Abstract
This study investigated the effect of astragalus polysaccharide (APS, an ingredient with hypoglycemic function in a traditional Chinese herbal medicine) on gut microbiota and metabolites of type 2 diabetes mellitus (T2DM) patients using a simulated fermentation model in vitro. The main components of APS were isolated, purified, and structure characterized. APS fermentation was found to increase the abundance of Lactobacillus and Bifidobacterium and decrease the Escherichia-Shigella level in the fecal microbiota of T2DM patients. Apart from increasing propionic acid, APS also caused an increase in all-trans-retinoic acid and thiamine (both have antioxidant properties), with their enrichment in the KEGG pathway associated with thiamine metabolism, etc. Notably, APS could also enhance fecal antioxidant properties. Correlation analysis confirmed a significant positive correlation of Lactobacillus with thiamine and DPPH-clearance rate, suggesting the antioxidant activity of APS was related to its ability to enrich some specific bacteria and upregulate their metabolites.
1. Introduction
With its incidence increasing year by year, diabetes mellitus has become a common metabolic disease and the third most chronic severe disease after cancer and cardiovascular disease. Diabetes mellitus can be divided into type 1 (T1DM) and type 2 diabetes mellitus (T2DM), with the latter accounting for more than 90% of all diabetic patients. T2DM is not only characterized by hyperglycemia induced by insulin deficiency or resistance [1], but also accompanied by various complications in the heart, blood vessels, kidneys, retina, and nervous system, thus posing a severe risk to human physical and mental health [2]. Acarbose, metformin, and α-glucosidase inhibitors are commonly used chemical hypoglycemic agents with good clinical applications, but they are expensive and have side effects [3], suggesting the need to find a stable, low-side-effect, and low-cost drug or food to improve and treat T2DM.
Intestinal microbiota disorders can lead to various chronic metabolic diseases, including obesity, diabetes, and hyperlipidemia [4]. Dysbiosis of the intestinal microbiota in patients with T2DM has been demonstrated, such as a decrease in the abundance of Firmicutes and short-chain fatty acid-generating bacteria (e.g., Bifidobacterium, Faecalibacterium, and Roseburia), and an increase in the abundance of conditionally pathogenic bacteria (e.g., Bacteroides caccae, Clostridium symbiosum Eggerthella lenta, and Escherichia coli) [5].
Conversely, supplementation with beneficial bacteria can alleviate diabetes. For example, supplementation with Lactobacillus paracasei [6] and Akkermansia muciniphila [7] improved glucose tolerance and insulin sensitivity in mice. Interestingly, diabetes was also shown to be influenced by the active metabolites of the intestinal microbiota. Specifically, ammonia, some amino acids, and amines (which are usually considered as the end products of protein degradation by harmful bacteria of gut microbiota) are positively associated with T2DM [8]. Additionally, short-chain fatty acids (SCFAs, the main metabolites of intestinal microorganisms) can improve insulin sensitivity and regulate pancreatic insulin secretion in T2DM patients [9]. These reports suggest that regulating intestinal microbiota and its metabolites is a new direction for T2DM treatment.
Plant polysaccharides, with their low toxicity and blood glucose lowering effects, have been a hot research topic in the treatment of T2DM in recent years. For example, Cyclocarya paliurus polysaccharide [10], Morus alba polysaccharide [11], and Dendrobium officinale polysaccharide [12] have been shown to stabilize glucose and reduce diabetic complications. The dried root of Astragalus membranaceus (Fisch.) Bunge (Fabaceae) (Astragali Radix) is one of the key ingredients in many herbal anti-diabetic formulations [13]. Astragalus polysaccharide (APS), the main active substance of astragali radix, has been demonstrated to have the function of controlling blood sugar [14], enhancing immunity [15], and antioxidant effects [16]. A recent work has shown that APS could improve glucose regulation in a diabetes mice model by elevating the abundance of Muribaculum, Faecalibaculum, and Lactobacillus, increasing the levels of acetic acid, butyric acid, and propionic acid, and decreasing the expression levels of tumor necrosis factor α (TNF-α) and pro-inflammatory factors interleukin 6 (IL-6) [17]. Similarly, Liu et al. demonstrated that APS stabilized glucose homeostasis in diabetic mice by altering the gut microbiota and regulating the level of short-chain lipoic acid [18]. However, most of these studies are based on animal models such as mice, and the effects of APS on the intestinal microbiota and intestinal metabolic profile in T2DM patients have not been systematically clarified. Therefore, this study aimed to elucidate the regulatory effects of APS on gut microbiota and its metabolic profile in T2DM patients by combining 16S sequencing technology and metabolomics.
2. Materials and Methods
2.1. Extraction, Purification, and Physicochemical Characterization
Astragali radix was purchased from Min County, Dingxi City, Gansu Province, China. APS was extracted from astragalus radix with boiling water and concentrated by rotary evaporator (Centron Technology Co., Shanghai, China), followed by precipitation in 80% ethanol. Next, the crude polysaccharide was deproteinized by the Sevag method and then filtered through a 3.5 KDa dialysis membrane to remove salt ions, followed by collecting the polysaccharide and lyophilization for further treatment (Marin Christ, Osterode, Germany). The main fractions (APS-0M, APS-0.1M, and APS-0.2M) were obtained from APS by DEAE Sepharose FF cellulose, followed by further isolation and purification of APS-1 and APS-2 from APS-0M and APS-0.2M by Sephadex G-100 column chromatography, respectively (Figure S1). Finally, the carbohydrate, total phenol, and protein contents of APS, APS-1, and APS-2 were assayed by the methods of phenol-sulfuric acid [19], Folin–Ciocalteu [20], and Bradford [21], respectively.
2.2. Structural Characterization
For UV-vis assay, APS, APS-1, and APS-2 solutions were scanned in a wavelength range of 360 to 190 nm using an UV spectrophotometer (ND-100C, Miulab, Hangzhou, China). The transmission assay was performed using a Vertex 70 FTIR spectrometer (Bruker, Mannheim, Germany) to collect the spectra of APS, APS-1, and APS-2 between 4000 and 400 cm−1.
The molecular weight distribution of APS, APS-1, and APS-2 was analyzed by high-pressure liquid chromatography (HPLC) (Agilent Technologies Inc., Palo Alto, CA, USA) equipped with a refractive index detector and a TSK gel G5000PWXL column (7.8 by 300 mm, 10 μm, TOSOH, Tokyo, Japan). Under the conditions of column temperature 30 °C, maximum column pressure 30 bar, and detector temperature 40 °C, 20 μL of sample was extracted and eluted with ultrapure water as mobile phase at 0.5 mL/min. The standard curve was generated using dextran standards (Mw: 2.7, 5.25, 9.75, 13.05, 36.8, 64.65, 135.35, 300.6, and 2000 kDa) and the relative molecular weight was estimated for each sample.
