Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture Conditions
2.3. Cell Viability Assay
2.4. Luciferase Reporter Assay
2.5. Quantitative Real-Time PCR Analysis
2.6. Nrf2 Activation Potential of PTS by Immunoblotting
2.7. Bisulfite Conversion and Primer Designing
2.8. Status of Nrf2 Promotor CpG Island Methylation and Its Reversal by PTS
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity of PTS in EA.hy929 Cells
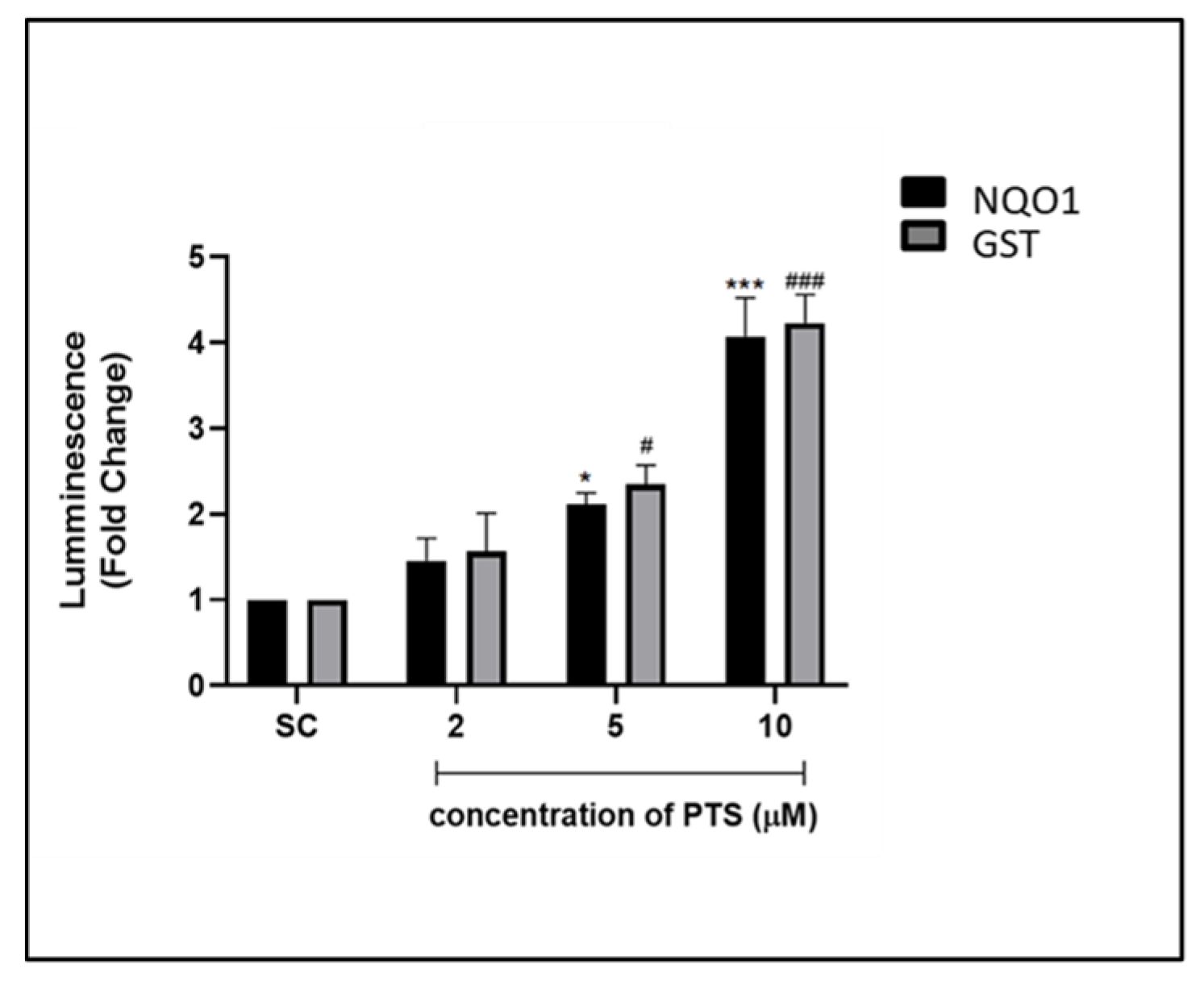
3.2. Nrf2 Activation Potential by PTS on Endothelial Cells by Luciferase Reporter Assay
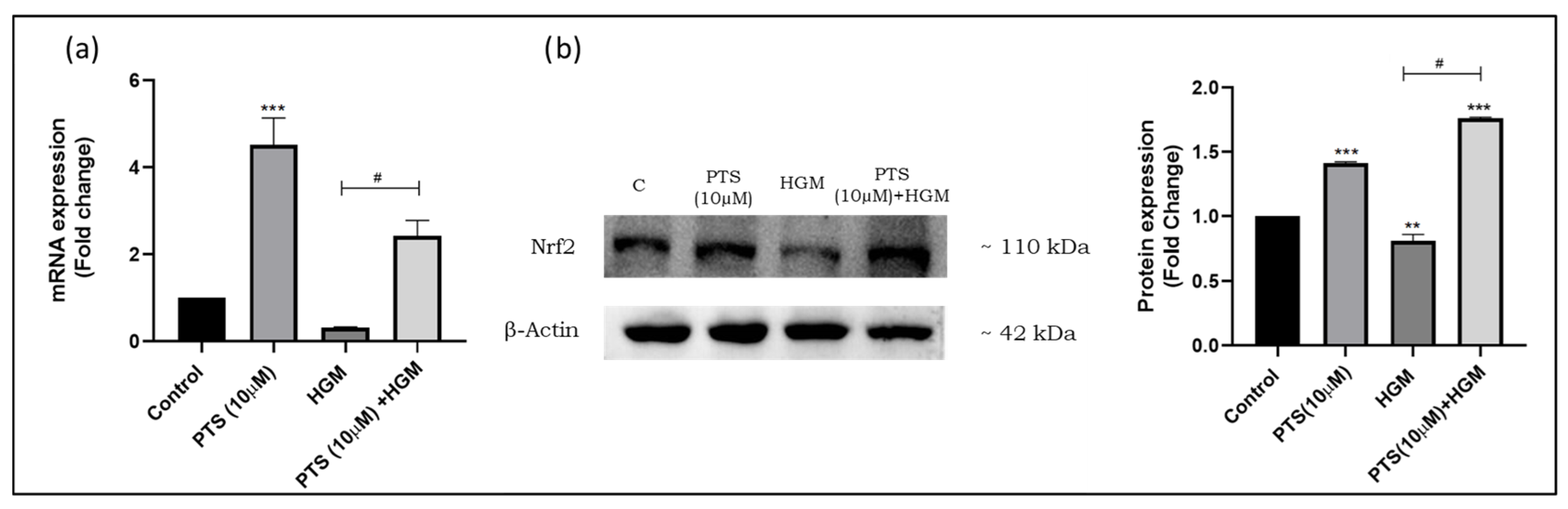
3.3. Effect of Pterostilbene on Nrf2 Activation and Nuclear Translocation in Endothelial Cells in a Hyperglycemic Microenvironment
3.4. Pterostilbene Modulates Nrf2 Expression in Endothelial Cells in an HGM
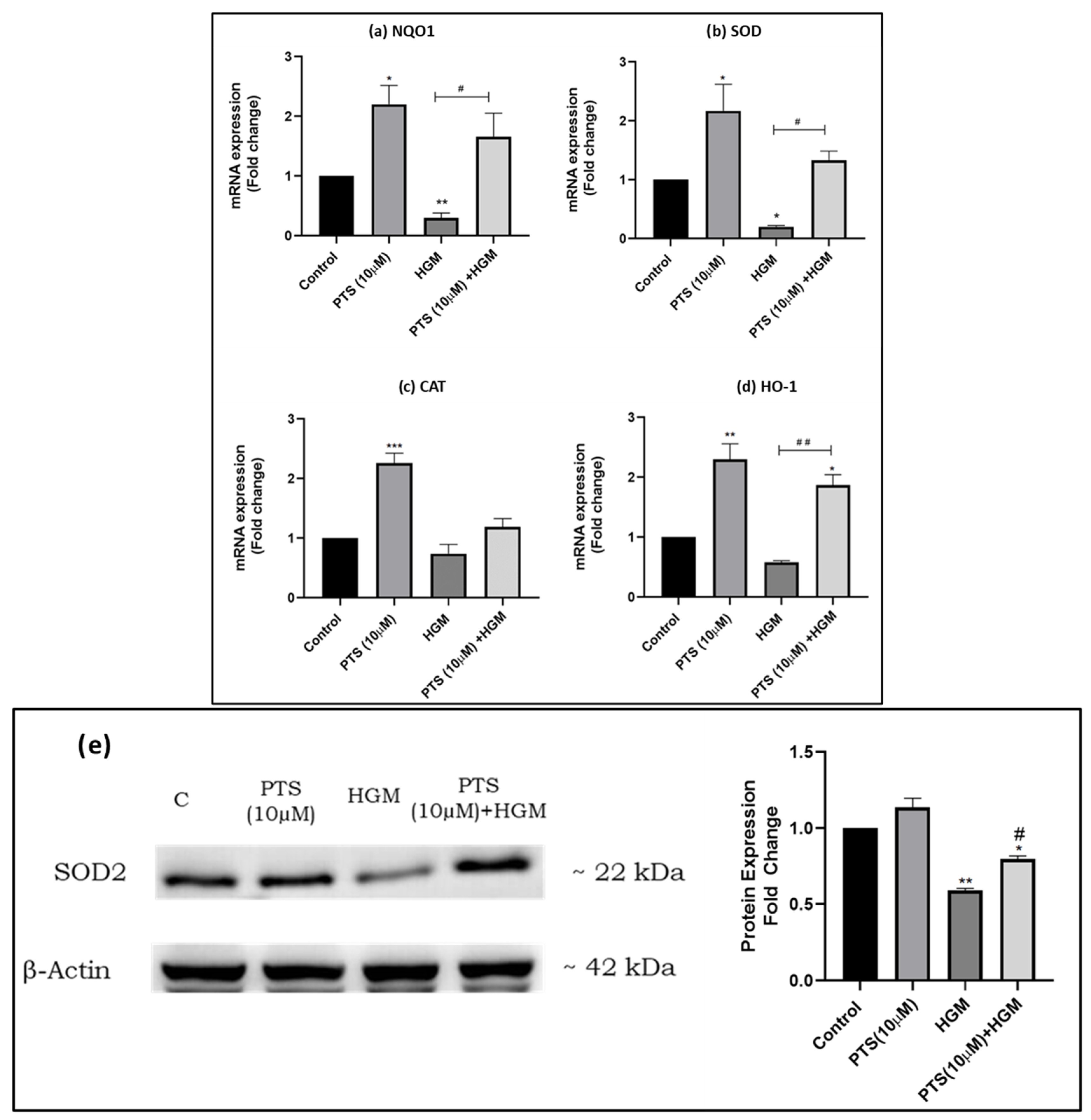
3.5. Pterostilbene Modulates the Expression of Nrf2 and Its Downstream Targets in Endothelial Cells in an HGM
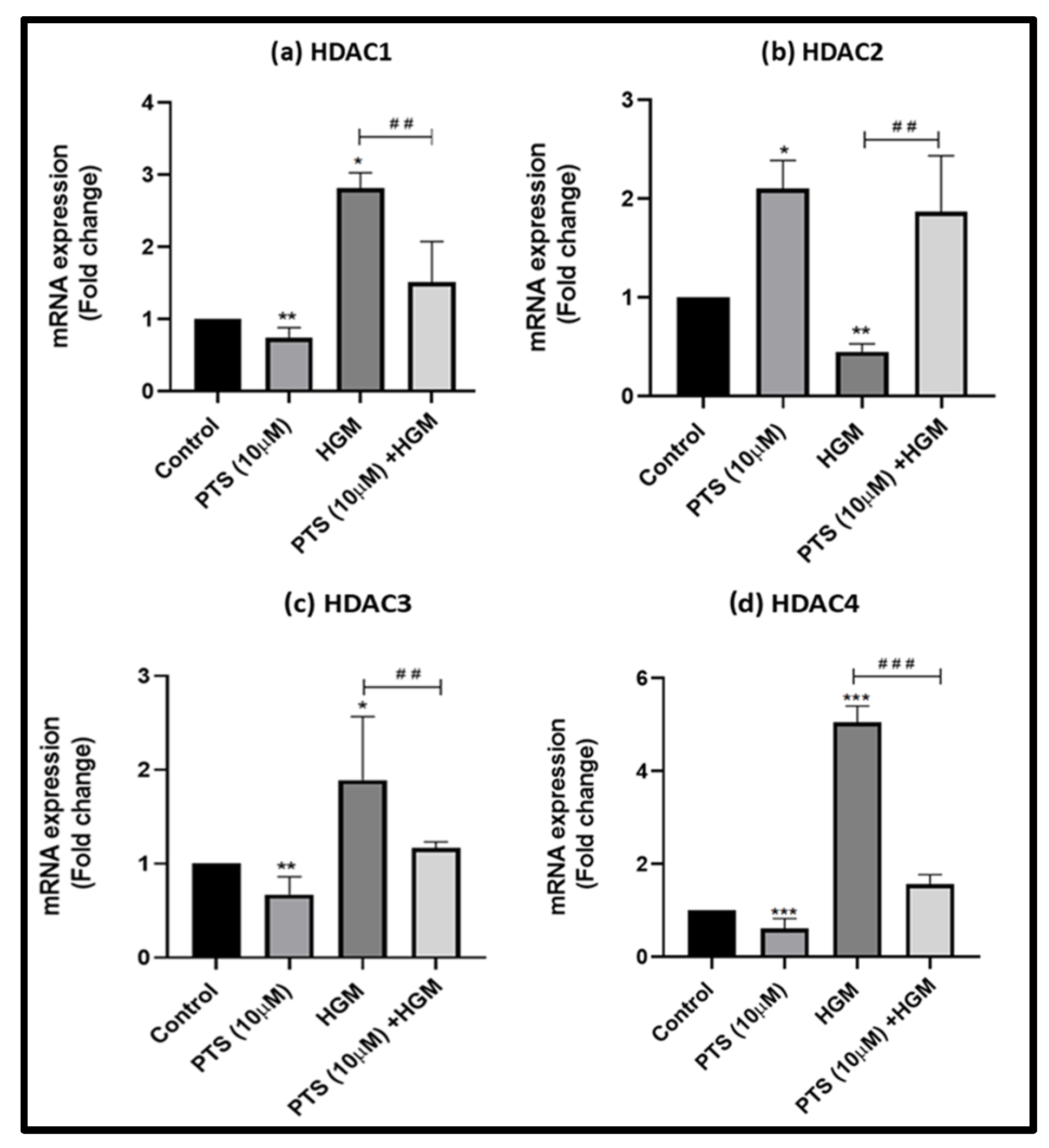
3.6. Pterostilbene Modulates the Expression of HDACs in Endothelial Cells in an HGM
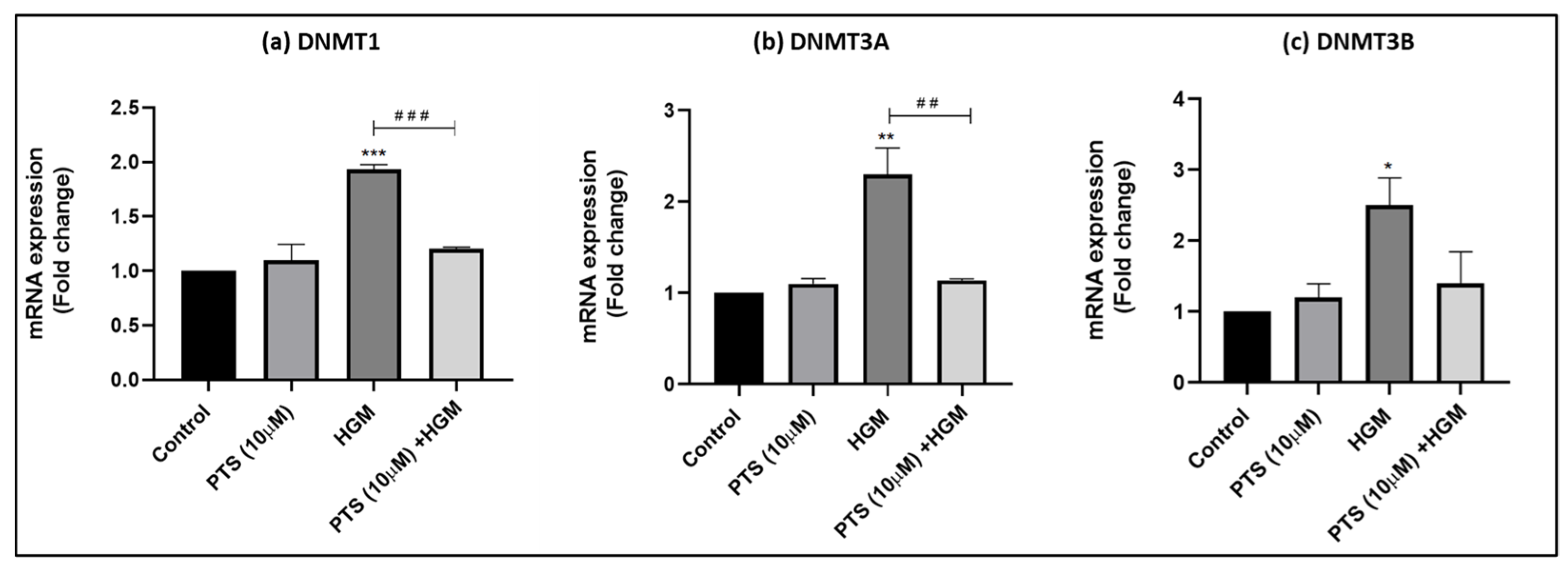
3.7. Effect of Pterostilbene in Regulating the Expression of Epigenetic Writers DNA Methyltransferase (DNMTs)
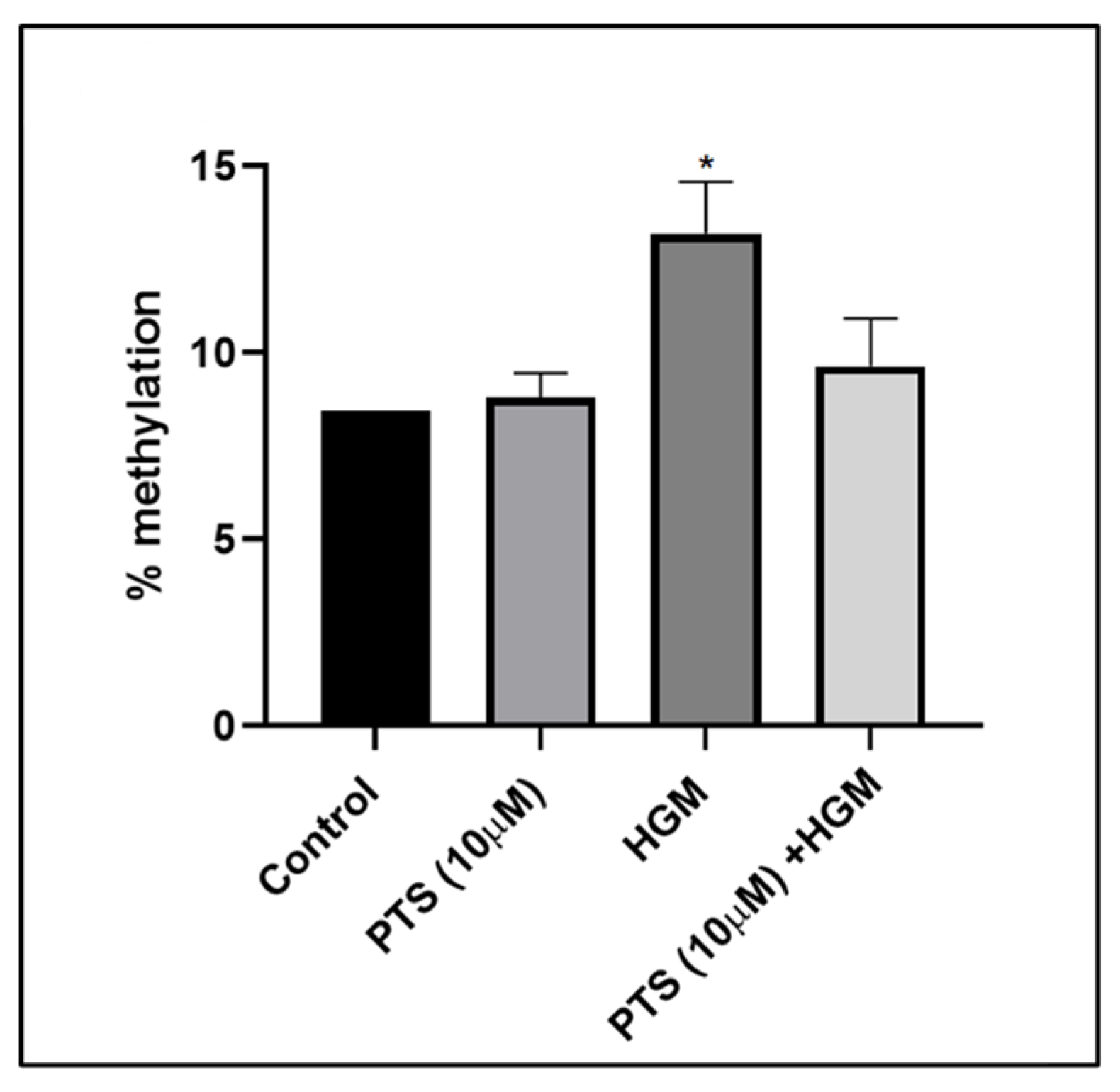
3.8. Pterostilbene Reverses Nrf2 Promoter CpG Island Methylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A. Endothelial dysfunction in patients with type 2 diabetes and the effects of thiazolidinedione antidiabetic agents. J. Diabetes Complicat. 2004, 18, 91–102. [Google Scholar] [CrossRef]
