Twelve Weeks of Daily Lentil Consumption Improves Fasting Cholesterol and Postprandial Glucose and Inflammatory Responses—A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Research Design
2.4. Anthropometrics
2.5. Elevated Postprandial Triglyceride Screening
2.6. Randomization
2.7. Lentil Sampling and Nutritional Analysis
2.8. Dietary Intervention
2.9. Diet Adherence
2.10. Recent and Habitual Diet Surveys
2.11. Satiety and Gastrointestinal Symptom Surveys
2.12. Blood Sampling
2.13. High-Fat Meal Challenge
2.14. Analysis of Blood Markers
2.15. Analysis of Inflammation Biomarkers
2.16. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Anthropometric Measures
3.3. Habitual Diet Analysis
3.4. Satiety during the Intervention
3.5. Gastrointestinal Symptoms during the Intervention
3.6. Fasting Lipid and Glycemic Measures
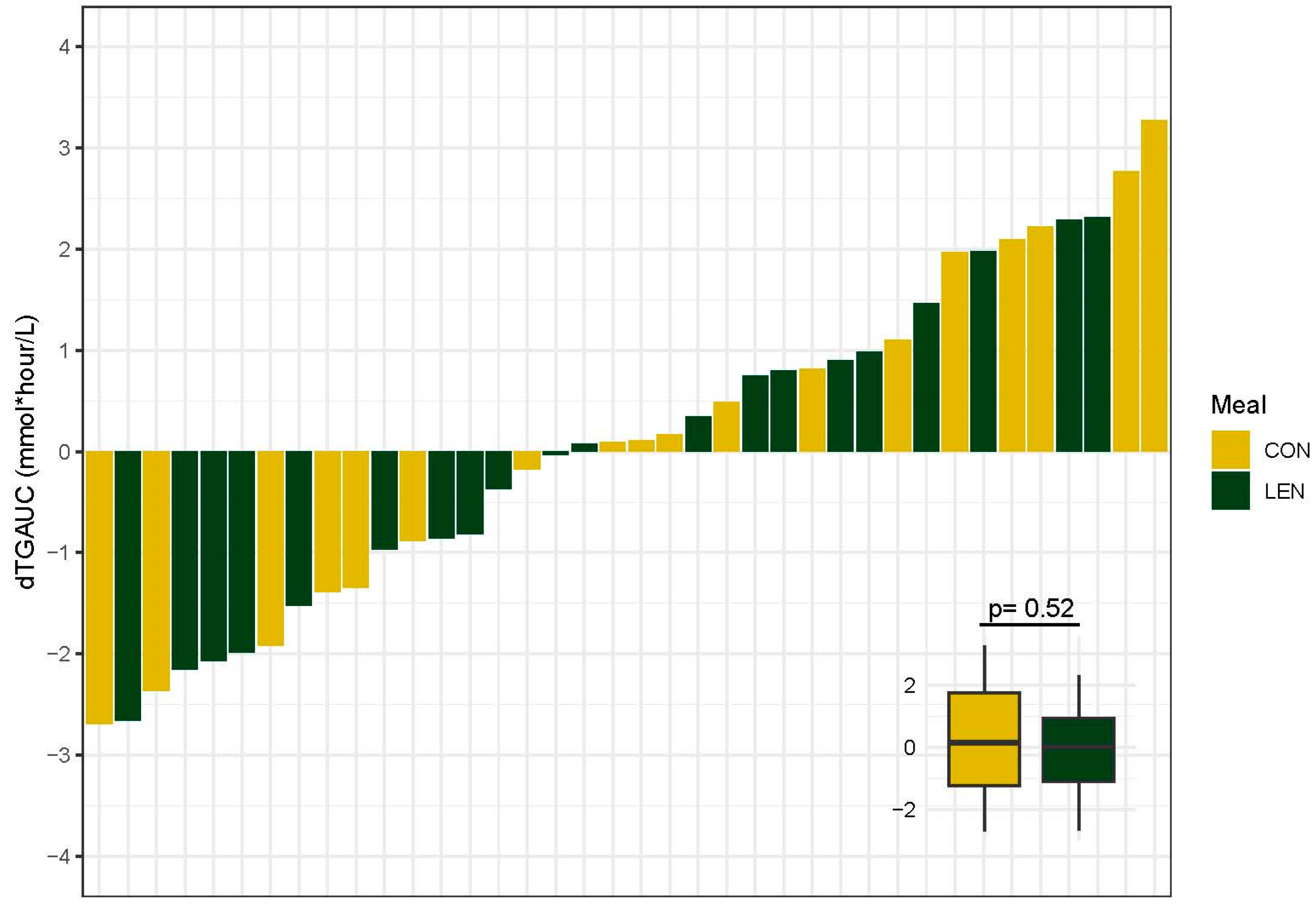
3.7. Postprandial Lipid Response
3.8. Postprandial Glycemic Response
3.9. Fasting and Postprandial Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Siren, R.; Eriksson, J.G.; Vanhanen, H. Waist circumference a good indicator of future risk for type 2 diabetes and cardiovascular disease. BMC Public. Health 2012, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Major, A.W. Pulses and lipaemia, short- and long-term effect: Potential in the prevention of cardiovascular disease. Br. J. Nutr. 2002, 88 (Suppl. 3), S263–S271. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, D.; Grafenauer, S.; Rangan, A. The Effects of Legume Consumption on Markers of Glycaemic Control in Individuals with and without Diabetes Mellitus: A Systematic Literature Review of Randomised Controlled Trials. Nutrients 2020, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, M.S.; Campbell, M.D.; O’Mahoney, L.L.; Holmes, M.; Orfila, C.; Boesch, C. Pulse consumption improves indices of glycemic control in adults with and without type 2 diabetes: A systematic review and meta-analysis of acute and long-term randomized controlled trials. Eur. J. Nutr. 2022, 61, 809–824. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, N.; Mousavi, S.M.; Santos, H.O.; Aune, D.; Hasani-Ranjbar, S.; Larijani, B.; Esmaillzadeh, A. Legume Consumption and Risk of All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2023, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Jurek, J.M. Implications of Plant Foods in Weight Management: Focus on Metabolic Health. J. Biomed. Res. Environ. Sci. 2022, 3, 1440–1450. [Google Scholar] [CrossRef]
