Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer
Abstract
1. Introduction
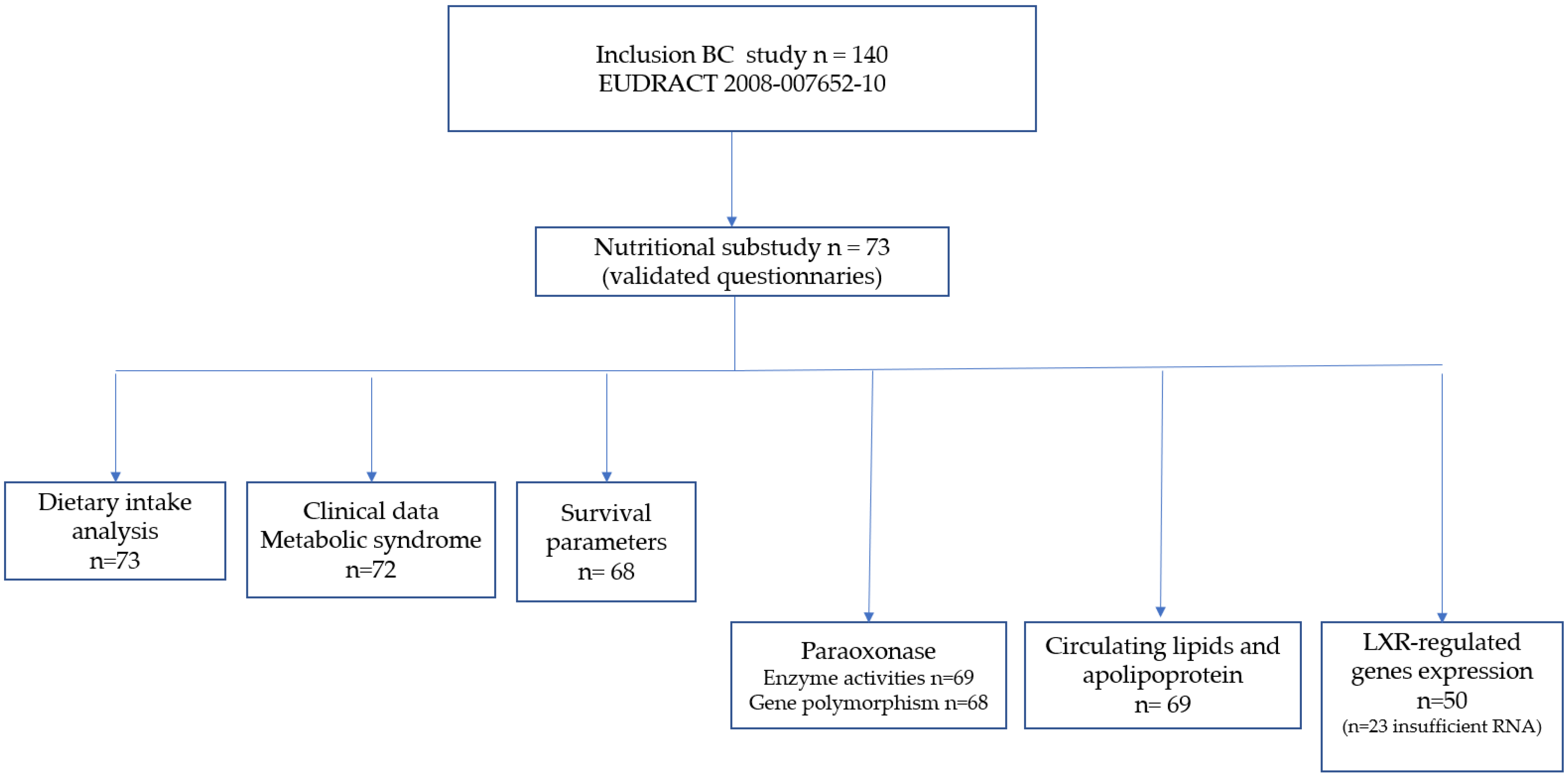
2. Materials and Methods
2.1. Patients
Study Design
2.2. Metabolic Syndrome
2.3. Dietary Lipids and Lipidic Biomarkers
2.3.1. Dietary Intake
2.3.2. Circulating Lipids and Apolipoproteins
