Abstract
Background: Among the dysfunctional eating behaviors associated with excessive food intake, a construct that is gaining increasing attention is grazing—the constant, continuous, compulsive, and repetitive consumption of small/moderate amounts of food. Furthermore, in some cases, grazing seems to indicate a dependence on food and/or eating. Currently, the Repetitive Eating Questionnaire (Rep(Eat)-Q) appears to be the only questionnaire that comprehensively measures grazing, including its repetitive and compulsive eating component. Therefore, in a sample of individuals with severe obesity, the objective of this study was twofold: (A) to evaluate the psychometric properties of the Italian version of the Rep(Eat)-Q, and (B) to analyze the association between grazing and food addiction (FA). Method: A cross-sectional research design was used. A total of 402 inpatients with severe obesity (BMI > 35) were recruited. Participants underwent a series of questionnaires to investigate structural validity and convergent validity and association with FA criteria. Results: The factorial structure of the Rep(Eat)-Q is robust and showed fit indexes: CFI = 0.973; RMSEA = 0.074; 90%CI [0.056–0.091]; and SRMR = 0.029. Also, it exhibited good internal consistency and convergent validity. Furthermore, logistic regression analysis highlights a specific association between certain FA criteria and grazing. Conclusions: The Rep(Eat)-Q can be considered to be a concise, robust, reliable, and statistically sound tool to assess repetitive eating, specifically grazing. Its strong psychometric properties offer significant advantages for both research and clinical applications. Furthermore, in a sample of individuals with severe obesity, the results suggest that individuals with problematic grazing exhibit a typical behavioral profile of subjects with FA, indicating that FA can manifest through problematic grazing as well.
1. Introduction
The prevalence of people with obesity worldwide continues to increase, particularly in industrialized countries, where it has been projected that more than 85% of adults will be affected by overweight or obesity in the coming years [1,2,3]. Consequently, healthcare costs associated with overweight and obesity account for almost 10% of national healthcare expenditure [4], and it is anticipated that these costs will continue to increase over the next 15 years [5,6].
Obesity is a multifactorial chronic disease influenced by various biological factors (e.g., genetic, medical), psychological factors, and situational factors that contribute to its development and persistence [1,5,7,8,9]. One potentially crucial aspect implicated in the development and persistence of obesity is the idea that certain foods may trigger a dependency response in some individuals [10,11,12]—namely, food addiction (FA). FA is a complex concept that involves compulsive overeating and a loss of control over food consumption, characterized by behaviors similar to those seen in substance use disorders (SUDs) [13,14,15,16,17].
It has, therefore, a dual nature [18]: a component associated with SUDs and a component related to the nature of eating disorders (EDs) [19,20,21,22,23,24].
Specifically, being driven by unhealthy eating patterns [25,26,27,28,29,30], the ease of access to highly processed foods (HPFs) [31,32,33]—which are highly palatable and psychologically rewarding [34,35,36,37,38]—may predispose people to develop an addiction. These foods have the potential to activate neural reward systems [34,35,36,37,38], requiring the individual to seek the same feelings of well-being and pleasure. If these cravings are not satisfied, this could lead to the appearance of withdrawal symptoms [39,40,41]. Furthermore, the sustained consumption of HPFs can lead the individual to develop tolerance to the substance, thus necessitating increased intake to achieve the same rewarding effect.
On the other hand, FA can promote dysfunctional eating behaviors, such as spending a significant amount of time thinking about food or, as observed in cases of emotional eating, using food as an external regulator for intense (often negative) and/or uncontrollable emotions [42,43,44,45]. Additionally, individuals with FA commonly experience significant social and psychological impairment [24,46] attributed to cravings for HPFs [35] and an inability to stop or moderate their intake, even when they recognize negative consequences [16,47]. Furthermore, when attempting to reduce or eliminate the consumption of addictive foods, people can experience withdrawal symptoms such as irritability, anxiety, or mood swings [48]. This could lead to the misconception that binge eating behavior could represent a prototypical manifestation of FA [49,50,51,52]. However, while some individuals who engage in binge eating may exhibit behaviors similar to those seen in substance addiction, such as engaging in episodes of compulsive overeating characterized by a loss of control and consuming large amounts of food in a short period of time, not all instances of binge eating can be attributed to FA.
Binge eating disorder (BED), for example, is a distinct psychological condition characterized by recurrent episodes of uncontrollably eating large amounts of food in a short period, often to the point of discomfort or distress. Individuals with BED may experience feelings of guilt, shame, or a loss of control during or after binge eating episodes. While there may be overlap in some symptoms with addictive behaviors, such as cravings and food preoccupation, BED is recognized as a separate disorder in the diagnostic and statistical manual of mental disorders (DSM-5). Notably, FA is instead not yet formally recognized as a diagnostic category in the DSM-5.
In addition, food addiction-like behaviors, such as eating large quantities of food followed by purging or compensatory behaviors, can be present in individuals with bulimia nervosa (BN), and, although less common, some individuals with anorexia nervosa (AN) may also experience FA during periods of binge eating or when attempting to reintroduce food after prolonged restriction.
Recent research findings also indicates an association between FA and other problematic eating patterns [53,54], such as grazing behavior [55,56,57]. It is defined as the constant, repetitive, compulsive, and unplanned consumption of small/moderate amounts of food throughout the day, commonly without structured meal times [55,58,59]. According to the conceptualization provided by Conceição and colleagues [58], there are two types of grazing. The first type of grazing (non-compulsive grazing/repetitive eating) represents a manner of eating distractedly and without particular thoughts [55,58]. This type of grazing is associated with a lesser loss of control and is also less predisposing to binge eating behavior [60]. The second type (compulsive grazing) would reflect the feeling that the individual cannot resist food and that the individual is tempted to eat again even when trying to resist [55,58]. This type of grazing is associated with a larger loss of control, predisposing to binge eating behavior, and subsequent negative emotional states. Compulsive grazing appears to be associated with greater psychological distress [55], including negative affect [61], anxiety, depression, and poorer mental health [49]. Furthermore, it is associated with a higher body mass index (BMI), food inhibition, and hunger [49], as well as the failure to lose weight after bariatric surgery interventions [49,60,62,63]. In this regard, evidence suggests that patients who exhibit binge eating behaviors before surgery might develop grazing behaviors after surgery, especially when large quantities of food cannot be consumed [49,64].
While not classified as a distinct diagnosis in the DSM-5, grazing behavior warrants attention in the assessment and treatment of eating disorders, particularly BED and other specified feeding or eating disorders, as it may also be observed in individuals with atypical presentations of eating disorders.
For the systematic screening and early identification of grazing behavior [55,58], Conceição and colleagues (2017) developed a brief, easily administered self-report tool: the Repetitive Eating Questionnaire (Rep(Eat)-Q) [55]. It comprises 12 easily interpretable items measured on a Likert scale, which refer to two distinct but highly correlated factors: the compulsivity factor and the repetitiveness factor. In the original validation study [55], the questionnaire was administered to two different populations: a community sample and a clinical sample of individuals enrolled in a bariatric surgery program.
