutral, Nutricia | 33.1 | Choline chloride |
| Neocate® Junior, Nutricia | 20 | Choline bitartrate |
| Infatrini 125 mL, Nutricia | 31.3 | Choline chloride |
| Infatrini Peptisorb, Nutricia | 32.1 | Choline chloride |
| Nutrini Multi Fibre, Nutricia | 20 | Choline chloride |
| NutriniDrink Smoothie, Nutricia | 19.8 | Choline chloride |
| NutriniDrink Compact Multi Fibre, Nutricia | 20 | Choline chloride |
| Nutrini creamy, Nutricia | 20 | Choline chloride |
| restoric® nephro prae, Vitasyn medical | 0 | ∅ |
| restoric® supportiv S (Malnutrition), Vitasyn medical | 0 | ∅ |
*, companies’ locations: Abbott: Wiesbaden, Germany; Fresenius KABI: Bad Homburg, Germany; other companies see legend to Table 3.
Appendix B
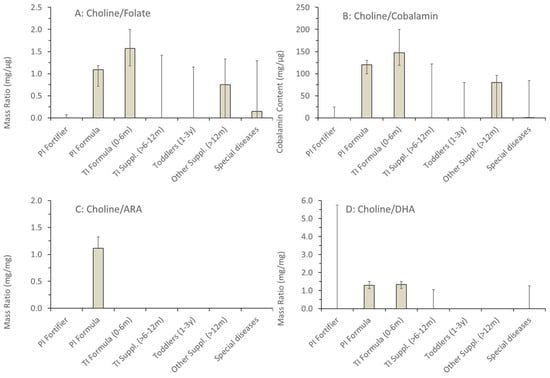
Figure A1.
Ratios in fortifiers, formula and supplements of added choline versus nutrients metabolically related to choline, namely folate (A), cobalamin (B), arachidonic acid (C) and docosahexaenoic acid (D). Data are medians and inter-quartile ranges of the numbers of samples indicated in Figure 1. Abbreviations: ARA, arachidonic acid, DHA, docosahexaenoic acid.
References
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary Reference Values for choline. EFSA J. 2016, 14, 4484. [Google Scholar] [CrossRef]
- Pynn, C.J.; Henderson, N.G.; Clark, H.; Koster, G.; Bernhard, W.; Postle, A.D. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J. Lipid Res. 2011, 52, 399–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhard, W. Choline in cystic fibrosis: Relations to pancreas insufficiency, enterohepatic cycle, PEMT and intestinal microbiota. Eur. J. Nutr. 2021, 60, 1737–1759. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, K.A.; Bernhard, W.; Minarski, M.; Shunova, A.; Wiechers, C.; Poets, C.F.; Franz, A.R. Choline supplementation for preterm infants: Metabolism of four Deuterium-labeled choline compounds. Eur. J. Nutr. 2023, 62, 1195–1205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhard, W.; Shunova, A.; Boriga, J.; Graepler-Mainka, U.; Hilberath, J. Low Plasma Choline in Cystic Fibrosis Patients Is Linked to Exocrine Pancreatic Insufficiency and Small Intestinal Bacterial Overgrowth. Nutrients 2025, 17, 868. [Google Scholar] [CrossRef]
- Song, J.; da Costa, K.A.; Fischer, L.M.; Kohlmeier, M.; Kwock, L.; Wang, S.; Zeisel, S.H. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005, 19, 1266–1271. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Choline and contribution to normal liver function of the foetus and exclusively breastfed infants: Evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2023, 21, 8115. [Google Scholar]
- Bernhard, W.; Lange, R.; Graepler-Mainka, U.; Engel, C.; Machann, J.; Hund, V.; Shunova, A.; Hector, A.; Riethmüller, J. Choline Supplementation in Cystic Fibrosis-The Metabolic and Clinical Impact. Nutrients 2019, 11, 656. [Google Scholar] [CrossRef]
- Buchman, A.L.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.H.; Rice, K.; Gornbein, J.; Ament, M.E.; Eckhert, C.D. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to betaine and contribution to normal homocysteine metabolism (ID 4325) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2052. [CrossRef]
- Bernhard, W.; Böckmann, K.A.; Minarski, M.; Wiechers, C.; Busch, A.; Bach, D.; Poets, C.F.; Franz, A.R. Evidence and Perspectives for Choline Supplementation during Parenteral Nutrition-A Narrative Review. Nutrients 2024, 16, 1873. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A. The addition of choline to parenteral nutrition. Gastroenterology 2009, 137 (Suppl. 5), S119–S128. [Google Scholar] [CrossRef] [PubMed]
- Kumpf, V.J. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr. Clin. Pract. 2006, 21, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Schön, C.; Derbyshire, E.; Jiang, X.; Mellott, T.J.; Blusztajn, J.K.; Zeisel, S.H. A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients 2024, 16, 260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaiswal, A.; Dewani, D.; Reddy, L.S.; Patel, A. Choline Supplementation in Pregnancy: Current Evidence and Implications. Cureus 2023, 15, e48538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhard, W.; Böckmann, K.; Maas, C.; Mathes, M.; Hövelmann, J.; Shunova, A.; Hund, V.; Schleicher, E.; Poets, C.F.; Franz, A.R. Combined choline and DHA supplementation: A randomized controlled trial. Eur. J. Nutr. 2020, 59, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Davidson, A.G.; Bay, B.N.; Slack, P.J.; Hasman, D. Plasma choline depletion is associated with decreased peripheral blood leukocyte acetylcholine in children with cystic fibrosis. Am. J. Clin. Nutr. 2011, 93, 564–568. [Google Scholar] [CrossRef]
- Bernhard, W.; Poets, C.F.; Franz, A.R. Choline and choline-related nutrients in regular and preterm infant growth. Eur. J. Nutr. 2019, 58, 931–945. [Google Scholar] [CrossRef]
