Abstract
Functional dyspepsia is a gastrointestinal disorder characterized by postprandial fullness, early satiation, epigastric pain, and epigastric burning. The pathophysiology of the disease is not fully elucidated and there is no permanent cure, although some therapies (drugs or herbal remedies) try to reduce the symptoms. Diet plays a critical role in either the reduction or the exacerbation of functional dyspepsia symptoms; therefore dietary management is considered to be of high importance. Several foods have been suggested to be associated with worsening functional dyspepsia, such as fatty and spicy foods, soft drinks, and others, and other foods are thought to alleviate symptoms, such as apples, rice, bread, olive oil, yogurt, and others. Although an association between functional dyspepsia and irregular eating habits (abnormal meal frequency, skipping meals, late-night snacking, dining out, etc.) has been established, not many dietary patterns have been reported as potential factors that influence the severity of functional dyspepsia. A higher adherence to Western diets and a lower adherence to FODMAPs diets and healthy patterns, such as the Mediterranean diet, can contribute to the worsening of symptoms. More research is needed on the role of specific foods, dietary patterns, or specific eating habits in the management of functional dyspepsia.
1. Introduction
Functional dyspepsia (FD) is considered to be one of the most common disorders in clinical practice [1]. It has a high prevalence that affects 10–30% of adults and 3.5–27% of children worldwide [2]. Despite its high prevalence, there are major uncertainties regarding its definition, pathophysiology, diagnosis, treatment, and prognosis [1].
1.1. Pathophysiology
Although the etiology of the disorder has not been fully elucidated, the main pathophysiological mechanisms that have been proposed throughout the years include motility alterations and psychosocial factors. The disruption of the microbiota–gut–brain axis, with abnormal central modulation, visceral hypersensitivity, and increased mucosal permeability contribute to the pathophysiology of FD. Increased intestinal permeability, immune activation, and gut dysbiosis caused by stress, which in turn affect the nervous system, suggests the concept of an impaired bidirectional communication of the “brain–gut axis” in FD [3]. In addition, acute enteric infections lead to colonic inflammation, recruitment of eosinophils and mast cells, lymphoid follicles, and duodenal mucosal bacterial loads, which affect the symptomatology of FD patients. Increased levels of inflammatory cytokines in the colonic mucosa are associated with anxiety and depression, which are related to the gut–brain axis [4]. Finally, genetic factors (such as polymorphisms in the genes related to gastrointestinal mobility and immune function), Helicobacter pylori infection, and impaired duodenal mucosal barrier function have been linked with worse FD symptoms [5,6,7] (Figure 1).
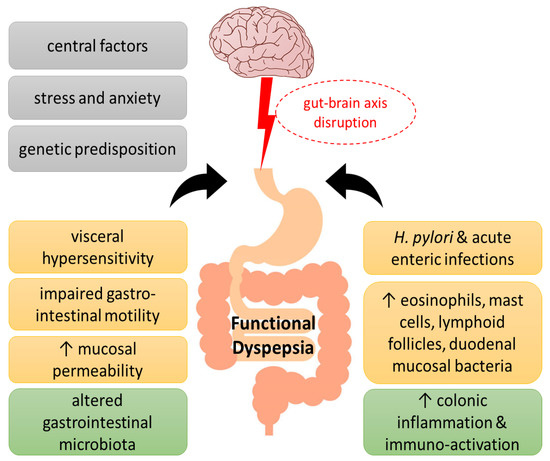
Figure 1.
Pathogenesis of functional dyspepsia (FD). A series of pathogenic factors have been proposed for FD, including central nervous system abnormalities and genetic predisposition, as well as psychological factors, which have been suggested to interfere with the gut–brain axis function. Visceral hypersensitivity, impaired gastrointestinal motility, increased epithelial barrier permeability of the duodenal mucosa and infections, such as Helicobacter pylori, have been associated with altered intestinal flora in FD towards immune activation, immune cell infiltration, and low-grade inflammation.
