Highlights
- The microbiota produces metabolites that influence gastrointestinal, metabolic, autoimmune, and neurodegenerative diseases.
- Dysbiosis biomarkers could indicate susceptibility to future health conditions, emphasizing the need for microbiota balance.
- Early-life microbiota development is shaped by gestational age, delivery method, antibiotics, and genetic/environmental factors.
- Targeting microbial metabolites offers potential for therapies aimed at inflammatory and chronic diseases.
Abstract
Research on the microbiome has progressed from identifying specific microbial communities to exploring how these organisms produce and modify metabolites that impact a wide range of health conditions, including gastrointestinal, metabolic, autoimmune, and neurodegenerative diseases. This review provides an overview of the bacteria commonly found in the intestinal tract, focusing on their main functional outputs. We explore biomarkers that not only indicate a well-balanced microbiota but also potential dysbiosis, which could foreshadow susceptibility to future health conditions. Additionally, it discusses the establishment of the microbiota during the early years of life, examining factors such as gestational age at birth, type of delivery, antibiotic intake, and genetic and environmental influences. Through a comprehensive analysis of current research, this article aims to enhance our understanding of the microbiota’s foundational development and its long-term implications for health and disease management.
1. Introduction
The human microbiome refers to the collective assembly of microorganisms, their genes, and metabolic products present in and on the human body [1]. However, it encompasses a complex ecosystem that impacts host biology, including metabolic and immune functions. In contrast, “microbiota” specifically denotes the community of these microorganisms themselves, residing in particular environments such as the skin, mouth, and notably, the gastrointestinal tract. This distinction highlights that while the microbiota includes the organisms, the microbiome also comprises the genetic elements and bioactive compounds they produce, which contribute to the host’s physiological landscape.
Since the year 2000, the advancement of sequencing techniques, especially next-generation sequencing, has enabled the development of the field of metagenomics, which studies the genetic material of microorganisms. This allows us to understand the diversity and functions of the microbiota, not only in humans but also in any environment that can be inhabited by them [2].
In terms of biological relevance, this community of bacteria, viruses, fungi, and other unicellular organisms plays a key role in metabolism and has been linked to a wide range of diseases, from digestive disorders to neurological and metabolic conditions.
But for a deeper understanding of microbial activity, it is advisable to consider the metabolites produced by microorganisms, providing a snapshot of the biochemical activities taking place within the microbiome. By combining this approach with metagenomics, it is possible to link genetic potential with metabolic function.
On the other hand, culturomics, a term first coined in 2012, represents a novel approach aimed at enriching our understanding of microbiomes. This method diversifies culture conditions to closely replicate the natural environments of bacteria, facilitating the growth of previously unculturable species [3]. Unlike metagenomics, which relies on DNA sequencing to analyze microbial communities directly from environmental samples without the need for culturing, culturomics focuses on growing microorganisms in the lab under various conditions that mimic their natural habitats. By integrating culturomics and metagenomics, we can understand not just what the microbiome is capable of, but also what it is actively doing [4]. In this way, alterations in the microbiome due to dietary changes, probiotic use, antibiotics, or disease can be revealed. Understanding how these factors affect metabolic pathways, leading to changes in the host’s health, is opening new avenues for personalized therapies. It can also help in identifying biomarkers for diseases and inform the development of microbiome-based therapies by linking specific microbial genes with their metabolic products that impact health.
This approach enhances our grasp of the intricate interactions between microbiota and host health, offering the potential for more tailored and effective interventions to maintain or restore a healthy gut microbiome [5].
Yet, the spectrum of functions that the microbiota actively performs and contributes to human physiology is still largely untapped. These functions include the microbiota’s involvement in the modulation of the nervous system [6], its influence on aging processes [7], and its role in the development of autoimmune diseases beyond the gut [8]. Additionally, the interactions between dietary components, microbiota metabolites, and genetic expression in the host are complex and not yet fully mapped [9]. Furthermore, the role of the microbiota extends far beyond what was previously understood, marking a significant shift in the scientific perspective. Historically overlooked, the microbiota is now recognized as a pivotal player in a myriad of physiological processes and pathologies. Ongoing research continues to uncover its critical functions across various health conditions, demonstrating its profound impact. For instance, recent studies have even identified correlations between the breast microbiota and various tumor characteristics, as well as prognostic clinicopathologic features [10]. This expanding understanding underscores the microbiota’s integral role in health and disease, highlighting the necessity for further exploration and the potential it holds for future medical advancements.
In this review, we will explore the search for biomarkers, specifically focusing on functional products produced by the microbiota. These biomarkers include not only indicators of a healthy microbiota but also signs of potential dysbiosis, which could predict susceptibility to future diseases. By identifying these functional products, we aim to provide a clearer understanding of how the microbiota contributes to health and disease states. We will also focus on how the microbiota is formed from birth up to 3 years of age—a critical period during which the microbiota appears to reach a composition that remains relatively stable throughout adult life. Finally, we will examine the main factors influencing this establishment.
2. Gut Microbiota
It is considered that the majority of bacteria present in humans are concentrated in the gastrointestinal tract (GI), especially in the colon.
The human gut microbiome exhibits a foundational similarity among family members due to shared environmental factors and genetic backgrounds [11]. Despite these commonalities, individual microbiomes display distinct differences, particularly in the specific lineages and species present. This variation underscores the personalized nature of each person’s gut microbiota, which is composed of a diverse array of bacteria ranging from 500 to 1000 species. The vast number of bacterial cells in the human gut, slightly outnumbering human cells, and a genomic presence that is 100 times larger than the human genome—approximately 38 trillion cells and 2 million bacterial genes—highlight the complex interplay of shared and unique microbial characteristics within families [12,13].
Figure 1 shows the bacteria that are commonly found in the gastrointestinal tract of a healthy person, classified according to their phylum, class, order, family, and genus.
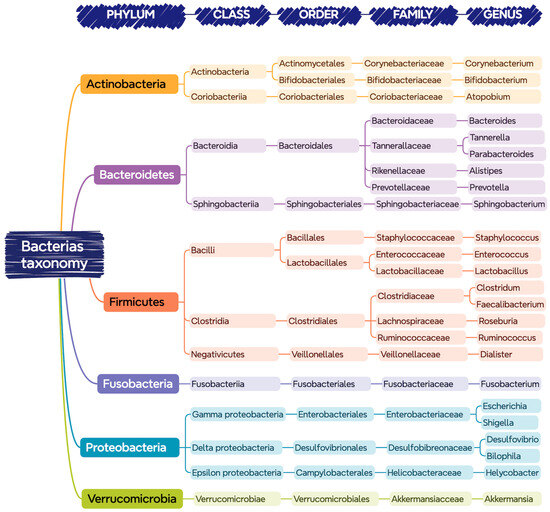
Figure 1.
Bacteria present in the gastrointestinal tract classified according to their taxonomy (phylum, class, order, family, and genus).