- Buranasin, P.; Kominato, H.; Mizutani, K.; Mikami, R.; Saito, N.; Takeda, K.; Iwata, T. Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients. Antioxidants 2023, 12, 1787. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Li, X.J.; Wang, X.; Channon, K.M.; Chen, A.F. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1423–E1429. [Google Scholar] [CrossRef][Green Version]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Jiang, Z.; Jiang, J.; Xu, L.; Tian, A.; Sun, X.; Meng, H.; Li, Y.; Huang, W.; et al. Protective role of NRF2 in macrovascular complications of diabetes. J. Cell Mol. Med. 2020, 24, 8903–8917. [Google Scholar] [CrossRef]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a Potential Mediator of Cardiovascular Risk in Metabolic Diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Long, M.; Rojo de la Vega, M.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2016, 65, 780–793. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, W.; Zhang, Y.; Tan, M.; Yin, Y.; Li, Y.; Zhang, L.; Han, L.; Bai, J.; Jiang, T.; et al. Comprehensive overview of Nrf2-related epigenetic regulations involved in ischemia-reperfusion injury. Theranostics 2022, 12, 6626–6645. [Google Scholar] [CrossRef]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020, 6, e03803. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Khaper, N.; Mercier, S.; Tai, T.C. The Role of DNMT and HDACs in the Fetal Programming of Hypertension by Glucocorticoids. Oxid. Med. Cell Longev. 2020, 2020, 5751768. [Google Scholar] [CrossRef]