1.2. Clinical Manifestations
According to the Rome IV criteria for the diagnosis of functional dyspepsia, the main clinical symptoms include bothersome postprandial fullness, early satiation, epigastric pain, and/or epigastric burning along with the absence of any structural disease that may explain the symptoms. Furthermore, the above symptomatology impairs the patient’s quality of life and emotional health, and creates significant financial burden due to increased medical expenses and reduced work productivity. [8]. FD symptoms must be present for a minimum of 3 days a week during the last 3 months, they must be chronic, and start at least 6 months before diagnosis [9]. FD diagnosis includes an evaluation of the clinical history, physical examination, minimal laboratory tests, and a normal upper endoscopy. It is further categorized into epigastric pain syndrome (EPS) and eating-related postprandial distress syndrome (PDS). PDS is defined by bothersome postprandial fullness, that can affect typical activities, and/or bothersome early satiation, that can prevent the completion of a regular-sized meal. EPS is defined by bothersome epigastric pain and/or epigastric burning, both severe enough to disturb usual activities. The Rome IV classification involves not only PDS and EPS, but also their overlap (PDS-EPS overlapped syndrome), which is observed more frequently in hospital than in the general population [8].
1.3. Medicines
As there is no standard treatment for FD, research on effective therapies is ongoing, but still needs further confirmation. The acid-suppressive therapy with proton pump inhibitors (PPIs) is the most common treatment method [10]. Treatment with the tetracyclic antidepressant mirtazapine improves the quality of life, and buspirone, a serotonin-1A receptor agonist, can alleviate FD symptoms [11]. Prokinetics facilitate the gastric emptying rate [12], while amitriptyline, a neuromodulator, seems to be less effective for the treatment of FD [13]. Finally, the antibiotic rifaximin can change the duodenal microenvironment and reduce FD symptoms [14].
1.4. Herbal Remedies
Several herbal remedies have been proven effective and safe in FD with comparable outcomes with conventional treatments, and can serve as complementary and alternative medicine, especially when first line therapeutic approaches fail or are inaccessible to patients [9]. Some herbal oils improve PDS and EPS, and improve gastrointestinal symptom rating scale (GSRS) numbers and quality of life scores [15]. Herbal treatments show anti-inflammatory effects and contribute to an improvement in the function of gut microbiota, immune system, central stimuli, and intestinal motility in FD [16]. A systematic review and meta-analysis of 23 randomized controlled trials (RCTs) comparing the effectiveness of herbal treatments versus a placebo or other standard treatments for FD found that the majority of participants (>60%) in the herbal treatment group experienced an improvement in symptomatology and quality of life, compared to participants in the placebo group [17]. Chinese herbal medicines have been considered an effective alternative to prokinetics, according to a meta-analysis of 28 RCTs showing that Chinese herbal remedies were more effective than prokinetics at reducing the overall symptoms [18]. A combination of three herbs (Trachyspermum ammi L., Anethum graveolens L., and Zataria multiflora Boiss) may be important in the treatment of FD, as the essential oils were proven more effective than omeprazole [15]. Similarly, the Japanese Yukgunja-tang, also known as Rikkunshito, is a mixture of eight herbs that is frequently prescribed in FD [19], and it was proven more effective in the total clinical efficacy rate in a meta-analysis of 10 studies with 1246 patients, when combined with Western medicine over the use of Western medicine alone [20]. Additionally, perilla/ginger nutraceuticals have been shown to ameliorate some FD symptoms, such as epigastric pain, heartburn, and gastric reflux, with minor adverse events [21]. Artichoke leaf extract supplementation resulted in a greater amelioration of the multiple correspondence analysis scale compared to a placebo [22], while ginger accelerated gastric emptying [23]. The use of peppermint and caraway oil, a combination with unique properties, showed a statistically significant effect in the global improvement of FD symptoms in a meta-analysis of five RCTs [24]. A unique Greek herbal remedy known as Chios mastic gum has been shown to alleviate the symptoms of FD when taken daily for three weeks over a placebo [25]. The Hong Kong index of dyspepsia was used to assess the efficacy of the mastic treatment.
