Abstract
Hypertensive diseases of pregnancy (HDPs) represent a global clinical challenge, affecting 5–10% of women and leading to complications for both maternal well-being and fetal development. At the heart of these complications is endothelial dysfunction, with oxidative stress emerging as a pivotal causative factor. The reduction in nitric oxide (NO) bioavailability is a vital indicator of this dysfunction, culminating in blood pressure dysregulation. In the therapeutic context, although antihypertensive medications are commonly used, they come with inherent concerns related to maternal–fetal safety, and a percentage of women do not respond to these therapies. Therefore, alternative strategies that directly address the pathophysiology of HDPs are required. This article focuses on the potential of the nitrate-nitrite-NO pathway, abundantly present in dark leafy greens and beetroot, as an alternative approach to treating HDPs. The objective of this review is to discuss the prospective antioxidant role of nitrate. We hope our discussion paves the way for using nitrate to improve endothelial dysfunction and control oxidative stress, offering a potential therapy for managing HDPs.
1. Introduction
A significant percentage of pregnant women worldwide suffer from hypertension, a common and potentially fatal pregnancy condition. It is estimated that approximately 5% to 10% of all pregnancies are complicated by hypertensive disorders of pregnancy (HDPs), making it one of the leading causes of maternal and fetal morbidity and mortality [1,2]. HDPs are categorized into four main categories: gestational hypertension, pre-eclampsia/eclampsia, chronic hypertension, and chronic hypertension with pre-eclampsia superimposed. Each has distinct characteristics and implications for the health of the mother and fetus [3].
HDPs can harm the placenta and affect the baby’s growth, leading to problems such as low birth weight, restricted growth, and premature birth [4,5]. A malfunctioning placenta is also a major contributor to the development of pre-eclampsia, placing both the mother and the baby at risk [5]. As a consequence, children born from pregnancies complicated by HDPs are more prone to conditions like heart disease and metabolic disorders later in life [6]. For mothers, the repercussions included renal and liver damage, thrombosis, and, in severe cases, cerebral hemorrhage [7].
Although HDPs are a common and potentially serious issue for both mothers and babies, there are limited therapeutic options. Managing conditions related to HDPs typically involves controlling blood pressure and preventing complications [8]. Commonly prescribed antihypertensive drugs include methyldopa, labetalol, and nifedipine [9], and aspirin supplementation may be recommended for specific cases [9]. In the most severe cases, when there is a threat to the life of the mother or the fetus, early delivery may be necessary, even if the baby has not yet reached full gestation maturity.
Managing high blood pressure during pregnancy requires reliable and effective therapeutic intervention. Research into complementary strategies, like dietary interventions, is essential alongside conventional treatments to ensure a safer and healthier pregnancy. Vitamins, minerals, and amino acids have been extensively studied for their potential effects on HDPs [10,11,12,13].
Among this exploration, dietary nitrate intake from natural sources shows promising potential. Many studies center on nitrates’ ability to elevate nitric oxide (NO) levels via the nitrate-nitrite-NO pathway, reducing blood pressure [14,15,16,17,18,19,20]. The nitrate-nitrite-NO pathway is recognized for its influence on vascular relaxation, and reviews published to date usually focus on these vasodilatory properties. However, the antioxidant effects of the nitrate-nitrite-NO pathway in the context of HDPs remain an underexplored avenue deserving further research. Therefore, this review discusses the potential implications of the nitrate-nitrite-NO pathway on HDPs, emphasizing its prospective antioxidant role and underlying mechanisms of the redox system.
Initially, we will discuss the foundational concepts of HDP pathophysiology: endothelial dysfunction and oxidative stress. From there, we will delve into the core of our discussion—the nitrate-nitrite-NO pathway’s potential as an antioxidant, exploring possible links with the pathophysiology of HDPs. We hope to uncover this pathway’s potential therapeutic value in a context beyond vasodilation, providing a deeper understanding of the mechanisms that might involve the modulation of the redox system.
2. Endothelial Dysfunction, Oxidative Stress, and Hypertensive Disorders of Pregnancy
The endothelium, a monolayer of endothelial cells lining the inner surface of blood vessels, regulates fluid and electrolyte flow between the bloodstream and tissues, aids vasodilation, and prevents blood clots in a healthy state [21]. Endothelial dysfunction, characterized by its inability to maintain vascular functions, is a hallmark of HDPs [22,23]. More than being a consequence, emerging evidence suggests that endothelial dysfunction may act as a primary etiology of HDPs [23]. Characteristic manifestations of this dysfunction encompass reduced NO bioavailability, increased inflammatory processes, and enhanced oxidative stress, forming a self-potentiating feedback cycle.
Oxidative stress occurs when there is an imbalance between reactive oxygen species (ROS) production and the body’s capacity to neutralize their harmful effects. Oxidative stress is associated with numerous cardiovascular diseases, including HDPs [24]. During pregnancy, oxidative stress plays a pivotal role in vascular physiological adaptations due to the increasing oxygen and metabolic demands of the growing fetus [25,26]. At physiological levels, ROS are important in cellular signaling and the modulation of vascular function [25] and contribute to the maturation and functionality of the placenta [27]. However, when levels become excessive during pregnancy, that can impair vascular equilibrium and disrupt endothelial harmony, resulting in the endothelial dysfunction characteristic of HDPs.
The endothelium’s vulnerability to elevated ROS levels is derived from various metabolic pathways activated in blood vessels during pregnancy, leading to free radical generation. The NADPH oxidase enzyme family is particularly identified as one of the primary sources of superoxide (O2•−) within the endothelium [28], and there is an upregulation in the expression of NADPH oxidase and its subunits in pre-eclampsia [29,30,31]. As the superoxide production driven by NADPH oxidase increases, this free radical rapidly reacts with vascular NO, forming peroxynitrite (ONOO−), an even more toxic radical [32] (Figure 1). More importantly, this fast reaction between superoxide anion and NO reduces NO bioavailability, feeding a detrimental cycle. Indeed, studies assessing peroxynitrite concentrations in pregnant women with hypertensive disorders have shown that elevated levels of peroxynitrite are related to increased vascular damage [33,34].
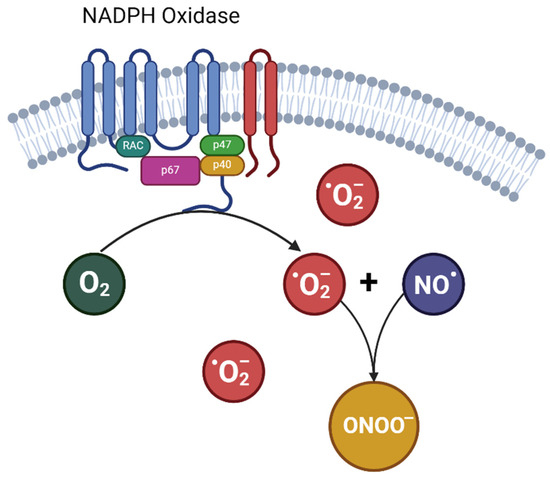
Figure 1.