However, despite its importance as a predictive factor for weight loss, to date the Rep(Eat)-Q is not available for use in the Italian population [62], and the relationship between grazing and FA requires further empirical confirmation. Therefore, considering a sample of individuals with severe obesity, the purpose of this study was twofold: (A) to evaluate the psychometric properties of the Italian version of the Rep(Eat)-Q (Part I) and (B) to shed new light on the association between grazing and FA (Part II).
2. Materials and Methods
2.1. Translation and Cultural Adaptation
The Rep(Eat)-Q underwent a process of translation and cultural adaptation conforming to international guidelines [65]. Thus, to ensure consistency across languages, a back-to-back translation process was carried out. The final translation (see Supplementary Material) was then administered to a sample of 10 individuals to assess the comprehensibility of the items. No further adjustments were needed. The Italian version of the Rep(Eat)-Q is reported in the Supplementary Materials.
2.2. Sample Size Determination
The decision regarding the sample size was made a priori. According to guidelines [66,67], the ‘n:q’ criterion (that is, the number of individuals per parameter) was adopted. A minimum sample of 10 individuals per parameter (=25) was enrolled. Consequently, a minimum sample of 250 participants was ensured.
2.3. Procedure
According to previous studies [68,69], participants were individually enrolled in the IRCCS Istituto Auxologico Italiano, San Giuseppe Hospital in Piancavallo, Verbania, Italy—a hospital for the treatment and rehabilitation of people with severe obesity (BMI > 35). The recruitment materials provided comprehensive information about eligibility criteria and further details to ensure that participants could make informed decisions. This included guarantees of anonymity of responses.
Inclusion criteria for participating in the study were as follows: (A) native-Italian speaker, (B) aged ≥18 years, and (C) having a BMI > 35. On the contrary, subjects were excluded from the study if (A) they were unable to complete the survey and (B) they did not provide informed consent.
This study received approval from the Ethics Committee of the IRCCS Istituto Auxologico Italiano (protocol no 2020_02_18_04).
2.4. Participants
A sample of 402 participants with severe obesity was enrolled. The sample was composed of 179 (44.5%) males and 223 (55.5%) females, aged 19 to 82 (mean = 55.25, SD = 12.92), with a body mass index (BMI) that ranged from 35.08 to 79.27 (mean = 42.28, SD = 6.46).
2.5. Measures
Respondents were asked to explain their main socio-demographic (i.e., age, sex, civil and educational status) and clinical characteristics (i.e., height and weight to compute the BMI).
2.5.1. The Repetitive Eating Questionnaire (Rep(Eat)-Q)
The Rep(Eat)-Q [55] is a brief self-report measure developed to assess the frequency of attitudinal and behavioral features of grazing over the past 28 days (see Supplementary Materials). It comprises 12 items answered on a 7-point Likert-type scale ranging from 0 (Never) to 6 (Every day), with higher scores representative of higher frequency. The Rep(Eat)-Q is composed of two scales. The first, repetitive eating (RE), measures grazing associated with eating in a distracted, disorderly, and unaware manner, which seems to be predisposed to binge eating. The second scale, compulsive grazing (CG), measures the behavior of taking small/moderate amounts of food through involuntary and compelling behaviors—which the individual cannot resist—and that can cause distress. The total score and two scales, CG and RE, are calculated by averaging the scale items. A cut-off score of 1.25 on the total score suggests the presence of problematic/pathological grazing. The Rep(Eat)-Q is currently the grazing measure with the strongest psychometric support in the literature [70].
2.5.2. The Modified Yale Food Addiction Scale 2.0 (mYFAS 2.0)
The mYFAS 2.0 [71,72] is a self-report tool designed to evaluate addictive eating behaviors through 13 items, each rated on an 8-point Likert-type scale. Of these items, 11 correspond to the DSM-5 diagnostic criteria for substance use disorder (SUD), while the other 2 specifically address the food-related deterioration or emotional distress experienced by the individual during the preceding 12 months. To diagnose FA, two scoring procedures must be considered: the symptom count score, which counts the diagnostic criteria met by the individual, and the diagnostic score, which considers the presence of impairment/emotional distress criteria (No FA; mild FA; moderate FA; and severe FA) [71]. In the current study, the mYFAS 2.0 shows a McDonald’s omega for categorical data equal to 0.908.
2.5.3. The Binge Eating Scale (BES)
The BES [73,74] is used as a self-report instrument to measure the intensity of binge eating in various settings, including both community and clinical ones [24,75]. It comprises 16 questions that describe both behavioral aspects of BED, such as fast food or consuming large quantities of food, and associated feelings and cognitions, such as the fear of not being unable to stop eating. Each item has three to four levels of symptom descriptions. For BED to be diagnosed, a cut-off point should be reached in the total score [76]. The reliability and validity of the BES as a measure of eating-related pathology are confirmed in several studies with both clinical and community samples [77,78]. The internal consistency (McDonald’s omega) of the BES in the present study was 0.909.
2.5.4. The Measure of Eating Compulsivity (MEC10)
The MEC10 [54,79] is a brief, feasible, solid, and extremely reliable tool consisting of 10 items answered on a 5-point Likert-type scale aimed at measuring compulsive eating behaviors and binge eating behaviors. High scores correspond to a high degree of eating compulsivity. A cut-off score suggests the presence of BED. The internal consistency (McDonald’s omega) of the MEC10-IT in the present study was equal to 0.949.
2.5.5. The Three-Factor Eating Questionnaire Revised—18 (TFEQ-R-18)
The TFEQ-R-18 [80,81] is a reliable, solid, and psychometrically sound questionnaire that consists of 18 items measured on a 4-point Likert-type scale designed to assess three main cognitive and behavioral domains of eating disorders: cognitive restraint (CR), uncontrolled eating (UE), and emotional eating (EE). High scores reflect a higher level of each dimension. In this study, the internal consistency (McDonald’s omega) of the IT-TFEQ-R-18 scales was 0.747 for the CR scale, 0.914 for the UE scale, and 0.874 for the EE scale.
2.6. Statistical Analysis
Statistical analyses were run with R software (v. 4.3.2) and the following packages: ggplot2 [82], lavaan [83], lme4 [84], psych and psychTools [85,86], semPlot [87], and tidyverse [88].
Considering the first objective of this study, a confirmatory factor analysis (CFA) was performed. According to the original validation study [55], a first-order model with a correlated factor was specified (see Figure 1).
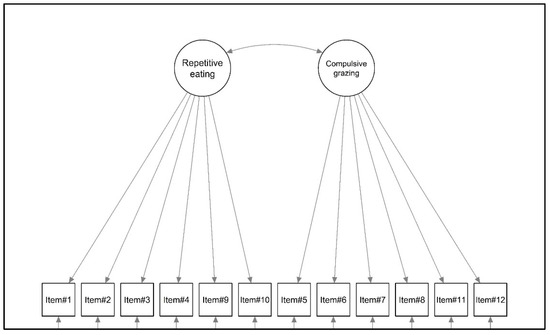
Figure 1.
Graphical representation of the factorial structure of the Rep(Eat)-Q.