- Saini, S.K.; Mangalhara, K.C.; Prakasam, G.; Bamezai, R.N.K. DNA Methyltransferase1 (DNMT1) Isoform3 methylates mitochondrial genome and modulates its biology. Sci. Rep. 2017, 7, 1525. [Google Scholar] [CrossRef]
- Zakeri, S.; Rahimi, Z.; Rezvani, N.; Vaisi-Raygani, A.; Alibakhshi, R.; Zakeri, S.; Yari, K. The influence of Nrf2 gene promoter methylation on gene expression and oxidative stress parameters in preeclampsia. BMC Med. Genom. 2024, 17, 64. [Google Scholar] [CrossRef]
- Bhatt, T.; Dey, R.; Hegde, A.; Ketkar, A.A.; Pulianmackal, A.J.; Deb, A.P.; Rampalli, S.; Jamora, C. Initiation of wound healing is regulated by the convergence of mechanical and epigenetic cues. PLoS Biol. 2022, 20, e3001777. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef]
- Amin, K.N.; Palanisamy, R.; Sarada, D.V.L.; Ali, D.; Suzuki, T.; Ramkumar, K.M. Effect of Rosolic acid on endothelial dysfunction under ER stress in pancreatic microenvironment. Free Radic. Res. 2021, 55, 698–713. [Google Scholar] [CrossRef]
- Ganesan, K.; Ramkumar, K.M.; Xu, B. Vitexin restores pancreatic beta-cell function and insulin signaling through Nrf2 and NF-kappaB signaling pathways. Eur. J. Pharmacol. 2020, 888, 173606. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Ramkumar, K.M. Mangiferin alleviates hyperglycemia-induced endothelial impairment via Nrf2 signaling pathway. Eur. J. Pharmacol. 2022, 936, 175359. [Google Scholar] [CrossRef]