Formation of peroxynitrite from nitric oxide and •O2−. NO•: nitric oxide; •O2−: superoxide; ONOO−: peroxynitrite.
The phenomenon of the uncoupling of endothelial nitric oxide synthase (eNOS) adds complexity to that scenario [35]. Typically, eNOS converts L-arginine into the vasodilator NO. Under stress conditions, however, its activity is redirected towards producing superoxide anion [36]. This shift is accentuated by the upregulation of NADPH oxidase, leading to excessive superoxide anions. These radicals, known for oxidizing tetrahydrobiopterin (BH4)—an indispensable cofactor for eNOS—can contribute to the onset of pre-eclampsia by affecting BH4 levels and thereby disrupting eNOS regulation [37]. A study by Chatre et al. (2022) showed the importance of BH4 to eNOS function, demonstrating that BH4 supplementation might promote eNOS coupling and potentially ameliorate pre-eclampsia symptoms in a mice model [38]. The oxidation of BH4 blocks its contribution to NO synthesis and further drives eNOS towards uncoupling, propelling the system further into a pronounced prooxidative state. In a rat model of pregnancy-induced hypertension, the authors observed an increase in superoxide generation and BH4 oxidation, indicating the connection between that pathway [39].
Xanthine dehydrogenase (XDH) and xanthine oxidase (XO) are interconvertible forms of an enzyme essential for purine metabolism. Ordinarily, XDH predominates, catalyzing the conversion of hypoxanthine to xanthine and, subsequently, to uric acid, producing NADH in the process [40]. Stressors, such as ischemia, can induce a transition from XDH to XO, which then utilizes molecular oxygen as its electron acceptor, producing superoxide and hydrogen peroxide [41]. Both pre-eclampsia and gestational hypertension have been linked to increased XO activity [42,43].
Lastly, mitochondria, essential to cellular bioenergetics, serve as an established source of ROS, particularly within the placental environment [44]. Acting as the cellular site for aerobic ATP synthesis via oxidative phosphorylation, the importance of mitochondrial function becomes accentuated during pregnancy due to the higher metabolic demands of the placenta [44,45]. The increased mitochondrial activity in the placenta directly correlates with a rise in ROS generation [46]. Inefficient electron transfer can facilitate partial oxygen reduction, leading to an excessive generation of superoxide radicals, as evidenced in HDPs [47,48].
A robust maternal antioxidant defense system regulates metabolic pathways, reducing the harmful effects of oxidative stress and protecting against endothelial dysfunction [49]. However, this appears not to be the case in pregnant women with HDPs. While some studies have reported a decline in maternal antioxidant capacity in HDPs, characterized by a decrease in the activity of enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) [50,51,52], others observed an upregulated antioxidant response [53,54], suggesting a compensatory mechanism to the heightened release of ROS. The indisputable fact is that there is an increase in ROS levels in HDP conditions. Additionally, elevated superoxide anions reduce the bioavailability of NO and have profound implications for endothelium-dependent vasodilation. Beyond these redox system alterations, evidence points to reduced nitrite and nitrate levels in HDPs [55,56]. Contemporary research increasingly postulates that a combination of endothelial dysfunction, oxidative stress, and failure of the NO system coordinate the onset and progression of pregnancy-associated hypertensive disorders.
A key proposition of this review is that the nitrate-nitrite-NO pathway could exhibit antioxidant properties and play a role in modulating oxidative stress. Although this perspective is little explored in the literature, it can have a significant impact on treating HDPs.
3. Nitrate-Nitrite-NO Pathway: Antioxidant Dynamics and Therapeutic Implications
NO is traditionally synthesized enzymatically via the nitric oxide synthase (NOS) pathway. However, research breakthroughs have shown alternative routes of NO generation, most notably by reducing nitrate and nitrite [57,58] (Figure 2). The nitrate-nitrite-NO pathway is increasingly recognized for its critical function in NO dynamic modulation [59]. Because of the volatile nature of NO and the myriad physiological processes that can deplete NO levels in the body, nitrate and nitrite are now perceived as functional reservoirs for stored NO.
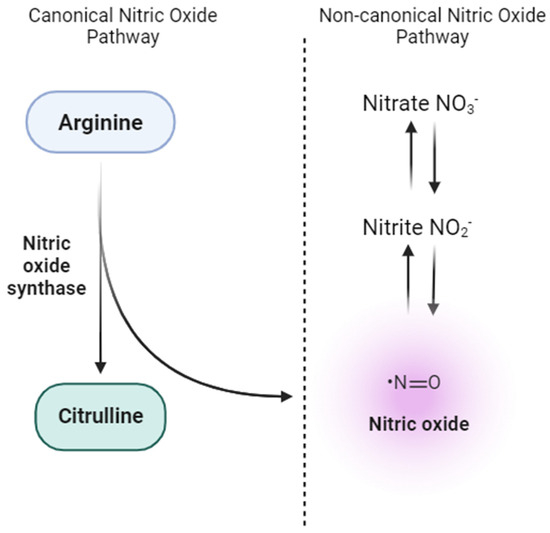
Figure 2.
Canonical and non-canonical pathways for nitric oxide (NO) production. The canonical NO pathway involves the enzymatic conversion of L-arginine to NO and citrulline by endothelial nitric oxide synthase (eNOS), predominantly in vascular endothelial cells. On the other hand, the non-canonical NO pathway highlights the stepwise reduction of dietary nitrate (NO3−) to nitrite (NO2−) and subsequently to NO in various tissues.
An equilibrium in NO is pivotal for maintaining vascular health, but it becomes vulnerable during detrimental conditions like endothelial dysfunction and oxidative stress. Reviews of clinical trials have underscored the role of nitrate in improving endothelial dysfunction and lowering blood pressure [19,60]. HDPs, such as pre-eclampsia, often exhibit reduced NO levels in the circulatory system, exacerbating vascular complications and elevating blood pressure [61,62]. A deficit in NO has been associated with endothelial dysfunction and overall oxidative stress. As a result, therapeutic interventions based on dietary modifications or supplements aimed at restoring NO balance could hold promise in managing HDPs by addressing endothelial dysfunction.
In exploring the nitrate-nitrite-NO pathway, it is essential to recognize the importance of nitrite, even though dietary sources often contain higher concentrations of nitrate. Following consumption, a substantial portion of the ingested nitrate is reduced to nitrite within the body [59]. Therefore, nitrite acts as an essential intermediate in the body’s nitrate metabolic pathway. To comprehensively discuss the nitrate-nitrite-NO pathway’s antioxidant properties, we must adopt a viewpoint encompassing nitrite-centric findings, understanding the synergistic relationship between nitrate and nitrite in the antioxidant mechanism.