Taking into account that some items should not be normally distributed, the MLR estimator (that is, robust maximum likelihood) was employed to evaluate the factorial structure of the Rep(Eat)-Q [66,67]. The fit was assessed using (A) the Yuan–Bentler chi-square statistic (YBχ2), (B) the approximation error of approximation (RMSEA), (C) the comparative fit index (CFI), and (D) the Standardized Root Mean Residual (SRMR). To assess the goodness of fit, cutoff criteria were used: (A) statistical nonsignificance of YBχ2, (B) an RMSEA lower than 0.08, (C) a CFI higher than 0.95, and (D) an SRMR lower than 0.08 [67,89,90].
Once the factorial structure of the Rep(Eat)-Q was tested, its internal consistency was evaluated with McDonald’s omega (ω) [91]. Additionally, the adjusted item-total correlation was calculated [92]. The assessment of convergence validity was performed using the Pearson correlation coefficient [92], with interpretations guided by Cohen’s benchmarks: r < 0.10, negligible; r ranging from 0.10 to 0.30, minimal; r ranging from 0.30 to 0.50, moderate; and r > 0.50, large [93].
Furthermore, considering the potential difference between males and females, a series of pairwise comparisons (independent sample t-tests) were conducted: independent variable, sex; dependent variables, Rep(Eat)-Q total score, Rep(Eat)-Q RE, and Rep(Eat)-Q CG. The results were interpreted using Cohen’s d and its benchmarks: small (d: 0.20 to 0.49), moderate (d: 0.50 to 0.79), and large (d > 0.80) [93].
Taking into account the second aim of this study, to test the association between problematic grazing and FA criteria, two different statistical analyses were performed. In each of them, grazing was used as a dichotomous dependent variable by dividing participants into two groups (0 = non-problematic grazing vs. 1 = problematic grazing) using the cut-off of the Rep(Eat)-Q (=1.25); meanwhile, FA criteria—measured with the mYFAS2.0 (0 = non-endorsed vs. 1 = endorsed)—were used as independent variable(s).
First, a series of simple bivariate chi-square tests (χ2) (2 × 2 contingency tables) were performed to test the simple bivariate association between the criteria of grazing and FA. The strength of the association was measured with the Phi (ϕ) coefficient, which was interpreted with Cohen’s benchmarks [93]: ϕ < 0.10, negligible; ϕ ranging from 0.10 to 0.30, minimal; ϕ from 0.30 to 0.50, moderate; and ϕ > 0.50, large. Furthermore, for each simple bivariate association, the odds ratio was also calculated: a positive odds ratio (OR) suggests that as the approval of FA criteria (independent variable) increases, there is a higher likelihood of problematic grazing (outcome).
Second, to verify the actual contribution of each FA criterion (controlling for all FA criteria) to the probability of presenting problematic grazing, a multiple logistic regression analysis was performed. The Hosmer–Lemeshow’s test was performed to test the goodness of model fit (a non-significant p-value is preferred). Cox and Snell’s PseudoR2 and Nagelkerke’s PseudoR2 coefficients were chosen as indices of the degree of variance explained. Furthermore, the OR for each predictor was calculated; even in this case, a positive odds ratio indicates that an increase in the acceptance of FA criteria (independent variable) is associated with a higher probability of experiencing problematic grazing (outcome). All regression coefficients (β) were not standardized.
3. Results
3.1. Part I: Psychometric Properties of the Italian Rep(Eat)-Q
3.1.1. Structural Validity
The two-factor model (Figure 1) showed a good fit to the data for the sample of patients with severe obesity; all of the fit indices revealed a good fit to the data: YBχ2 (53) = 121.750, p < 0.001, the CFI = 0.973, RMSEA = 0.074, 90%CI [0.056–0.091], p (RMSEA < 0.05) < 001, and SRMR = 0.029. The standardized covariance between latent factors was equal to 0.926. Standardized factor loadings ranged from 0.689 (item#1—Repetitive eating) to 0.899 (item#10—Repetitive eating). All statistics are shown in Table 1.

Table 1.
Item descriptive statistics, item psychometric properties, and confirmation factor analysis.
3.1.2. Internal Consistency
Internal consistency analysis shows excellent values for the two repeat scales (RE McDonald’s omega = 0.938; CG McDonald’s omega = 0.927) and for all aggregate items (McDonald’s omega = 0.960).
3.1.3. Convergent Validity
As shown in Table 2, large correlations were found among the Rep(Eat)-Q scales and the total score. It should be noted that the Rep(Eat)-Q scales exhibit high associations with convergent validity scales that are linked to excessive food intake and uncontrolled behaviors.

Table 2.
Correlations among variables.
Considering the Rep(Eat)-Q total score, a large association was found with the TFEQ-R-18 UE scale (r = 0.753, p < 0.001), the BES (r = 0.694, p < 0.001), and the mYFAS2.0 symptom count (r = 0.694, p < 0.001). Moreover, considering the Rep(Eat)-Q repetitive eating scale, a large association was found with the TFEQ-R-18 UE scale (r = 0.690, p < 0.001), the mYFAS2.0 symptom count (r = 0.639, p < 0.001), and the BES (r = 0.633, p < 0.001). Lastly, considering the Rep(Eat)-Q compulsive grazing scale, a large association was found with the TFEQ-R-18 UE scale (r = 0.762, p < 0.001), the MEC10 (r = 0.737, p < 0.001), and the BES (r = 0.706, p < 0.001)
3.1.4. Differences between Males and Females
As shown in Figure 2, small-to-moderate differences were found between males and females, with the female sample scoring higher on all scales. Specifically, considering the total grazing scale (Rep(Eat)-Q total), males (M = 1.404; SD = 1.310) reported slightly lower scores compared to females (M = 1.962; SD = 1.652): t = −3.685; p < 0.001; d = |0.370|. Furthermore, considering the RE scale, males (M = 1.417; SD = 1.373) reported slightly lower scores compared to females (M = 1.814; SD = 1.675): t = −2.559; p = 0.011; d = |0.275|. Lastly, also considering the CG scale, males (M = 1.392; SD = 1.367) reported slightly lower scores compared to females (M = 2.110; SD = 1.725): t = −4.540; p < 0.001; d = |0.456|.
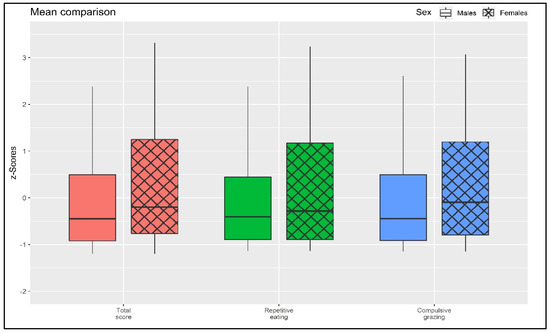
Figure 2.
Mean comparison.
3.2. Part II: Association between Grazing and Food Addiction Criteria
3.2.1. Bivariate Associations
A series of chi-square tests were conducted to assess the association between grazing (non-problematic vs. problematic) and meeting the criteria for FA. As reported in Table 3 and Figure 3, all criteria for FA are statistically significantly associated (all p-values < 0.001) with grazing, with an effect size ranging from small (ϕ = 0.198; Criterion C, “Social activities given up or reduced”) to moderate/high (ϕ = 0.480; Criterion F, “Use despite knowledge of adverse consequences”). Furthermore, a simple bivariate OR indicates that endorsing Criterion C for FA is associated with a 4.863 times higher risk of having problematic grazing compared to those who do not endorse it. Also, endorsing Criterion F for FA is associated with a 15.972 times higher risk of having problematic grazing compared to those who do not endorse it.

