1-Acetoxypinoresinol, a Lignan from Olives: Insight into Its Characterization, Identification, and Nutraceutical Properties
Abstract
1. Introduction
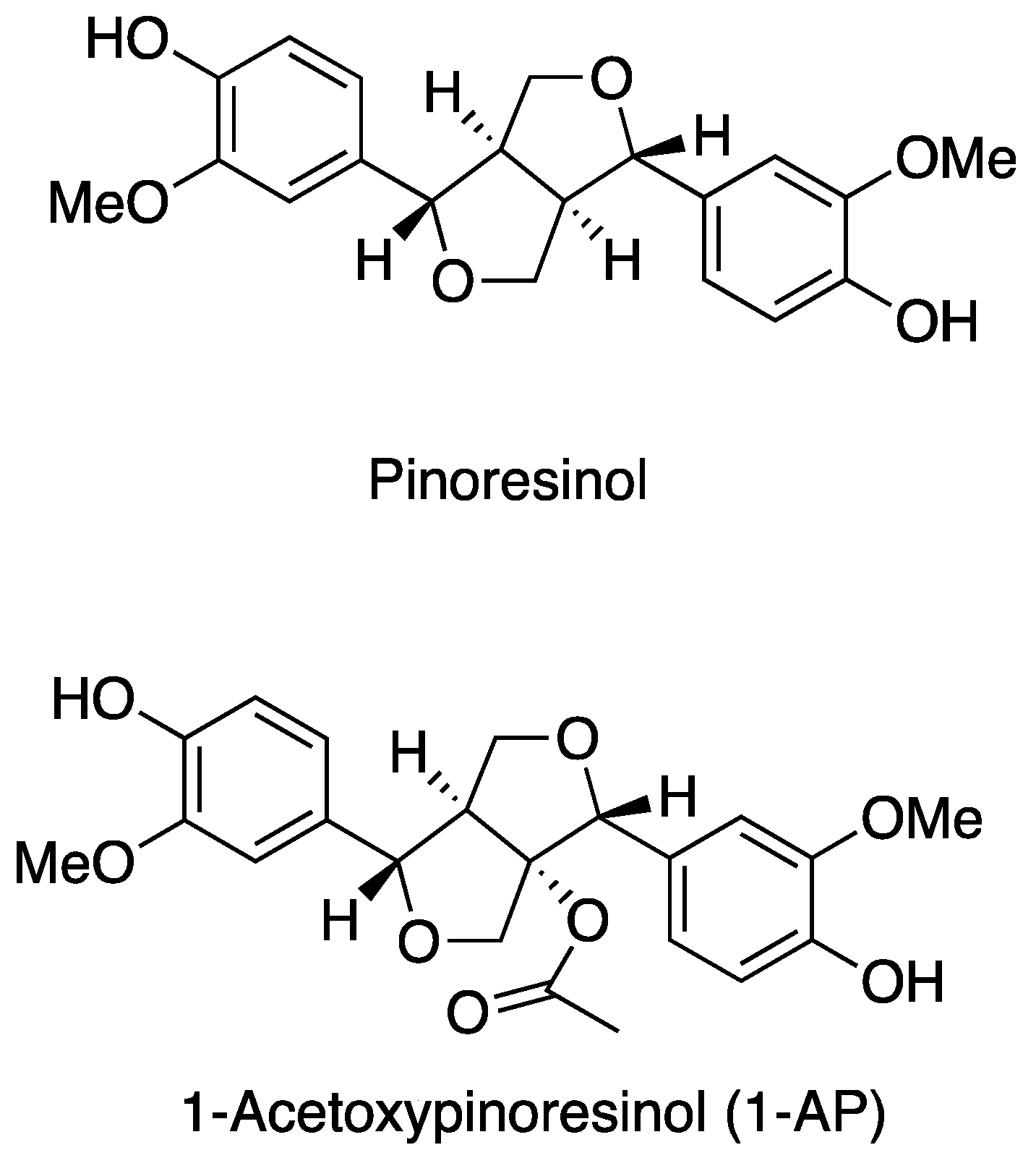
2. 1-Acetoxypinoresinol
3. Distribution of 1-AP on Different Olive Cultivars in Main Producing Countries
4. Extraction of 1-AP from EVOO
5. Purification of 1-AP
6. Identification and Quantification of 1-AP
7. Nutraceutical Properties of 1-AP
7.1. Antioxidant Effect
7.2. Antidiabetic Effect
7.3. Other Activities
8. 1-AP as Marker of EVOO Quality
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Serra-Majem, L.; Ferro-Luzzi, A.; Bellizzi, M.; Salleras, L. Nutrition Policies in Mediterranean Europe. Nutr. Rev. 1997, 55, S42–S57. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martinez-Gonzalez, M.A. Olive Oil and Prevention of Chronic Diseases: Summary of an International Conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ngo de la Cruz, J.; Ribas, L.; Tur, J.A. Olive Oil and the Mediterranean Diet: Beyond the Rhetoric. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Wahrburg, U.; Kratz, M.; Cullen, P. Mediterranean Diet, Olive Oil and Health. Eur. J. Lipid Sci. Technol. 2002, 104, 698–705. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R. Anti-Cancer Properties of Olive Oil Secoiridoid Phenols: A Systematic Review of in Vivo Studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [PubMed]
- Bazal, P.; Gea, A.; de la Fuente-Arrillaga, C.; Barrio-López, M.T.; Martinez-González, M.A.; Ruiz-Canela, M. Olive Oil Intake and Risk of Atrial Fibrillation in the SUN Cohort. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 450–457. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Lampousi, A.-M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive Oil in the Prevention and Management of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies and Intervention Trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-Virgin Olive Oil for Potential Prevention of Alzheimer Disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Liu, G.; Li, Y.; Sampson, L.; Manson, J.E.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Willett, W.C.; Sun, Q.; et al. Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J. Am. Coll. Cardiol. 2020, 75, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. [Google Scholar] [CrossRef] [PubMed]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef]
- Cuffaro, D.; Digiacomo, M.; Macchia, M. Dietary Bioactive Compounds: Implications for Oxidative Stress and Inflammation. Nutrients 2023, 15, 4966. [Google Scholar] [CrossRef]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of Health-Promoting Phenolic Compounds in Two Extra Virgin Olive Oils and Diversity of Associated Yeasts. J. Food Compos. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Brenes, M.; Hidalgo, F.J.; García, A.; Rios, J.J.; García, P.; Zamora, R.; Garrido, A. Pinoresinol and 1-Acetoxypinoresinol, Two New Phenolic Compounds Identified in Olive Oil. J. Am. Oil Chem. Soc. 2000, 77, 715–720. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef]
- Verpoorte, R. Pharmacognosy in the New Millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2000, 52, 253–262. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, H.-L.; Huang, J.; Chu, T.-Z.; Peng, C.; Zhang, H.; Chen, H.-L.; Xiong, Y.-A.; Tan, Y.-Z. Review of Lignans from 2019 to 2021: Newly Reported Compounds, Diverse Activities, Structure-Activity Relationships and Clinical Applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef]