In 2011, Montenegro and colleagues conducted the first study that showed a clear antioxidant effect from the treatment with sodium nitrite treatment in hypertensive animals [63]. By that time, the emerging role of the novel nitrate-nitrite-NO pathway was taking shape, and the first evidence that this newer path could be used to control human blood pressure was shown in 2008 by Andrew Webb and his collaborators [18]. In an elegant study, they demonstrated the influence of organic nitrate from a dietary source (beetroot juice) on human blood pressure and the role of the bioconversion of nitrate to nitrite [18]. However, only healthy volunteers were included. Montenegro’s study questioned the role of the newer pathway in disease scenarios such as hypertension, where the increased oxidative stress could reduce NO bioavailability. They used an experimental model of hypertension 2K1C known for its high activation of the renin-angiotensin system that leads to overexpression of NADPH oxidase. Surprisingly, in addition to the antihypertensive effects induced by nitrite, confirming the potential of the newer pathway even during high oxidative stress conditions induced by hypertension, nitrite treatment dramatically reduced oxidative stress, including in the sham-treated animals [63]. Further investigation depicted a role of nitrite inhibiting the vascular NADPH oxidase, and these experimental findings have been confirmed in several subsequent studies [64,65,66].
Experimental studies offer insights into the potential mechanisms of nitrate and nitrite in controlling oxidative stress [66,67,68]. Nitrate has been shown to reduce ROS production, decreasing oxidative markers tied to lipid oxidation, such as TBARS adducts and 4-hydroxynonenal (4-HNE) [69,70,71]. Moreover, short-term nitrite therapy has shown promise in reversing age-related vascular endothelial dysfunction and reducing oxidative stress. This is evident from the restoration of endothelium-dependent dilation and significant mitigation of arterial superoxide production and inflammation in aged male C57BL6J mice [72].
Beyond the effects on lipid oxidation, nitrate and nitrite have exhibited protective mechanisms against protein oxidations—a crucial aspect of oxidative stress. Such modifications can lead to irreversible changes to protein side chains, often compromising cellular function by affecting protein stability and enzymatic activity [73]. In studies spanning both humans and animals, nitrate and nitrite supplementation has demonstrated a dual benefit: it not only ameliorated endothelial function but also resulted in a notable decrease in nitrotyrosine levels—a marker of protein oxidation [74,75,76].
In this sense, the potential therapeutic benefits of nitrate for HDPs appear particularly promising. The ability of nitrate-nitrite-NO to mitigate oxidative stress markers suggests its potential in reducing such markers and alleviating endothelial dysfunction, placing nitrate supplementation as a viable path avenue for HDP management.
Elevated markers of lipid and protein oxidation in pregnant women with pre-eclampsia and gestational hypertension are central contributors to the endothelial dysfunction characteristic of HDPs. Studies carry out in women with pre-eclampsia show a pronounced elevation of lipid peroxidation markers [77,78,79,80,81]. Similarly, plasma from women with HDPs consistently reveals heightened levels of protein oxidation, contrasting with samples from women experiencing normotensive pregnancies [82]. Although DNA oxidation markers are less frequently examined, they are also altered in women with HDPs [83,84].
The exact mechanism by which nitrate-nitrite-NO exerts these protective roles remains unclear. Nonetheless, based on existing studies, there is growing speculation that these compounds regulate the production of the superoxide radical, targeting its primary sources, namely NADPH oxidase, XOR, and mitochondria. Additionally, considering nitrate and nitrite as reservoirs for NO, it is essential to emphasize their potential to enhance NO levels or their bioavailability.
Research across various animal models has consistently shown that the potential mechanism behind nitrate’s action and its intermediary nitrite might reside in the inhibition of NADPH oxidase activity [63,66,67]. Although direct neutralization of free radicals by these compounds has been suggested, the potential therapeutic role of the nitrate-nitrite-NO pathway seems to be derived from blocking NADPH oxidase activity and the synthesis of O2•− [63,64]. These results align with in vitro studies; in macrophages activated with LPS, the NO derived from nitrite reduced the NOX-dependent superoxide production, further emphasizing the role of nitrite-NO in this mechanism [85]. Such results become especially relevant considering the elevated NADPH oxidase activity observed in HDP contexts [86].
Hypotheses about the underlying mechanisms by which the nitrate-nitrite-NO pathway reduces NADPH oxidase activity and superoxide production have gained prominence in the scientific community. A central hypothesis delves into the role of NO in facilitating protein modifications via S-nitrosylation. S-nitrosylation represents a post-translational protein modification in which a NO group is covalently attached to a cysteine residue in a protein, and this process plays a pivotal role in how NO regulates various proteins [87]. Intriguingly, S-nitrosylation appears to be a crucial mechanism by which NO might govern NADPH oxidase activity. In a cellular model, Qian et al. (2012) uncovered that NO could facilitate the reversible post-translational modification of Nox5, leading to reduced ROS production [88].
Beyond the influence of nitrate and nitrite on NADPH oxidase, we cannot dismiss the potential mechanisms for controlling oxidative stress via XOR. Amaral et al. (2015) utilized the deoxycorticosterone-salt (DOCA-salt) hypertensive model and found that sodium nitrite supplementation displayed antioxidant properties [64]. In a separate study centered on the activated macrophage, nitrite supplementation appeared to suppress NADPH oxidase activity, although not through the previously hypothesized S-nitrosation mechanism [89]. The authors suggest that this result might be attributed to changes in Nox2 and XOR [89]. The decrease in XOR activity emphasizes the evolving understanding of the effects of the nitrate-nitrite-NO pathway.
The results of nitrate as an antioxidant are also promising regarding mitochondria control. A study involving rats assessed dietary nitrate supplementation’s ability to mitigate oxidative stress in a hypoxia model. In this research, nitrate provided protection against the reduction in complex I activity and alleviated oxidative stress, thereby boosting NO bioavailability within the myocardium [90].
It is interesting to note that even though nitrate is bioactivated to nitrite, which exerts antioxidant effects, both nitrite and nitrate may also regulate eNOS activity and affect endogenous NO formation [91]. Nitrate supplementation dose-dependently reduced vascular eNOS Ser1177 phosphorylation and increased Thr495 phosphorylation, thus decreasing global eNOS activity, with corresponding attenuation of vascular responses [91]. Similar alterations were observed when nitrite was directly added to vascular preparations, thus indicating a direct inhibitory effect of nitrite on endogenous NO formation [91]. In contrast, there is evidence that nitrite may directly promote eNOS-derived NO formation in hypertensive disorders. Nitrite administration improved endothelium-dependent relaxation in spontaneously hypertensive rats by mechanisms involving eNOS activation to produce NO [92]. These studies lead to contrasting conclusions. However, it is possible that both nitrate and nitrite may exert opposite vascular effects when we compare healthy [91] and hypertensive [92] vessels, with significant differences especially with respect to redox conditions, so that nitrite may exert beneficial effects in disease conditions. Adding more complexity to nitrite/nitrate biology, it is also possible that nitrate may inhibit some of the responses found with nitrite, as previously shown [93]. Both nitrate and nitrite are bioactivated by XOR, and they compete for the catalytic site of the enzyme, and these effects may indeed translate a protection by nitrate against excess nitrite bioactivation to NO by XOR [93].