Table 3.
Contingency tables.
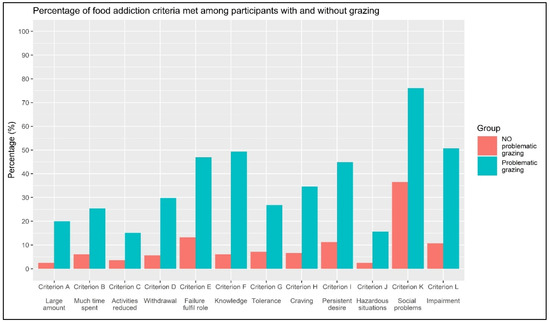
Figure 3.
Percentage of food addiction criteria met among participants with and without problematic grazing.
3.2.2. Logistic Regression Analysis
A binary logistic regression analysis was conducted, regressing the FA criteria (independent variables: 0 = not endorsed vs. 1 = endorsed) onto grazing (dependent variable: 0 = non-problematic grazing vs. 1 = problematic grazing). The model demonstrated a good fit to the data: Hosmer–Lemeshow’s test = 4.134; p = 0.530 ns. When considering all FA criteria simultaneously, many of the previously observed associations are no longer statistically significant (Table 4 and Figure 4). However, it should be noted that four criteria were still significantly associated with grazing. Specifically, these were Criterion F (“Use despite knowledge of adverse consequences”: β = 1.463; OR = 4.319, 95%CIOR [1.922; 9.707]), Criterion I (“Persistent desire or unsuccessful attempts to quit”: B = 0.779; OR = 2.180, 95%CIOR [1.126; 4.221]), Criterion K (“Continued use despite social or interpersonal problems”: β = 0.844; OR = 2.325, 95%CIOR [1.389, 3.892]), and Criterion L (“Use causes clinically significant impairment or distress”: β = 0.755, OR = 2.127, 95%CIOR [1.040, 4.348]). The degree of explained variance (PseudoR2) according to Cox and Snell was 0.320, and according to Nagelkerke, it was 0.426. Results are reported in Table 4 and Figure 3.

Table 4.
Logistic regression analysis.
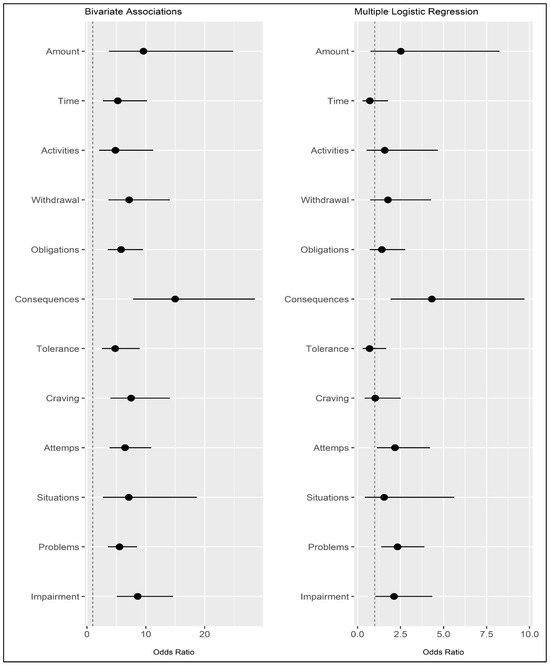
Figure 4.
Odds ratios and their confidence intervals (95%) for bivariate associations (left) and multiple logistic regression (right). Note: Dependent variable: grazing (0 = non-problematic vs. 1 = problematic). Independent variable: FA criteria measured with the mYFAS2.0 (0 = absent vs. 1 = present). The dotted line is positioned at the value of 1 (OR not statistically significant).
4. Discussion
The global prevalence of obesity is on the rise [2,3], and a contributing factor may be the concept that certain foods can trigger a dependency response, known as FA [10,11,12]. FA is associated with dysfunctional eating behaviors [53], including excessive thoughts about food and the use of food as a regulator of intense emotions [42,43,44,45]—which can lead individuals to experience a loss of control, leading to binge eating behaviors. While binge eating is often associated with FA, recent research suggests that FA is also linked to other problematic eating patterns, such as grazing behavior [55]—the constant, repetitive, compulsive, and unplanned consumption of small quantities of food [55,58].
FA and grazing represent distinct eating behaviors that often intersect and reinforce each other, contributing to unhealthy eating patterns and potential weight-related issues. Grazing on addictive foods can, indeed, reinforce cravings associated with FA, as each instance of grazing provides an opportunity for individuals to consume more of the foods they are addicted to, thus perpetuating the cycle of cravings and consumption. Moreover, grazing can contribute to a loss of control over eating, particularly when individuals continuously consume foods they find addictive. The lack of structured mealtimes and constant access to food can further make it challenging for individuals to regulate their intake and resist cravings. Moreover, the combination of FA and grazing can create a negative reinforcement loop, where individuals consume addictive foods in response to cravings, which in turn reinforces the addictive behaviors and leads to further grazing and overeating. This can exacerbate symptoms of BED, with individuals experiencing intense cravings for specific foods and engaging in frequent episodes of compulsive overeating, and also contribute to weight gain and obesity, as individuals consume excessive calories from addictive foods throughout the day without regard for hunger cues or nutritional balance.
Still, more empirical confirmation is needed to establish the relationship between grazing and FA.
This cross-sectional study had two main objectives. First, (A) aimed to investigate the psychometric properties of the Italian version of the Rep(Eat)-Q, designed to measure grazing in a large sample of patients with severe obesity. Additionally, (B) aimed to examine the association between the presence of problematic grazing and the acceptance of FA criteria in a large sample of patients with severe obesity.
Considering the first aim of the study, the results of the CFA showed that the Rep(Eat)-Q has a first-order two-factor structure with a good fit to the data. Furthermore, all items loaded in a high (λs ≥ 0.69) and statistically significant way on their respective factors, namely repetitive eating and compulsive eating. Moreover, the internal consistency was good for all the grazing scales. In summary, the Rep(Eat)-Q emerges as a short, useful, reliable, and statistically valid instrument for evaluating repetitive eating behaviors, particularly grazing among people with obesity. Its robust psychometric characteristics present notable benefits for both research aims and clinical evaluations.
Furthermore, these findings support the convergent validity of the Rep(Eat)-Q, as it was positively associated in a statistically significant way with measures of uncontrolled eating, binge eating, compulsive eating, and FA. In particular, these findings are consistent with the current scientific literature showing that repetitive eating might be more strongly related to uncontrolled and binge eating behaviors that make individuals more prone to excessive food intake [55,58,94]. On the one hand, these results support evidence showing that repetitive eating could be a potential predictor of binge eating behaviors [55,58,60]. On the other hand, the compulsive grazing scale shows a strong positive association with compulsive eating, suggesting that the grazing behavior may be the result of an urge to eat that cannot be postponed [54,55,58,61]. In the end, all grazing scales (total, RE, CG) are strongly associated with the FA symptom count score.