Despite the indicated diversity, it seems that there is an optimal balance of this core of bacteria for each person, in which those belonging to the phyla Bacteroidetes and Firmicutes are predominant [14].
Next, we will describe main functions associated with each of the mentioned phyla in the gut. It is worth considering that there is variability in the specific functions, with the potential for distinct or complementary roles in the colon ecosystem at the family or genus level within each phylum.
2.1. Actinobacteria
This phylum is divided into six classes: Actinobacteria, Acidimicrobiia, Coriobacteriia, Nitriliruptoria, Rubrobacteria, and Thermoleophilia. They are primarily Gram-positive filamentous bacteria, most of them aerobic and saprophytic in nature [15]. They have the ability to compete with pathogens for nutrients and adhesion sites on the intestinal mucosa, producing antimicrobial substances that inhibit the growth of harmful bacteria, especially the genus Bifidobacterium, a lactic acid producer, considered as a positive stimulus for intestinal health and immune system development and widely used as a probiotic [16].
Moreover, like Firmicutes, Actinobacteria break down fibers and complex carbohydrates that are not digested in the small intestine, producing SCFAs [17]. In addition, they can synthesize certain B complex vitamins, such as biotin (B7) and folic acid (B9), important for cellular metabolism and DNA production [18].
2.2. Bacteroidetes
This phylum is comprised of six classes of Gram-negative bacteria that are non-spore-forming, non-motile, and mostly anaerobic. The Bacteroidetes break down complex polysaccharides and dietary fiber ingested through the diet. As a result of their metabolism, a wide range of metabolites is produced, such as isopedopeptins, related to antibiotic resistance; pigments, with antioxidant function; or short linear peptides, many of them with immunogenic characteristics [19].
2.3. Firmicutes
The phylum Firmicutes includes Gram-positive bacteria, many of which are spore-forming. They can be obligate anaerobes, as well as aerobes or facultative anaerobes. Bacteria belonging to this phylum can adopt different shapes, such as cocci, bacilli, or filaments. Additionally, it includes genera beneficial to health as well as those with potential pathogenic effects [20].
Gut Firmicutes are known to contain numerous genes that facilitate the fermentation of dietary fiber. Additionally, they may interact with the intestinal mucosa, contributing to the maintenance of homeostasis. [21]. As a result of their metabolism, they generate short-chain fatty acids (SCFAs), including acetate, propionate, butyrate, valerate and isovalerate, among others [22].
They are also capable of producing vitamin K2, important for blood clotting and bone health, as well as several B complex vitamins, including riboflavin (B2), essential for energy metabolism; cobalamin (B12), necessary for nerve function and blood formation; folic acid (B9), crucial for DNA synthesis and cell division; and biotin (B7), involved in the metabolism of lipids, proteins, and carbohydrates [23].
2.4. Fusobacteria
Fusobacteria are generally Gram-negative bacilli, predominantly anaerobes, considered opportunistic pathogens in humans. Some exhibit a fusiform morphology, hence their name [24].
Their role in the gastrointestinal tract is not fully defined. It seems they interact with the rest of the microbiota’s microorganisms, activating the inflammatory response designed to protect against pathogens that promote tumor growth. In fact, a higher abundance of these microorganisms has been shown in the presence of colorectal cancer (CRC) [25,26]. Tumors characterized by a high level of inflammation, such as those enriched with granulocytes and Fusobacterium, have the worst prognosis [27]. Furthermore, resistance to chemotherapy is a major cause of tumor recurrence and poor prognosis in patients with CRC. Studies have reported a higher abundance of Fusobacterium nucleatum in the CRC tissues of patients with post-chemotherapy recurrence compared to those without recurrence, suggesting that Fusobacteria might play a role in chemoresistance. Additionally, Fusobacterium nucleatum has been shown to inhibit the recruitment of anti-cancer tumor-infiltrating T cells, further complicating therapeutic interventions [28].
2.5. Proteobacteria
This phylum of Gram-negative bacteria is highly diverse, consisting of more than 200 genera that include both beneficial and inherently pathogenic bacteria. Although Proteobacteria are less abundant in the intestine compared to the phyla Bacteroidetes and Firmicutes, they exhibit extensive metabolic diversity. This allows them to participate in a variety of biochemical processes within the intestinal ecosystem and to metabolize a broad range of organic compounds, thus contributing to the complexity and dynamism of the intestinal microbiome. While beneficial Proteobacteria contribute to nutrient processing and support the immune system, certain genera such as Shigella spp. or Vibrio cholerae are primarily recognized for their pathogenic potential. In some studies, an increase in the proportion of Proteobacteria in the intestine has been associated with states of dysbiosis, potentially serving as an indicator of inflammatory bowel disease, obesity, and other related diseases [29,30].
2.6. Verrucomicrobia
These are Gram-negative bacteria that present a diversity of shapes such as cocci, bacilli, and spirals. Some of them have a type of appendage that gives them a warty appearance—the reason for their name.
Within this phylum, the genus Akkermansia has the capacity to degrade mucin, a component of intestinal mucus. This activity aids in the renewal and maintenance of the mucus layer’s thickness, essential for preventing the passage of harmful substances and microorganisms from the intestine into the bloodstream. Indeed, its abundance has been inversely associated with people with cardiovascular diseases and certain types of cancer, suggesting a potential probiotic function [31]. Specifically, Akkermansia muciniphila helps preserve a healthy gut barrier, which in turn regulates immunity and curbs the onset of inflammation. This inflammation is a fundamental factor in the development of many diseases [32].
3. Functional Products of the Intestinal Microbiota
By functional products of the microbiota, we refer to small bioactive molecules resulting from the metabolic activity of intestinal bacteria. These substances have both local and systemic effects, significantly contributing to metabolism regulation, the maintenance of intestinal barrier integrity, the modulation of the immune system, and protection against pathogens.
The complex interaction between these functional products and the host organism underscores the importance of a balanced microbiome for overall well-being and highlights the therapeutic potential of manipulating the microbiota to prevent and treat diseases [33].
Regarding these studied functional products, they have been categorized into six groups: short-chain fatty acids, bioactive lipids, vitamins, amino acids and bioactive peptides, signaling gases, and secondary bile acids (Figure 2).
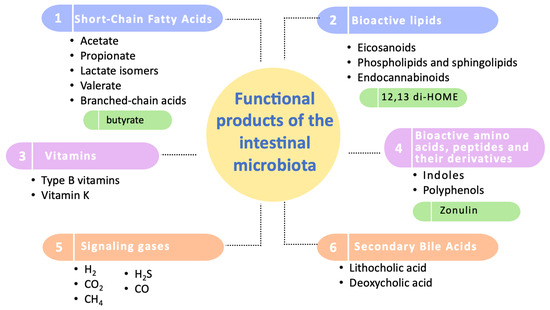
Figure 2.