- Vanitha, P.; Senthilkumar, S.; Dornadula, S.; Anandhakumar, S.; Rajaguru, P.; Ramkumar, K.M. Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells. Eur. J. Pharmacol. 2017, 801, 9–18. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yanez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Dysregulation of Nrf2 redox pathway in macrophages under diabetic microenvironment. Exp. Gerontol. 2021, 152, 111479. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Pterostilbene attenuates hemin-induced dysregulation of macrophage M2 polarization via Nrf2 activation in experimental hyperglycemia. Inflammopharmacology 2023, 31, 2133–2145. [Google Scholar] [CrossRef]
- Sireesh, D.; Ganesh, M.R.; Dhamodharan, U.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J. Nutr. Biochem. 2017, 44, 11–21. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Sekar, T.V.; Foygel, K.; Elango, B.; Paulmurugan, R. Reporter protein complementation imaging assay to screen and study Nrf2 activators in cells and living animals. Anal. Chem. 2013, 85, 7542–7549. [Google Scholar] [CrossRef]
- Prasad, M.K.; Victor, P.S.; Ganesh, G.V.; Juttada, U.; Kumpatla, S.; Viswanathan, V.; Ramkumar, K.M. Sodium-Glucose Cotransporter-2 Inhibitor Suppresses Endoplasmic Reticulum Stress and Oxidative Stress in Diabetic Nephropathy Through Nrf2 Signaling: A Clinical and Experimental Study. J. Clin. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Fabrizio, F.P.; Costantini, M.; Copetti, M.; la Torre, A.; Sparaneo, A.; Fontana, A.; Poeta, L.; Gallucci, M.; Sentinelli, S.; Graziano, P.; et al. Keap1/Nrf2 pathway in kidney cancer: Frequent methylation of KEAP1 gene promoter in clear renal cell carcinoma. Oncotarget 2017, 8, 11187–11198. [Google Scholar] [CrossRef]