Furthermore, it is worth emphasizing that the results show how females have (slightly/moderately) higher levels of grazing in all its facets, namely, repetitive eating and compulsive grazing. In particular, it appears that the levels of compulsive grazing are higher (d = |0.456|) in this regard, suggesting that females may be (slightly) more prone to the inability to resist feelings and sensations related to food.
Considering the second objective of this study, the frequency analysis (Table 3 and Figure 2) shows that there is a systematic association between the presence of all FA criteria and the presence of problematic grazing. In particular, subjects who meet the FA criteria are those with problematic grazing and vice versa—it should be noted that it is not possible to establish temporal/causal relationships between variables.
Taking into account the bivariate association and not controlling for all independent variables, people with problematic grazing appear to be more prone to consume food despite knowledge of adverse effects (Criterion F; ϕ = 0.480), to eat despite social or interpersonal problems (Criterion K; ϕ = 0.399), to try unsuccessfully try to stop eating (Criterion I; ϕ = 0.374), to not be able to fulfill major role obligations (Criterion E; ϕ = 3.66), and to develop craving symptoms (Criterion H, ϕ = 0345).
Consistent with these results, the logistic regression analysis, controlling for all independent variables, appears to confirm the previously observed results. It suggests that, among all the criteria for food addiction, only four appear to play a statistically significant role in the association with problematic grazing. Specifically, individuals with problematic grazing appear to have a 4.32 times higher risk of eating despite the awareness that such behavior will have negative consequences (Criterion F). Simultaneously, people with problematic grazing would also show a 2.33 times higher risk of encountering social and interpersonal problems (Criterion K); a 2.18 times higher risk of being unable to stop during substance use (Criterion I); and a 2.13 times higher risk of having clinically significant problems or distress (Criterion L).
These results suggest that problematic grazing is not only correlated with aspects intuitively linked to excessive food intake, which can lead the individual to develop obesity. Problematic grazing is also related to aspects and symptoms of FA more closely associated with addiction itself, such as craving, social and interpersonal problems, and the inability to stop using the substance (food). In particular, in this last aspect, the overlap between symptoms of FA and grazing is evident, specifically in the compulsive eating component (grazing) that the individual is unable to interrupt (addiction).
Thus, considering patients with severe obesity, the association between problematic grazing and Criteria I could be particularly important. It is commonly believed that people with FA predominantly engage in binge eating behaviors, consuming excessive amounts of food in a short period [35,53,54]. Consequently, there is a tendency to think that bariatric surgery can prevent the behavioral manifestation of FA, such as loss of control, binge eating, compulsive overeating, and severe obesity [95,96,97,98,99,100]. However, it is not often considered that FA can also manifest as the compulsive eating of small amounts of food over a long (but consistent) period—namely, compulsive grazing [49,55]. Supporting this hypothesis, the literature has shown that individuals with problematic grazing undergoing bariatric surgery appear to be more prone to failing in maintaining weight lost immediately after surgery, suggesting that the absence/presence of grazing could be a central predictor of the success/failure of these interventions [49,60,62,63]. Therefore, the demonstrated association suggests that FA can also manifest through problematic grazing. Therefore, those who show problematic grazing have the typical behavioral profile of subjects with FA.
4.1. Limitations and Strengths
Regarding the limitations of this study, the cross-sectional research design did not allow for the testing of the stability of the results over time; the sample consisted only of patients with obesity, thus limiting the generalizability of results to other populations; and only self-report assessment tools were used despite their potential proneness to social desirability biases. Furthermore, factors such as dieting practices, medication use, and socioeconomic status were not considered. Future research may try to overcome these limitations by extending this study to other populations (e.g., community samples) and by using a longitudinal study design monitoring the levels of psychological variables over time [101]. This will allow for the testing of the measurement invariance of the Rep(Eat)-Q across various countries and longitudinally. Future studies will also test the discriminant validity of the Rep(Eat)-Q with other measures related to eating [102].
Regarding the strengths of this research, this is the first Italian study that provides the validation of the Rep(Eat)-Q which is widely used as it can predict the failure of bariatric surgery in weight reduction [49,60,62,63]. If regularly included in the assessment batteries, the Rep(Eat)-Q can lead to several benefits, which are both clinical and economic. The other strengths of this research are the wide sample that allowed accurate estimates in the statistical models, the good psychometric properties of the tool, and the use of rigorous and well-established statistical methods according to current guidelines. Furthermore, the demonstrated associations seem to suggest that FA may manifest not only through loss of control and excessive food intake but also through compulsive and constant behaviors. This implies alternative pathways for conceptualizing the constructs of grazing and FA.
4.2. Clinical Implications
The Rep(Eat)-Q and the results previously showed have crucial clinical implications. Providing validation of a questionnaire measuring grazing and showing its significant association with FA can raise awareness about the importance of these constructs, grazing, and FA among healthcare professionals.
Indeed, the absence of specific criteria for diagnosing FA and grazing can make it challenging for clinicians to identify these behaviors within the framework of traditional psychiatric diagnoses. Professionals, therefore, need to rely on measures of established validity and reliability to identify and address these behaviors and the underlying contributors.
Furthermore, considering that many people with obesity undergo bariatric surgery to achieve weight reduction, the majority experience weight gain after an initial decrease, with the inability to consume large amounts of food quickly leading them to adopt grazing as an eating strategy, which is potentially influenced by an underlying FA. Thus, these findings show that grazing is strongly associated with FA criteria, suggesting that individuals exhibiting problematic grazing demonstrate a typical behavioral profile of subjects with FA. Thus, targeting grazing may indirectly lower FA levels, consequently helping patients with obesity to recover functional eating behaviors.
5. Conclusions
In conclusion, evaluating grazing habits emerges as a worthwhile strategy to improve weight management in individuals with severe obesity. Identifying this problematic eating pattern can pose challenges due to its minimal psychological impact, which is often overlooked by both patients and clinicians. However, adopting the Rep(Eat)-Q, a brief self-report questionnaire with good psychometric properties, offers valuable clinical insights to inform practitioners’ efforts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16070949/s1; File S1: The Italian version of the Rep(Eat)-Q.