For monosaccharide analysis, APS, APS-1, and APS-2 were hydrolyzed by the trifluoroacetic acid (TFA) method. Next, the hydrolyzed products of APS, APS-1, and APS-2 were analyzed using Dionex ICS 5000 ion chromatograph (Dionex, Sunnyvale, CA, USA) equipped with a Dionex CarboPac-PA20 analytical column. The retention times and response values of standards were used to draw standard curves and determine the monosaccharide composition and content of samples.
2.3. In Vitro Simulated Gastric-Intestinal Digestion
Preparation of simulated gastric buffer (SGF) followed the description in Table S1, followed by prewarming 4 mL of SGF in a 37 °C water bath, and then supplementation with CaCl2 solution (0.3 M, 0.025 mL), pepsin (3000 U/mL, 0.33 mL), polysaccharide solution (8 mg/mL, 5 mL), and ultrapure water to a 10 mL total volume (pH = 3.0). After 2 h of incubation, samples were collected and inactivated, followed by simulated small intestinal digestion. The simulated small intestine buffer (SIF) was configured as shown in Table S1. After prewarming in a 37 °C water bath, SIF was supplemented with CaCl2 solution (0.3 M, 0.1 mL), trypsin (4000 U/mL, 0.25 mL), bile salt solution (100 mg/mL, 0.4 mL), gastric digestive fluid of APS (5 mL), and ultrapure water to a 10 mL total volume (pH = 7.0). After 3 h of incubation, samples were collected and inactivated.
2.4. Fermentation In Vitro
Fresh feces for this experiment were provided by fourteen volunteers, including 7 healthy volunteers aged 27 to 58 years (3 females and 4 males) and 7 T2DM volunteers aged 50 to 73 years (3 females and 4 males), who had not taken antibiotics or prebiotics for at least 3 months. All experiments in this study were performed following the Institutional Guidelines of the Biomedical Ethics Committee of West China Hospital of Sichuan University (2018(286)). Each fresh stool was collected and processed into a stool suspension using an automatic stool processor (HALO-P100, Hunan Hailu Biotechnology Co., Changsha, China). Next, the fecal slurry samples were mixed with yeast extract-casein hydrolyzed fatty acid (YCFA) medium or YCFA medium containing 1% (w/v) APS at 1:10, followed by anerobic incubation at 37 °C for 24 h. In this study, all the samples were divided into 3 groups: in vitro medium-fermented feces of healthy control groups (HC), in vitro medium-fermented feces of T2DM patients (T2DM), and in vitro APS-fermented feces of T2DM patients (APS-T2DM).
2.5. Gut Microbiota Analysis
Briefly, the gut microbiota in the fermentation solution were collected by centrifugation at 4 °C and 12,000 rpm for 10 min, followed by bacterial DNA extraction and 16S rDNA sequencing in the Major Biotechnology Co., Ltd. (Shanghai, China). Species sequence comparisons were annotated based on the Ribosome Database Project (RDP). Alpha diversity was analyzed by QIIME software (v1.9.1), and the species diversity of alpha diversity was characterized by Shannon and Simpson indices. Shannon’s index is used to describe the disorder and uncertainty in the occurrence of individuals of a species, the higher the uncertainty, the higher the diversity. Simpson’s index reflects the size of species richness by analyzing the number of individuals in the same population. The value of this index ranges from 0 to 1, with larger values indicating lower species identification and higher concentration of species numbers. It is worth noting that the two indicators are calculated differently and with different emphasis, so they are considered together to avoid differences in the results of individual indicators [16]. Finally, the gut microbiota data were analyzed using the Majorbio Cloud platform (https://cloud.majorbio.com, 13 January 2023).
2.6. Metabolomic Analysis
GC-MS. After centrifugal collection of the supernatant of fermentation samples, 200 µL of supernatant was supplemented with 20 µL of ribitol (1 mg/mL) as an internal standard and then lyophilized in a freeze dryer. Next, the silane derivatization of the samples was performed as previously reported [22]. Gas phase conditions: Agilent HP-5 column (30 m × 0.25 μm × 0.25 mm, Agilent, Santa Clara, CA, USA), helium carrier gas, 1 μL injection volume, and 250 °C injection port temperature. The heating procedure was performed with 50 °C as the initial temperature for 2 min, up to 270 °C at 5 °C/min, 290 °C at 2.5 °C/min, and 310 °C at 10 °C/min and held for 4 min. Mass spectral conditions: 50–700 m/z scanning range, 230 °C ion source temperature, 280 °C interface temperature, and 10 min solvent delay time.
For HPLC/MS analysis, the samples were pre-treated as follows. After centrifuging the fermentation broth at 10,000 rpm for 15 min, the supernatant was extracted by SPE column, followed by elution with methanol. Next, the eluate was blown dry at 37 °C under nitrogen, followed by dissolution and methanol washing. Meanwhile, the instrument stability during the experiment was assessed with quality control samples (QC, an aliquot mixture of all fermentation samples). Liquid phase conditions: Xbridge C18 column (3.5 μm, 2.1 × 100 mm), 30 °C column temperature, 10 μL injection volume, and 0.25 mL/min flow rate. The mobile phase consisted of 0.1% formic acid water (A) and acetonitrile (B), with the gradient elution program of 10% B at 0–1 min, 10–95% B at 1–12 min, 95% B at 12–15 min, and 10% B at 15–0 min.
Electrospray ionization (ESI) mass spectral analysis was performed using ESI source in a positive ion mode with an Agilent 6230 accurate mass time-of-flight (TOF) mass spectrometer (Santa Clara, CA, USA). The parameters were set as follows: 4000.0 V capillary voltage, 120 °C source temperature, 35 psi nebulizer pressure, 350 °C desolvation temperature, 9 L/min desolvation gas flow rate, 40 V cone voltage, 121.050873 and 922.009798 reference ion m/z, 175 V collision energy voltage, and helium collision gas.
2.7. Quantification of Fecal SCFAs
Briefly, each fermented sample was mixed with crotone-phosphite solution, acidified for 24 h, and filtered into a sample vial. Next, the contents of short-chain fatty acids in each fermented sample (such as acetic, butyric, propionic, isobutyric, isovaleric, and valeric acids) was determined using an Agilent 7890 detector (Santa Clara, CA, USA) with an Agilent FFAP column 30 mm × 0.25 mm × 0.25 μm (Santa Clara, CA, USA). Trans-2-butenoic acid was used as the internal standard for this experiment.
2.8. Antioxidant Analysis
Briefly, each sample was mixed with 2,2-diphenyl-1-picrylhydrazyl (DPPH) ethanol solution (0.15 mM) in an equal volume and incubated for 30 min under light-proof conditions. Next, the absorbance value of each solution was measured at 517 nm using a multifunctional enzyme marker (Tecan, Salzburg, Austria). Finally, DPPH removal efficiency is calculated by the following formula:
where A control 517 nm is the absorbance of the control (ethanol instead of DPPH) and A blank 517 nm is the absorbance of the blank (distilled water instead of the samples).