- He, Y.; Taylor, R.L., Jr.; Bai, H.; Ashwell, C.M.; Zhao, K.; Li, Y.; Sun, G.; Zhang, H.; Song, J. Transgenerational epigenetic inheritance and immunity in chickens that vary in Marek’s disease resistance. Poult. Sci. 2023, 102, 103036. [Google Scholar] [CrossRef]
- Camina, N.; Penning, T.M. Genetic and epigenetic regulation of the NRF2-KEAP1 pathway in human lung cancer. Br. J. Cancer 2022, 126, 1244–1252. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Muller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Activation of Nrf2 by Natural Bioactive Compounds: A Promising Approach for Stroke? Int. J. Mol. Sci. 2020, 21, 4875. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper, W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorg Med. Chem. 2016, 24, 3378–3386. [Google Scholar] [CrossRef]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef]
- Amarnath Satheesh, M.; Pari, L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006, 58, 1483–1490. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- He, X.; Ma, Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol. Pharmacol. 2009, 76, 1265–1278. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin Protects H9C2 Cardiomyocytes from High-Glucose and Hypoxia/Reoxygenation Injury via Inhibition of Reactive Oxygen Species Generation and Inflammatory Responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef]
- Zubair, M.; Malik, A.; Ahmad, J. Plasma adiponectin, IL-6, hsCRP, and TNF-alpha levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian. J. Endocrinol. Metab. 2012, 16, 769–776. [Google Scholar]