Categorization of the functional products of the intestinal microbiota. This diagram illustrates the primary categories of functional products produced by the intestinal microbiota, highlighting the most extensively studied compounds within each category. Key biomarkers of significant influence on human health are emphasized in green. It should be noted that zonulin, although included, is primarily produced by human cells and not by the microbiota.
3.1. Short-Chain Fatty Acids (SCFAs)
As previously indicated, SCFAs are produced by anaerobic bacteria present in the intestine through the bacterial fermentation of fiber and other non-digestible carbohydrates. These dietary compounds, such as resistant starch, fructooligosaccharides (FOSs), and galactooligosaccharides (GOSs), escape digestion and absorption in the small intestine, reaching the colon, where they are utilized for their own metabolism by these bacteria [22].
SCFAs are, by definition, organic molecules composed of a chain of less than six carbon atoms. Thus, they include acetate (C2), propionate (C3), and butyrate (C4). Their short carbon chain endows them with unique physicochemical properties—they are water-soluble and play crucial roles in various biological processes in the human body [34].
In the intestine, among all SCFAs present, acetate is the most abundant (60%), while propionate and butyrate are found at 20%. Additionally, lactate isomers, valerate, and branched-chain SCFAs such as isobutyrate and isovalerate are detected, but their levels are noticeably lower than the rest [35].
Despite the abundance of acetate, the role of butyrate stands out, as it is considered the main food source for colonocytes [36]—the cells that inhabit the colon, whose main functions are:
- Maintain the water and electrolyte balance of the body, being responsible for the absorption of water and electrolytes (like sodium and chloride) from waste materials passing through the colon and for the formation of feces of adequate consistency.
- Act as a physical and biochemical barrier that protects against the invasion of pathogens and the entry of toxic substances from the intestinal lumen into the bloodstream. This barrier function is maintained by tight junctions between epithelial cells, as well as by mucus production by neighboring goblet cells.
- Contribute to the regulation of the immune response in the intestine. Through interaction with immune cells and the production of cytokines and chemokines, colonocytes help maintain immunological tolerance and prevent excessive inflammatory responses.
- Maintain the integrity of the intestinal lining against daily wear and/or after injuries or inflammation.
SCFAs can induce both differentiation and the apoptosis of colonocytes, potentially playing a fundamental role in the prevention of colon cancer [37].
Regarding the specific functions of these fatty acids, SCFAs, mainly butyrate, stimulate the concentration of tight junctions by activating genes that encode these proteins. Additionally, they stimulate the formation of Mucin 2, essential for maintaining the mucus layer of the intestinal epithelium [38]. These actions help preserve the permeability and integrity of the intestinal barrier.
Butyrate also intervenes in the modulation of oxidative stress, reducing DNA damage induced by H2O2 by restoring the levels of the antioxidant glutathione [39].
Furthermore, they are involved in various physiological processes of the nervous system. For example, propionate can enter the portal circulation, activating the free fatty acid receptor 3 (FFAR3) on the surface of afferent periportal neurons and inducing intestinal gluconeogenesis. SCFAs also regulate the inhibition of histone deacetylase (HDAC), implicated in several neurological diseases like depression, schizophrenia, and Alzheimer’s [40]. Additionally, they participate in systemic neuroinflammation and serotonin biosynthesis, affecting emotions, cognition, and mental disorders [41].
In animal models, a higher presence of SCFAs, especially acetate, has been linked to reduced body weight and decreased appetite [42].
In the cardiovascular area, high levels of butyrate and propionate have been associated with a decrease in blood pressure and levels of plasminogen activator inhibitor-1 (PAI-1), a pro-thrombotic factor [43].
Lastly, the functions of SCFAs regarding immune function are being studied [44]. They seem to act directly on neutrophils, decreasing the production of reactive oxygen species (ROS) and myeloperoxidase (MPO), as well as favoring their apoptosis. Moreover, they reduce the chemotaxis of inflammatory cells by decreasing the expression of MCP-1 (monocyte chemoattractant protein-1), VCAM1 (vascular cell adhesion molecule-1), and chemokines. In this way, they prevent exacerbated inflammatory reactions. Regarding adaptive immunity, they could increase the number and activity of Treg cells, inhibit CD4+ T lymphocytes, decrease NF-κB signaling, and increase IL-10 and other anti-inflammatory cytokines [45], favoring an anti-inflammatory environment. Thus, they are considered as a protective factor against pathologies associated with excessive inflammation such as inflammatory bowel diseases (IBDs).
3.2. Bioactive Lipids
Besides short-chain fatty acids (SCFAs), eicosanoids, phospholipids, sphingolipids and endocannabinoids have important functions in regulating the immune system, inflammation, and metabolism [46].
Eicosanoids, primarily derived from arachidonic acid (AA), a polyunsaturated ω-6 fat, include prostaglandins (PGs), leukotrienes, thromboxanes, and lipoxins. These entities play a role in various physiological and homeostatic functions such as regulating vascular tone, controlling platelet aggregation, and managing pain perception. However, they are primarily recognized for their involvement in immunity and inflammation, where they serve as initiators for initiating the inflammatory response. [47]. In fact, when the integrity of the intestinal barrier is lost, some PGs, lipoxins, and leukotrienes amplify the signal of certain pro-inflammatory cytokines (TNF-α, IL-1β, and Il-6 among others), exacerbating the inflammatory response and being associated with chronic inflammatory diseases [48]. As an example, regarding inflammatory bowel disease (IBD), the study by Gobbetti et al. [49] demonstrated that a systemic treatment with ω-3 docosapentaenoic acid DPA-derived protectin D1 and ω-3 DPA-derived resolvin D5 protected against colitis and intestinal ischemia/reperfusion-induced inflammation in mice.
Additionally, a molecule that has been gaining importance is 12-13-diHOME (12,13-dihydroxy-9Z-octadecenoic acid). It is an oxylipin, a product of the metabolism of linoleic acid, a type of omega-6 fatty acid, formed through mono- or dioxygenase action. Gut bacteria, such as Enterococcus faecalis and Bifidobacterium spp., encode epoxide hydrolase enzymes capable of generating 12,13-diHOME [50]. This compound can act on dendritic cells, promoting a Th2-type inflammatory response, with its elevation being related to the development of allergic processes. On the other hand, an increase in this molecule in plasma has been found in response to physical exercise and exposure to cold, stimulating the conversion of brown adipose tissue (BAT) into white adipose tissue (WAT); thus, it is considered protective against obesity [51].
Regarding phospholipids and sphingolipids, these are fundamental components of cell membranes, and their functions are thus primarily related to inflammation, vesicular trafficking, and endocytosis [52]. A study conducted in mice by Brown et al. [53] demonstrated that patients with inflammatory bowel disease exhibit lower levels of Bacteroides-derived sphingolipids in their feces and elevated levels of host sphingolipids, which inversely correlate with the abundance of Bacteroidetes. These findings underscore their role as targets for the treatment of diseases characterized by chronic inflammatory processes.