The current literature review was conducted with the aim of mapping the development of research on the nutritional aspects of FD during the period beginning on 1 January 2010 and ending on 31 December 2022. The research studies used fulfilled the following criteria: (1) they assessed the impact of foods, dietary patterns, eating behaviors, and botanicals on FD, including the relief of FD symptoms as the main outcome, (2) the literature was published in English, and (3) published from 2010 onwards. Duplicate/in vitro/animal studies, book chapters, study protocols, case reports, comments, and letters were excluded, as well as studies that involved multifactorial lifestyle interventions, in which the effects of dietary factors could not be distinguished from either genetic or lifestyle factors. To be accurate and trustworthy, the literature search was carried out using the PubMed-MEDLINE and Scopus databases. The search strategy included the following keywords: “Functional dyspepsia”, “Epigastric pain syndrome”, “Postprandial distress syndrome” “Nutrition”, “Diet”, “Foods”, “Dietary patterns”, “Dietary intervention”, “Nutrients”, “Macronutrients”, and “Micronutrients”. References from the extracted articles and reviews were also used to complete the data bank. The relevance of the studies was based on the title, abstract, and the full manuscript, which was reviewed. The search for duplicate publications was performed using an electronic database, and then the full text of each potentially relevant study was reviewed to ensure that it was consistent with the search criteria. A specific table was constructed by the same independent reviewers to facilitate the data extraction and selection, including the following information: article title, authors’ names, year of publication, participants’ characteristics, study design, duration of intervention, type of intervention, study outcomes, major findings, and limitations. Additionally, the studies were grouped according to their design (i.e., food-based intervention and dietary counseling interventions), and secondly according to the outcome.
2. Certain Foods as Inducers or Suppressants of Functional Dyspepsia Symptoms
Food consumption is the main triggering factor for dyspepsia in most FD patients. Several studies have reported that the consumption of specific foods may trigger or suppress FD symptoms. Most commonly reported triggering foods include fatty and spicy foods, soft drinks, wheat products, products containing caffeine, and alcohol [9]. Studies investigating foods that may trigger or alleviate symptoms of FD are presented in Table 1.

Table 1.
Studies investigating foods that may trigger or alleviate symptoms of FD.
In the study of Akhondi-Meybodi and colleagues, three hundred and eighty four patients with FD, who had previously undergone endoscopy, were examined for their response to 114 foods in terms of relief or aggravation of their symptoms. Symptoms were most aggravated by sausage and bologna, pickles, vinegar, soft drinks, grains, tea, salt, pizza, watermelon, red pepper, and macaroni, as well as soft drinks, and acidic fruits [26]. Moreover, the foods that most frequently led to an alleviation of symptoms were apples, rice, rock candy, bread, caraway seeds, dates, honey, yogurt, quince, and walnuts. The evidence that particularly spicy foods stimulate FD symptoms has been confirmed by other studies. A spicy food’s burning sensation is caused by capsaicin found in high concentrations in chili peppers. Patients with FD who consume capsaicin-containing foods experience more symptoms than healthy controls or those who consume placebos [27,28]. It has been reported that transient receptor potential vanilloid-1 receptors (TRPV1) interact with capsaicin to cause the burning sensations, however the G315 polymorphism of the TRPV1 gene is inversely correlated with FD [29]. The prevalence of FD subtypes did not vary with the consumption of spicy foods or TRPV1 genotypes in a comparison of symptom generation according to these two factors, and TRPV1 polymorphisms were not associated with scores on symptom severity questionnaires, but eating spicy food was associated with higher scores for retching and stomach fullness [30]. When comparing the nutritional habits of 168 adults with FD to 135 healthy control subjects, spicy, but also fatty foods, and carbonated drinks were the most frequently reported food items to cause symptoms. In postprandial distress syndrome FD, symptoms were more likely to be brought on by carbonated beverages and legumes [31]. Similarly, the consumption of spicy, hot, raw, or cold foods, and dairy foods or products has been associated with FD symptoms, while tea was associated with FD prevention [32]. Canned foods, fast foods, and alcoholic beverages have also been linked with FD symptoms [33].
Patients with FD commonly report food hypersensitivities to wheat and gluten, a protein found in wheat. According to an Australian population-based study [34], 29% of people with FD avoided gluten, and self-reported gluten sensitivity had a significant association with FD diagnosis. In some cases, gluten-free diets have helped FD patients to improve their symptoms. Furthermore, in a randomized double-blind placebo-controlled trial in FD patients, the application of a gluten-free diet resulted in an improvement of symptoms in 35% of patients, yet only 18% of those patients were confirmed to have this sensitivity to gluten, with symptoms reoccurring following a blind gluten challenge [35]. This suggests that other components in wheat-based foods, such as fructans, may induce FD symptoms.