- Uruno, A.; Yagishita, Y.; Yamamoto, M. The Keap1-Nrf2 system and diabetes mellitus. Arch. Biochem. Biophys. 2015, 566, 76–84. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Reisman, S.A.; Yeager, R.L.; Goedken, M.J.; Klaassen, C.D. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J. Pharmacol. Exp. Ther. 2010, 333, 140–151. [Google Scholar] [CrossRef]
- Bellot, G.L.; Dong, X.; Lahiri, A.; Sebastin, S.J.; Batinic-Haberle, I.; Pervaiz, S.; Puhaindran, M.E. MnSOD is implicated in accelerated wound healing upon Negative Pressure Wound Therapy (NPWT): A case in point for MnSOD mimetics as adjuvants for wound management. Redox Biol. 2019, 20, 307–320. [Google Scholar] [CrossRef]
- Teena, R.; Dhamodharan, U.; Jayasuriya, R.; Ali, D.; Kesavan, R.; Ramkumar, K.M. Analysis of the Exonic Single Nucleotide Polymorphism rs182428269 of the NRF2 Gene in Patients with Diabetic Foot Ulcer. Arch. Med. Res. 2021, 52, 224–232. [Google Scholar] [CrossRef]
- Deng, J.Y.; Wu, X.Q.; He, W.J.; Liao, X.; Tang, M.; Nie, X.Q. Targeting DNA methylation and demethylation in diabetic foot ulcers. J. Adv. Res. 2023, 54, 119–131. [Google Scholar] [CrossRef]
- Carlos-Reyes, A.; Lopez-Gonzalez, J.S.; Meneses-Flores, M.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernandez de la Cruz, O.N.; Lopez-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Silva-Llanes, I.; Shin, C.H.; Jimenez-Villegas, J.; Gorospe, M.; Lastres-Becker, I. The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis. Antioxidants 2023, 12, 641. [Google Scholar] [CrossRef]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Kim, Y.; Li, J.; Huang, S.; Saleem, S.; Li, R.C.; Xu, Y.; Dore, S.; Cao, W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic. Biol. Med. 