2.3.3. LXR-Regulated Genes of Cholesterol Trafficking
2.3.4. Paraoxonase
2.4. Statistical Considerations
3. Results
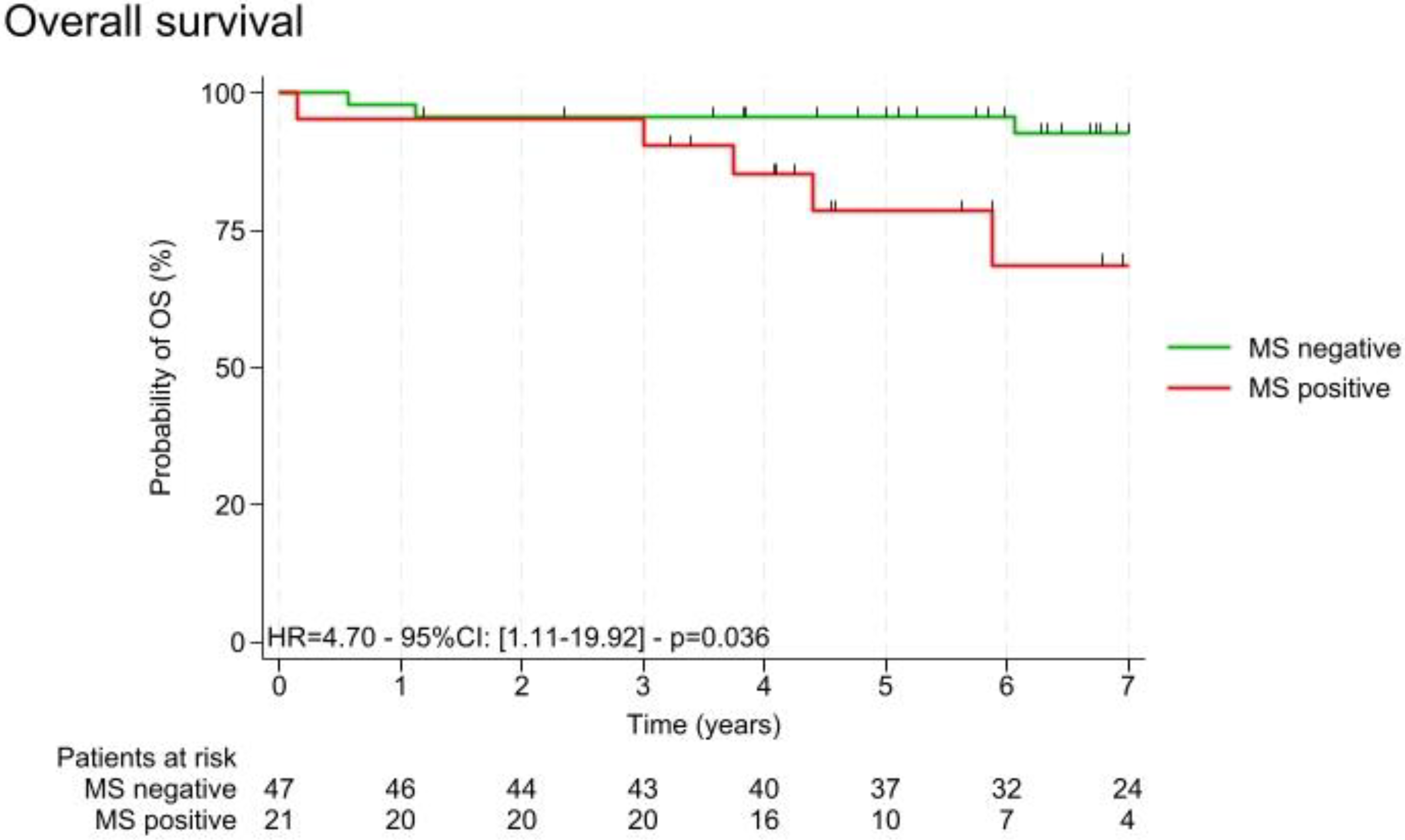
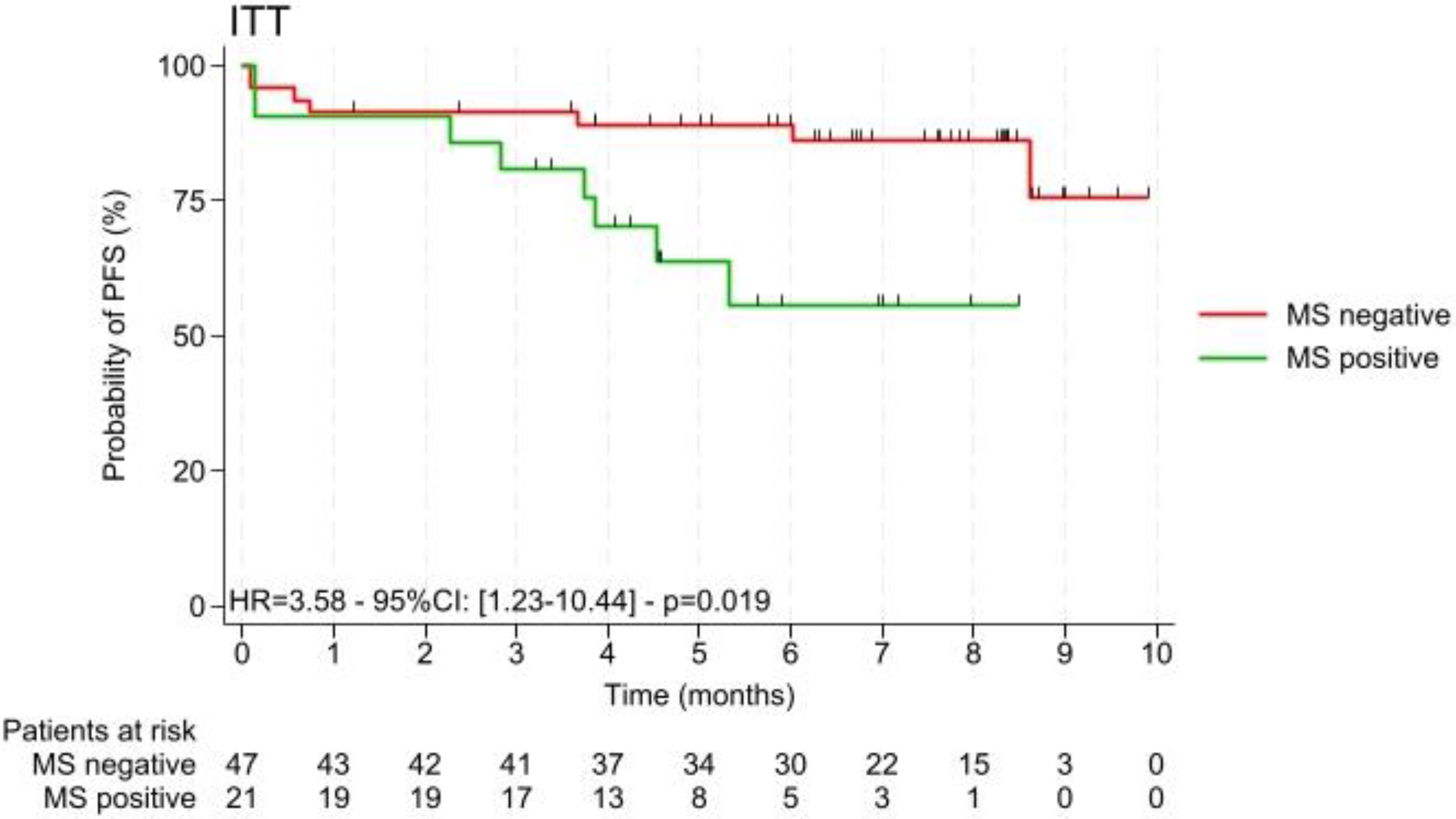
3.1. Description of the Study Cohort and Metabolic Syndrome
3.2. Dietary Habits and Dietary Inflammatory Index