Author Contributions
Conceptualization, A.A.R.; methodology, A.A.R.; formal analysis, A.A.R.; writing—original draft preparation, G.P. and A.A.R.; writing—review and editing, M.S.; supervision, S.M. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the IRCCS Istituto Auxologico Italiano (protocol no. 2020_02_18_04).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on a reasonable request due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Corsica, J.A.; Perri, M.G. Obesity. In Handbook of Psychology: Health Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Volume 9, pp. 121–145. ISBN 978-0-471-38514-1. [Google Scholar]
- Liu, Y.; Song, Y.; Hao, Q.; Wu, J. Global Prevalence of Osteosarcopenic Obesity amongst Middle Aged and Older Adults: A Systematic Review and Meta-Analysis. Arch. Osteoporos. 2023, 18, 60. [Google Scholar] [CrossRef]
- Heeren, F.A.N.; Darcey, V.L.; Deemer, S.E.; Menon, S.; Tobias, D.; Cardel, M.I. Breaking down Silos: The Multifaceted Nature of Obesity and the Future of Weight Management. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220215. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Serdula, M.K.; Dietz, W.H.; Bowman, B.A.; Marks, J.S.; Koplan, J.P. The Continuing Epidemic of Obesity in the United States. JAMA 2000, 284, 1650–1651. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will All Americans Become Overweight or Obese? Estimating the Progression and Cost of the US Obesity Epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef]
- Sturmberg, J.P. Obesity—A Multifaceted Approach: One Problem—Different Models—Different Insights and Solutions. In Health System Redesign: How to Make Health Care Person-Centered, Equitable, and Sustainable; Sturmberg, J.P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 213–232. ISBN 978-3-319-64605-3. [Google Scholar]
- Mandlik, M.; Oetzel, J.G.; Kadirov, D. Obesity and Health Care Interventions: Substantiating a Multi-Modal Challenge through the Lens of Grounded Theory. Health Promot. J. Austr. 2021, 32, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Schultes, B.; Ernst, B.; Hallschmid, M.; Bueter, M.; Meyhöfer, S.M. The ‘Behavioral Balance Model’: A New Perspective on the Aetiology and Therapy of Obesity. Diabetes Obes. Metab. 2023, 25, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Neff, K.M.H.; Fay, A.; Saules, K.K. Foods and Nutritional Characteristics Associated with Addictive-Like Eating. Psychol. Rep. 2022, 125, 1937–1956. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; DiFeliceantonio, A.G. The Risks of Misclassifying Addictive Food Substances as Non-Addictive. Addiction 2023, 118, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; DiFeliceantonio, A.G. Highly Processed Foods Can Be Considered Addictive Substances Based on Established Scientific Criteria. Addiction 2023, 118, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Hebebrand, J. The Concept of “Food Addiction” Helps Inform the Understanding of Overeating and Obesity: Debate Consensus. Am. J. Clin. Nutr. 2021, 113, 274–276. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An Examination of the Food Addiction Construct in Obese Patients with Binge Eating Disorder. Int. J. Eat. Disord. 2012, 45, 657–663. [Google Scholar] [CrossRef]
- Schulte, E.M.; Joyner, M.A.; Potenza, M.N.; Grilo, C.M.; Gearhardt, A.N. Current Considerations Regarding Food Addiction. Curr. Psychiatry Rep. 2015, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Gearhardt, A.N. Food Addiction in the Light of DSM-5. Nutrients 2014, 6, 3653–3671. [Google Scholar] [CrossRef]
- Meule, A.; Hermann, T.; Kübler, A. Food Addiction in Overweight and Obese Adolescents Seeking Weight-Loss Treatment. Eur. Eat. Disord. Rev. 2015, 23, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Davis, C. Compulsive Overeating as an Addictive Behavior: Overlap Between Food Addiction and Binge Eating Disorder. Curr. Obes. Rep. 2013, 2, 171–178. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Five Years of the Yale Food Addiction Scale: Taking Stock and Moving Forward. Curr. Addict. Rep. 2014, 1, 193–205. [Google Scholar] [CrossRef]
- Schulte, E.M.; Wadden, T.A.; Allison, K.C. An Evaluation of Food Addiction as a Distinct Psychiatric Disorder. Int. J. Eat. Disord. 2020, 53, 1610–1622. [Google Scholar] [CrossRef]
- Gordon, E.L.; Ariel-Donges, A.H.; Bauman, V.; Merlo, L.J. What Is the Evidence for “Food Addiction?” A Systematic Review. Nutrients 2018, 10, 477. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating Addiction”, Rather than “Food Addiction”, Better Captures Addictive-like Eating Behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef]
- Albayrak, Ö.; Wölfle, S.M.; Hebebrand, J. Does Food Addiction Exist? A Phenomenological Discussion Based on the Psychiatric Classification of Substance-Related Disorders and Addiction. Obes. Facts 2012, 5, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, G.M.; Rossi, A.; Pietrabissa, G.; Mannarini, S.; Fabbricatore, M.; Imperatori, C.; Innamorati, M.; Gearhardt, A.N.; Castelnuovo, G. Structural Validity, Measurement Invariance, Reliability and Diagnostic Accuracy of the Italian Version of the Yale Food Addiction Scale 2.0 in Patients with Severe Obesity and the General Population. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 345–366. [Google Scholar] [CrossRef]
- Abiri, B.; Valizadeh, M.; Nasreddine, L.; Hosseinpanah, F. Dietary Determinants of Healthy/Unhealthy Metabolic Phenotype in Individuals with Normal Weight or Overweight/Obesity: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5856–5873. [Google Scholar] [CrossRef] [PubMed]
- Al-Jawaldeh, A.; Abbass, M.M.S. Unhealthy Dietary Habits and Obesity: The Major Risk Factors Beyond Non-Communicable Diseases in the Eastern Mediterranean Region. Front. Nutr. 2022, 9, 817808. [Google Scholar] [CrossRef] [PubMed]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary Patterns Associated with Obesity Outcomes in Adults: An Umbrella Review of Systematic Reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef] [PubMed]
- Brytek-Matera, A.; Obeid, S.; Akel, M.; Hallit, S. How Does Food Addiction Relate to Obesity? Patterns of Psychological Distress, Eating Behaviors and Physical Activity in a Sample of Lebanese Adults: The MATEO Study. Int. J. Environ. Res. Public. Health 2021, 18, 10979. [Google Scholar] [CrossRef] [PubMed]
- Romero-Blanco, C.; Hernández-Martínez, A.; Parra-Fernández, M.L.; Onieva-Zafra, M.D.; Prado-Laguna, M.d.C.; Rodríguez-Almagro, J. Food Addiction and Lifestyle Habits among University Students. Nutrients 2021, 13, 1352. [Google Scholar] [CrossRef]
- Pachucki, M.A. Food Pattern Analysis over Time: Unhealthful Eating Trajectories Predict Obesity. Int. J. Obes. 2012, 36, 686–694. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which Foods May Be Addictive? The Roles of Processing, Fat Content, and Glycemic Load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Schulte, E.M.; Smeal, J.K.; Lewis, J.; Gearhardt, A.N. Development of the Highly Processed Food withdrawal Scale. Appetite 2018, 131, 148–154. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- García-García, I.; Horstmann, A.; Jurado, M.A.; Garolera, M.; Chaudhry, S.J.; Margulies, D.S.; Villringer, A.; Neumann, J. Reward Processing in Obesity, Substance Addiction and Non-Substance Addiction. Obes. Rev. 2014, 15, 853–869. [Google Scholar] [CrossRef]