In sixty patients with FD, a 1-month retrospective food consumption frequency questionnaire was used to examine fifty-one foods that may stimulate FD symptoms [36]. The consumption of broccoli, radish, celery, green olives, and olive oil was lower in patients with increased postprandial fullness. Participants who had more pain in the stomach reported a lower consumption of dried fruits, green olives, butter, fast food, and alcohol, but the consumption of sunflower oil was higher. Overall, foods high in fat are often blamed by patients with FD for making their symptoms worse. Unknown pathophysiological mechanisms are responsible for high-fat foods’ ability to cause FD symptoms. However, cholecystokinin seems to be an important mediator [37]. Consuming fatty foods can slow down gastric emptying, disrupt gastric motility, and make dyspeptic patients feel fuller after meals [38]. Despite the known evidence regarding fatty foods, almonds do not appear to worsen FD symptoms as expected [38]. This effect may be related to the high content of tryptophan, a precursor of serotonin. Serotonin (5-hydroxytryptamine) is a key neurotransmitter involved in the regulation of gastrointestinal motility and sensory function. Indeed, stimulation of 5-HT1 serotonergic receptors induces smooth gastric muscle contractions that enhances gastric emptying, and appears to improve abdominal symptoms in patients [39]. However, when examining the brain activity using functional magnetic resonance imaging (fMRI) following the consumption of high- and low-fat foods with accurate or inaccurate fat information, researchers discovered that FD patients displayed more pronounced FD symptoms than healthy controls. These symptoms were less relieved following the consumption of high-fat yogurt than low-fat yogurt, regardless of the actual fat content. This suggests that low-fat foods may have a placebo effect or that high-fat foods may have a nocebo effect on symptom expression [40]. Interestingly, extra-virgin olive oil enriched with probiotics or antioxidants incorporated blindly into the regular diet of subjects with FD for seven days, induced a significant improvement in dyspeptic symptoms in those receiving the probiotic- or antioxidant-enriched oil diet, with the probiotic-enriched oil having a greater impact [41].
The relationship between coffee consumption and gastroesophageal reflux has been widely investigated [42,43,44]. Coffee has been considered a beverage that should be avoided, as it has been shown to worsen the symptoms of FD [8,26]. Chinese people with FD have been reported to be more likely to have a preference for coffee [45]. In the study by Correia et al., the effects of removing and substituting caffeinated or decaffeinated coffee with a non-caffeinated coffee alternative in the diet of fifty-one patients with FD were investigated [46]. Using a self-reported questionnaire, this descriptive, quasi-experimental pre/post intervention study looked at the relationship between functional dyspepsia and non-caffeinated coffee consumption. A statistically significant reduction in FD symptoms was reported following a month of consuming the coffee substitute.
3. Dietary Patterns and Eating Behaviors; Proportions, Variety, Frequency, and Cooking Habits
The dietary patterns and behavioral parameters examined constitute a multidimensional construct, including diet composition spanning from alimentary selection to the subsequent development of healthy or disordered eating habits, and ranging from meal frequency and regularity to cooking techniques. To the best of our knowledge, only a small percentage of studies provide a solution for to what extent do specific dietary behaviors influence and ultimately cause susceptibility to dyspeptic symptoms (Table 2).

Table 2.
Studies investigating dietary patterns and eating behaviors associated with FD.
Despite the difficulty of adhering to a FODMAP diet, Staudacher et al. noticed that individuals with underlying symptoms of FD and coexisting irritable bowel syndrome, and who followed a diet limited to FODMAPs, showed a higher reduction in the epigastric and total symptom score in comparison with those who received personalized dietary counseling at the discretion of the dietitians who participated in the research [47]. Similarly to Staudacher et al., Goyal and his team studied the effects of a low FODMAP diet in a panel of people with persistent FD, and subsequently they compared them to a slightly restricted standard diet that included an avoidance to spices, soft drinks, tea, coffee, and alcohol. Both interventions showed a significant remission of symptom severity and quality of life improvement, although one sub-group analysis of volunteers with postprandial distress syndrome or bloating were better responders to a low FODMAP regime [48].