- Gómez Caravaca, A.M.; Carrasco Pancorbo, A.; Cañabate Díaz, B.; Segura Carretero, A.; Fernández Gutiérrez, A. Electrophoretic Identification and Quantitation of Compounds in the Polyphenolic Fraction of Extra-Virgin Olive Oil. Electrophoresis 2005, 26, 3538–3551. [Google Scholar] [CrossRef]
- Arslan, D.; Karabekir, Y.; Schreiner, M. Variations of Phenolic Compounds, Fatty Acids and Some Qualitative Characteristics of Sarıulak Olive Oil as Induced by Growing Area. Food Res. Int. 2013, 54, 1897–1906. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Sakar, E.H.; Khtira, A.; Aalam, Z.; Zeroual, A.; Gagour, J.; Gharby, S. Variations in Physicochemical Characteristics of Olive Oil (Cv ‘Moroccan Picholine’) According to Extraction Technology as Revealed by Multivariate Analysis. AgriEngineering 2022, 4, 922–938. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Scarpa, E.S.; Frati, A.; Ninfali, P. Quantity and Quality of Secoiridoids and Lignans in Extra Virgin Olive Oils: The Effect of Two- and Three-Way Decanters on Leccino and Raggiola Olive Cultivars. Int. J. Food Sci. Nutr. 2016, 67, 9–15. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Lucchetti, S.; Forte, V.; Pastore, G. α-Tocopherol, β-Carotene, Lutein, Squalene and Secoiridoids in Seven Monocultivar Italian Extra-Virgin Olive Oils. Int. J. Food Sci. Nutr. 2017, 68, 538–545. [Google Scholar] [CrossRef]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, Separation, and Identification of Phenolic Compounds in Virgin Olive Oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548–562. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Lanza, B.; Cellini, M.; Di Marco, S.; D’Amico, E.; Simone, N.; Giansante, L.; Pompilio, A.; Di Loreto, G.; Bacceli, M.; Del Re, P.; et al. Olive Pâté by Multi-Phase Decanter as Potential Source of Bioactive Compounds of Both Nutraceutical and Anticancer Effects. Molecules 2020, 25, 5967. [Google Scholar] [CrossRef] [PubMed]
- García-González, D.L.; Romero, N.; Aparicio, R. Comparative Study of Virgin Olive Oil Quality from Single Varieties Cultivated in Chile and Spain. J. Agric. Food Chem. 2010, 58, 12899–12905. [Google Scholar] [CrossRef] [PubMed]
- Agiomyrgianaki, A.; Petrakis, P.V.; Dais, P. Influence of Harvest Year, Cultivar and Geographical Origin on Greek Extra Virgin Olive Oils Composition: A Study by NMR Spectroscopy and Biometric Analysis. Food Chem. 2012, 135, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Pacchiarotta, T.; Carrasco-Pancorbo, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Deelder, A.M.; Mayboroda, O.A. Gas Chromatography-Atmospheric Pressure Chemical Ionization-Time of Flight Mass Spectrometry for Profiling of Phenolic Compounds in Extra Virgin Olive Oil. J. Chromatogr. A 2011, 1218, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-López, M.J.; Innocenti, M.; Giaccherini, C.; Ieri, F.; Romani, A.; Mulinacci, N. Study of the Phenolic Composition of Spanish and Italian Monocultivar Extra Virgin Olive Oils: Distribution of Lignans, Secoiridoidic, Simple Phenols and Flavonoids. Talanta 2007, 73, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Pacetti, D.; Boarelli, M.C.; Giovannetti, R.; Ferraro, S.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; et al. Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region. Antioxidants 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Selvaggini, R.; Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; Montedoro, G. Evaluation of Phenolic Compounds in Virgin Olive Oil by Direct Injection in High-Performance Liquid Chromatography with Fluorometric Detection. J. Agric. Food Chem. 2006, 54, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Terracone, C.; Viggiani, I.; Nobile, M.A.D. Effects of Cultivars and Location on Quality, Phenolic Content and Antioxidant Activity of Extra-Virgin Olive Oils. J. Am. Oil Chem. Soc. 2013, 90, 103–111. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Molinu, M.G.; Dore, A.; Culeddu, N.; Azara, E.; Naziri, E.; Tsimidou, M.Z. Can All the Sardinian Varieties Support the PDO “Sardegna” Virgin Olive Oil? Eur. J. Lipid Sci. Technol. 2019, 121, 1800135. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From Olive Fruits to Olive Oil: Phenolic Compound Transfer in Six Different Olive Cultivars Grown under the Same Agronomical Conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; Rios, J.J.; García, P.; Garrido, A. Use of 1-Acetoxypinoresinol to Authenticate Picual Olive Oils. Int. J. Food Sci. Technol. 2002, 37, 615–625. [Google Scholar] [CrossRef]
- Christophoridou, S.; Dais, P. Detection and Quantification of Phenolic Compounds in Olive Oil by High Resolution 1H Nuclear Magnetic Resonance Spectroscopy. Anal. Chim. Acta 2009, 633, 283–292. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Sevim, D.; Bouaziz, M. Fatty Acid and Triacyglycerid as Markers of Virgin Olive Oil from Mediterranean Region: Traceability and Chemometric Authentication. Eur. Food Res. Technol. 2022, 248, 1749–1764. [Google Scholar] [CrossRef]
- Manganiello, R.; Pagano, M.; Nucciarelli, D.; Ciccoritti, R.; Tomasone, R.; Di Serio, M.G.; Giansante, L.; Del Re, P.; Servili, M.; Veneziani, G. Effects of Ultrasound Technology on the Qualitative Properties of Italian Extra Virgin Olive Oil. Foods 2021, 10, 2884. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A Simple and Rapid Electrophoretic Method to Characterize Simple Phenols, Lignans, Complex Phenols, Phenolic Acids, and Flavonoids in Extra-Virgin Olive Oil. J. Sep. Sci. 2006, 29, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive Oil Polyphenols: A Quantitative Method by High-Performance Liquid-Chromatography-Diode-Array Detection for Their Determination and the Assessment of the Related Health Claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of Extraction Methods for the Identification and Quantification of Polyphenols in Virgin Olive Oil by Ultra-HPLC-QToF Mass Spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and Hydrolyzable Compounds in Virgin Olive Oil. 3. Spectroscopic Characterizations of the Secoiridoid Derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative 1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Ebrahim, H.; Mohyeldin, M.; Qusa, M.; Batarseh, Y.; Fayyad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; Sayed, K.E. Novel Liquid-Liquid Extraction and Self-Emulsion Methods for Simplified Isolation of Extra-Virgin Olive Oil Phenolics with Emphasis on (-)-Oleocanthal and Its Oral Anti-Breast Cancer Activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Identification of Lignans as Major Components in the Phenolic Fraction of Olive Oil. Clin. Chem. 2000, 46, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic Compounds from Virgin Olive Oil Obtained by Natural Deep Eutectic Solvent (NADES): Effect of the Extraction and Recovery Conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Qusa, M.H.; Abdelwahed, K.S.; Meyer, S.A.; El Sayed, K.A. Olive Oil Lignan (+)-Acetoxypinoresinol Peripheral Motor and Neuronal Protection against the Tremorgenic Mycotoxin Penitrem A Toxicity via STAT1 Pathway. ACS Chem. Neurosci. 2020, 11, 3575–3589. [Google Scholar] [CrossRef] [PubMed]