2012, 52, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Mizuno, W.; Oda, M.; Ide, N.; Morita, T.; Hara, Y.; Yamawaki, H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1894–H1904. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, G.; Sun, J.; Chen, Y.; Wang, N.; Dong, Y.; Shen, E.; Hu, Z.; Gong, W.; Jin, L.; et al. Histone deacetylase 3 inhibition alleviates type 2 diabetes mellitus-induced endothelial dysfunction via Nrf2. Cell Commun. Signal. 2021, 19, 35. [Google Scholar] [CrossRef]
- Choi, J.D.; Lee, J.S. Interplay between Epigenetics and Genetics in Cancer. Genom. Inform. 2013, 11, 164–173. [Google Scholar] [CrossRef]
- Khor, T.O.; Fuentes, F.; Shu, L.; Paredes-Gonzalez, X.; Yang, A.Y.; Liu, Y.; Smiraglia, D.J.; Yegnasubramanian, S.; Nelson, W.G.; Kong, A.N. Epigenetic DNA methylation of antioxidative stress regulator NRF2 in human prostate cancer. Cancer Prev. Res. 2014, 7, 1186–1197. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Mai, A.; Altucci, L. Epi-drugs to fight cancer: From chemistry to cancer treatment, the road ahead. Int. J. Biochem. Cell Biol. 2009, 41, 199–213. [Google Scholar] [CrossRef]
- Lou, D.; Wei, X.; Xiao, P.; Huo, Q.; Hong, X.; Sun, J.; Shuai, Y.; Tao, G. Demethylation of the NRF2 Promoter Protects Against Carcinogenesis Induced by Nano-SiO2. Front. Genet. 2020, 11, 818. [Google Scholar] [CrossRef]


| S. No. | Gene | Forward Primer | Reverse Primer | Tm (°C) |
|---|---|---|---|---|
| 1 | Nrf2 | TGTAGATGACAATGAGGTTTC | ACTGAGCCTGATTAGTAGCAA | 56 °C |