The body’s defense against oxidative stress is complex, and nitrate emerges as a potential contributor to enhancing antioxidant capacities. A study by Cui et al. (2019), utilizing a model of ischemia-reperfusion injury-induced post-skin flap in mice, showed that nitrate restored the enzymatic activity of SOD, GPx, and CAT [94]. Extending these findings to humans, research has explored nitrate’s impact on the enzymatic defense system, especially in the context of exercise, given NO’s vasodilatory role [95]. Another study involving patients with metabolic syndrome noted that acute exercise, combined with the intake of nitrate-rich juice, amplified the expression of MnSOD, GPx, and CAT [96].
In HDP conditions, increased oxidative stress can overload antioxidant enzymes, causing the antioxidant system to operate at or beyond its capacity to neutralize the excessive free radicals, leading to an apparent decline in enzyme activity. Women diagnosed with pre-eclampsia typically display lowered plasma concentrations of enzymes like GPx and SOD, a fact that is observed at the mRNA level for these enzymes [97]. Furthermore, CAT activity appears to be suboptimal in women with gestational hypertension than in those with normotensive pregnancies [98]. Building on this, a study by Qiu et al. (2021) highlighted reduced activities of CAT, SOD, and GPx in preeclamptic placental tissues compared to their regular counterparts [99]. While many studies report a decline in antioxidant activity, findings indicate increased enzyme activity as the body attempts to restore normal homeostasis. That idea is supported by the work of Caldeiras-Dias et al. (2021), where women with pre-eclampsia demonstrated heightened activity in the antioxidant response element (ARE) compared to normal pregnancies [100]. Intriguingly, resveratrol, a well-known antioxidant, enhanced ARE activity [100]. This underscores that, regardless of whether there is a decline or increase in activity, adding compounds with antioxidant potential can bolster antioxidant capabilities.
Delving into the molecular complexity of the antioxidant defense, the Nrf2 pathway needs attention. The ARE region is a direct downstream target of Nrf2 and encompasses a diverse array of enzymes essential for antioxidant and detoxification processes [101]. Nrf2 is pivotal for protecting against oxidative stress, orchestrating the coordinated expression of an array of antioxidant genes [101]. Under basal conditions, Nrf2 is anchored in the cytoplasm, bound closely to the Kelch like ECH-associated protein 1 (Keap1) [101]. In oxidative stress, Nrf2 detaches from Keap1, allowing it to translocate to the nucleus, initiating its protective transcriptional program. Given the conceptual role of Nrf2, one might expect its pathway to be increased in conditions like HDPs. However, studies indicate that the oxidative stress signaling pathway, mediated by Nrf2-Keap1, is compromised in the placentas of women with pre-eclampsia [102,103,104]. Studies in animals have shown the potential of nitrites to influence Nrf2 [75,105]. Fascinatingly, Amaral et al. (2019), utilizing a two-kidney one-clip hypertensive rat model, highlighted nitrite’s capability—as an NO donor—to modulate the transcription factor Nrf2 [105]. Mechanistic investigations suggest that NO might enact its effect-inducing modifications in Keap1, subsequently releasing Nrf2 or through the NO-cGMP pathway, bolstering the notion of NO’s central role in orchestrating the antioxidant response.
In light of evidence highlighting the antioxidant abilities of the nitrate-nitrite-NO pathway, as well as the potential influence on transcription factors like Nrf2, it seems pertinent to explore the therapeutic potential of these compounds in the context of HDPs. The idea of supplementing with nitrate or nitrite to enhance antioxidant defenses in HDPs warrants thorough investigation. Such an approach might not only mitigate oxidative stress but could also offer a new path in the management of HDP by addressing the core oxidative perturbations intrinsic to these conditions. Achieving control over oxidative stress offers a dual advantage: (1) augments the bioavailability of NO and (2) prevents the combination of superoxide with NO, which would lead to the production of the potent oxidant peroxynitrite. In the HDP context, enhancing vasodilation through NO, especially when considering nitrate and nitrite supplementation, is pivotal for blood pressure control and improving endothelial dysfunction. This idea is supported by an intriguing study conducted on a pregnant eNOS−/− mice model, where supplementation with beetroot juice—a known rich nitrate source—reduced blood pressure and improved endothelial function [106]. This outcome is in line with the inhibitory role of nitrite on NADPH oxidase, which reduces oxidative stress, thus increasing NO availability.
Drawing upon dietary sources for nitrate consumption, rather than synthetic derivatives, presents both safety and bioavailability benefits. Foods naturally rich in nitrates, like beetroot and green vegetables, may confer a synergistic health advantage, given the multitude of nutritive constituents they encompass. Although the literature has explored the antihypertensive facets of nitrate-rich foods, especially in the HDP context [107,108], there remains a notable gap in in-depth research on the antioxidant potential of dietary nitrate in this specific cohort.
Endothelial dysfunction and oxidative stress are unequivocal hallmarks of HDPs. However, most human studies concerning nitrate or dietary sources thereof are predominantly concerned with their capacity to modulate blood pressure. We do not intend to minimize the importance of blood pressure control in HDP conditions. Instead, we want to discuss its role beyond the vasodilator action. The outcomes of animal studies addressed in this review reinforce the antioxidant potential of the nitrate-nitrite-NO pathway, as summarized in Table 1.

Table 1.
Summary of antioxidant effects on animal studies.
A point of debate that needs to be highlighted here is the ideal level of nitrate intake to obtain the expected results. Although there is no consensus regarding the necessary amount, most studies in humans have employed amounts ranging from 400 to 2400 mg. These intakes exceed the average nitrate intake, estimated to be between 0.4 and 2.6 mg/kg [109,110]. Such daily intake levels can be easily achieved by consuming dark green vegetables and beetroot. However, supplementation often becomes necessary to attain higher nitrate levels. Innovations in the industry have addressed this need by increasing nitrate concentration and obtaining feasible results through different food preparations using beetroot [111]. For example, concentrated beetroot juice (containing 400 mg of nitrate in 70 mL) is commercially available and has been used in studies on pregnant women without adverse effects [107,112].
Another topic warranting brief exploration is the association of nitrate and nitrite with the formation of nitrosamines—compounds linked to cancer [113]. Often, nitrate and nitrite are utilized as preservatives in processed foods, including sausages, ham, and specific meat types [114]. Consuming high quantities of meat and processed foods has been associated with increased cancer risk [115]. On the other hand, the Mediterranean diet, lauded for its numerous health benefits, is rich in nitrates sourced from leafy green vegetables and beetroot [116]. Beyond nitrate content, these dietary patterns also have high amounts of antioxidants, fiber, and other beneficial compounds that contribute to a balanced redox environment.