Schnabel et al., within the NutriNet-Santé prospective observational cohort study, examined the interaction of consuming ultra-processed foods in people with various functional gastrointestinal disorders. Results showed that subjects suffering from FD and concurrent irritable bowel syndrome have a higher risk of being affected because of the increased daily amount of ultra-processed foods consumed [49]. In 2034 children and adolescents with functional gastrointestinal disorders, including FD, approximately 90% of the subjects reported fast food consumption, and there was a significantly higher prevalence of a history of functional disorder in fast food consumers, compared to non-consumers [50]. The increased risk of functional disorders was associated with the regular fast food intake. Ultra-processed and fast foods consist of the core of the Westernized dietary pattern. The popularity of ultra-processed and fast foods is partly due to their inexpensive and easy to find nature. However, the high fat content, particularly the trans fatty acid content and the presence of additives or reaction products owed to processing [51,52,53,54] may be linked with the augmentation of FD symptoms. Similarly, the prevalence of FD in Asia has increased during the last decades due to the change towards a more Westernized diet, higher in fat- and lower in carbohydrate-rich foods, whereas the spicy foods in Asian diets are associated with a higher risk of developing FD [55].
Quite the opposite, a dietary pattern that appears to be closely related to FD symptom amelioration is one that contains fruits and vegetables. According to the cross-sectional study by Tabibian et al., people on a diet rich in fruit have a 32% lower risk of FD, as well as a lower risk of early satiation and postprandial fullness in comparison with those on a low fruit diet. Moreover, a high vegetable intake seems to have a beneficial effect on FD, however, only in male subjects [56]. The same pattern was observed by Zito and his associates, who also emphasized through their results the importance of adopting a balanced diet, such as the Mediterranean diet due to its preventive function against FD. Specifically, a low adherence to the Mediterranean diet is associated with FD mainly in younger people [57].
Regarding the eating habits of FD subjects, a confusion about the frequency of main meals and snacks per day prevails in the bibliography. Göktaş et al. did not manage to demonstrate a significant difference in the frequency of main meals among FD and control subjects, as both groups had three main meals per day (68.5% and 70.4%, respectively). Likewise for snack consumption, researchers found no difference in their frequency between the two groups [31]. These findings corroborate those of Çolak et al., who recently extrapolated the conclusion that meal frequency had no impact on the triggering of FD symptoms among FD subjects [36]. Moreover, in a study involving 1139 volunteers, of whom 936 were healthy subjects and 203 had been diagnosed with FD, Xu et al. [45] demonstrated that the vast majority of the latter had by far more unhealthy nutritional habits than the control group (75.86% versus 37.50%, respectively; p < 0.001). It appears that the number of meals during the day shows an inversely independent relationship with the occurrence of FD. Hassanzadeh et al. [58] concluded that regularly consuming at least three main meals per day was not only indissolubly related with a 52% lower chance of developing FD compared to eating one meal, but also was inversely associated with the prevalence of early satiety. Similar findings were observed in Yamamoto et al.’s [59] cross-sectional study, where the risk of FD in subjects who ate one, two, and three meals daily was 4.8%, 2.2%, and 1.7%, respectively.
Correspondingly, the literature review shows that, three to five snacks per day are found to be a determinant factor for low FD incidence data, inhibiting alongside both the occurrence of postprandial discomfort (42%) and epigastric pain symptoms (43%) [58].
Concerning late-night snacking, the findings are controversial, with Xu et al. arguing that it is an independent triggering factor of postprandial fullness, while Yamamoto et al. state that there is no correlation with the development of FD. Carvalho et al. claim that the duration of the overnight fast was significantly longer in subjects with FD than in the controls, as the former tended to eat dinner earlier. On the contrary, this difference is equated to daytime fasting [38].