Scavenging effect (%) = [1 − (A sample 517nm − A control 517nm)/A blank517nm] × 100%
Additionally, 2 mL of each fermentation liquid sample was mixed with 2 mL of FeSO4 (6 mM), 2 mL of H2O2 (6 mM), and 2 mL of sodium salicylate (6 mM), with distilled water used as a positive control. Next, the absorbance value of each reaction product at 510 nm was measured spectrophotometrically. The OH•-scavenging activity was expressed as:
%HO• scavenged = (1 − (A sample 510nm − A control 510nm)/A blank510nm) × 100%
Among them, control 510 nm is the absorbance of control (distilled water replaced sodium salicylate), and blank 510 nm is the absorbance of blank (distilled water replaced the sample).
The reducing power was determined according to Chen et al. [23]. An aliquot of 3 μL of fermentation liquid sample was mixed with 247 μL of phosphate buffer solution (pH 6.6, 0.2 mol/L) and 250 μL 1% potassium ferricyanide (w/v), and incubated for 30 min at 50 °C. Next, 250 μL 10% trichloroacetic acid (w/v) was added and centrifuged. Then 100 μL of the supernatant was aspirated and mixed with 100 μL of distilled water and 20 μL 0.1% ferric chloride (w/v). Finally, these mixtures were analyzed at an absorbance of 700 nm taking distilled water as a reference.
2.9. Statistical Analysis
All data were analyzed using SPSS 22 (IBM, New York, NY, USA) and the results were presented as mean ± standard deviation (SD). Data normal distribution and homogeneity of variance were assessed by Shapiro–Wilk and Brown–Forsythe tests, respectively. When the data satisfied normal distribution, differences between three groups were analyzed, and post hoc tests were conducted through one-way ANOVA and Tukey’s test (equal variances) or Dunnet-T3 (unequal variances). If data were not normally distributed, Kruskal–Wallis analysis was performed. Graphs were made with GraphPad Prism 9 software (San Diego, CA, USA). Metabolic data were analyzed using Metaboanalyst 5.0 (https://metaboanalyst.ca, 21 December 2022) and SIMCA 14 (v14.1, Sartorius, Göttingen, Germany). Correlation analysis between differential metabolites and fecal microbiota was performed using Spearman correlation.
3. Results
3.1. Chemical and Structural Characterization of APS
As shown in Figure S1A,B, the three components of crude APS (APS-0M, APS-0.1M, and APS-0.2M) were separated by DEAE Sepharose FF, with their proportion accounting for 82.72 ± 4.10%, 2.65 ± 0.01%, and 13.14 ± 0.02% of the total polysaccharide, respectively. Finally, APS-1 and APS-2 were further isolated and purified from APS-0M and APS-0.2M, respectively (Figure S1C,D).
Table S2 shows the basic physicochemical properties of APS, APS-1, and APS-2. The yield was about 9.48 ± 0.54% (w/w), 59.92 ± 0.70% (w/w), and 32.21 ± 0.20% (w/w) for APS, APS-1, and APS-2 extracts, and their carbohydrate content was 75.73 ± 0.72% (w/w), 95.71 ± 1.42% (w/w), and 92.5 ± 1.25% (w/w), respectively. Meanwhile, the protein and total phenol content was found to be extremely low in APS, APS-1, and APS-2, with only about 0.08% of proteins found in APS, while no detectable proteins and phenolics in both APS-1 and APS-2, suggesting the virtual absence of proteins and phenolics in APS-1 and APS-2.
In the UV spectra of Figure 1A, APS, APS-1, and APS-2 exhibited no absorption peak at 260 and 280 nm, implying the presence of little or no nucleic acid and protein. In Figure 1B, HPGPC analysis revealed that four significant peaks were observed in APS and the average molecular weight of these four components were about 1928.54 kDa, 396.31 kDa, 239.92 kDa, and 4.87 kDa, respectively. The average molecular weights were about 4.39 and 1915.15 kDa for the purified APS-1 and APS-2, respectively.
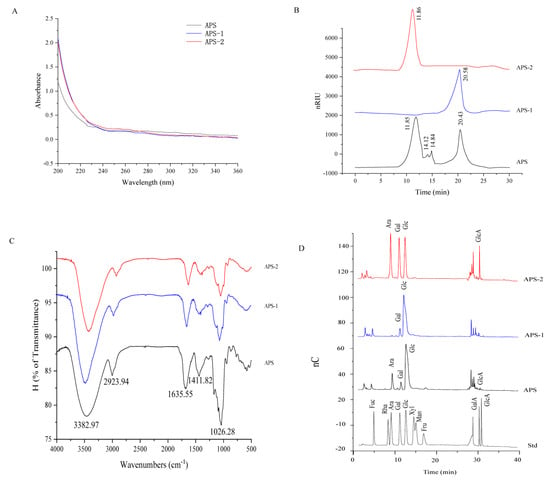
Figure 1.
Structure analysis of APS, AP-1, and APS-2. (A) UV spectra. (B) Molecular weight distribution of APS. (C) FT-IR spectra. (D) Monosaccharide composition analysis by ion chromatography. Fuc, Fucose; Rha, Rhamnose; Ara, Arabinose; Gal, Galactose; Glc, Glucose; Xyl, Xylose; Man, mannose; Fru, Fructose; GalA, Galacturonic acid; GlcA, Glucuronic acid.
In Figure 1C, APS, APS-1, and APS-2 were seen to have similar FT-IR spectra. The strong absorption peak around 3382.97 cm−1 can be ascribed to the -OH stretching vibration of sugars, the weak absorption peak around 2923.94 cm−1 is attributed to the C–H stretching vibration [24], the weak peaks around 1635.55 and 1411.82 cm−1 correspond to the presence of carboxyl groups [25], and the strong absorption peak near 1026.28 is assigned to the presence of a pyran ring in the polysaccharide [26]. The monosaccharide composition in APS was determined by ion chromatography. In Figure 1D, the major monosaccharides were seen to consist of arabinose, galactose, glucose, and galacturonic acid at a molarity ratio of 1:0.33:4.36:0.02 in APS, galactose and glucose at a molar ratio of 1:9.25 in APS-1, and arabinose, galactose, glucose, and galacturonic acid at a molar ratio of 1:0.85:0.96:0.46 in APS-2.
3.2. APS Altered the Fecal Microbiota Composition of T2DM Patients
The degradation rate of APS was 1.52% and 2.36% in simulated gastric and simulated intestinal fluids, respectively, indicating that APS can hardly be digested by the stomach and small intestine, so it can be utilized by gut microbes. In addition, Supplementary Figure S2 shows that APS concentration at 50–400 µg/mL had no significant effect on Caco2 activity. Consistent with our results, Ying et al. [27] and Wang et al. [28] also found that APS was almost non-toxic to epithelial cells. In Figure S3, the sequencing depth of samples was seen to sufficiently reflect the composition of most microorganisms in the samples. In Figure 2A,B, the Shannon index was shown to be lower in the T2DM group than in the HC group, in contrast to an opposite result in the Simpson index, despite no significant difference. Meanwhile, APS addition did not significantly affect the Shannon and Simpson indices in the feces of T2DM patients. Further PCoA and PLS-DA analyses confirmed a notable and consistent separation between the groups of HC, T2DM, and APS-T2DM (Figure 2C,D). These results suggest that APS fermentation may have promoted the proliferation of some specific bacteria, but with no effect on microbial diversity.