Another type of lipid produced by the gut microbiota are the endocannabinoids, which are not exclusive to microbial action and are present in many organs and tissues. The most studied of these ligands are N-arachidonoylethanolamide (AEA), 2-arachidonoylglycerol (2-AG), O-arachidonoylethanolamine (EA), N-oleoylethanolamine (OEA), and N-palmitoylethanolamine (PEA) [54]. They play a role in both the innate and adaptive immune responses, exerting a potent anti-inflammatory effect. Among them, PEA has been associated with a protective effect in patients suffering from Alzheimer’s and Parkinson’s diseases. Furthermore, it is considered a fat sensor by mediating the response to high-fat diets and regulating thermogenic processes through the activation of PPAR-α [55]. Additionally, endocannabinoids like AEA and 2-AG are known to influence appetite regulation, primarily through their interaction with cannabinoid receptor-1 (CNR1). These interactions tend to stimulate hunger and increase food intake, whereas OEA appears to inhibit hunger [56].
3.3. Vitamins
Bacterial species such as Bacteroides spp., Bifidobacterium spp., and Enterococcus spp. are capable of synthesizing several vitamins such as type B vitamins (such as thiamine, folate, riboflavin, pantothenic acid) or vitamin K. These vitamins are involved in several essential functions such as blood coagulation, DNA synthesis, or energy metabolism. Interestingly, the molecular structure of vitamins synthesized by bacteria occasionally diverges from that of identical components consumed in the diet, influencing, for instance, the necessity of different transporters for their absorption [57].
Kang et al. [58] conducted a study using the Caenorhabditis elegans model, demonstrating that vitamin B12-producing bacteria that colonize the intestine can reduce cholinergic signaling in the nervous system through a process of rewiring the methionine cycle in the intestine. The vitamin B12 produced reduces cholinergic signaling by limiting the availability of free choline, which is needed by neurons for the synthesis of acetylcholine.
It is noteworthy that other gut bacteria, such as butyrate-producing bacteria from the Firmicutes genus, require vitamins for their growth, which can come from both the diet and their production by other bacteria in the colon [59]. This fact suggests the need to supplement prototrophic bacteria with these vitamins to stimulate the proliferation of butyrate-producing bacteria. Moreover, it highlights that fermentable fiber is not the only nutrient that affects microbial composition [60].
3.4. Bioactive Amino Acids, Peptides and Their Derivatives
It has been established that the colonic microbiota exhibits proteolytic power, particularly the Bacteroides, which are capable of breaking down both endogenous and exogenous proteins into peptides, amino acids, and derivatives [61,62].
Bioactive peptides are recognized for their wide range of beneficial activities, such as antimicrobial, antioxidant, antihypertensive, immunomodulatory, hypocholesterolemic, opiate-like, mineral-binding, and antithrombotic properties [63,64,65,66]. Highlighting the practical impact of such peptides, Liu et al. [66] conducted studies demonstrating that the fermentation of the peptide fraction from Dendrobium aphyllum with human fecal microbiota not only released antioxidant peptides but also promoted the proliferation of intestinal bacteria.
Zonulin, an enterotoxin secreted by intestinal epithelial cells in response to dietary or microbiota stimuli, is garnering significant interest for its role in regulating the competency and function of tight junctions (TJs) in the intestinal epithelium. Elevated levels of zonulin have been linked to enhanced intestinal permeability, thereby increasing susceptibility to potentially harmful substances entering the bloodstream. This increased permeability has been associated with various autoimmune diseases, such as celiac disease and type 1 diabetes, highlighting its potent regulatory role in intestinal barrier function [67,68].
Additionally, gut microbiota can metabolize tryptophan to produce indole, which can subsequently be transformed into other metabolites such as indole-3-acetic acid (IAA), indolepropionic acid, and indole-3-lactate [69]. These compounds have been proposed as therapeutic targets as they activate nuclear receptors and regulate intestinal hormones, thereby aiding in the maintenance of intestinal homeostasis and positively impacting liver metabolism and immune response [70,71].
Polyphenols are also being studied for their potent anti-inflammatory and antioxidant properties. The intestinal microbiota can produce some of these compounds through the endogenous metabolism of aromatic amino acids. Additionally, more complex polyphenols derived from plant sources enter the intestinal tract through dietary intake and are subsequently metabolized in the colon by the resident microbiota. This fermentation process results in the production of a wide range of metabolites, which play a crucial role in mitigating chronic diseases [72,73,74,75].
3.5. Signaling Gases
Among the products resulting from the fermentation of carbohydrates, proteins, and other compounds by intestinal bacteria, gases are also produced. More than 99% of intestinal gas is composed of hydrogen (H2), carbon dioxide (CO2), and methane (CH4). The remainder includes other gases and volatile elements such as hydrogen sulfide (H2S) and carbon monoxide (CO) [72]. Bacteroides and Clostridium species are considered major producers of these microbial gases [76].
These gases are not merely byproducts; they can also perform significant functions. Some possess antimicrobial properties, can act as signaling molecules or neurotransmitters, stimulate intestinal transit, protect against oxidative damage, and even influence the immune response [77].
In their review, Ichikaea et al. [78] emphasize the benefits of hydrogen-producing bacteria, noting that a reduction in the number of these bacteria has been linked to dysbiosis. They highlight the bidirectional gut–brain relationship where hydrogen molecules play a protective role both in the gut against hepatitis induced by concanavalin A and in the brain, where they help mitigate neuronal changes associated with conditions such as depression and dementia.
3.6. Secondary Bile Acids
Bile acids are synthesized in the liver from cholesterol, secreted into the intestine to function as detergents, and emulsify dietary lipids and cholesterol to facilitate their absorption. Once their function is completed, they are reabsorbed in the ileum and circulate back to the liver, where they are re-secreted to establish their enterohepatic circulation [79].
Bile acids are not synthesized by the intestinal microbiota. Nonetheless, the microbiota plays a vital role in metabolizing and transforming these bile acids after their secretion into the intestine. Present bacteria can modify these primary bile acids, converting them into secondary bile acids through processes such as dihydroxylation; thus, they are considered to be metabolites derived from intestinal microbial activity [80].
These transformations affect the solubility and reabsorption capacity of bile acids, as well as their biological functions, resulting in them being considered more than mere detergents and extensively studied for their significant role in cellular signaling [81].
Therefore, the composition and activity of the intestinal microbiota can have a significant impact on the bile acid profile in the body and, by extension, on various physiological and pathological functions, including the regulation of lipid metabolism, inflammation, and resistance to metabolic and hepatic diseases. In fact, it has been described in murine models how secondary bile acids, such as lithocholic acid and deoxycholic acid, reduce the risk of hypertension. These acids act as agonists for farnesoid X receptors and TGR5, leading to a reduction in inflammation and fibrosis [82].