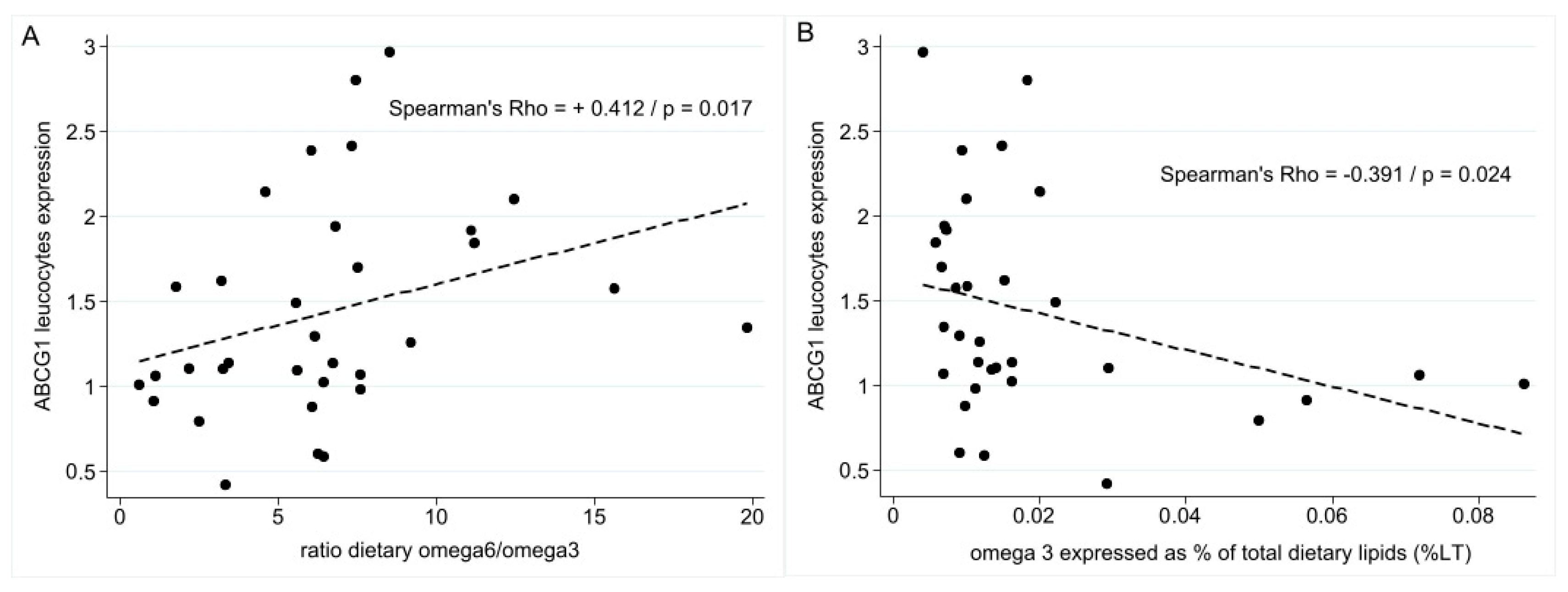
3.3. LXR-Regulated Genes of Cholesterol Trafficking, Lipidic Dietary Habits, and Survival Parameters
3.4. Paraoxonase
3.4.1. Link Between Enzyme Activities and Paraoxonase Genotypes
3.4.2. Link Between Paraoxonase Activities and Lipid Dietary Habits
3.4.3. Link Between Paraoxonase Activities and Survival Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breast Cancer Statistics|World Cancer Research Fund International. WCRF International. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/ (accessed on 24 July 2024).