- Nestel, P.; Cehun, M.; Chronopoulos, A. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am. J. Clin. Nutr. 2004, 79, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Kendall, C.W.; Esfahani, A.; Wong, J.M.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Niero, M.; Bartoli, G.; De Colle, P.; Scarcella, M.; Zanetti, M. Impact of Dietary Fiber on Inflammation and Insulin Resistance in Older Patients: A Narrative Review. Nutrients 2023, 15, 2365. [Google Scholar] [CrossRef]
- Henn, K.; Goddyn, H.; Olsen, S.B.; Bredie, W.L.P. Identifying behavioral and attitudinal barriers and drivers to promote consumption of pulses: A quantitative survey across five European countries. Food Qual. Prefer. 2022, 98, 104455. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Wilson, S.M.G.; Peterson, E.J.; Gaston, M.E.; Kuo, W.Y.; Miles, M.P. Eight weeks of lentil consumption attenuates insulin resistance progression without increased gastrointestinal symptom severity: A randomized clinical trial. Nutr. Res. 2022, 106, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.E.; Stamler, J.; Neaton, J.D. Relation of Triglyceride Levels, Fasting and Nonfasting, to Fatal and Nonfatal Coronary Heart Disease. Arch. Intern. Med. 2003, 163, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Department of Agriculture and US Department of Health and Human Services: Washington, DC, USA, 2020.
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Schautz, B.; Later, W.; Kehayias, J.J.; Gallagher, D.; Muller, M.J. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur. J. Clin. Nutr. 2013, 67, S14–S21. [Google Scholar] [CrossRef]
- Uschner, D.; Schindler, D.; Hilgers, R.-D.; Heussen, N. randomizeR: An R Package for the Assessment and Implementation of Randomization in Clinical Trials. J. Stat. Softw. 2018, 85, 1–22. [Google Scholar] [CrossRef]
- Lee, S.C. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov (accessed on 10 October 2023).
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of he Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.L. Ways to Fit a PK Model with Some Data Below the Quantification Limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Fox, J. Effect Displays in R for Generalised Linear Models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef]
- Peterson, R.A. Finding Optimal Normalizing Transformations via bestNormalize. R J. 2021, 13, 294–313. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Couillard, C.; Bergeron, N.; Prud’homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriège, P.; Després, J.-P. Gender Difference in Postprandial Lipemia, Importance of Visceral Adipose Tissue Accumulation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2448–2455. [Google Scholar] [CrossRef]
- Slipher, S. SCRS-MSU/Auctime. Available online: https://github.com/scrs-msu/auctime (accessed on 10 October 2023).