- Rogers, P.J. Food and Drug Addictions: Similarities and Differences. Pharmacol. Biochem. Behav. 2017, 153, 182–190. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Food Additives, Food and the Concept of ‘Food Addiction’: Is Stimulation of the Brain Reward Circuit by Food Sufficient to Trigger Addiction? Pathophysiology 2018, 25, 263–276. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The Dopamine Motive System: Implications for Drug and Food Addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Loxton, N.J.; Levitan, R.D.; Kaplan, A.S.; Carter, J.C.; Kennedy, J.L. ‘Food Addiction’ and Its Association with a Dopaminergic Multilocus Genetic Profile. Physiol. Behav. 2013, 118, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Parnarouskis, L.; Leventhal, A.M.; Ferguson, S.G.; Gearhardt, A.N. withdrawal: A Key Consideration in Evaluating Whether Highly Processed Foods Are Addictive. Obes. Rev. 2022, 23, e13507. [Google Scholar] [CrossRef] [PubMed]
- Parnarouskis, L.; Gearhardt, A.N. Preliminary Evidence That Tolerance and withdrawal Occur in Response to Ultra-Processed Foods. Curr. Addict. Rep. 2022, 9, 282–289. [Google Scholar] [CrossRef]
- Gilbert, D.G.; Gilbert, B.O.; Schultz, V.L. withdrawal Symptoms: Individual Differences and Similarities across Addictive Behaviors. Pers. Individ. Differ. 1998, 24, 351–356. [Google Scholar] [CrossRef]
- Dingemans, A.; Danner, U.; Parks, M. Emotion Regulation in Binge Eating Disorder: A Review. Nutrients 2017, 9, 1274. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Richard, A.; Schnepper, R.; Reichenberger, J.; Georgii, C.; Naab, S.; Voderholzer, U.; Blechert, J. Emotion Regulation and Emotional Eating in Anorexia Nervosa and Bulimia Nervosa. Eat. Disord. 2021, 29, 175–191. [Google Scholar] [CrossRef]
- Reichenberger, J.; Schnepper, R.; Arend, A.-K.; Blechert, J. Emotional Eating in Healthy Individuals and Patients with an Eating Disorder: Evidence from Psychometric, Experimental and Naturalistic Studies. Proc. Nutr. Soc. 2020, 79, 290–299. [Google Scholar] [CrossRef]
- Konttinen, H. Emotional Eating and Obesity in Adults: The Role of Depression, Sleep and Genes. Proc. Nutr. Soc. 2020, 79, 283–289. [Google Scholar] [CrossRef]
- Brewerton, T.D. Food Addiction as a Proxy for Eating Disorder and Obesity Severity, Trauma History, PTSD Symptoms, and Comorbidity. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2017, 22, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Gearhardt, A.N.; Castelnuovo, G.; Mannarini, S. Different Methods of Assessment, Food Addiction, Emotional Eating, and Binge Eating Behaviors: Comparing the Total Model Effects of Sequential Mediation Analysis. CEUR Workshop Proc. 2020, 2730. [Google Scholar]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and Loss of Control Related to Eating: Two High-Risk Factors Following Bariatric Surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 2017, 42, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Chapter 3—Dissecting Compulsive Eating Behavior into Three Elements. In Compulsive Eating Behavior and Food Addiction; Cottone, P., Sabino, V., Moore, C.F., Koob, G.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 41–81. ISBN 978-0-12-816207-1. [Google Scholar]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Chapter 4—Habitual Overeating. In Compulsive Eating Behavior and Food Addiction; Cottone, P., Sabino, V., Moore, C.F., Koob, G.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 83–95. ISBN 978-0-12-816207-1. [Google Scholar]
- Rossi, A.A.; Mannarini, S.; Castelnuovo, G.; Pietrabissa, G. Disordered Eating Behaviors Related to Food Addiction/Eating Addiction in Inpatients with Obesity and the General Population: The Italian Version of the Addiction-like Eating Behaviors Scale (AEBS-IT). Nutrients 2023, 15, 104. [Google Scholar] [CrossRef]
- Rossi, A.A.; Pietrabissa, G.; Gearhardt, A.N.; Musetti, A.; Castelnuovo, G.; Mannarini, S. Eating Compulsivity in Inpatients with Severe Obesity and the General Population: The Italian Version of the Measure of Eating Compulsivity (MEC10-IT). Nutrients 2023, 15, 1378. [Google Scholar] [CrossRef]
- Conceição, E.M.; Mitchell, J.E.; Machado, P.P.P.; Vaz, A.R.; Pinto-Bastos, A.; Ramalho, S.; Brandão, I.; Simões, J.B.; de Lourdes, M.; Freitas, A.C. Repetitive Eating Questionnaire [Rep(Eat)-Q]: Enlightening the Concept of Grazing and Psychometric Properties in a Portuguese Sample. Appetite 2017, 117, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bonder, R.; Davis, C.; Kuk, J.L.; Loxton, N.J. Compulsive “Grazing” and Addictive Tendencies towards Food. Eur. Eat. Disord. Rev. 2018, 26, 569–573. [Google Scholar] [CrossRef]
- Heriseanu, A.I.; Hay, P.; Touyz, S. The Short Inventory of Grazing (SIG): Development and Validation of a New Brief Measure of a Common Eating Behaviour with a Compulsive Dimension. J. Eat. Disord. 2019, 7, 4. [Google Scholar] [CrossRef]
- Conceição, E.M.; Mitchell, J.E.; Engel, S.G.; Machado, P.P.P.; Lancaster, K.; Wonderlich, S.A. What Is “Grazing”? Reviewing Its Definition, Frequency, Clinical Characteristics, and Impact on Bariatric Surgery Outcomes, and Proposing a Standardized Definition. Surg. Obes. Relat. Dis. 2014, 10, 973–982. [Google Scholar] [CrossRef]
- Heriseanu, A.I.; Hay, P.; Touyz, S. Grazing Behaviour and Associations with Obesity, Eating Disorders, and Health-Related Quality of Life in the Australian Population. Appetite 2019, 143, 104396. [Google Scholar] [CrossRef]
- Conceição, E.M.; Utzinger, L.M.; Pisetsky, E.M. Eating Disorders and Problematic Eating Behaviours Before and After Bariatric Surgery: Characterization, Assessment and Association with Treatment Outcomes. Eur. Eat. Disord. Rev. 2015, 23, 417–425. [Google Scholar] [CrossRef]
- Poole, N.A.; Atar, A.A.; Kuhanendran, D.; Bidlake, L.; Fiennes, A.; McCluskey, S.; Nussey, S.; Bano, G.; Morgan, J.F. Compliance with Surgical After-Care Following Bariatric Surgery for Morbid Obesity: A Retrospective Study. Obes. Surg. 2005, 15, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity 2020, 28, O1–O58. [Google Scholar] [CrossRef] [PubMed]
- Conceição, E.; Mitchell, J.E.; Vaz, A.R.; Bastos, A.P.; Ramalho, S.; Silva, C.; Cao, L.; Brandão, I.; Machado, P.P.P. The Presence of Maladaptive Eating Behaviors after Bariatric Surgery in a Cross Sectional Study: Importance of Picking or Nibbling on Weight Regain. Eat. Behav. 2014, 15, 558–562. [Google Scholar] [CrossRef]
- Conceição, E.M.; Mitchell, J.E.; Pinto-Bastos, A.; Arrojado, F.; Brandão, I.; Machado, P.P.P. Stability of Problematic Eating Behaviors and Weight Loss Trajectories after Bariatric Surgery: A Longitudinal Observational Study. Surg. Obes. Relat. Dis. 2017, 13, 1063–1070. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling; The Guilford Press: New York, NY, USA, 2023. [Google Scholar]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research, 2nd ed.; The Guilford Press: New York, NY, USA, 2015; p. xvii+462. ISBN 978-1-4625-1779-4. [Google Scholar]