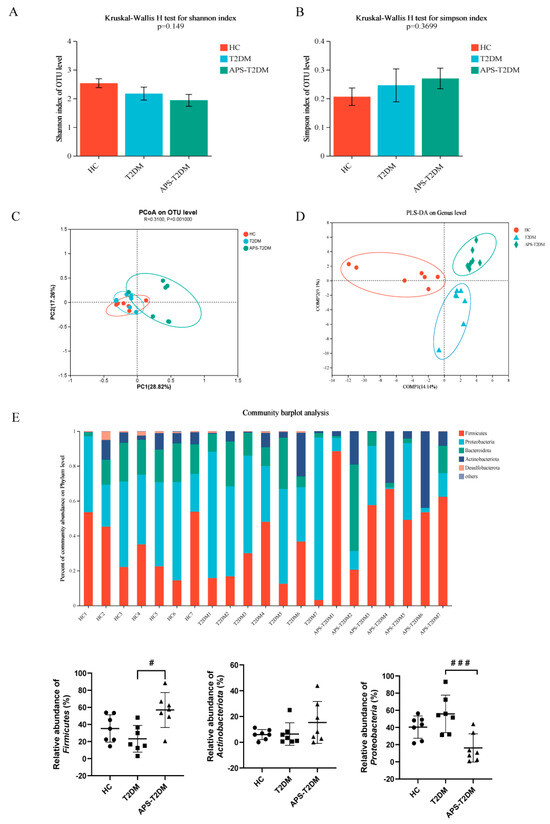
Figure 2.
Effects of APS on gut microbiota structure of T2DM patients. (A) Shannon index. (B) Simpson index. (C) PCoA analysis. (D) PLS-DA analysis. (E) Taxonomic analysis of samples at phylum levels. # p < 0.05, ### p < 0.01 vs. T2DM group, n = 7. Black Circles represent HC, black squares represent T2DM, and black triangles represent APS-T2DM.
In Figure 2E, the microbiota of each group at the phylum level were shown to consist mainly of Firmicutes, Bacteroidota, Actinobacteria, and Proteobacteria. Specifically, the T2DM group was lower than the HC group in the relative abundance of Firmicutes, but higher in the relative abundance of Proteobacteria. However, the abundance of Firmicutes and Proteobacteria was significantly reversed by APS fermentation. The different groups of gut microbial taxa were displayed from phylum to genus using LEfSe (Figure S4), with the dominance of Clostridia, Acidaminococcaceae, Lachnospiraceae, and Phascolarctobacterium in the HC group, and the dominance of Proteobacteria, Enterobacteriaceae, and Escherichia-Shigella in the T2DM group. Meanwhile, Firmicutes and Lactobacillaceae showed significant enrichment in the APS-T2DM group.
The distributions of microorganisms at both family and genus levels are shown in Figure 3A–D. Compared with the HC group, the T2DM group showed higher relative abundances of Enterobacteriaceae, Escherichia-Shigella, and Parabacteroides. However, the APS addition caused a decrease in the relative abundance of Enterobacteriaceae, Escherichia-Shigella, and Parabacteroides, and an increase in the relative abundance of Bifidobacterium and Lactobacillus in T2DM patients, suggesting that APS could regulate the intestinal microbiota structure of T2DM patients.
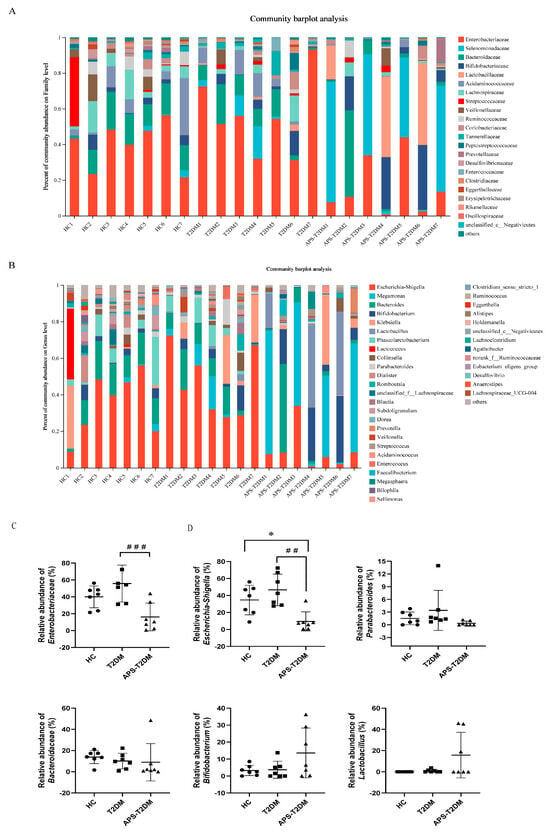
Figure 3.
Effects of APS on intestinal microbiota composition in T2DM patients at family and genus levels. (A) Gut microbial composition at the family level. (B) Gut microbial composition at the genus level. (C) Relative abundance analysis of gut microbiota at the family level. (D) Relative abundance analysis of gut microbiota at the genus level. ## p < 0.01, ### p < 0.001 vs. T2DM group, and * p < 0.05 vs. HC group, n = 7. Black Circles represent HC, black squares represent T2DM, and black triangles represent APS-T2DM.
3.3. Metabolic Analysis of APS Fermentation Samples
Table S3 shows the GC-MS and HPLC-MS results of metabolites in fermentation samples, and a total of 113 substances were detected. As shown in Figure 4A, the three groups of samples were clearly separated, indicating the presence of differential metabolites in them. In Figure 4B, the results of orthogonal partial least squared discriminant analysis (OPLS-DA) revealed a significant separation between two pairs in the HC, T2DM, and APS-T2DM groups. All the OPLS-DA models were confirmed to be valid by the permutation test (n = 200) (Figure 4C). These results suggest the differences of metabolic profiles among the three groups of samples.
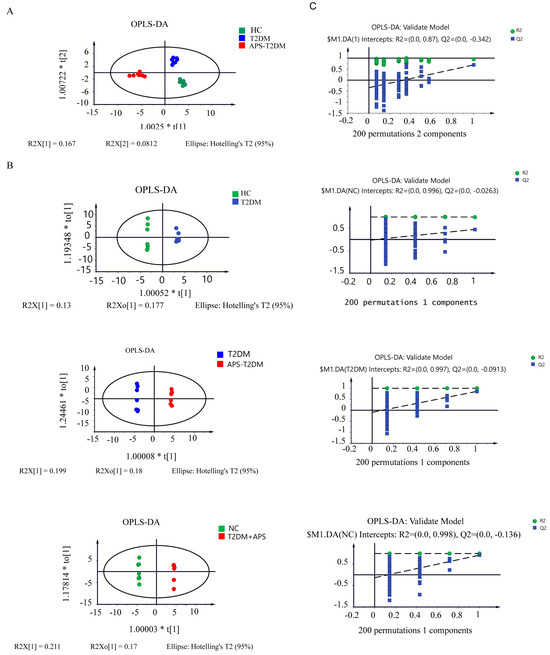
Figure 4.