- White, K.T.; Moorthy, M.V.; Akinkuolie, A.O.; Demler, O.; Ridker, P.M.; Cook, N.R.; Mora, S. Identifying an Optimal Cutpoint for the Diagnosis of Hypertriglyceridemia in the Nonfasting State. Clin. Chem. 2015, 61, 1156–1163. [Google Scholar] [CrossRef]
- Langsted, A.; Freiberg, J.J.; Nordestgaard, B.G. Fasting and nonfasting lipid levels: Influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008, 118, 2047–2056. [Google Scholar] [CrossRef]
- Mollard, R.C.; Luhovyy, B.L.; Panahi, S.; Nunez, M.; Hanley, A.; Anderson, G.H. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br. J. Nutr. 2012, 108 (Suppl. 1), S111–S122. [Google Scholar] [CrossRef] [PubMed]
- Abeysekara, S.; Chilibeck, P.D.; Vatanparast, H.; Zello, G.A. A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. Br. J. Nutr. 2012, 108 (Suppl. 1), S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Oakenfull, D.G. A mechanism for the hypocholesterolaemic activity of saponins. Br. J. Nutr. 1986, 55, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.T.; Sarfaraz, S.; Qi, X.; Ramdath, D.G.; Fougere, G.C.; Ramdath, D.D. A Review of the Relationship between Lentil Serving and Acute Postprandial Blood Glucose Response: Effects of Dietary Fibre, Protein and Carbohydrates. Nutrients 2022, 14, 849. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.C.R.; Lousley, S.; Geekie, M.; Simpson, R.W.; Carter, R.D.; Hockaday, T.D.R.; Mann, J.I. A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet 1981, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Horowitz, M.; Jones, K.L.; Marathe, C.S. Gut-Based Strategies to Reduce Postprandial Glycaemia in Type 2 Diabetes. Front. Endocrinol. 2021, 12, 661877. [Google Scholar] [CrossRef]
- Higgins, J.A. Whole grains, legumes, and the subsequent meal effect: Implications for blood glucose control and the role of fermentation. J. Nutr. Metab. 2012, 2012, 829238. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.; Muir, J.; Proietto, J. Carbohydate Fermentation Decreases Hepatic Glucose Output in Healthy Subjects. Metabolism 1993, 42, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K.; Ferchaud-Roucher, V.; Preston, T.; Small, A.C.; Henckaerts, L.; Krempf, M.; Wang, H.; Vonk, R.J.; Priebe, M.G. Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur. J. Clin. Nutr. 2010, 64, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P.; Lairon, D.; Maraninchi, M.; Valero, R. Effect of Nutrient and Micronutrient Intake on Chylomicron Production and Postprandial Lipemia. Nutrients 2019, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Gharaaghaji, F.; Gargari, B.P. The effects of legumes on metabolic features, insulin resistance and hepatic function tests in women with central obesity: A randomized controlled trial. Int. J. Prev. Med. 2014, 5, 710–720. [Google Scholar] [PubMed]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Hedayati, M.; Azizi, F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: A cross-over randomized clinical trial. Eur. J. Clin. Nutr. 2015, 69, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Hansen, L.T.; Sørensen, A. Pea Fiber Lowers Fasting and Postprandial Blood Triglyceride Concentrations in Humans. J. Nutr. 1994, 124, 2386–2396. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Wilson, S.M.; Maes, A.P.; Yeoman, C.J.; Walk, S.T.; Miles, M.P. Determinants of the postprandial triglyceride response to a high-fat meal in healthy overweight and obese adults. Lipids Health Dis. 2021, 20, 107. [Google Scholar] [CrossRef]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems 2021, 6, e00115-21. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Säemann, M.D.; Bohmig, G.A.; Osterreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stockl, J.; Horl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Monk, J.M.; Wu, W.; Wellings, H.R.; Robinson, L.E.; Power, K.A. Red lentil supplementation reduces the severity of dextran sodium sulfate-induced colitis in C57BL/6 male mice. J. Funct. Foods 2020, 64, 103625. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thevenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Winham, D.M.; Hutchins, A.M. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr. Res. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Zahradka, P.; Wright, B.; Weighell, W.; Blewett, H.; Baldwin, A.; Karmin, O.; Guzman, R.P.; Taylor, C.G. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis 2013, 230, 310–314. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio 2021, 12, e0102821. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D.; Lu, Z.H.; Maharaj, P.L.; Winberg, J.; Brummer, Y.; Hawke, A. Proximate Analysis and Nutritional Evaluation of Twenty Canadian Lentils by Principal Component and Cluster Analyses. Foods 2020, 9, 175. [Google Scholar] [CrossRef]
- Perera, T.; Russo, C.; Takata, Y.; Bobe, G. Legume Consumption Patterns in US Adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014 and Beans, Lentils, Peas (BLP) 2017 Survey. Nutrients 2020, 12, 1237. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Wang, C.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant starch and its nanoparticles: Recent advances in their green synthesis and application as functional food ingredients and bioactive delivery systems. Trends Food Sci. Technol. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Bednar, G.E.; Patil, A.R.; Murray, S.M.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentability in vitro in a canine model. J. Nutr. 2001, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Colee, J.; Langkamp-Henken, B.; Dahl, W.J. Assessing Gastrointestinal Tolerance in Healthy Adults: Reliability and Validity of a Weekly Questionnaire. Curr. Dev. Nutr. 2023, 7, 101976. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.M.; Duncan, A.M.; Cryne, C.N.; Deschambault, B.R.; Boye, J.I.; Benali, M.; Marcotte, M.; Tosh, S.M.; Farnworth, E.R.; Wright, A.J. Effect of pulse consumption on perceived flatulence and gastrointestinal function in healthy males. Food Res. Int. 2010, 43, 553–559. [Google Scholar] [CrossRef]



| Meal | CON—0 g/week | LEN—980 g/week |
|---|---|---|
| Bolognese | Turkey Bolognese + ½ cup cooked rotini pasta | Lentil Bolognese + ½ cup cooked rotini pasta |
| Curry | Chicken curry + 1/3 cup cooked basmati rice | Lentil curry + 1/3 cup cooked basmati rice |
| Loaf | Turkey loaf + ¼ cup mashed potatoes + ½ cup cooked zucchini | Lentil loaf + ¼ cup mashed potatoes + ½ cup cooked zucchini |
| Taco | Turkey taco filling + ½ oz shredded cheddar cheese + 1 Tbsp salsa + 1 Tbsp sour cream + 2 street flour tortillas | Lentil taco filling + ½ oz shredded cheddar cheese + 1 Tbsp salsa + 1 Tbsp sour cream + 2 street flour tortillas |
| Soup | Chicken soup + 3 packets saltine crackers | Lentil soup |
| Shepherd’s Pie | Chicken shepherd’s pie + 2 dinner rolls + 1 pat butter | Lentil shepherd’s pie + 1 dinner roll + 2 pats butter |
| Chili | Turkey chili + 3 packets saltine crackers | Lentil chili + 1 packet saltine crackers |
| CON (n = 18) | LEN (n = 20) | p-Value | |
|---|---|---|---|
| Age (years) | 43.2 (14.0) | 50.6 (11.5) | 0.08 |
| Sex (M/F) | 1/17 | 2/18 | 0.58 |
| BMI (kg/m2) | 35.9 (7.6) | 33.1 (5.1) | 0.19 |
| Fat mass (%) | 45.1 (6.2) | 44.0 (6.1) | 0.57 |