- Consoli, S.; Rossi, A.; Thompson, L.Y.; Volpi, C.; Mannarini, S.; Castelnuovo, G.; Molinari, E. Assessing Psychometric Properties of the Italian Version of the Heartland Forgiveness Scale. Front. Psychol. 2020, 11, 596501. [Google Scholar] [CrossRef]
- Rossi, A.A.; Manzoni, G.M.; Pietrabissa, G.; Di Pauli, D.; Mannarini, S.; Castelnuovo, G. Weight Stigma in Patients with Overweight and Obesity: Validation of the Italian Weight Self-Stigma Questionnaire (WSSQ). Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2022, 27, 2459–2472. [Google Scholar] [CrossRef]
- Conceição, E.M.; de Lourdes, M.; Neufeld, C.B. Assessment of Grazing. In Assessment of Eating Behavior; Psychological assessment—Science and practice; Hogrefe: Göttingen, Germany, 2023; pp. 82–96. ISBN 978-0-88937-616-8. [Google Scholar]
- Schulte, E.M.; Gearhardt, A.N. Development of the Modified Yale Food Addiction Scale Version 2.0. Eur. Eat. Disord. Rev. 2017, 25, 302–308. [Google Scholar] [CrossRef]
- Imperatori, C.; Fabbricatore, M.; Lester, D.; Manzoni, G.M.; Castelnuovo, G.; Raimondi, G.; Innamorati, M. Psychometric Properties of the Modified Yale Food Addiction Scale Version 2.0 in an Italian Non-Clinical Sample. Eat. Weight Disord. 2019, 24, 37–45. [Google Scholar] [CrossRef]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The Assessment of Binge Eating Severity among Obese Persons. Addict. Behav. 1982, 7, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ricca, V.; Mannucci, E.; Moretti, S.; Di Bernardo, M.; Zucchi, T.; Cabras, P.L.; Rotella, C.M. Screening for Binge Eating Disorder in Obese Outpatients. Compr. Psychiatry 2000, 41, 111–115. [Google Scholar] [CrossRef]
- Imperatori, C.; Innamorati, M.; Lamis, D.A.; Contardi, A.; Continisio, M.; Castelnuovo, G.; Manzoni, G.M.; Fabbricatore, M. Factor Structure of the Binge Eating Scale in a Large Sample of Obese and Overweight Patients Attending Low Energy Diet Therapy. Eur. Eat. Disord. Rev. 2016, 24, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.D.; Wing, R.R.; Hopkins, J. Obese Binge Eaters: Affect, Cognitions, and Response to Behavioral Weight Control. J. Consult. Clin. Psychol. 1988, 56, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Pinto-Gouveia, J.; Ferreira, C. Expanding Binge Eating Assessment: Validity and Screening Value of the Binge Eating Scale in Women from the General Population. Eat. Behav. 2015, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.M.; Grupski, A.E.; Hall, B.J.; Ivan, I.; Corsica, J. Factor Structure and Predictive Utility of the Binge Eating Scale in Bariatric Surgery Candidates. Surg. Obes. Relat. Dis. 2013, 9, 942–948. [Google Scholar] [CrossRef]
- Schroder, R.; Sellman, J.D.; Adamson, S. Development and Validation of a Brief Measure of Eating Compulsivity (MEC). Subst. Use Misuse 2017, 52, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Persson, L.-O.; Sjöström, L.; Sullivan, M. Psychometric Properties and Factor Structure of the Three-Factor Eating Questionnaire (TFEQ) in Obese Men and Women. Results from the Swedish Obese Subjects (SOS) Study. Int. J. Obes. 2000, 24, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.A.; Pietrabissa, G.; Castelnuovo, G.; Mannarini, S. Cognitive Restraint, Uncontrolled Eating, and Emotional Eating. The Italian Version of the Three Factor Eating Questionnaire-Revised 18 (TFEQ-R-18): A Three-step Validation Study. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2024, 29, 16. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Elsevier B.V.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Revelle, W. PsychTools: Tools to Accompany the “Psych” Package for Psychological Research; Elsevier B.V.: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Epskamp, S. semPlot: Unified Visualizations of Structural Equation Models. Struct. Equ. Model. Multidiscip. J. 2015, 22, 474–483. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Yu, C.-Y. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models with Binary and Continuous Outcomes. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2002. [Google Scholar]
- McDonald, R.P. Test Theory: A Unified Treatment; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; p. xi+485. ISBN 978-0-8058-3075-0. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics; Pearson: Harlow, UK, 2014. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Pietrabissa, G.; Castelnuovo, G.; Semonella, M.; Mannarini, S.; Rossi, A.A. Measuring Motivations to Eat Palatable Foods: Adaptation and Psychometric Properties of the Italian Version of the Palatable Eating Motives Scale (PEMS-IT). Healthcare 2024, 12, 574. [Google Scholar] [CrossRef]
- Meany, G.; Conceição, E.; Mitchell, J.E. Binge Eating, Binge Eating Disorder and Loss of Control Eating: Effects on Weight Outcomes after Bariatric Surgery. Eur. Eat. Disord. Rev. 2014, 22, 87–91. [Google Scholar] [CrossRef]
- Niego, S.H.; Kofman, M.D.; Weiss, J.J.; Geliebter, A. Binge Eating in the Bariatric Surgery Population: A Review of the Literature. Int. J. Eat. Disord. 2007, 40, 349–359. [Google Scholar] [CrossRef]
- Smith, K.E.; Orcutt, M.; Steffen, K.J.; Crosby, R.D.; Cao, L.; Garcia, L.; Mitchell, J.E. Loss of Control Eating and Binge Eating in the 7 Years Following Bariatric Surgery. Obes. Surg. 2019, 29, 1773–1780. [Google Scholar] [CrossRef]
- Nasirzadeh, Y.; Kantarovich, K.; Wnuk, S.; Okrainec, A.; Cassin, S.E.; Hawa, R.; Sockalingam, S. Binge Eating, Loss of Control over Eating, Emotional Eating, and Night Eating After Bariatric Surgery: Results from the Toronto Bari-PSYCH Cohort Study. Obes. Surg. 2018, 28, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Cassin, S.; Leung, S.; Hawa, R.; Wnuk, S.; Jackson, T.; Sockalingam, S. Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy. Nutrients 2020, 12, 2905. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porat, T.; Weiss, R.; Sherf-Dagan, S.; Rottenstreich, A.; Kaluti, D.; Khalaileh, A.; Abu Gazala, M.; Zaken Ben-Anat, T.; Mintz, Y.; Sakran, N.; et al. Food Addiction and Binge Eating During One Year Following Sleeve Gastrectomy: Prevalence and Implications for Postoperative Outcomes. Obes. Surg. 2021, 31, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, A.; DeVita, M.; Di Rosa, E.; Bottesi, G.; Brundisini, V.; Guarrera, C.; Ravelli, A.; Ponza, I.; Cattelan, A.; Volpe, B.; et al. Trauma Shaping the Psychopathological Correlates of Patients with Long-COVID: A 6-Months Longitudinal Study with Repeated Measures Mixed Models. Psychiatry Res. 2023, 330, 115609. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, A.; Castelnuovo, G.; Spoto, A. Assessing Discriminant Validity through Structural Equation Modeling: The Case of Eating Compulsivity. Nutrients 2024, 16, 550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).