4. Development of the Microbiota in the First Years of Life
The first 1000 days of life, spanning from conception to approximately three years of age, are considered a critical window for the development of the intestinal microbiota. The composition and diversity of the microbiota established during this period can have lasting effects on an individual’s health, including predisposition to diseases such as obesity, allergies, autoimmune diseases, and metabolic disorders [83].
After birth, the infant’s intestinal microbiota is initially dominated by Enterobacteriaceae and Staphylococcus spp., which are subsequently replaced by Bifidobacterium spp. and other lactic acid-producing bacteria. This composition, known as ‘Bifidus flora’ persists until complementary foods are introduced into the baby’s diet. The weaning process triggers a progressive increase in the presence of Bacteroides, leading to the displacement of Bifidobacterium. By the age of 2–3 years, the microbial community begins to resemble that of adults, primarily composed of species such as Bacteroides spp, Prevotella spp, Ruminococcus spp., Clostridium spp., and Veillonella spp. Accompanying these microbiota changes are shifts in the production and diversity of SCFAs. During the early stages of life, the concentration of acetate is high, primarily produced by the Bifidobacterium strains that characterize the infant intestinal microbiota. These strains metabolize human milk oligosaccharides as an energy source [84]. Following the cessation of breastfeeding and the introduction of complementary foods, intestinal levels of propionate increase, driven by a larger proportion of Firmicutes, particularly from the Clostridia class. This entire process is depicted in Figure 3.
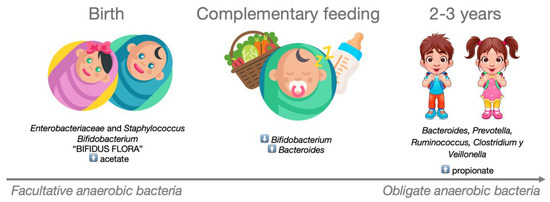
Figure 3.
Development of the microbiota in the early years of life. During the first two years of life, the composition of a child’s intestinal microbiota evolves from being dominated by facultative anaerobic bacteria to predominantly obligate anaerobic bacteria.
4.1. Initial Colonization
Until recently, it was thought that the bacterial colonization of the intestinal tract began during childbirth and was a dynamic process. However, recent studies suggest that such colonization begins in the uterine environment [83] and does not end after the transfer of intestinal and vaginal microbiota following birth [85]. Breast milk is also considered a source of microbiota transfer to the newborn [86,87,88]. In fact, the enteric–mammary axis has been described [89], meaning that microorganisms and/or their functional products can be carried from the maternal intestine to the mammary glands, directly influencing the initial colonization of the newborn.
During the first years of life, the microbial population inhabiting the gastrointestinal tract includes facultative anaerobic bacteria. Actinobacteria, especially Bifidobacterium, are among the first colonizers of the intestinal tract.
Below, the main modulating factors of the microbiota during the early years of life are indicated (Figure 4).
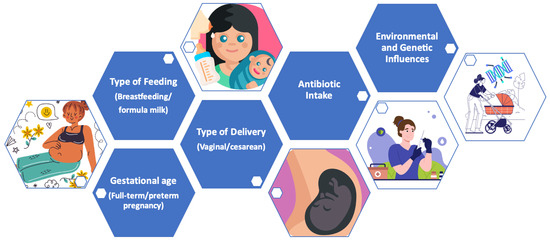
Figure 4.
Main modulating factors of the microbiota in early life.
4.2. Gestational Age
It should be noted that babies born at full term reach an adult microbiota more quickly, compared with preterm infants, which are characterized by a lower diversity of the intestinal microbiota compared to full-term babies. In fact, differences in the vaginal microbiota have been noted between women who gave birth to full-term babies compared to those who had preterm babies, pointing out that mothers of preterm children had dysbiosis during pregnancy [90].
4.3. Type of Delivery
Childbirth plays a crucial role in establishing the initial colonization of the newborn’s intestinal microbiota [91,92,93]. Previously, it was believed that the fetal gastrointestinal system was sterile. However, recent research indicates that the transfer of commensal bacteria from mother to child might occur before birth. Studies have found small quantities of bacteria in initial meconium samples from newborns delivered at full term and in good health [94].
In infants born through vaginal delivery, Lactobacillus spp. is found on their skin, in their mouths, and intestines, being one of the most abundant microorganisms in the maternal vaginal flora. Besides vaginal bacteria, uterine and placental bacteria swept along to the newborn seem to foster tolerance towards microorganisms that promote postnatal well-being.
Conversely, infants born via cesarean section possess a microbiota similar to maternal skin, with Staphylococcus spp. dominating, followed by Propionibacterium spp. and Corynebacterium. Cesarean section has been associated with a lesser abundance and diversity of the phyla Actinobacteria and Bacteroidetes and a greater abundance and diversity of the phylum Firmicutes from birth to 3 months of life [95]. At the colonization level, the genera Bifidobacterium and Bacteroides seem to be significantly more common in vaginally delivered infants compared to those delivered by cesarean section, which are more colonized by the genera Clostridium and Lactobacillus. Consequently, babies born via cesarean section present a lower diversity and abundance of microorganisms considered beneficial compared to those born vaginally [96].
4.4. Type of Feeding
The type of feeding is another primary modulator of the microbiota. Breast milk provides all the initial nutritional requirements for the newborn’s rapid growth during the first few months of life, in addition to containing a wide variety of protective factors, such as immunoglobulin A, cytokines, fatty acids, oligosaccharides, lysozymes, or lactoferrin [97]. Although the composition of breast milk varies according to the mother’s lifestyle and diet [98], certain commensal bacteria (Lactobacilli, Bacteroides spp., and Bifidobacterium spp.) [99] have been associated with healthy milk. Furthermore, breast milk contains specific oligosaccharides that serve as prebiotics, selectively promoting the growth of Bifidobacterium spp. and the production of SCFAs. Indeed, breastfeeding has been associated with a reduced risk of developing allergies, asthma, obesity, and metabolic diseases in the future.
In contrast, babies fed with formula milk show a more diverse microbiota similar to that of adults, with higher proportions of Bacteroides spp., Clostridium spp., and a lesser predominance of Bifidobacterium spp. Although greater microbial diversity is generally considered beneficial in adults, in babies, a microbiota dominated by Bifidobacterium spp. associated with breastfeeding is deemed optimal for the early development of the immune and metabolic system [100].
4.5. Antibiotic Exposure
Exposure to antibiotics is another potential modulator of the microbiota described [101]. This exposure can affect the fetus’s microbiota, whether it occurs during pregnancy, at the moment of delivery, or during the breastfeeding period. Antibiotics can alter the composition and diversity of the maternal microbiota, including vaginal, intestinal, breast milk, and potentially placental microbiota [102]. These changes can influence the microbiota that the fetus encounters and acquires during delivery and birth, potentially resulting in a reduced or altered initial colonization of beneficial bacteria such as Bifidobacterium spp. and Lactobacillus spp., which are important for the development of the baby’s immune system and protection against pathogens [103].