- De Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of Phenolic Compounds in Virgin Olive Oil by Response Surface Methodology with Particular Focus on Flavonoids and Lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- García, A.; Brenes, M.; Martínez, F.; Alba, J.; García, P.; Garrido, A. High-performance Liquid Chromatography Evaluation of Phenols in Virgin Olive Oil during Extraction at Laboratory and Industrial Scale. J. Americ Oil Chem. Soc. 2001, 78, 625–629. [Google Scholar] [CrossRef]
- Bakhouche, A.; Lozano-Sánchez, J.; Beltrán-Debón, R.; Joven, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic Characterization and Geographical Classification of Commercial Arbequina Extra-Virgin Olive Oils Produced in Southern Catalonia. Food Res. Int. 2013, 50, 401–408. [Google Scholar] [CrossRef]
- Caligiani, A.; Acquotti, D.; Palla, G.; Bocchi, V. Identification and Quantification of the Main Organic Components of Vinegars by High Resolution 1H NMR Spectroscopy. Anal. Chim. Acta 2007, 585, 110–119. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Edible Oil Analysis by High-Resolution Nuclear Magnetic Resonance Spectroscopy: Recent Advances and Future Perspectives. Trends Food Sci. Technol. 2003, 14, 499–506. [Google Scholar] [CrossRef]
- Khallouki, F.; Hull, W.E.; Würtele, G.; Haubner, R.; Erben, G.; Owen, R.W. Isolation of the Major Phenolic Compounds in the Pits of Brined Green Olive Drupes: Structure Elucidation by Comprehensive 1H/13C-NMR Spectroscopy. Nat. Product. Commun. 2019, 14, 1934578X19857365. [Google Scholar] [CrossRef]
- Olmo-Cunillera, A.; López-Yerena, A.; Lozano-Castellón, J.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Pérez, M. NMR Spectroscopy: A Powerful Tool for the Analysis of Polyphenols in Extra Virgin Olive Oil. J. Sci. Food Agric. 2020, 100, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Bajoub, A.; Monasterio, R.P.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Metabolic Profiling Approach to Determine Phenolic Compounds of Virgin Olive Oil by Direct Injection and Liquid Chromatography Coupled to Mass Spectrometry. Food Chem. 2017, 231, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Neusüß, C.; Pelzing, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. CE- and HPLC-TOF-MS for the Characterization of Phenolic Compounds in Olive Oil. ELECTROPHORESIS 2007, 28, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive Properties of Pinoresinol-Rich Olive Oil Involve a Selective Activation of the ATM-P53 Cascade in Colon Cancer Cell Lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-Pinoresinol Exerts Antitumor Activity in Breast Cancer Cells with Different Oestrogen Receptor Statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Morozzi, G.; Fabiani, R. Oleuropein Inhibits Tumour Growth and Metastases Dissemination in Ovariectomised Nude Mice with MCF-7 Human Breast Tumour Xenografts. J. Funct. Foods 2014, 8, 269–273. [Google Scholar] [CrossRef]