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- González-Palacios Torres, C.; Barrios-Rodríguez, R.; Muñoz-Bravo, C.; Toledo, E.; Dierssen, T.; Jiménez-Moleón, J.J. Mediterranean Diet and Risk of Breast Cancer: An Umbrella Review. Clin. Nutr. 2023, 42, 600–608. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Borgard, H.; Jijiwa, M.; Nasu, M.; He, M.; Deng, Y. The Function and Mechanism of Lipid Molecules and Their Roles in The Diagnosis and Prognosis of Breast Cancer. Molecules 2020, 25, 4864. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.G.; Peterson, L.B.; Adams, A.D.; Lam, M.-H.N.; Burton, C.A.; Chin, J.; Guo, Q.; Huang, S.; Latham, M.; Lopez, J.C.; et al. Different Roles of Liver X Receptor α and β in Lipid Metabolism: Effects of an α-Selective and a Dual Agonist in Mice Deficient in Each Subtype. Biochem. Pharmacol. 2006, 71, 453–463. [Google Scholar] [CrossRef]
- Nazih, H.; Bard, J.M. Cholesterol, Oxysterols and LXRs in Breast Cancer Pathophysiology. Int. J. Mol. Sci. 2020, 21, 1356. [Google Scholar] [CrossRef]
- Bacchetti, T.; Ferretti, G.; Sahebkar, A. The Role of Paraoxonase in Cancer. Semin. Cancer Biol. 2019, 56, 72–86. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human Paraoxonase-1 (PON1): Gene Structure and Expression, Promiscuous Activities and Multiple Physiological Roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T. Effect of Dietary Lipids on Paraoxonase-1 Activity and Gene Expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.M.; Gharib, A.F.; Etewa, R.L.; ElSawy, W.H. Association of L55M and Q192R Polymorphisms in Paraoxonase 1 (PON1) Gene with Breast Cancer Risk and Their Clinical Significance. Mol. Cell Biochem. 2011, 351, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Farmohammadi, A.; Momeni, A.; Bahmani, B.; Ghorbani, H.; Ramzanpour, R. Association of PON1-L55M Genetic Variation and Breast Cancer Risk: A Case-Control Trial. Asian Pac. J. Cancer Prev. 2020, 21, 255–258. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.; Drouet, L.; Lairon, D.; Cazaubiel, M.; Marmonier, C.; Ninio, E.; Bal Dit Sollier, C.; Martin, J.; Boyer, C.; Bobin-Dubigeon, C. Effect of Milk Fat on LDL Cholesterol and Other Cardiovascular Risk Markers in Healthy Humans: The INNOVALAIT Project. Eur. J. Clin. Nutr. 2020, 74, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ciqual Table de Composition Nutritionnelle Des Aliments. Available online: https://ciqual.anses.fr/ (accessed on 27 March 2023).
- Black, A.E. The Sensitivity and Specificity of the Goldberg Cut-off for EI:BMR for Identifying Diet Reports of Poor Validity. Eur. J. Clin. Nutr. 2000, 54, 395–404. [Google Scholar] [CrossRef]
- Phillips, C.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J. Dietary Inflammatory Index and Biomarkers of Lipoprotein Metabolism, Inflammation and Glucose Homeostasis in Adults. Nutrients 2018, 10, 1033. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.-W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef]
- Kranz, S.; Hasan, F.; Kennedy, E.; Zoellner, J.; Guertin, K.A.; Shivappa, N.; Hébert, J.R.; Anderson, R.; Cohn, W. Diet Quality and Dietary Inflammatory Index Score among Women’s Cancer Survivors. Int. J. Environ. Res. Public Health 2022, 19, 1916. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Nazih, H.; Croyal, M.; Bard, J.-M. Link between Omega 3 Fatty Acids Carried by Lipoproteins and Breast Cancer Severity. Nutrients 2022, 14, 2461. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Chauvin, A.; Brillaud-Meflah, V.; Boiffard, F.; Joalland, M.; Bard, J. Liver X Receptor (LXR)-Regulated Genes of Cholesterol Trafficking and Breast Cancer Severity. Anticancer Res. 2017, 37, 5495–5498. [Google Scholar] [CrossRef] [PubMed]