4.6. Environmental and Genetic Influences
The influence of genetics on the human resident microbiota has been demonstrated, although the mechanisms remain elusive. A recent study in mice has linked specific bacterial strains to particular chromosomes, significantly advancing our understanding of how genetics shapes microbiota development. Additionally, the substantial impact of environmental factors has been consistently observed in various previous studies, underscoring their role alongside genetics in influencing microbiota composition [104,105]. Specifically, among the environmental factors that most influence this early stage of life, environmental pollution and the presence of siblings and pets stand out [106,107].
The geographic area of residence will be determinant in this environmental exposure, varying considerably between urban and rural areas [108]. In environments with significant air pollution, a depletion of Firmicutes, especially of the genus Bifidobacterium, has been detected, which is associated with inflammatory diseases [109].
Regarding living with siblings, the study by Talavoire et al. [110] demonstrated that siblings living in the same house shared a greater similarity of microbiota, even if they were not genetically related. This cohabitation with siblings has been linked to the development of a more diverse microbiota in the early years of life [111]. Specifically, Christensen et al. [112] found that children with older siblings (regardless of the number) exhibited a lower abundance of Veillonella spp. and Enterobacteriaceae such as Escherichia spp. and Shigella spp., along with a higher abundance of Prevotella spp. This fact was associated with a reduced risk of developing atopic diseases and asthma during the early stage of life.
Regarding exposure to animals, evidence has been found that direct and prolonged contact shapes the microbiota. In the study by Song et al. [113], adults cohabiting with a dog not only showed similar microbiota among themselves but also exhibited overlap in microbial communities with their dog. This exposure has been associated with a lower predisposition to allergies in the future [114].
4.7. Microbiota Stabilization
Over time, the initial facultative anaerobic colonizers are gradually displaced by conventional anaerobic bacteria. When the child is weaned or when solid foods are introduced, their microbiota begins to resemble that of adults, with an increase in the number and variety of bacterial species and strains; they harbor around 500 to 1000 species, mainly bacteria of the phyla Bacteroidetes (25%) and Firmicutes (60%) [115].
This intestinal microbiota eventually achieves a state of maturity similar to that of an adult, approximately by three years of age [116]. During this period, the microbiota undergoes further diversification and becomes more stable.
5. Conclusions
Studies on the microbiota have expanded in recent years, evolving from merely identifying the presence and quantity of specific microbial communities to understanding the production and modification of metabolites by these communities.
These metabolites are indicators of the bacterial activity occurring at any given time. Thus, understanding the mechanisms leading to their production will allow us to comprehend what is happening and how to modulate their effects according to our interests. We have observed that the range of metabolites is broad, serving various functions. They have been linked to gastrointestinal diseases such as Crohn’s disease, ulcerative colitis, irritable bowel syndrome, and colorectal cancer; metabolic diseases including obesity, type II diabetes, and dyslipidemias; autoimmune diseases such as rheumatoid arthritis, allergies, and asthma; neurodegenerative diseases like Alzheimer’s and Parkinson’s; depression and anxiety; hypertension and other cardiovascular diseases; and skin diseases such as acne, eczema, and psoriasis.
All these conditions share the development of an uncontrolled inflammatory state. However, the metabolites produced, as well as the microbiota involved, must be appropriately selected for each study and disease involved. There remains a long way to go in understanding how this microbiota affects our health. More studies are needed that provide as complete a view as possible of all the interactions that occur in this complex scenario.
It should be noted that the microbial community is not composed only of bacteria. Fungi, viruses, and archaea can also play significant roles, despite their scarce presence compared to bacteria. Additionally, it is important to consider that the microbial composition varies depending on the location studied, which will affect the type and quantity of functional products produced by it.
In summary, this is a challenging yet promising field of study where biomarkers and therapeutic targets can be identified for both healthy microbiota and states of dysbiosis that may prevent future diseases. Since the establishment of the microbiota occurs in the early years of life, studies should be aimed at understanding these initial interactions that will shape our future microbial trajectory.
Author Contributions
Conceptualization, A.B.M.-M.; methodology, A.B.M.-M.; validation, A.B.M.-M., A.R., and J.M.A.-M.; writing—original draft preparation, A.B.M.-M.; writing—review and editing, B.M.L.-P., M.L., A.R., and J.M.A.-M.; visualization, A.B.M.-M.; supervision, J.M.A.-M.; project administration, A.B.M.-M.; funding acquisition, A.B.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Bancaria Ibercaja, grant number 216198 (JIUZ2022-SAL-03). J.M.A-M.’s research was supported by grants from the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional “ERDF, A way of making Europe” (PI22/01366), and the Fondo Social Europeo-Gobierno de Aragón (B03_23R).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Ovidi and Alvaro Tormo for their contribution to research on healthy microbiome.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Recent Advances in Basic Science Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.X.; Zeng, Z.; Xu, M.; Sun, F.; Yang, L.; Bi, X.; Lin, Y.; Gao, Y.J.; Hao, H.X.; et al. Advances in Metagenomics and Its Application in Environmental Microorganisms. Front. Microbiol. 2021, 12, 766364. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Khelaifia, S.; Tidjani Alou, M.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Ibrahima Traore, S.; Hadji Seck, E.; et al. Culture of Previously Uncultured Members of the Human Gut Microbiota by Culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Tidjani Alou, M.; Khelaifia, S.; Michelle, C.; Andrieu, C.; Armstrong, N.; Bittar, F.; Sokhna, C.; Diallo, A.; Fournier, P.E.; Raoult, D.; et al. Anaerococcus Rubiinfantis sp. Nov., Isolated from the Gut Microbiota of a Senegalese Infant with Severe Acute Malnutrition. Anaerobe 2016, 40, 85–94. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain Modulation by the Gut Microbiota: From Disease to Therapy. J. Adv. Res. 2023, 53, 153–173. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Basnet, T.B.; GC, S.; Basnet, R.; Fatima, S.; Safdar, M.; Sehar, B.; Alsubaie, A.S.R.; Zeb, F. Interaction between Gut Microbiota Metabolites and Dietary Components in Lipid Metabolism and Metabolic Diseases. Access Microbiol. 2023, 5, 000403. [Google Scholar] [CrossRef]