Analyses of metabolites in in vitro fermentation samples by GC-MS and HPLC-MS. (A) The OPLS-DA plot of three groups. (B) OPLS-DA plots of T2DM vs. HC, APS-T2DM vs. T2DM, and APS-T2DM vs. HC. (C) OPLS-DA validation plot (n = 200).
Compounds with significant differences were further screened (Table S4), and some differential metabolites are shown in Figure 5. The T2DM group was significantly lower than the HC group in butyric acid and biotin and was significantly higher in L-threonine, L-glutamic acid, glycine, and guanine. In contrast with the T2DM group, APS fermentation significantly decreased the levels of L-valine, L-proline, L-threonine and L-glutamic acid, and spermidine, while the levels of all-trans-retinoic acid, glutamine, thiamine, butyric acid, and propanoic acid were elevated. The functions of these differential metabolites were determined by KEGG pathway enrichment analysis. In Figure 5B, L-valine, L-threonine, L-proline, and L-tyrosine were shown to be enriched in the pathways of aminoacyl-tRNA biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis, and proline metabolism, while butyric acid and thiamine were found to be mainly enriched in the pathways of butanoate metabolism and thiamine metabolism.
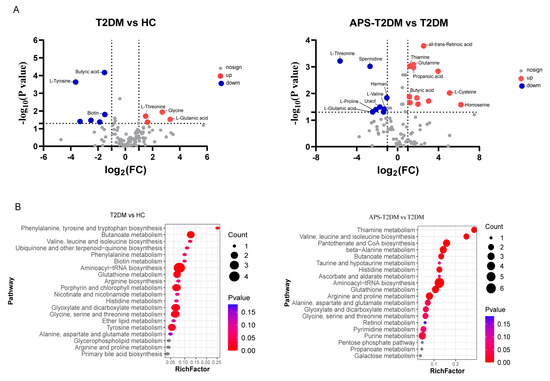
Figure 5.
Differential metabolite analysis. (A) Volcano plots of metabolites, with blue dots for downregulated metabolites (p < 0.05; FC < 0.5 and VIP > 1), red dots for upregulated metabolites (p < 0.05; FC > 2 and VIP > 1), and gray dots for metabolites with no significant differences (p > 0.05; FC < 2 and VIP < 1). (B) KEGG enrichment analysis of different metabolites.
3.4. APS Upregulated the Levels of SCFAs in Feces of T2DM Patients
Figure 6A–F show the levels of SCFAs in the three groups detected by GC. The T2DM group was shown to be lower than the HC group in the levels of all SCFAs. The fermentation of APS in the feces of T2DM patients was seen to increase the propionic acid content (p < 0.01) and partially recover both acetic and butyric acid levels. However, the three groups showed no significant difference in isobutyric, valeric, and isovaleric acids (Figure 6D–F).
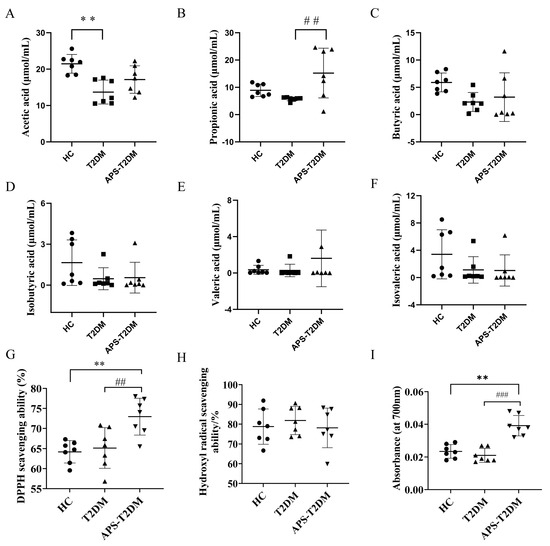
Figure 6.
Effects of APS on the levels of SCFAs and antioxidant activities in the feces of T2DM patients after in vitro fermentation. (A) Acetic acid. (B) Propionic acid. (C) Butyric acid. (D) Isobutyric acid. (E) Valeric acid. (F) Isovaleric acid. (G) DPPH- scavenging rate. (H) Hydroxyl radical scavenging rate. (I) Reduction ability. ## p < 0.01 vs. ### p < 0.001 vs. T2DM group. ** p < 0.01 vs. HC group; Black Circles represent HC, black squares represent T2DM, and black triangles represent APS-T2DM. n = 7.
3.5. APS Fermentation Enhanced Antioxidant Activity in Feces of T2DM Patients
Oxidative stress is one of the key pathogenic factors that induce T2DM, and the APS antioxidant activity was investigated by analyzing its ability to scavenge DPPH-, hydroxyl radicals (HO•-), and reduction ability in this study. As shown in Figure 6G–I, compared to the T2DM group, the APS-T2DM group was significantly (p < 0.05) higher in the scavenging ability of DPPH- and reduction ability, but with no significant difference between them in the scavenging ability of HO•.
3.6. Correlations between Gut Microbiota and Differential Metabolites or Oxidative Stress
The results of Spearman correlation analysis between differential metabolites and gut microbiota are shown in Figure 7A,B. Escherichia-Shigella was negatively correlated with all-trans-retinoic acid and glutamine, while positively correlated with L-threonine and spermidine. Lactobacillus had a positive correlation with thiamin, valeric acid, and DPPH- clearance rate, and a negative correlation with dopamine. Bifidobacterium and Faecalibacterium exhibited a positive correlation with butyric acid. Parabacteroides showed a negative correlation with propionic acid and a positive correlation with spermidine, L-valine, and L-threonine.
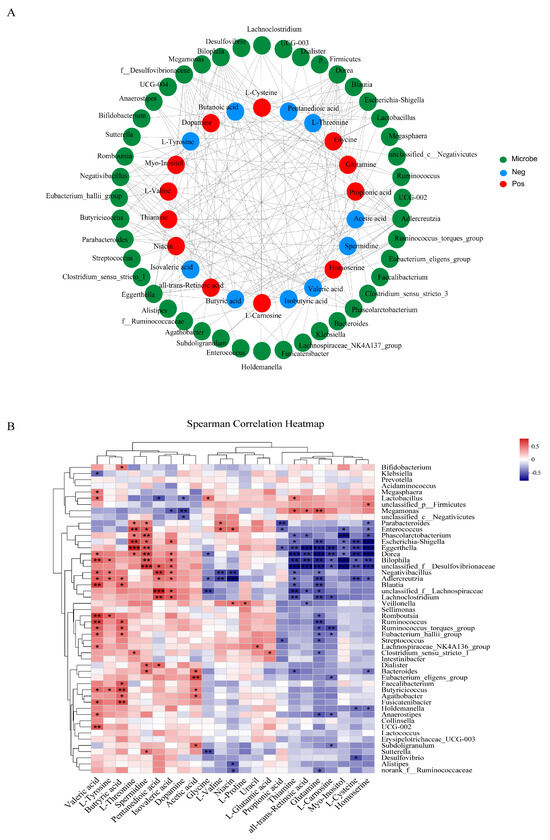
Figure 7.