We believe it is necessary to consider the intrinsic nature of nitrate versus its source and intake conditions. The nitrate debate calls for caution, considering the importance of dietary and physiological context. An illustrative example comes from a clinical study on using isolated compounds: while antioxidants like vitamin C and E might be beneficial when consumed through natural sources like fruits—due to their interactions with other compounds—their isolated forms did not yield positive results in pre-eclampsia patients [117]. This discrepancy underscores the complexity of bioactive compounds and their interactions. The mechanisms of action of these antioxidants, including potential synergies with other compounds present in whole foods, might play a pivotal role in their beneficial effects. Interestingly, a review addressing the role of nutraceuticals in HDPs highlights how various components present in foods can benefit blood pressure, including improving vascular function, reducing inflammation, and modulating oxidative stress [118]. Therefore, rather than viewing nitrate as a singular antagonist, it is more judicious to analyze the contexts in which it is introduced and the potential synergistic outcomes that may arise.
4. Perspectives and Conclusions
In HDPs, the vascular endothelium’s role in ensuring vasodilator, anti-inflammatory, and anti-thrombotic functions becomes severely compromised. A healthy endothelium produces NO in a well-regulated manner. However, this synthesis is hampered in hypertensive conditions. With the increased oxidative stress and endothelial dysfunction that characterize HDPs, the health and well-being of both the mother and fetus are jeopardized. The potential of nitrate, mainly from natural food sources, to reduce oxidative stress, improve endothelial function, and possibly lower blood pressure through the nitrate-nitrite-NO pathway presents a promising avenue for exploration in HDPs.
The potential of the nitrate-nitrite-NO pathway holds considerable promise regarding both safety and bioavailability. Natural nitrate-rich foods, such as beetroot and leafy greens, provide a matrix of nutrients and compounds that may improve dietary nitrate uptake and metabolic conversion to bioactive forms. Aside from the intrinsic safety of dietary consumption, there is the added benefit that certain foods may provide synergistic health benefits due to the presence of other nutritive constituents. While preliminary evidence is promising [107,108], more comprehensive, well-designed clinical trials are required before recommending nitrate-rich diets or supplements as a complementary treatment in HDPs. The physiological alterations during pregnancy may affect the impact of nitrate and nitrite. Dosage, administration, frequency, and the overall nutritional environment are pivotal factors determining both efficacy and safety. Additionally, the long-term effects on fetal development and maternal health post-partum demand extensive research.
Finally, a few caution notes regarding nitrate or nitrite use during pregnancy should be considered. To our knowledge, no information is available concerning the amounts of nitrate and nitrite that may cross the placental barrier and interact with the biology of the fetus. Nitrate from dietary sources leads to a high plasma concentration (90–152 μM) and nitrite is finely controlled and is kept in lower levels [119]. No previous study has simultaneously assessed nitrite and nitrate concentrations in the mother and the newborn. However, it is known that fetal hemoglobin is more easily oxidized to form methemoglobin by nitrite than adult hemoglobin [120]. Methemoglobin is generated when the ferrous component (Fe2+) of hemoglobin is oxidized by oxidants, rendering it incapable of binding O2 [121]. Moreover, there is evidence that NADH-methemoglobin reductase activity, which reconverts methemoglobin back to hemoglobin, is lower in infants than in adults [122], which could be looked at with caution. Whether maternal nitrate therapy increases newborns’ methemoglobin concentrations remains to be determined, and further studies are needed.
In conclusion, this review exploits a novel perspective of the nitrate-nitrite NO pathway’s potential as an antioxidant agent in the context of HDPs—a topic previously underexplored. Our work shines a light on future investigations against the context of limited research on the interplay between nitrate supplementation and its interplay with controlling oxidative stress, endothelial dysfunction, and HDPs.
Author Contributions
Conceptualization, P.O.B. and V.C.S.; Writing—Original Draft Preparation, P.O.B., J.E.T.-S. and V.C.S.; Writing and critical review and Editing, P.O.B., R.d.C.C., V.C.S., T.B. and M.F.M.; Supervision, R.d.C.C. and V.C.S.; Funding Acquisition, V.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the São Paulo Research Foundation, Brazil (FAPESP) grant numbers 21/12010-7 and 23/07589-1.
Acknowledgments
The authors thank Priscila Rezeck Nunes for reading this manuscript and commenting critically. All figures were generated using BioRender (BioRender.com).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomstrom-Lundqvist, C.; Cifkova, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Coggins, N.; Lai, S. Hypertensive Disorders of Pregnancy. Emerg. Med. Clin. N. Am. 2023, 41, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; An, H.; Li, Z.; Zhang, L.; Li, H.; Zhang, Y.; Ye, R. Impact of gestational hypertension and preeclampsia on low birthweight and small-for-gestational-age infants in China: A large prospective cohort study. J. Clin. Hypertens. 2021, 23, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, D.D.; Avagliano, L.; Ferrazzi, E.; Fuse, F.; Sterpi, V.; Parasiliti, M.; Stampalija, T.; Zullino, S.; Farina, A.; Bulfamante, G.P.; et al. Hypertensive Disorders of Pregnancy and Fetal Growth Restriction: Clinical Characteristics and Placental Lesions and Possible Preventive Nutritional Targets. Nutrients 2022, 14, 3276. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacos, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W., Jr.; LaMarca, B. Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R521–R528. [Google Scholar] [CrossRef]
- Metoki, H.; Iwama, N.; Hamada, H.; Satoh, M.; Murakami, T.; Ishikuro, M.; Obara, T. Hypertensive disorders of pregnancy: Definition, management, and out-of-office blood pressure measurement. Hypertens. Res. 2022, 45, 1298–1309. [Google Scholar] [CrossRef]
- Brown, C.M.; Garovic, V.D. Drug treatment of hypertension in pregnancy. Drugs 2014, 74, 283–296. [Google Scholar] [CrossRef]
- Jiang, X.; Wei, Y. Effect of vitamin D3 supplementation during pregnancy on high risk factors—A randomized controlled trial. J. Perinat. Med. 2021, 49, 480–484. [Google Scholar] [CrossRef]
- Camarena Pulido, E.E.; Garcia Benavides, L.; Panduro Baron, J.G.; Pascoe Gonzalez, S.; Madrigal Saray, A.J.; Garcia Padilla, F.E.; Totsuka Sutto, S.E. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: A double-blind, randomized, clinical trial. Hypertens. Pregnancy 2016, 35, 217–225. [Google Scholar] [CrossRef]