- Actis, S.; Cazzaniga, M.; Bounous, V.E.; D’Alonzo, M.; Rosso, R.; Accomasso, F.; Minella, C.; Biglia, N. Emerging Evidence on the Role of Breast Microbiota on the Development of Breast Cancer in High-Risk Patients. Carcinogenesis 2023, 44, 718–725. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2008, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 4, 116. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745. [Google Scholar] [CrossRef] [PubMed]
- Ayakdaş, G.; Ağagündüz, D. Microbiota-Accessible Carbohydrates (MACs) as Novel Gut Microbiome Modulators in Noncommunicable Diseases. Heliyon 2023, 9, e19888. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, S.; Spohn, M.S.; Schäberle, T.F.; Schäberle, S. Bioactive Natural Products from Bacteroidetes. Nat. Prod. Rep. 2022, 39, 1045–1065. [Google Scholar] [CrossRef]
- Hashmi, I.; Bindschedler, S.; Junier, P. Firmicutes. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut Firmicutes: Relationship with Dietary Fiber and Role in Host Homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal Microbiota as a Route for Micronutrient Bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F. Infections Caused by Anaerobic Microorganisms. Encycl. Infect. Immun. 2022, 1, 614–627. [Google Scholar] [CrossRef]
- Kelly, D.; Yang, L.; Pei, Z. Gut Microbiota, Fusobacteria, and Colorectal Cancer. Diseases 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.N.; Araújo-Pérez, F.; Azcárate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium Is Associated with Colorectal Adenomas. PLoS ONE 2013, 8, 0053653. [Google Scholar] [CrossRef]
- Qiao, H.; Li, H.; Wen, X.; Tan, X.; Yang, C.; Liu, N. Multi-Omics Integration Reveals the Crucial Role of Fusobacterium in the Inflammatory Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Microbiol. Spectr. 2022, 10, e0106822. [Google Scholar] [CrossRef] [PubMed]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium Nucleatum and Cancer. Periodontol. 2000 2022, 89, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 253607. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L. Short-Chain Fatty Acids Produced by Intestinal Bacteria. Asia Pac. J. Clin. Nutr. 1996, 5, 15–19. [Google Scholar] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte Metabolism Shapes the Gut Microbiota Single Sentence Summary. Science 2018, 362, 6418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, Y.; Gu, Y.; Ma, Q. Butyrate Facilitates Immune Clearance of Colorectal Cancer Cells by Suppressing STAT1-Mediated PD-L1 Expression. Clinics 2023, 78, 100303. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut Microbiota-Derived Butyrate Regulates Gut Mucus Barrier Repair by Activating the Macrophage/WNT/ERK Signaling Pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Kautenburger, T.; Beyer-Sehlmeyer, G.; Festag, G.; Haag, N.; Kühler, S.; Küchler, A.; Weise, A.; Marian, B.; Peters, W.H.M.; Liehr, T.; et al. The Gut Fermentation Product Butyrate, a Chemopreventive Agent, Suppresses Glutathione S-Transferase Theta (HGSTT1) and Cell Growth More in Human Colon Adenoma (LT97) than Tumor (HT29) Cells. J. Cancer Res. Clin. Oncol. 2005, 131, 692–700. [Google Scholar] [CrossRef]
- Chen, H.; Meng, L.; Shen, L. Multiple Roles of Short-Chain Fatty Acids in Alzheimer Disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef]
- Bruun, C.F.; Hansen, T.H.; Vinberg, M.; Kessing, L.V.; Coello, K. Associations between Short-Chain Fatty Acid Levels and Mood Disorder Symptoms: A Systematic Review. Nutr. Neurosci. 2023, 17, 1–14. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331. [Google Scholar] [CrossRef] [PubMed]
- Cookson, T.A. Bacterial-Induced Blood Pressure Reduction: Mechanisms for the Treatment of Hypertension via the Gut. Front. Cardiovasc. Med. 2021, 8, 721393. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The Role of Short-Chain Fatty Acids in Immunity, Inflammation and Metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed]
- Salsinha, A.S.; Pintado, M. Impact of Bioactive Lipids on Gut Microbiota. In Bioactive Lipids; Elsevier Science: Amsterdam, The Netherlands, 2023; pp. 191–207. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 11, 511–523. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 308893. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, T.; Dalli, J.; Colas, R.A.; Canova, D.F.; Aursnes, M.; Bonnet, D.; Alric, L.; Vergnolle, N.; Deraison, C.; Hansen, T.V.; et al. Protectin D1n-3 DPA and Resolvin D5n-3 DPA Are Effectors of Intestinal Protection. Proc. Natl. Acad. Sci. USA 2017, 114, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, A.D.; Bazzi, M.H.; Lukacs, N.W.; Huffnagle, G.B. Interkingdom Communication and Regulation of Mucosal Immunity by the Microbiome. J. Infect. Dis. 2021, 223, S236–S240. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-DiHOME: An Exercise-Induced Lipokine That Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-Brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2017, 25, 3930–3952. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Eric, A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2020, 25, 668–680. [Google Scholar] [CrossRef]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting Cannabinoid Signaling in the Immune System: “High”-Ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 302153. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.C.; Sun, Y.; Carter, C.S.; Buford, T.W. Crosstalk Between the Gut Microbiome and Bioactive Lipids: Therapeutic Targets in Cognitive Frailty. Front. Nutr. 2020, 7, 508131. [Google Scholar] [CrossRef] [PubMed]
- Aguilera Vasquez, N.; Nielsen, D.E. The Endocannabinoid System and Eating Behaviours: A Review of the Current State of the Evidence. Curr. Nutr. Rep. 2022, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.K.; Florman, J.T.; Araya, A.; Fox, B.W.; Thackeray, A.; Schroeder, F.C.; Walhout, A.J.M.; Alkema, M.J. Vitamin B12 Produced by Gut Bacteria Modulates Cholinergic Signalling. Nat. Cell Biol. 2024, 26, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Soto-Martin, E.C.; Warnke, I.; Farquharson, F.M.; Christodoulou, M.; Horgan, G.; Derrien, M.; Faurie, J.M.; Flint, H.J.; Duncan, S.H.; Louis, P. Vitamin Biosynthesis by Human Gut Butyrate-Producing Bacteria and Cross-Feeding in Synthetic Microbial Communities. MBio 2020, 11, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 409606. [Google Scholar] [CrossRef]
- Guo, Z.; Yi, D.; Hu, B.; Shi, Y.; Xin, Y.; Gu, Z.; Liu, H.; Zhang, L. The Alteration of Gut Microbiota by Bioactive Peptides: A Review. Syst. Microbiol. Biomanufacturing 2021, 1, 363–377. [Google Scholar] [CrossRef]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive Peptides in the Management of Lifestyle-Related Diseases: Current Trends and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606. [Google Scholar] [CrossRef] [PubMed]