- Böckmann, K.A.; Franz, A.R.; Minarski, M.; Shunova, A.; Maiwald, C.A.; Schwarz, J.; Gross, M.; Poets, C.F.; Bernhard, W. Differential metabolism of choline supplements in adult volunteers. Eur. J. Nutr. 2022, 61, 219–230. [Google Scholar] [CrossRef]
- Grothe, J.; Riethmüller, J.; Tschürtz, S.M.; Raith, M.; Pynn, C.J.; Stoll, D.; Bernhard, W. Plasma phosphatidylcholine alterations in cystic fibrosis patients: Impaired metabolism and correlation with lung function and inflammation. Cell Physiol. Biochem. 2015, 35, 1437–1453. [Google Scholar] [CrossRef]
- Cohen, D.E. Hepatocellular transport and secretion of biliary phospholipids. Semin. Liver Dis. 1996, 16, 191–200. [Google Scholar] [CrossRef]
- Schmitz, G.; Muller, G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991, 32, 1539–1570. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.M. The role of factors that regulate the synthesis and secretion of very-low-density lipoprotein by hepatocytes. Crit. Rev. Clin. Lab. Sci. 1998, 35, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Goss, K.C.W.; Goss, V.M.; Townsend, J.P.; Koster, G.; Clark, H.W.; Postle, A.D. Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: Implications for choline and PUFA metabolism. Am. J. Clin. Nutr. 2020, 112, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewell, L.; Mason, A.; Vance, D.E. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, C.B. An introduction to the nutrition and metabolism of choline. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 100–113. [Google Scholar] [CrossRef]
- Bernhard, W.; Raith, M.; Shunova, A.; Lorenz, S.; Böckmann, K.; Minarski, M.; Poets, C.F.; Franz, A.R. Choline Kinetics in Neonatal Liver, Brain and Lung-Lessons from a Rodent Model for Neonatal Care. Nutrients 2022, 14, 720. [Google Scholar] [CrossRef]
- Grassmé, H.; Carpinteiro, A.; Edwards, M.J.; Gulbins, E.; Becker, K.A. Regulation of the inflammasome by ceramide in cystic fibrosis lungs. Cell Physiol. Biochem. 2014, 34, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and function of the cholinergic system in immune cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Vance, D.E. Choline redistribution during adaptation to choline deprivation. J. Biol. Chem. 2007, 282, 10283–10289. [Google Scholar] [CrossRef]
- Bernhard, W.; Maas, C.; Shunova, A.; Mathes, M.; Böckmann, K.; Bleeker, C.; Vek, J.; Poets, C.F.; Schleicher, E.; Franz, A.R. Transport of long-chain polyunsaturated fatty acids in preterm infant plasma is dominated by phosphatidylcholine. Eur. J. Nutr. 2018, 57, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, Å.; Duan, R.D. Pancreatic and mucosal enzymes in choline phospholipid digestion. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G425–G445. [Google Scholar] [CrossRef] [PubMed]
- Northfield, T.C.; Hofmann, A.F. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut 1975, 16, 1–11. [Google Scholar] [CrossRef]
- Chen, A.H.; Innis, S.M.; Davidson, A.G.; James, S.J. Phosphatidylcholine and lysophosphatidylcholine excretion is increased in children with cystic fibrosis and is associated with plasma homocysteine, S-adenosylhomocysteine, and S-adenosylmethionine. Am. J. Clin. Nutr. 2005, 81, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Shunova, A.; Machann, J.; Grimmel, M.; Haack, T.B.; Utz, P.; Graepler-Mainka, U. Resolution of severe hepatosteatosis in a cystic fibrosis patient with multifactorial choline deficiency: A case report. Nutrition 2021, 89, 111348. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods-Release Two. 2008. Available online: https://data.nal.usda.gov/system/files/Choln02.pdf (accessed on 11 June 2020).
- Shunova, A.; Böckmann, K.A.; Minarski, M.; Franz, A.R.; Wiechers, C.; Poets, C.F.; Bernhard, W. Choline Content of Term and Preterm Infant Formulae Compared to Expressed Breast Milk-How Do We Justify the Discrepancies? Nutrients 2020, 12, 3815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-on Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32016R0127 (accessed on 14 February 2025).
- Cho, C.E.; Aardema, N.D.J.; Bunnel, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants: A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS guideline on nutrition care for cystic fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Maas, C.; Franz, A.R.; Shunova, A.; Mathes, M.; Bleeker, C.; Poets, C.F.; Schleicher, E.; Bernhard, W. Choline and polyunsaturated fatty acids in preterm infants’ maternal milk. Eur. J. Nutr. 2017, 56, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Nierle, W.; el Wahab el Bayâ, A. Untersuchung und Zusammensetzung einiger Leguminosen [Examination and composition of some legume seeds (author’s transl)]. Z. Lebensm. Unters. Forsch. 1977, 164, 23–27. (In German) [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).