- Bobin-Dubigeon, C.; Lefrançois, A.; Classe, J.; Joalland, M.; Bard, J. Paired Measurement of Serum Amyloid A (SAA) and Paraoxonase 1 (PON1) as Useful Markers in Breast Cancer Recurrence. Clin. Biochem. 2015, 48, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Torres, J.A.; García-Domínguez, M.L.; Cruz, M.; Aradillas-García, C. Q192R Polymorphism of Paraoxonase 1 Gene Associated with Insulin Resistance in Mexican Children. Arch. Med. Res. 2015, 46, 78–83. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Nazih, H.; Blanchard, V.; Croyal, M.; Bard, J.-M. Circulating HDL and Non-HDL Associated Apolipoproteins and Breast Cancer Severity. J. Clin. Med. 2022, 11, 1345. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Y.; Li, Y.; Jiang, C.; Wu, Y.; Shang, L.; Huang, Y.; Cheng, S. Metabolic Syndrome Is a Risk Factor for Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: A Case-Control Study. Front. Oncol. 2023, 12, 1080054. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Caklili, O.T.; Oguz, A. Association between Metabolic Syndrome and Cancer. ANM 2016, 68, 173–179. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A Systematic Review and Meta-Analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, T.; Lukanova, A.; Jonsson, H.; Tretli, S.; Ulmer, H.; Manjer, J.; Stocks, T.; Selmer, R.; Nagel, G.; Almquist, M.; et al. Metabolic Syndrome and Breast Cancer in the Me-Can (Metabolic Syndrome and Cancer) Project. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Gathirua-Mwangi, W.G.; Song, Y.; Monahan, P.; Champion, V.L.; Zollinger, T. Associations of Metabolic Syndrome and C-Reactive Protein with Mortality from Total Cancer, Obesity-Linked Cancers and Breast Cancer among Women in NHANES III. Int. J. Cancer 2018, 143, 535–542. [Google Scholar] [CrossRef]
- Pan, K.; Aragaki, A.K.; Neuhouser, M.L.; Simon, M.S.; Luo, J.; Caan, B.; Snetselaar, L.; Mortimer, J.E.; Manson, J.E.; Kroenke, C.; et al. Low-Fat Dietary Pattern and Breast Cancer Mortality by Metabolic Syndrome Components: A Secondary Analysis of the Women’s Health Initiative (WHI) Randomised Trial. Br. J. Cancer 2021, 125, 372–379. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Pan, K.; Simon, M.S.; Neuhouser, M.L.; Haque, R.; Rohan, T.E.; Wactawski-Wende, J.; Orchard, T.S.; Mortimer, J.E.; et al. Breast Cancer Incidence and Mortality by Metabolic Syndrome and Obesity: The Women’s Health Initiative. Cancer 2024, 130, 3147–3156. [Google Scholar] [CrossRef]
- Bergman, R.; Berko, Y.A.; Sanchez, V.; Sanders, M.E.; Gonzalez-Ericsson, P.I.; Arteaga, C.L.; Rexer, B.N. Obesity and Metabolic Syndrome Are Associated with Short-Term Endocrine Therapy Resistance in Early ER + Breast Cancer. Breast Cancer Res. Treat. 2023, 197, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, K.; Lawrence, M.A.; McNaughton, S.A. A Systematic Review of the Methods Used to Assess and Report Dietary Patterns. Front. Nutr. 2022, 9, 892351. [Google Scholar] [CrossRef]
- Rapport d’Expertise Collective. Avis de l’ANSES. Etude Individuelle des Consommations Alimentaires 3 (INCA3) Juin 2017. Available online: https://www.anses.fr/fr/content/avis-et-rapport-de-lanses-sur-lactualisation-de-la-base-de-donnees-des-consommations (accessed on 24 July 2024).