- Tsafack, P.B.; Li, C.; Tsopmo, A. Food Peptides, Gut Microbiota Modulation, and Antihypertensive Effects. Molecules 2022, 27, 8806. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Oh, D.H. The Functional Interplay between Gut Microbiota, Protein Hydrolysates/Bioactive Peptides, and Obesity: A Critical Review on the Study Advances. Antioxidants 2022, 11, 333. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Yin, Z.; Wu, H. Characteristic Analysis of Peptide Fraction Extracted from Dendrobium Aphyllum After In Vitro Gastrointestinal Digestion and Fermentation by Human Fecal Microbiota. Int. J. Pept. Res. Ther. 2019, 25, 573–582. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Macfarlane, G.T. Enumeration of Human Colonic Bacteria Producing Phenolic and Indolic Compounds: Effects of PH, Carbohydrate Availability and Retention Time on Dissimilatory Aromatic Amino Acid Metabolism. J. Appl. Microbiol. 1996, 81, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Mutuyemungu, E.; Singh, M.; Liu, S.; Rose, D.J. Intestinal Gas Production by the Gut Microbiota: A Review. J. Funct. Foods 2023, 100, 105367. [Google Scholar] [CrossRef]
- Fagone, P.; Mazzon, E.; Bramanti, P.; Bendtzen, K.; Nicoletti, F. Gasotransmitters and the Immune System: Mode of Action and Novel Therapeutic Targets. Eur. J. Pharmacol. 2018, 834, 92–102. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Yamamoto, H.; Hirano, S.I.; Sato, B.; Takefuji, Y.; Satoh, F. The Overlooked Benefits of Hydrogen-Producing Bacteria. Med. Gas Res. 2023, 13, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.M.; Albers, S.; Trautwein, C. Role of Bile Acids in the Gut-Liver Axis. J. Hepatol. 2018, 68, 1083–1085. [Google Scholar] [CrossRef]
- Sun, R.; Xu, C.; Feng, B.; Gao, X.; Liu, Z. Critical Roles of Bile Acids in Regulating Intestinal Mucosal Immune Responses. Therap. Adv. Gastroenterol. 2021, 14. [Google Scholar] [CrossRef]
- Tremblay, S.; Romain, G.; Roux, M.; Chen, X.L.; Brown, K.; Gibson, D.L.; Ramanathan, S.; Menendez, A. Bile Acid Administration Elicits an Intestinal Antimicrobial Program and Reduces the Bacterial Burden in Two Mouse Models of Enteric Infection. Infect. Immun. 2017, 85, 10-1128. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef] [PubMed]
- Wicinski, M.; Sawicka, E.; Gebalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C. Gut Microbiota: Next Frontier in Understanding Human Health and Development of Biotherapeutics. Biol. Targets Ther. 2011, 5, 71–86. [Google Scholar]
- Doare, K.L.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 325188. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Fernández, L.; Verhasselt, V. The Gut-breast Axis: Programming Health for Life. Nutrients 2021, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and Development of Intestinal Microbiota in Preterm Neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The Mode of Delivery Affects the Diversity and Colonization Pattern of the Gut Microbiota during the First Year of Infants’ Life: A Systematic Review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Lyu, L.; Zhou, X.; Zhang, M.; Liu, L.; Niu, H.; Zhang, J.; Chen, S.; Gong, P.; Jiang, S.; Pan, J.; et al. Delivery Mode Affects Intestinal Microbial Composition and the Development of Intestinal Epithelial Cells. Front. Microbiol. 2021, 12, 626144. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of Delivery Mode-Associated Gut Microbiota Dynamics on Health in the First Year of Life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Pekmez, C.T.; Dragsted, L.O.; Brahe, L.K. Gut Microbiota Alterations and Dietary Modulation in Childhood Malnutrition—The Role of Short Chain Fatty Acids. Clin. Nutr. 2018, 38, 615–630. [Google Scholar] [CrossRef]
- Wang, S.; Egan, M.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. A Good Start in Life Is Important-Perinatal Factors Dictate Early Microbiota Development and Longer Term Maturation. FEMS Microbiol. Rev. 2020, 30, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Pinto Coelho, G.D.; Arial Ayres, L.F.; Barreto, D.S.; Henriques, B.D.; Cardoso Prado, M.R.M.; Dos Passos, C.M. Acquisition of Microbiota According to the Type of Birth: An Integrative Review. Rev. Lat. Am. Enfermagem 2021, 29, e3446. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of Human Breast Milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; De Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper From the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef]
- Łubiech, K.; Twarużek, M. Lactobacillus Bacteria in Breast Milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Layuk, N.; Sinrang, A.W.; Asad, S. Early Initiation of Breastfeeding and Gut Microbiota of Neonates: A Literature Review. Med. Clin. Pract. 2021, 4, 100222. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in Early Life: Dysbiosis and the Damage Done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, G.; Xu, S.; Xiang, Q.; Huang, M.; Zhao, M.; Bai, X. Antibiotic Treatments to Mothers during the Perinatal Period Leaving Hidden Trouble on Infants. Eur. J. Pediatr. 2022, 181, 3459. [Google Scholar] [CrossRef] [PubMed]
- Friswell, M.K.; Gika, H.; Stratford, I.J.; Theodoridis, G.; Telfer, B.; Wilson, I.D.; Mcbain, A.J. Site and Strain-Specific Variation in Gut Microbiota Profiles and Metabolism in Experimental Mice. PloS ONE 2010, 5, e8584. [Google Scholar] [CrossRef] [PubMed]
- Hufeldt, M.R.; Nielsen, D.S.; Vogensen, F.K.; Midtvedt, T.; Hansen, A.K. Variation in the Gut Microbiota of Laboratory Mice Is Related to Both Genetic and Environmental Factors. Comp. Med. 2010, 60, 336–342. [Google Scholar] [PubMed]
- Jeong, S. Factors Influencing Development of the Infant Microbiota: From Prenatal Period to Early Infancy. Clin. Exp. Pediatr. 2022, 65, 438. [Google Scholar] [CrossRef] [PubMed]
- Vallès, Y.; Francino, M.P. Air Pollution, Early Life Microbiome, and Development. Curr. Environ. Health Rep. 2018, 5, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Tyakht, A.V.; Alexeev, D.G.; Popenko, A.S.; Kostryukova, E.S.; Govorun, V.M. Rural and Urban Microbiota: To Be or Not to Be? Gut Microbes 2014, 5, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational Exposure to Particulate Matter Air Pollution Alters the Composition of Microbiome in Small Bowel and Colon. Environ. Pollut. 2018, 240, 817. [Google Scholar] [CrossRef] [PubMed]
- Tavalire, H.F.; Christie, D.M.; Leve, L.D.; Ting, N.; Cresko, W.A.; Bohannan, B.J.M. Shared Environment and Genetics Shape the Gut Microbiome after Infant Adoption. mBio 2021, 12, 10-1128. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.V.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Christensen, E.D.; Hjelmsø, M.H.; Thorsen, J.; Shah, S.; Redgwell, T.; Poulsen, C.E.; Trivedi, U.; Russel, J.; Gupta, S.; Chawes, B.L.; et al. The Developing Airway and Gut Microbiota in Early Life Is Influenced by Age of Older Siblings. Microbiome 2022, 10, 106. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting Family Members Share Microbiota with One Another and with Their Dogs. Elife 2013, 2013, e00458. [Google Scholar] [CrossRef] [PubMed]
- Ownby, D.R.; Johnson, C.C.; Peterson, E.L. Exposure to Dogs and Cats in the First Year of Life and Risk of Allergic Sensitization at 6 to 7 Years of Age. JAMA 2002, 288, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 10-1128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).