Spearman correlation analysis. (A) Network analysis of correlations between metabolites and the top 45 most abundant gut microorganisms at the genus level, with green for microbe, red for positive correlation, and blue for negative correlation. (B) Heatmap analysis of correlations between metabolites and microbiota at the genus level, with the color from red to blue in the heatmap representing changes of the R values of Spearman’s correlations from greater to lower. * 0.01 < p ≤ 0.05, ** 0.001 < p ≤ 0.01, and *** p ≤ 0.0001.
4. Discussion
Gastrointestinal simulation and in vitro fermentation are commonly used in vitro to study food digestion and influence on the structure of gut microbiota. For example, Hu et al. [29] used gastrointestinal simulation and in vitro fermentation to study the molecular weight changes of extracellular polysaccharides of Corynebacterium parapsilosis during gastrointestinal digestion and their effects on human intestinal microbiota. Yi et al. [30] used gastrointestinal simulation and in vitro fermentation to investigate the digestive characteristics of brown rice gel and its effects on intestinal microbiota, and Xie et al. [31] used gastrointestinal simulation and in vitro fermentation to study the catabolic metabolism of polyphenols of mung bean hull and their effects on intestinal microbiota. Gastrointestinal simulation and in vitro fermentation have the advantages of being fast, cheap, without ethical restrictions, and are widely used. However, these do not fully mimic the in vivo environment and requires in vivo experimental validation compared to human trials [32]. The gastrointestinal simulation results in this paper suggest that APS is barely digested in the gastrointestinal simulation solution, which indicates that it can reach the intestine and be utilized by the gut microbiota without any problem.
Previous studies have confirmed that hyperglycemia and insulin resistance in T2DM patients are strongly associated with gut dysbiosis. High enrichment of conditionally pathogenic bacteria such as Escherichia coli, Helicobacter pylori, Salmonella, and Vibrio was reported to induce or promote T2DM development and progression [33,34]. Conversely, commensal or beneficial bacteria can improve glucolipid metabolism and favor health. Firmicutes, represented by Ruminococcaceae, Clostridium spp., and Lactobacillaceae, can hydrolyze starch and other sugars to produce butyric acid and other SCFAs, which are often used as probiotics [35]. Studies have also shown that Bifidobacterium and Lactobacillus can regulate lipid metabolism, lower blood glucose, and reduce T2DM complications [36,37]. In the present study, APS fermentation was shown to increase the abundance of Firmicutes, Bifidobacterium, and Lactobacillus in T2DM feces and decrease the levels of Proteobacteria, Escherichia-Shigella, and Parabacteroides. Our results are consistent with a previous report that Astragalus membranaceus polysaccharides could improve glucolipid metabolism disorders and alleviate symptoms in T2DM mice by increasing Allobaculum and Lactobacillus abundance and decreasing Escherichia coli abundance [38].
Metabolites produced by intestinal microorganisms have been shown to impact the host’s gut and health [39]. In this study, APS fermentation was observed to significantly reduce the levels of threonine, L-valine, L-threonine, L-proline, and spermidine, and increase the levels of all-trans-retinoic acid, thiamine, glutamine, propanoic acid, and butyric acid in the feces of T2DM patients. This agreed well with the findings in previous studies. For instance, T2DM patients were reported to have higher levels of L-threonine, glutamine, L-valine, L-proline, and spermidine, with these amino acids as potential biomarkers in T2DM patients [40,41]. All-trans-retinoic acid is a biologically active metabolite of retinoic acid that can significantly reduce blood glucose levels in diabetic rats [42]. Thiamine is commonly deficient in diabetic patients and its reduction can impair the endocrine function of the pancreas, thereby exacerbating insulin deficiency and hyperglycemia [43]. Glutamine can prevent or delay T2DM onset by reducing the inflammatory response and promoting insulin sensitivity in skeletal muscle [44]. The differential metabolite-based metabolic pathways are aminoacyl-tRNA biosynthesis, butanoate metabolism, and thiamine metabolism, which are dysregulated in T2DM patients [45,46]. Based on these reports and our results, APS can be concluded to significantly regulate glucolipid metabolism and exert lipid-lowering effects through fecal metabolites and metabolite pathways.
SCFAs are important gut microbiota-generated metabolites and their levels are usually very low in T2DM or obesity patients [47]. SCFAs, especially acetic acid, butyric acid, and propionic acid, have the function of improving glucose regulation and insulin sensitivity in T2DM patients by triggering glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) production, inhibiting β-cell apoptosis, and stimulating insulin secretion [48,49]. In this study, APS was found to upregulate the contents of acetic, propionic, and butyric acids in the stools of T2DM patients, probably due to the reason that APS can promote the proliferation of Firmicutes, Lactobacillus, and Bifidobacterium. Similarly, oral administration of APS was reported to augment the abundance of Muribaculum, Lactobacillus, and Faecalibaculum and raise the levels of acetic and propionic acids in a diabetic mice model [17].
Chronic oxidative stress can lead to mitochondrial damage, pancreatic β-cell apoptosis, and insufficient insulin secretion [50]. The antioxidant effect of APS has been demonstrated to contribute to the remission and treatment of diabetes [51]. In another study, some antioxidants that are difficult to digest and absorb were found to be fermented by intestinal microorganisms into antioxidant metabolites upon arrival in the intestine, resulting in antioxidant properties in feces [52]. In the present study, the fermentation of APS was found to significantly increase reduction ability and the clearance rate of DPPH- in the feces of T2DM patients, which may be related to the fact that APS can regulate the intestinal microbiota and promote the production of antioxidant metabolites, such as glutamine [53], thiamine [54], and all-trans-retinoic acid [42].
The correlations between intestinal microbiota and differential metabolites or chronic oxidative stress were further confirmed by correlation analysis. Interestingly, Lactobacillus was significantly positively associated with thiamine and DPPH- clearance. This is consistent with previous reports that Lactobacillus can produce thiamine [55] and have the potential to enhance DPPH- clearance [56]. Therefore, APS can be assumed to increase thiamine levels by promoting Lactobacillus content, which in turn improves fecal DPPH- clearance in T2DM patients.