| 2 | NQO1 | AGGATGGAAGAAACGCCTGG | TCAGTTGGGATGGACTTGCC | 60 °C |
| 3 | SOD | GGCATCATCAATTTCGAG | CCGTAGTAGTTAAAGCTC | 59 °C |
| 4 | CAT | ATCCGTGTAACCCGCTCATC | ACCTTCATTTTCCCCTGGGG | 61 °C |
| 5 | HO-1 | GGGAATTCTCTTGGCTGGCT | AACTGAGGATGCTGAAGGGC | 59 °C |
| 6 | HDAC1 | GGCTGGCAAAGGCAAGTAT | CGCACTAGGCTGGAACATCT | 58 °C |
| 7 | HDAC2 | ATTGGGGAACAGGTGGTG | GGGGCGAGGGATAAAAGA | 56 °C |
| 8 | HDAC3 | GTATGAAGTCGGGGCAGAGA | CGTGGGTTGGTAGAAGTCC | 55.5 °C |
| 9 | HDAC4 | GCACAGTCCTTGGTTGGT | AGAAACTGCTGATGCTGCT | 56 °C |
| 10 | DNMT1 | TCAAGACTGATGGGAAGAAGAGTT | CGTGACCCTTGCTAGATACAGC | 56 °C |
| 11 | DNMT3A | GATGACGAGCCAGAGTACGA | CTTCTCAACACACACCACTGA | 56 °C |
| 12 | DNMT3B | CGACCTCACAGACGACACAG | TCCAAACTCCTTCCCATCCT | 56.2 °C |
| 13 | GAPDH | AAGAAGGTGGTGAAGCAGGC | GTCAAAGGTGGAGGAGTGGG | 60 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harithpriya, K.; Ganesan, K.; Ramkumar, K.M. Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment. Nutrients 2024, 16, 2045. https://doi.org/10.3390/nu16132045
Harithpriya K, Ganesan K, Ramkumar KM. Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment. Nutrients. 2024; 16(13):2045. https://doi.org/10.3390/nu16132045
Chicago/Turabian StyleHarithpriya, Kannan, Kumar Ganesan, and Kunka Mohanram Ramkumar. 2024. "Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment" Nutrients 16, no. 13: 2045. https://doi.org/10.3390/nu16132045
APA StyleHarithpriya, K., Ganesan, K., & Ramkumar, K. M. (2024). Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment. Nutrients, 16(13), 2045. https://doi.org/10.3390/nu16132045