- Monari, F.; Menichini, D.; Pignatti, L.; Basile, L.; Facchinetti, F.; Neri, I. Effect of L-arginine supplementation in pregnant women with chronic hypertension and previous placenta vascular disorders receiving Aspirin prophylaxis: A randomized control trial. Minerva Obstet. Gynecol. 2021, 73, 782–789. [Google Scholar] [CrossRef]
- Mesdaghinia, E.; Shahin, F.; Ghaderi, A.; Shahin, D.; Shariat, M.; Banafshe, H. The Effect of Selenium Supplementation on Clinical Outcomes, Metabolic Profiles, and Pulsatility Index of the Uterine Artery in High-Risk Mothers in Terms of Preeclampsia Screening with Quadruple Test: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial: Selenium and preeclampsia. Biol. Trace Elem. Res. 2023, 201, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide 2013, 35, 123–130. [Google Scholar] [CrossRef]
- Jovanovski, E.; Bosco, L.; Khan, K.; Au-Yeung, F.; Ho, H.; Zurbau, A.; Jenkins, A.L.; Vuksan, V. Effect of Spinach, a High Dietary Nitrate Source, on Arterial Stiffness and Related Hemodynamic Measures: A Randomized, Controlled Trial in Healthy Adults. Clin. Nutr. Res. 2015, 4, 160–167. [Google Scholar] [CrossRef]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Wong, M.; Jirangrat, W.; Teh, K.H.; Ali, A. Acute Supplementation with Nitrate-Rich Beetroot Juice Causes a Greater Increase in Plasma Nitrite and Reduction in Blood Pressure of Older Compared to Younger Adults. Nutrients 2019, 11, 1683. [Google Scholar] [CrossRef]
- Bahra, M.; Kapil, V.; Pearl, V.; Ghosh, S.; Ahluwalia, A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide 2012, 26, 197–202. [Google Scholar] [CrossRef]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelium as an organ system. Crit. Care Med. 2004, 32, S271–S279. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aranguren, L.C.; Prada, C.E.; Riano-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. Biomed. Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, H.; Qiu, Y.; Liu, Y.; Wen, L.; Fu, Y.; Qi, H.; Baker, P.N.; Tong, C. Reactive Oxygen Species are Essential for Placental Angiogenesis During Early Gestation. Oxidative Med. Cell. Longev. 2022, 2022, 4290922. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Dechend, R.; Viedt, C.; Muller, D.N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: Involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Myatt, L.; Rosenfield, R.B.; Eis, A.L.; Brockman, D.E.; Greer, I.; Lyall, F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension 1996, 28, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Roggensack, A.M.; Zhang, Y.; Davidge, S.T. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension 1999, 33, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Phoswa, W.N.; Khaliq, O.P. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxidative Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef]
- Kukor, Z.; Valent, S.; Toth, M. Regulation of nitric oxide synthase activity by tetrahydrobiopterin in human placentae from normal and pre-eclamptic pregnancies. Placenta 2000, 21, 763–772. [Google Scholar] [CrossRef]
- Chatre, L.; Ducat, A.; Spradley, F.T.; Palei, A.C.; Chereau, C.; Couderc, B.; Thomas, K.C.; Wilson, A.R.; Amaral, L.M.; Gaillard, I.; et al. Increased NOS coupling by the metabolite tetrahydrobiopterin (BH4) reduces preeclampsia/IUGR consequences. Redox Biol. 2022, 55, 102406. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.M.; Cook, L.G.; Danchuk, S.; Puschett, J.B. Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. Am. J. Hypertens. 2007, 20, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, I.; Talosi, G.; Papp, A.; Boda, D. Xanthine oxidase activation in mild gestational hypertension. Hypertens. Pregnancy 2002, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Kafkasli, A.; Burak, F.; Gozukara, E.M. Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialdehyde levels in pre-eclampsia. Cell Biochem. Funct. 2005, 23, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.J.; Hickey, A.J.R.; Alvsaker, A.; Moran, S.; Hedges, C.; Chamley, L.W.; Perkins, A.V. Changes in mitochondrial respiration in the human placenta over gestation. Placenta 2017, 57, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Gupta, M.B.; Myatt, L.; Powell, T.L.; Glenn, J.P.; Cox, L.; Jansson, T. Mechanistic Target of Rapamycin Complex 1 Promotes the Expression of Genes Encoding Electron Transport Chain Proteins and Stimulates Oxidative Phosphorylation in Primary Human Trophoblast Cells by Regulating Mitochondrial Biogenesis. Sci. Rep. 2019, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Aiken, C.E.; Charnock-Jones, D.S.; Smith, G.C.S. Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S928–S944. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.J.; Cuffe, J.S.M.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K.A.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Freire, V.A.F.; Melo, A.D.; Santos, H.L.; Barros-Pinheiro, M. Evaluation of oxidative stress markers in subtypes of preeclampsia: A systematic review and meta-analysis. Placenta 2023, 132, 55–67. [Google Scholar] [CrossRef]
- Yalcin, S.; Ulas, T.; Eren, M.A.; Aydogan, H.; Camuzcuoglu, A.; Kucuk, A.; Yuce, H.H.; Demir, M.E.; Vural, M.; Aksoy, N. Relationship between oxidative stress parameters and cystatin C levels in patients with severe preeclampsia. Medicina 2013, 49, 19. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, M.; Aref, A.; Salem, M.E.; Sheiba, M. Radical-scavenging antioxidants in pre-eclampsia and eclampsia. Int. J. Gynaecol. Obstet. 1998, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.F.; Palei, A.C.; Machado, J.S.; da Silva, L.M.; Montenegro, M.F.; Jordao, A.A.; Duarte, G.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Assessment of oxidative status markers and NO bioavailability in hypertensive disorders of pregnancy. J. Hum. Hypertens. 2013, 27, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Adiga, U.; D’Souza, V.; Kamath, A.; Mangalore, N. Antioxidant activity and lipid peroxidation in preeclampsia. J. Chin. Med. Assoc. 2007, 70, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Palei, A.C.; Metzger, I.F.; Cavalli, R.C.; Duarte, G.; Tanus-Santos, J.E. Interethnic differences in ADMA concentrations and negative association with nitric oxide formation in preeclampsia. Clin. Chim. Acta 2010, 411, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Stankiewicz, M.; Zolniezewicz, K.; Puk, O.; Wartega, M. Decrease in Nitric Oxide Production as a Key Mediator in the Pathogenesis of Preeclampsia and a Potential Therapeutic Target: A Case-Control Study. Biomedicines 2022, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Sobko, T.; Reinders, C.I.; Jansson, E.; Norin, E.; Midtvedt, T.; Lundberg, J.O. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide 2005, 13, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Carlstrom, M.; Montenegro, M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Intern. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef]