- Iguacel, I.; Perez-Cornago, A.; Schmidt, J.A.; Van Puyvelde, H.; Travis, R.; Casagrande, C.; Nicolas, G.; Riboli, E.; Weiderpass, E.; Ardanaz, E.; et al. Evaluation of Protein and Amino Acid Intake Estimates from the EPIC Dietary Questionnaires and 24-h Dietary Recalls Using Different Food Composition Databases. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 80–89. [Google Scholar] [CrossRef]
- Shivappa, N.; Sandin, S.; Löf, M.; Hébert, J.R.; Adami, H.-O.; Weiderpass, E. Prospective Study of Dietary Inflammatory Index and Risk of Breast Cancer in Swedish Women. Br. J. Cancer 2015, 113, 1099–1103. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Mo, X.-F.; Ye, Y.-B.; Shivappa, N.; Lin, F.-Y.; Huang, J.; Hébert, J.R.; Yan, B.; Zhang, C.-X. A Higher Dietary Inflammatory Index Score Is Associated with a Higher Risk of Breast Cancer among Chinese Women: A Case-Control Study. Br. J. Nutr. 2017, 117, 1358–1367. [Google Scholar] [CrossRef]
- Fowler, M.E.; Akinyemiju, T.F. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Cancer Outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Zucchetto, A.; Serraino, D.; Shivappa, N.; Hébert, J.R.; Stocco, C.; Puppo, A.; Falcini, F.; Panato, C.; Dal Maso, L.; Polesel, J. Dietary Inflammatory Index before Diagnosis and Survival in an Italian Cohort of Women with Breast Cancer. Br. J. Nutr. 2017, 117, 1456–1462. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Caan, B.; Chlebowski, R.T.; Freudenheim, J.L.; Hou, L.; Mossavar-Rahmani, Y.; et al. Association between Dietary Inflammatory Potential and Breast Cancer Incidence and Death: Results from the Women’s Health Initiative. Br. J. Cancer 2016, 114, 1277–1285. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct Validation of the Dietary Inflammatory Index among Postmenopausal Women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Y.; Pan, Q.; Chen, H.; Chen, F.; Wu, J.; Di, D. Expression of LXR-β, ABCA1 and ABCG1 in Human Triple-Negative Breast Cancer Tissues. Oncol. Rep. 2019, 42, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Bobin-Dubigeon, C.; Jaffré, I.; Joalland, M.; Classe, J.; Campone, M.; Hervé, M.; Bard, J. Paraoxonase 1 (PON1) as a Marker of Short Term Death in Breast Cancer Recurrence. Clin. Biochem. 2012, 45, 1503–1505. [Google Scholar] [CrossRef]
- Balci, H.; Genc, H.; Papila, C.; Can, G.; Papila, B.; Yanardag, H.; Uzun, H. Serum Lipid Hydroperoxide Levels and Paraoxonase Activity in Patients With Lung, Breast, and Colorectal Cancer. J. Clin. Lab. Anal. 2012, 26, 155–160. [Google Scholar] [CrossRef]
- Camps, J.; Marsillach, J.; Joven, J. Measurement of Serum Paraoxonase-1 Activity in the Evaluation of Liver Function. World J. Gastroenterol. 2009, 15, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Mazzuferi, G.; Bacchetti, T.; Islam, M.O.; Ferretti, G. High Density Lipoproteins and Oxidative Stress in Breast Cancer. Lipids Health Dis. 2021, 20, 143. [Google Scholar] [CrossRef]
- Pan, X.; Huang, L.; Li, M.; Mo, D.; Liang, Y.; Liu, Z.; Huang, Z.; Huang, L.; Liu, J.; Zhu, B. The Association between PON1 (Q192R and L55M) Gene Polymorphisms and Risk of Cancer: A Meta-Analysis Based on 43 Studies. BioMed Res. Int. 2019, 2019, 5897505. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [PubMed]