| Visceral adipose (L) | 3.6 (2.1) | 3.1 (1.8) | 0.45 |
| MetS Presence (Y/N) 1 | 11/7 | 4/16 | <0.01 |
| HbA1c (%) | 5.4 (0.3) | 5.5 (0.2) | 0.48 |
| Fasting Glucose (mmol/L) | 5.5 (0.5) | 5.4 (0.3) | 0.43 |
| HOMA-IR | 3.8 (2.6) | 4.2 (8.2) | 0.86 |
| Total Cholesterol (mmol/L) | 4.4 (0.7) | 5.1 (0.7) | 0.01 |
| HDL Cholesterol (mmol/L) | 1.2 (0.2) | 1.5 (0.4) | 0.01 |
| LDL Cholesterol (mmol/L) | 2.85 (0.64) | 3.28 (0.64) | 0.05 |
| Triglycerides (mmol/L) | 1.7 (0.8) | 1.6 (0.7) | 0.52 |
| Blood Pressure (mmHg) | |||
| Systolic | 117.8 (11.9) | 111.2 (11.4) | 0.09 |
| Diastolic | 82.6 (10.8) | 76.5 (9.4) | 0.08 |
| Component | Maximum Points | Score | |||
|---|---|---|---|---|---|
| CON (n = 18) | LEN (n = 20) | ||||
| Pre | Post | Pre | Post | ||
| Adequacy | |||||
| Total fruits | 5 | 3.6 ± 1.4 | 2.8 ± 1.5 | 3.3 ± 1.6 | 3.2 ± 1.6 |
| Whole fruits | 5 | 4.4 ± 1.0 | 3.9 ± 1.5 | 4.0 ± 1.5 | 3.9 ± 1.6 |
| Total vegetables | 5 | 4.0 ± 1.3 | 4.1 ± 1.0 | 3.9 ± 1.3 | 4.5 ± 1.0 * |
| Greens and beans | 5 | 4.3 ± 1.2 | 4.2 ± 1.4 | 4.0 ± 1.5 | 4.8 ± 0.8 |
| Whole grains | 10 | 2.7 ± 1.1 | 3.3 ± 1.5 | 3.3 ± 1.6 | 2.9 ± 2.2 |
| Dairy | 10 | 7.4 ± 2.0 | 6.3 ± 2.0 * | 6.8 ± 2.1 | 5.9 ± 2.5 |
| Total protein foods | 5 | 4.9 ± 0.4 | 4.9 ± 0.2 | 4.7 ± 0.9 | 5.0 ± 0.2 |
| Seafood and plant proteins | 5 | 4.4 ± 1.0 | 4.7 ± 0.7 | 4.2 ± 1.1 | 4.8 ± 0.8 |
| Fatty acids | 10 | 3.7 ± 2.4 | 4.1 ± 2.2 | 4.9 ± 2.5 | 5.0 ± 2.5 |
| Moderation | |||||
| Refined grains | 10 | 9.1 ± 1.4 | 7.1 ± 2.4 * | 8.5 ± 2.2 | 8.2 ± 2.7 |
| Sodium | 10 | 3.4 ± 3.3 | 1.9 ± 1.8 | 4.1 ± 2.7 | 2.2 ± 2.1 * |
| Added sugars | 10 | 8.0 ± 2.3 | 8.5 ± 1.6 | 7.9 ± 2.5 | 8.7 ± 1.5 |
| Saturated fats | 10 | 3.3 ± 2.9 | 4.0 ± 2.0 | 4.7 ± 2.9 | 5.5 ± 2.8 |
| Total | 100 | 63.1 ± 8.1 | 59.6 ± 7.7 | 64.1 ± 10.8 | 64.4 ± 7.5 |
| CON (n = 18) | LEN (n = 20) | p-Value | |
|---|---|---|---|
| ∆ Total Cholesterol (mmol/L) | 0.36 (0.43) | −0.11 (0.50) | <0.01 |
| ∆ HDL Cholesterol (mmol/L) | 0.10 (0.14) | −0.07 (0.15) | <0.001 |
| ∆ LDL Cholesterol (mmol/L) | 0.30 (0.36) | −0.03 (0.43) | 0.02 |
| ∆ Triglycerides (mmol/L) | −0.21 (0.54) | −0.06 (0.73) | 0.51 |
| ∆ GLU (mmol/L) | −0.05 (0.44) | −0.06 (0.34) | 0.56 |
| ∆ INS (mmol/L) | −2.40 (8.84) | −3.83 (22.35) | 0.85 |
| ∆ HbA1c (%) | 0.06 (0.38) | 0.03 (0.17) | 0.56 |
| ∆ HOMA-IR | −0.59 (2.29) | −0.95 (5.23) | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamberlin, M.L.; Wilson, S.M.G.; Gaston, M.E.; Kuo, W.-Y.; Miles, M.P. Twelve Weeks of Daily Lentil Consumption Improves Fasting Cholesterol and Postprandial Glucose and Inflammatory Responses—A Randomized Clinical Trial. Nutrients 2024, 16, 419. https://doi.org/10.3390/nu16030419
Chamberlin ML, Wilson SMG, Gaston ME, Kuo W-Y, Miles MP. Twelve Weeks of Daily Lentil Consumption Improves Fasting Cholesterol and Postprandial Glucose and Inflammatory Responses—A Randomized Clinical Trial. Nutrients. 2024; 16(3):419. https://doi.org/10.3390/nu16030419
Chicago/Turabian StyleChamberlin, Morgan L., Stephanie M.G. Wilson, Marcy E. Gaston, Wan-Yuan Kuo, and Mary P. Miles. 2024. "Twelve Weeks of Daily Lentil Consumption Improves Fasting Cholesterol and Postprandial Glucose and Inflammatory Responses—A Randomized Clinical Trial" Nutrients 16, no. 3: 419. https://doi.org/10.3390/nu16030419
APA StyleChamberlin, M. L., Wilson, S. M. G., Gaston, M. E., Kuo, W.-Y., & Miles, M. P. (2024). Twelve Weeks of Daily Lentil Consumption Improves Fasting Cholesterol and Postprandial Glucose and Inflammatory Responses—A Randomized Clinical Trial. Nutrients, 16(3), 419. https://doi.org/10.3390/nu16030419