- Choi, J.W.; Im, M.W.; Pai, S.H. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann. Clin. Lab. Sci. 2002, 32, 257–263. [Google Scholar] [PubMed]
- Schiessl, B.; Strasburger, C.; Bidlingmaier, M.; Mylonas, I.; Jeschke, U.; Kainer, F.; Friese, K. Plasma- and urine concentrations of nitrite/nitrate and cyclic Guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch. Gynecol. Obstet. 2006, 274, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Amaral, J.H.; Pinheiro, L.C.; Sakamoto, E.K.; Ferreira, G.C.; Reis, R.I.; Marcal, D.M.; Pereira, R.P.; Tanus-Santos, J.E. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radic. Biol. Med. 2011, 51, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.H.; Ferreira, G.C.; Pinheiro, L.C.; Montenegro, M.F.; Tanus-Santos, J.E. Consistent antioxidant and antihypertensive effects of oral sodium nitrite in DOCA-salt hypertension. Redox Biol. 2015, 5, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Tian, R.; Lu, N. NADPH oxidase is a primary target for antioxidant effects by inorganic nitrite in lipopolysaccharide-induced oxidative stress in mice and in macrophage cells. Nitric Oxide 2019, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, T.; Liu, M.; Peleli, M.; Zollbrecht, C.; Weitzberg, E.; Lundberg, J.O.; Persson, A.E.; Carlstrom, M. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension 2015, 65, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, X.M.; Tarnawski, L.; Peleli, M.; Zhuge, Z.; Terrando, N.; Harris, R.A.; Olofsson, P.S.; Larsson, E.; Persson, A.E.G.; et al. Dietary nitrate attenuates renal ischemia-reperfusion injuries by modulation of immune responses and reduction of oxidative stress. Redox Biol. 2017, 13, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; McCann Haworth, S.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; Cruz, J.C.; et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 217–226. [Google Scholar] [CrossRef]
- Brunetta, H.S.; Politis-Barber, V.; Petrick, H.L.; Dennis, K.; Kirsh, A.J.; Barbeau, P.A.; Nunes, E.A.; Holloway, G.P. Nitrate attenuates high fat diet-induced glucose intolerance in association with reduced epididymal adipose tissue inflammation and mitochondrial reactive oxygen species emission. J. Physiol. 2020, 598, 3357–3371. [Google Scholar] [CrossRef]
- DesOrmeaux, G.J.; Petrick, H.L.; Brunetta, H.S.; Holloway, G.P. Independent of mitochondrial respiratory function, dietary nitrate attenuates HFD-induced lipid accumulation and mitochondrial ROS emission within the liver. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E217–E228. [Google Scholar] [CrossRef]
- Carlstrom, M.; Persson, A.E.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011, 89, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Sindler, A.L.; Fleenor, B.S.; Calvert, J.W.; Marshall, K.D.; Zigler, M.L.; Lefer, D.J.; Seals, D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 2011, 10, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.C.; Mustafa, M.R.; Vanhoutte, P.M.; Murugan, D.D. Chronic administration of sodium nitrite prevents hypertension and protects arterial endothelial function by reducing oxidative stress in angiotensin II-infused mice. Vasc. Pharmacol. 2018, 102, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Gioscia-Ryan, R.A.; Santos-Parker, J.R.; Ziemba, B.P.; Lubieniecki, K.L.; Johnson, L.C.; Poliektov, N.E.; Bispham, N.Z.; Woodward, K.A.; Nagy, E.E.; et al. Inorganic Nitrite Supplementation Improves Endothelial Function with Aging: Translational Evidence for Suppression of Mitochondria-Derived Oxidative Stress. Hypertension 2021, 77, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Chowdhury, S.; Oggioni, C.; Qadir, O.; Brandt, K.; Ishaq, A.; Mathers, J.C.; Saretzki, G.; Siervo, M. Inorganic Nitrate Supplementation in Young and Old Obese Adults Does Not Affect Acute Glucose and Insulin Responses but Lowers Oxidative Stress. J. Nutr. 2016, 146, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Chamy, V.M.; Lepe, J.; Catalan, A.; Retamal, D.; Escobar, J.A.; Madrid, E.M. Oxidative stress is closely related to clinical severity of pre-eclampsia. Biol. Res. 2006, 39, 229–236. [Google Scholar] [CrossRef]
- Surmiak, P.; Wojnarowicz, O.; Szymkowiak, M. Malondialdehyde and Neutrophil Gelatinase-Associated Lipocalin as Markers of Oxidative Stress in Small for Gestational Age Newborns from Hypertensive and Preeclamptic Pregnancies. Biomed. Res. Int. 2022, 2022, 9246233. [Google Scholar] [CrossRef]
- Gratacos, E.; Casals, E.; Deulofeu, R.; Cararach, V.; Alonso, P.L.; Fortuny, A. Lipid peroxide and vitamin E patterns in pregnant women with different types of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 1072–1076. [Google Scholar] [CrossRef]
- Noris, M.; Todeschini, M.; Cassis, P.; Pasta, F.; Cappellini, A.; Bonazzola, S.; Macconi, D.; Maucci, R.; Porrati, F.; Benigni, A.; et al. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension 2004, 43, 614–622. [Google Scholar] [CrossRef]
- Walsh, S.W.; Vaughan, J.E.; Wang, Y.; Roberts, L.J., 2nd. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.M.P.; Harish, S.; Pai, V.R.; Shriyan, C. Increased Oxidatively Modified Forms of Albumin in Association with Decreased Total Antioxidant Activity in Different Types of Hypertensive Disorders of Pregnancy. Indian J. Clin. Biochem. 2017, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kimura, C.; Watanabe, K.; Iwasaki, A.; Mori, T.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J. Matern. Fetal Neonatal Med. 2013, 26, 491–496. [Google Scholar] [CrossRef]
- Hilali, N.; Kocyigit, A.; Demir, M.; Camuzcuoglu, A.; Incebiyik, A.; Camuzcuoglu, H.; Vural, M.; Taskin, A. DNA damage and oxidative stress in patients with mild preeclampsia and offspring. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peleli, M.; Zollbrecht, C.; Giulietti, A.; Terrando, N.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radic. Biol. Med. 2015, 83, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Quinn, P.A.; Jennings, S.C.; Ng, L.L. NADPH oxidase activity in preeclampsia with immortalized lymphoblasts used as models. Hypertension 2003, 41, 925–931. [Google Scholar] [CrossRef]
- Rezeck Nunes, P.; Cezar Pinheiro, L.; Zanetoni Martins, L.; Alan Dias-Junior, C.; Carolina Taveiros Palei, A.; Cristina Sandrim, V. A new look at the role of nitric oxide in preeclampsia: Protein S-nitrosylation. Pregnancy Hypertens. 2022, 29, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, F.; Kovalenkov, Y.; Pandey, D.; Moseley, M.A.; Foster, M.W.; Black, S.M.; Venema, R.C.; Stepp, D.W.; Fulton, D.J. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic. Biol. Med. 2012, 52, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Zollbrecht, C.; Persson, A.E.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Nitrite-mediated reduction of macrophage NADPH oxidase activity is dependent on xanthine oxidoreductase-derived nitric oxide but independent of S-nitrosation. Redox Biol. 2016, 10, 119–127. [Google Scholar] [CrossRef]