5. Conclusions
In this study, the impacts of APS on the intestinal microbiota and its metabolites in T2DM patients were investigated using an in vitro simulated fermentation model. APS addition was shown to improve the imbalance of intestinal microbiota by increasing the abundance of Bifidobacterium and Lactobacillus and decreasing the abundance of Escherichia-Shigella. Additionally, based on metabolomics results, APS could increase all-trans-retinoic acid, thiamine, glutamine, and propanoic acid levels and decrease L-threonine L-valine, L-proline, and spermidine levels, with these metabolites mainly enriched in the pathways of aminoacyl-tRNA biosynthesis, butanoate metabolism, and thiamine metabolism. Moreover, APS could upregulate the levels of acetic, butyric, and propionic acids in the stools of T2DM patients and improve the corresponding antioxidant properties. Furthermore, based on correlation analysis results, Escherichia-Shigella was negatively correlated with all-trans-retinoic acid and glutamine while positively correlated with L-threonine and spermidine; Bifidobacterium and Lactobacillus had a positive correlation with butyric acid and thiamine, respectively. All these results indicate that APS may attenuate type 2 diabetes by modulating gut microbes and metabolites. This study has laid a theoretical foundation for the development of APS as an anti-diabetic drug.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16111698/s1, Table S1. Preparation of simulated digestion solution stock solution. Table S2. Basic physicochemical properties of APS, APS-1, and APS-2. Table S3. Metabolites of fermentation samples. Table S4. Screening and identification of differential metabolites in different treatment groups. Figure S1. Purification of APS. (A) Purification procedure of APS. (B) Stepwise elution curve of APS on DEAE Sepharose FF chromatography column. (C) Elution curve of APS-1 on Sephadex G-100 chromatography column. (D) Elution curve of APS-2 on Sephadex G-100 chromatography column. Figure S2. The effects of APS on the viability of Caco-2 cells for 24 h in different concentrations (50, 100, 200, 400 μg/mL). Cell viability was determined using the MTT assay. Figure S3. Dilution curve. Figure S4. Linear discriminant analysis (LDA) score f gut microbial taxa among groups.
Author Contributions
Formal analysis, Writing—original draft, Writing—review and editing, X.Z. (Xin Zhang); Methodology, Formal analysis, Investigation, L.J. and W.Q.; Methodology, Investigation, Q.M.; Investigation, X.Z. (Xiaoyuan Zhang), M.C., F.L. and L.Z.; Conceptualization, Formal analysis, Supervision, Resources, T.Z. and W.J.; Supervision, Writing—review and editing, Resources, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Jinan Innovation Team Project (No. 202228040), the National Natural Science Foundation of China (No. U20A20400), and the National Key R&D Program of China (No. 2017YFD0400304). The authors declare that no competing financial interests influenced the work reported in this paper.
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the West China Hospital of Sichuan University, with approval code as 2018(286) on 23 November 2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data for this study are available in the article and the Supplementary Materials.
Acknowledgments
We thank Wei Qi and Weiguo Jia for their guidance in this project.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
APS, Astragalus polysaccharide; APS-T2DM, APS group of T2DM fecal slurry; GLP-1, glucagon-like peptide-1; HC, healthy original control group; HPGPC, high-performance gel permeation chromatography; OTUs, operational taxonomic units; PYY, peptide YY; RDP, Ribosome Database Project; SCFAs, short-chain fatty acids; IR, insulin resistance; SD, standard deviation; SIF, simulated small intestine buffer; T2DM, type 2 diabetes mellitus; TFA, trifluoroacetic acid; SGF, simulated gastric buffer; YCFA, yeast extract-casein hydrolysate-fatty acids; Pos: positive; Neg: negative. IL-6, factors interleukin 6; DPPH, 2,2-diphenyl-1-picrylhydrazyl; TNF-α, tumor necrosis factor α.
References
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, Y.; Su, Y.; Li, J.; Ren, W.C.; Wang, Q.H.; Kuang, H.X. Probiotics with anti-type 2 diabetes mellitus properties: Targets of polysaccharides from traditional Chinese medicine. Chin. J. Nat. Med. 2022, 20, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ogasawara, N.; Funaki, Y.; Mizuno, M.; Iida, A.; Goto, C.; Koikeda, S.; Kasugai, K.; Joh, T. Transglucosidase improves the gut microbiota profile of type 2 diabetes mellitus patients: A randomized double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J. Mol. Endocrinol. 2016, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef]
- Zhang, K.; Qi, J.X.; Li, Y.Y.; Gao, H.S.; Shao, X.Y.; Su, C.Y.; Wang, M.Q.; Ouyang, J. Antioxidative and immunological effects of Cyclocarya paliurus polysaccharides on the spleen injury of diabetic rat. J. Tradit. Chin. Med. 2021, 41, 739–746. [Google Scholar] [CrossRef]
- Li, J.S.; Ji, T.; Su, S.L.; Zhu, Y.; Chen, X.L.; Shang, E.X.; Guo, S.; Qian, D.W.; Duan, J.A. Mulberry leaves ameliorate diabetes via regulating metabolic profiling and AGEs/RAGE and p38 MAPK/NF-kappaB pathway. J. Ethnopharmacol. 2022, 283, 114713. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, H.; Nie, Q.; Huang, X.; Nie, S. Dendrobium officinale polysaccharide ameliorates the liver metabolism disorders of type II diabetic rats. Int. J. Biol. Macromol. 2020, 164, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, W.; Bian, J.; Gao, Y.; Hao, Z.; Tan, J. Recent advances in medicinal and edible homologous polysaccharides: Extraction, purification, structure, modification, and biological activities. Int. J. Biol. Macromol. 2022, 222, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-M.; Wang, Y.; Chen, S.-Y. Astragalus polysaccharide alleviates type 2 diabetic rats by reversing the glucose transporters and sweet taste receptors/GLP-1/GLP-1 receptor signaling pathways in the intestine-pancreatic axis. J. Funct. Foods 2021, 76, 104310. [Google Scholar] [CrossRef]
- Wang, D.; Cui, Q.; Yang, Y.J.; Liu, A.Q.; Zhang, G.; Yu, J.C. Application of dendritic cells in tumor immunotherapy and progress in the mechanism of anti-tumor effect of Astragalus polysaccharide (APS) modulating dendritic cells: A review. Biomed. Pharmacother. 2022, 155, 113541. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiong, Z.; Lin, M.; Yang, Y.; Chen, Y.; Zeng, J.; Jia, X.; Feng, L. Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 2023, 234, 123767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Li, J.; Tang, S.; Wang, M.; Huang, W.; Yao, W.; Gao, X. A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydr. Polym. 2019, 205, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Hu, B.; Sun, Y.; Ye, H.; Ma, D.; Zeng, X. TPC in the leaves of 116 sweet potato (Ipomoea batatas L.) varieties and Pushu 53 leaf extracts. J. Food Compos. Anal. 2010, 23, 599–604. [Google Scholar] [CrossRef]