| SM+ (n = 22) | SM− (n = 50) | p-Value | |
|---|---|---|---|
| Age (years) * | 65 (54–75) | 52 (44–63) | 0.006 |
| BMI (kg m−2) * | 28.5 (25.7–30.9) | 22.5 (20.6–24.9) | <0.001 |
| Menopausal status ** | 18 (81.8%) | 23 (46%) | 0.005 |
| Performance status = 0 | 17 (77.3%) | 50 (100%) | 0.002 |
| Type of cancer ** | 0.03 | ||
| Invasive carcinoma of no special type (ductal) | 13 (59.1%) | 41 (82.0%) | |
| Invasive lobular carcinoma | 9 (40.9%) | 7 (14.0%) | |
| Other | 0 (0%) | 2 (4.0%) | |
| Overexpression of estrogen receptor | 22 (100%) | 50 (100%) | |
| HER2+ | 0% | 0% | |
| Circulating lipids (mmol/L) | |||
| Plasma cholesterol | 5.42 (4.74–5.94) | 5.68 (4.94–6.67) | 0.144 |
| Plasma triglycerides | 1.07 (0.74–1.59) | 0.94 (0.72–1.30) | 0.309 |
| HDL cholesterol | 1.26 (1.15–1.51) | 1.81 (1.58–2.03) | <0.001 |
| LDL cholesterol | 3.26 (2.73–3.86) | 3.42 (2.91–4.21) | 0.303 |
| Genes | AU (n = 50) | ||
|---|---|---|---|
| Baseline | Week 5 | p-Value | |
| ABCG1 | 1.47 [1.06–1.85] | 1.23 [0.94–1.70] | 0.72 |
| ABCA1 | 1.40 [1.01–2.10] | 1.40 [1.01–2.10] | 0.61 |
| PON2 | 0.58 [0.50–0.73] | 0.65 [0.52–0.70] | 0.47 |
| LXRb | 1.46 [0.95–2.34] | 1.49 [1.01–2.30] | 0.98 |
| Activities | L/L (n = 30) | M/L (n = 34) | M/M (n = 5) | P Global | LL vs. ML | P ML vs. MM | LL vs. MM |
|---|---|---|---|---|---|---|---|
| ARE mmol/L/min | 20.5 [17.4–25.9] | 23.9 [15.9–38.2] | 50.9 [18.2–59.0] | 0.18 | ns | ns | ns |
| LAC µmol/L/min | 0.177 [0.16–0.187] | 0.174 [0.15–0.20] | 0.171 [0.13–0.177] | 0.71 | ns | ns | ns |
| PON µmol/L/min | 109 [64–165] | 123.5 [71–209] | 81 [51–112] | 0.34 | ns | ns | ns |
| Activities | Q/Q | R/Q | R/R | P | |||
|---|---|---|---|---|---|---|---|
| (n = 34) | (n = 25) | (n = 9) | P Global | QQ vs. RQ | RQ vs. RR | QQ vs. RR | |
| ARE mmol/L/min | 27.9 [18.5–38.0] | 19.3 [14.8–23.9] | 18.2 [14.8–31] | 0.036 | 0.016 | 1 | 0.11 |
| LAC µmol/L/min | 0.172 [0.143–0.18] | 0.174 [0.16–0.20] | 0.187 [0.18–0.21] | 0.032 | 0.27 | 0.081 | 0.01 |
| PON µmol/L/min | 69 [52.2–99.5] | 168 [120–218] | 242 [69–285] | <0.0001 | <0.0001 | 0.61 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobin-Dubigeon, C.; Campion, L.; Bossard, C.; Rossignol, E.; Frenel, J.-S.; Campone, M.; Bard, J.-M. Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer. Nutrients 2024, 16, 3579. https://doi.org/10.3390/nu16213579
Bobin-Dubigeon C, Campion L, Bossard C, Rossignol E, Frenel J-S, Campone M, Bard J-M. Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer. Nutrients. 2024; 16(21):3579. https://doi.org/10.3390/nu16213579
Chicago/Turabian StyleBobin-Dubigeon, Christine, Loic Campion, Clémence Bossard, Elsa Rossignol, Jean-Sébastien Frenel, Mario Campone, and Jean-Marie Bard. 2024. "Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer" Nutrients 16, no. 21: 3579. https://doi.org/10.3390/nu16213579
APA StyleBobin-Dubigeon, C., Campion, L., Bossard, C., Rossignol, E., Frenel, J.-S., Campone, M., & Bard, J.-M. (2024). Link Between Metabolic Syndrome, Blood Lipid Markers, Dietary Lipids, and Survival in Women with Early-Stage Breast Cancer. Nutrients, 16(21), 3579. https://doi.org/10.3390/nu16213579