- Ashmore, T.; Fernandez, B.O.; Branco-Price, C.; West, J.A.; Cowburn, A.S.; Heather, L.C.; Griffin, J.L.; Johnson, R.S.; Feelisch, M.; Murray, A.J. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J. Physiol. 2014, 592, 4715–4731. [Google Scholar] [CrossRef]
- Carlstrom, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.; et al. Cross-talk Between Nitrate-Nitrite-NO and NO Synthase Pathways in Control of Vascular NO Homeostasis. Antioxid. Redox Signal. 2015, 23, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.C.; Murugan, D.D.; Lau, Y.S.; Vanhoutte, P.M.; Mustafa, M.R. Sodium nitrite exerts an antihypertensive effect and improves endothelial function through activation of eNOS in the SHR. Sci. Rep. 2016, 6, 33048. [Google Scholar] [CrossRef] [PubMed]
- Damacena-Angelis, C.; Oliveira-Paula, G.H.; Pinheiro, L.C.; Crevelin, E.J.; Portella, R.L.; Moraes, L.A.B.; Tanus-Santos, J.E. Nitrate decreases xanthine oxidoreductase-mediated nitrite reductase activity and attenuates vascular and blood pressure responses to nitrite. Redox Biol. 2017, 12, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Feng, Y.; Shu, C.; Yuan, R.; Bu, L.; Jia, M.; Pang, B. Dietary Nitrate Protects Against Skin Flap Ischemia-Reperfusion Injury in Rats via Modulation of Antioxidative Action and Reduction of Inflammatory Responses. Front. Pharmacol. 2019, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.F.; Peixoto, L.G.; Teixeira, R.R.; Justino, A.B.; Puga, G.M.; Espindola, F.S. Potential Benefits of Nitrate Supplementation on Antioxidant Defense System and Blood Pressure Responses after Exercise Performance. Oxidative Med. Cell. Longev. 2019, 2019, 7218936. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Capo, X.; Reynes, C.; Quetglas, M.; Salaberry, E.; Tonolo, F.; Suau, R.; Mari, B.; Tur, J.A.; Sureda, A.; et al. Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients. J. Clin. Med. 2021, 10, 2618. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J. Soc. Gynecol. Investig. 1996, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Osunkalu, V.O.; Taiwo, I.A.; Makwe, C.C.; Akinsola, O.J.; Quao, R.A. Methylenetetrahydrofolate Reductase Enzyme Level and Antioxidant Activity in Women with Gestational Hypertension and Pre-eclampsia in Lagos, Nigeria. J. Obstet. Gynaecol. India 2019, 69, 317–324. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, J.; Li, M.; Wang, L.; Zhu, X.; Chen, Y. Impaction of factors associated with oxidative stress on the pathogenesis of gestational hypertension and preeclampsia: A Chinese patients based study. Medicine 2021, 100, e23666. [Google Scholar] [CrossRef]
- Caldeira-Dias, M.; Viana-Mattioli, S.; de Souza Rangel Machado, J.; Carlstrom, M.; de Carvalho Cavalli, R.; Sandrim, V.C. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021, 23, 205–210. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Kweider, N.; Huppertz, B.; Wruck, C.J.; Beckmann, R.; Rath, W.; Pufe, T.; Kadyrov, M. A role for Nrf2 in redox signalling of the invasive extravillous trophoblast in severe early onset IUGR associated with preeclampsia. PLoS ONE 2012, 7, e47055. [Google Scholar] [CrossRef] [PubMed]
- Chigusa, Y.; Tatsumi, K.; Kondoh, E.; Fujita, K.; Nishimura, F.; Mogami, H.; Konishi, I. Decreased lectin-like oxidized LDL receptor 1 (LOX-1) and low Nrf2 activation in placenta are involved in preeclampsia. J. Clin. Endocrinol. Metab. 2012, 97, E1862–E1870. [Google Scholar] [CrossRef] [PubMed]
- Khadir, F.; Rahimi, Z.; Ghanbarpour, A.; Vaisi-Raygani, A. Nrf2 rs6721961 and Oxidative Stress in Preeclampsia: Association with the Risk of Preeclampsia and Early-Onset Preeclampsia. Int. J. Mol. Cell. Med. 2022, 11, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.H.; Rizzi, E.S.; Alves-Lopes, R.; Pinheiro, L.C.; Tostes, R.C.; Tanus-Santos, J.E. Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radic. Biol. Med. 2019, 141, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tropea, T.; Renshall, L.J.; Nihlen, C.; Weitzberg, E.; Lundberg, J.O.; David, A.L.; Tsatsaris, V.; Stuckey, D.J.; Wareing, M.; Greenwood, S.L.; et al. Beetroot juice lowers blood pressure and improves endothelial function in pregnant eNOS−/− mice: Importance of nitrate-independent effects. J. Physiol. 2020, 598, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Ormesher, L.; Myers, J.E.; Chmiel, C.; Wareing, M.; Greenwood, S.L.; Tropea, T.; Lundberg, J.O.; Weitzberg, E.; Nihlen, C.; Sibley, C.P.; et al. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: A randomised, double-blind, placebo-controlled feasibility trial. Nitric Oxide 2018, 80, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Willmott, T.; Ormesher, L.; McBain, A.J.; Humphreys, G.J.; Myers, J.E.; Singh, G.; Lundberg, J.O.; Weitzberg, E.; Nihlen, C.; Cottrell, E.C. Altered Oral Nitrate Reduction and Bacterial Profiles in Hypertensive Women Predict Blood Pressure Lowering Following Acute Dietary Nitrate Supplementation. Hypertension 2023, 80, 2397–2406. [Google Scholar] [CrossRef]
- Gangolli, S.D.; van den Brandt, P.A.; Feron, V.J.; Janzowsky, C.; Koeman, J.H.; Speijers, G.J.; Spiegelhalder, B.; Walker, R.; Wisnok, J.S. Nitrate, nitrite and N-nitroso compounds. Eur. J. Pharmacol. 1994, 292, 1–38. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Baiao, D.D.S.; Silva, D.; Paschoalin, V.M.F. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Said Abasse, K.; Essien, E.E.; Abbas, M.; Yu, X.; Xie, W.; Sun, J.; Akter, L.; Cote, A. Association between Dietary Nitrate, Nitrite Intake, and Site-Specific Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 666. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Patarata, L.; Lorenzo, J.M.; Fraqueza, M.J. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods 2021, 10, 3019. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef]
- Shannon, O.M.; Stephan, B.C.M.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Nitric Oxide Boosting Effects of the Mediterranean Diet: A Potential Mechanism of Action. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 902–904. [Google Scholar] [CrossRef]
- Spinnato, J.A., 2nd; Freire, S.; Pinto, E.S.J.L.; Cunha Rudge, M.V.; Martins-Costa, S.; Koch, M.A.; Goco, N.; Santos Cde, B.; Cecatti, J.G.; Costa, R.; et al. Antioxidant therapy to prevent preeclampsia: A randomized controlled trial. Obstet. Gynecol. 2007, 110, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, S.; Fogacci, F.; Cicero, A.F.G. Nutraceuticals and Hypertensive Disorders in Pregnancy: The Available Clinical Evidence. Nutrients 2020, 12, 378. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free Radic. Biol. Med. 2014, 77, 353–362. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef]
- Martinez, A.; Sanchez-Valverde, F.; Gil, F.; Clerigue, N.; Aznal, E.; Etayo, V.; Vitoria, I.; Oscoz, M. Methemoglobinemia induced by vegetable intake in infants in northern Spain. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Spyker, D.A. NADH-methemoglobin reductase activity: Adult versus child. Clin. Toxicol. 2018, 56, 866–868. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).