- BRADFORD, M.M. A rapid and sensitive method for the quantitation of microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Huo, W.; Zhu, W. Use of pyrosequencing to characterize the microbiota in the ileum of goats fed with increasing proportion of dietary grain. Curr. Microbiol. 2013, 67, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Haxaire, K.; Marechal, Y.; Milas, M.; Rinaudo, M. Hydration of polysaccharide hyaluronan observed by IR spectrometry. I. Preliminary experiments and band assignments. Biopolymers 2003, 72, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.Q.; Lu, C.Q.; Cui, S.H.; Pan, L.H.; Zhang, H.L.; Wang, J.H.; Luo, J.P. Structural identification and immunostimulating activity of a Laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 78, 429–438. [Google Scholar] [CrossRef]
- Ying, Y.; Song, L.Y.; Pang, W.L.; Zhang, S.Q.; Yu, J.Z.; Liang, P.T.; Li, T.G.; Sun, Y.; Wang, Y.Y.; Yan, J.Y.; et al. Astragalus polysaccharide protects experimental colitis through an aryl hydrocarbon receptor-dependent autophagy mechanism. Br. J. Pharmacol. 2024, 181, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Yang, X.; Yao, J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, C.; Jiang, W.; Zhu, H.; Zhang, H.; Qian, H.; Zhang, W. Chronic in vitro fermentation and in vivo metabolism: Extracellular polysaccharides from Sporidiobolus pararoseus regulate the intestinal microbiome of humans and mice. Int. J. Biol. Macromol. 2021, 192, 398–406. [Google Scholar] [CrossRef]
- Yi, C.; Xu, L.; Luo, C.; He, H.; Ai, X.; Zhu, H. In vitro digestion, fecal fermentation, and gut bacteria regulation of brown rice gel prepared from rice slurry backfilled with rice bran. Food Hydrocoll. 2022, 133, 107986. [Google Scholar] [CrossRef]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of polyphenols released from mung bean coat and its effects on gut microbiota during in vitro simulated digestion and colonic fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, A.J.; Hinojosa-Nogueira, D.; Lopez-Maldonado, A.; Pastoriza, S.; Rufian-Henares, J.A. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Raquinio, P.; Kristal, B.S.; Setiawan, V.W.; Wilkens, L.R.; Franke, A.A.; Lim, U.; Le Marchand, L.; Randolph, T.W.; Lampe, J.W.; et al. The gut microbiome and type 2 diabetes status in the Multiethnic Cohort. PLoS ONE 2021, 16, e0250855. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.M.; Shata, A.; Hassan, H.M.; El-Sokkary, M.M.A.; Khodir, A.E. The therapeutic role of lactobacillus and montelukast in combination with metformin in diabetes mellitus complications through modulation of gut microbiota and suppression of oxidative stress. Int. Immunopharmacol. 2021, 96, 107757. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fu, X. Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota-mucosal barrier”. Food Funct. 2022, 13, 10121–10133. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Al-Aama, J.Y.; Al Mahdi, H.B.; Salama, M.A.; Bakur, K.H.; Alhozali, A.; Mosli, H.H.; Bahijri, S.M.; Bahieldin, A.; Willmitzer, L.; Edris, S. Detection of Secondary Metabolites as Biomarkers for the Early Diagnosis and Prevention of Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2019, 12, 2675–2684. [Google Scholar] [CrossRef]
- Fernandez-Garcia, J.C.; Delpino-Rius, A.; Samarra, I.; Castellano-Castillo, D.; Munoz-Garach, A.; Bernal-Lopez, M.R.; Queipo-Ortuno, M.I.; Cardona, F.; Ramos-Molina, B.; Tinahones, F.J. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial. J. Clin. Med. 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moreno, A.; Quintanar Escorza, M.A.; Garcia Garza, R.; Hady, K.; Melendez Valenzuela, A.; Marszalek, J.E.; Sharara-Nunez, I.; Delgadillo-Guzman, D. All-trans retinoic acid improves pancreatic cell proliferation on induced type 1 diabetic rats. Fundam. Clin. Pharmacol. 2020, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Page, G.L.; Laight, D.; Cummings, M.H. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 2011, 65, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Dollet, L.; Kuefner, M.; Caria, E.; Rizo-Roca, D.; Pendergrast, L.; Abdelmoez, A.M.; Karlsson, H.K.R.; Bjornholm, M.; Dalbram, E.; Treebak, J.T.; et al. Glutamine Regulates Skeletal Muscle Immunometabolism in Type 2 Diabetes. Diabetes 2022, 71, 624–636. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, D.; Xu, Y.; Peng, F.; Zhao, S.; Li, H.; Jin, D.; Xu, X.; Xia, Z.; Che, M.; et al. High-Coverage Serum Metabolomics Reveals Metabolic Pathway Dysregulation in Diabetic Retinopathy: A Propensity Score-Matched Study. Front. Mol. Biosci. 2022, 9, 822647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Xie, C.; Hu, Z.; Zhang, Y.; Peng, S.; He, Y.; Kang, J.; Gao, H.; Yuan, H.; et al. Shenqi compound ameliorates type-2 diabetes mellitus by modulating the gut microbiota and metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1194, 123189. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Shen, G.X. Oxidative stress and diabetic cardiovascular disorders: Roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol. 2010, 88, 241–248. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fu, X. Hypoglycemic activity in vitro and vivo of a water-soluble polysaccharide from Astragalus membranaceus. Food Funct. 2022, 13, 11210–11222. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Porras, B.; Perez-Burillo, S.; Hinojosa-Nogueira, D.; Douros, K.; Pastoriza, S.; Rufian-Henares, J.A. The Gut Microbiota of Obese Children Releases Lower Antioxidant Capacity from Food than That of Lean Children. Nutrients 2022, 14, 2829. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A.; Akhigbe, T.M.; Akhigbe, R.E.; Aremu, A.O.; Oyedokun, P.A.; Gbadamosi, J.A.; Anifowose, P.E.; Adewole, M.A.; Aboyeji, O.O.; Yisau, H.O.; et al. Glutamine restores testicular glutathione-dependent antioxidant defense and upregulates NO/cGMP signaling in sleep deprivation-induced reproductive dysfunction in rats. Biomed. Pharmacother. 2022, 148, 112765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, C.; Zheng, J.; Zhao, J.; Wei, S.; Xiong, Y.; Limbu, S.M.; Kong, Y.; Cao, F.; Ding, Z. Dietary thiamine modulates carbohydrate metabolism, antioxidant status, and alleviates hypoxia stress in oriental river prawn Macrobrachium nipponense (de Haan). Fish. Shellfish. Immunol. 2022, 131, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.; Santos, F.; Roos, S.; Mistretta, T.A.; Spinler, J.K.; Molenaar, D.; Teusink, B.; Versalovic, J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 2011, 6, e18783. [Google Scholar] [CrossRef] [PubMed]
- Chooruk, A.; Piwat, S.; Teanpaisan, R. Antioxidant activity of various oral Lactobacillus strains. J. Appl. Microbiol. 2017, 123, 271–279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).