- Morelló, J.-R.; Vuorela, S.; Romero, M.-P.; Motilva, M.-J.; Heinonen, M. Antioxidant Activity of Olive Pulp and Olive Oil Phenolic Compounds of the Arbequina Cultivar. J. Agric. Food Chem. 2005, 53, 2002–2008. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Morelló, J.R.; Motilva, M.J. Enrichment of Refined Olive Oil with Phenolic Compounds: Evaluation of Their Antioxidant Activity and Their Effect on the Bitter Index. J. Agric. Food Chem. 2006, 54, 6079–6088. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The Antioxidant/Anticancer Potential of Phenolic Compounds Isolated from Olive Oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Mwakalukwa, R.; Ashour, A.; Amen, Y.; Niwa, Y.; Tamrakar, S.; Miyamoto, T.; Shimizu, K. Anti-Allergic Activity of Polyphenolic Compounds Isolated from Olive Mill Wastes. J. Funct. Foods 2019, 58, 207–217. [Google Scholar] [CrossRef]
- Mwakalukwa, R.; Amen, Y.; Nagata, M.; Shimizu, K. Postprandial Hyperglycemia Lowering Effect of the Isolated Compounds from Olive Mill Wastes—An Inhibitory Activity and Kinetics Studies on α-Glucosidase and α-Amylase Enzymes. ACS Omega 2020, 5, 20070–20079. [Google Scholar] [CrossRef] [PubMed]
- Artajo, L.-S.; Romero, M.-P.; Suárez, M.; Motilva, M.-J. Partition of Phenolic Compounds during the Virgin Olive Oil Industrial Extraction Process. Eur. Food Res. Technol. 2007, 225, 617–625. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Bertini, S.; Ricci, C.; Cascone, M.G.; Danti, S.; Saba, A.; Macchia, M.; Digiacomo, M. Olive Mill Wastewater as Source of Polyphenols with Nutraceutical Properties. Nutrients 2023, 15, 3746. [Google Scholar] [CrossRef] [PubMed]
- Eringa, E.C.; Serne, E.H.; Meijer, R.I.; Schalkwijk, C.G.; Houben, A.J.H.M.; Stehouwer, C.D.A.; Smulders, Y.M.; van Hinsbergh, V.W.M. Endothelial Dysfunction in (Pre)Diabetes: Characteristics, Causative Mechanisms and Pathogenic Role in Type 2 Diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among Plant Lignans, Pinoresinol Has the Strongest Antiinflammatory Properties in Human Intestinal Caco-2 Cells, 3. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary Lignans: Physiology and Potential for Cardiovascular Disease Risk Reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, E.; Yoshida, Y.; Nitoda, T.; Baba, N.; Nakajima, S. (−)-Olivil and (+)-1-Acetoxypinoresinol from the Olive Tree (Olea Europaea L INNE; Oleaceae) as Feeding Stimulants of the Olive Weevil (Dyscerus Perforatus). Biosci. Biotechnol. Biochem. 2003, 67, 415–419. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Melani, F.; Migliorini, M.; Conte, L.; Mulinacci, N. New Isobaric Lignans from Refined Olive Oils as Quality Markers for Virgin Olive Oils. Food Chem. 2017, 219, 148–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nescatelli, R.; Bonanni, R.C.; Bucci, R.; Magrì, A.L.; Magrì, A.D.; Marini, F. Geographical Traceability of Extra Virgin Olive Oils from Sabina PDO by Chromatographic Fingerprinting of the Phenolic Fraction Coupled to Chemometrics. Chemom. Intell. Lab. Syst. 2014, 139, 175–180. [Google Scholar] [CrossRef]

| Cultivar | Geographical Origin | Concentration (mg/kg) | References |
|---|---|---|---|
| Taggiasca | Liguria, Italy | 48–160 | [36] |
| Leccino | Marche, Italy | 14.40–18.40 | [28] |
| Raggiola | Marche, Italy | 19.40–39.10 | [28] |
| Ascolana Tenera | Marche, Italy | 5.98–8.94 | [37] |
| Coroncina | Marche, Italy | 5.52–8.86 | [37] |
| Mignola | Marche, Italy | 8.13–10.30 | [37] |
| Piantone di Mogliano | Marche, Italy | 5.31–9.03 | [37] |
| Raggia | Marche, Italy | 31.77–35.47 | [37] |
| Canino | Marche, Italy | 2.0–2.9 | [38] |
| Coratina | Puglia, Italy | 3.6–33.9 | [39,40] |
| Nocellara | Puglia, Italy | 2.7–26.2 | [39,41] |
| Ogliarola | Puglia, Italy | 2.9–4.3 | [39] |
| Peranzana | Puglia, Italy | 3.6–3.9 | [39] |
| Bosana | Sardinia, Italy | 7.5–14.8 | [40] |
| Semidana | Sardinia, Italy | 10.9–49.4 | [40] |
| Maiorca | Sardinia, Italy | 19.9 | [40] |
| Paschixedda | Sardinia, Italy | 21.5 | [40] |
| Terza Grande | Sardinia, Italy | 23.3 | [40] |
| Terza Piccola | Sardinia, Italy | 22.5 | [40] |
| Corsicana da Olio | Sardinia, Italy | 56.9 | [40] |
| Pizz’e Carroga | Sardinia, Italy | 17.5 | [40] |
| Sivigliana da Olio | Sardinia, Italy | 36.5 | [40] |
| Frantoio | Tuscany, Italy | 23.1 | [40] |
| Seggianese | Tuscany, Italy | 40 | [36] |
| Moraiolo | Tuscany, Italy | 8.3–11.5 | [38] |
| Sikitita | Cordoba, Spain | 8.27 | [42] |
| Arbosana | Cordoba, Spain | 11.70 | [42] |
| Changlot Real | Cordoba, Spain | 7.00 | [42] |
| Arbequina | Catalonia, Granada and Cordoba, Spain | 26.1–66.9 | [19,41,43] |
| Empeltre | Huezca, Spain | 31.5–94.2 | [19,43] |
| Hojiblanca | Lucena, Spain | 24.4–50.1 | [19,41,43] |
| Picudo | Jaen, Spain | 6.8–12.5 | [19] |
| Cornicabra | Jaen, Spain | 10.2 | [19] |
| Picual | Jaen, Granada and Cordoba, Spain | 1.9–16.9 | [19,41,43] |
| Athinolia | Lakonia, Greece | 2.29–11.10 | [44] |
| Koroneiki | Messinia, Peloponnese, Greece | 20.1–26.2 | [44] |
| Tsunati | Messinia, Peloponnese, Greece | 21.3–28.7 | [44] |
| Athinolia | Lakonia, Greece | 2.29–11.10 | [44] |
| Nutraceutical Effect | Biological Process | References |
|---|---|---|
| Antioxidant | Antioxidant activity toward oxidation of liposomes and bulk lipids | [69] |
| increase in oxidative stability | [70] | |
| potent response against ROS attack | [71] | |
| DPPH assay | [47] | |
| Protection against neurotoxicity | Mediation of PA toxicity on Schwann cells | [56] |
| Anti-allergic | Decrease in the expression of calcium channel proteins in RBL-2H3 cells | [72] |
| Anti-diabetes | Inhibitory activity towards α-glucosidase and α-amylase enzymes | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, G.Y.A.; Cuffaro, D.; Bertini, S.; Digiacomo, M.; Macchia, M. 1-Acetoxypinoresinol, a Lignan from Olives: Insight into Its Characterization, Identification, and Nutraceutical Properties. Nutrients 2024, 16, 1474. https://doi.org/10.3390/nu16101474
Wijaya GYA, Cuffaro D, Bertini S, Digiacomo M, Macchia M. 1-Acetoxypinoresinol, a Lignan from Olives: Insight into Its Characterization, Identification, and Nutraceutical Properties. Nutrients. 2024; 16(10):1474. https://doi.org/10.3390/nu16101474
Chicago/Turabian StyleWijaya, Ganesha Yanuar Arief, Doretta Cuffaro, Simone Bertini, Maria Digiacomo, and Marco Macchia. 2024. "1-Acetoxypinoresinol, a Lignan from Olives: Insight into Its Characterization, Identification, and Nutraceutical Properties" Nutrients 16, no. 10: 1474. https://doi.org/10.3390/nu16101474
APA StyleWijaya, G. Y. A., Cuffaro, D., Bertini, S., Digiacomo, M., & Macchia, M. (2024). 1-Acetoxypinoresinol, a Lignan from Olives: Insight into Its Characterization, Identification, and Nutraceutical Properties. Nutrients, 16(10), 1474. https://doi.org/10.3390/nu16101474