Based on the current literature, another causative agent for the generation of dyspeptic symptoms is the skipping of one or even more main meals during the day, and depending on which meal (breakfast, lunch, dinner), there are different impacts on FD and its subtypes. In particular, Yamamoto et al. claim that FD subjects were more prone to skip lunch and dinner than the controls, and the habit of omitting breakfast and/or lunch was independently inversely related to a high incidence of FD symptoms (breakfast: adjusted OR, 1.60 [95% CI, 1.10–2.32] and lunch: adjusted OR, 2.52 [95% CI, 1.04–5.18]). Contrarily, researchers did not prove any relation between FD prevalence and skipping dinner [59]. A more comprehensive description can be found in Xu et al.’s study, that not only confirms the analogous findings, but also reveals the close association between skipping breakfast and PDS (18/203) and EPS (13/203) subtypes, but not with their overlapping type (9/203) [45].
Apropos irregular meals and the tendency of dining out, which are positively connected to the generation of FD symptoms, investigators claim that both correlated with PDS, but only an erratic dinner time was related to EPS and the overlapping subtype [45]. Furthermore, in respect to the possible correlation between sleep and the development of FD symptoms, researchers did not prove any statistically significant difference between FD and control subjects who go to bed following a meal [38].
In regard to meal time or chewing efficiency, and their role in triggering FD symptoms, Çolak et al. [36] claim that this assertion has no merit, although some clinical studies demonstrate that subjects with FD are more likely to self-report themselves as fast eaters than the controls [38,60]. In the same cross-sectional study, the findings indicated that roasting was the most usual cooking method in subjects experiencing the postprandial fullness symptoms [36]. Furthermore, compared to both PDS and EPS subtypes, as well as the control group, the overlapping group appeared to have a significantly higher intra-meal fluid consumption [31] (Figure 2).
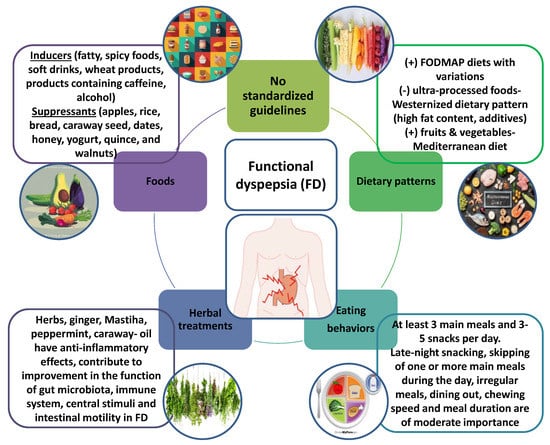
Figure 2.
Functional dyspepsia (FD) is considered one of the most common disorders in clinical practice. Standardized guidelines for the nutritional approach and eating habits of patients with FD do not exist. However, assessing the impact of foods, dietary patterns, eating behaviors and botanicals on FD can help, on the one hand, to elucidate the pathophysiology of the disease, and on the other hand, to improve palliative treatments.
4. Conclusions
Well-structured and standardized guidelines for the nutritional approach and eating habits of patients with FD do not exist, and randomized controlled trials are few, while most available evidence comes from observational studies. Hence, although the contribution of specific foods identified as triggers by FD patients varies, some causal relationships between specific foods and symptoms have been demonstrated. The retrospective nature of most studies and the lack of a standardized method for verifying the food-symptom association accounts for the difficulty in accumulating a definitive list of foods to avoid or foods to favor. Some of the most frequently reported foods to avoid are fatty foods, processed foods, and wheat products. Alike, processed foods and overall the Western dietary pattern, which includes fatty foods, as these are considered inducers of FD symptoms. Eating small and frequent meals may be a reasonable suggestion to reduce symptoms, however the evidence is inadequate and inconsistent. In the future, the improvement in our understanding of the pathophysiological mechanisms underlying FD might lead to well-designed and target-driven clinical trials on the effects of foods, dietary patterns, or specific eating habits in the management of FD.
Author Contributions
All authors contributed equally to writing this review article. All authors have read and agreed to the published version of the manuscript.
Funding
This work received funding by the Chios Mastiha Research and Development Center.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; Accarino, A.; Barbara, G.; Bor, S.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. United Eur. Gastroenterol. J. 2021, 9, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Meroni, G.; Pistone, D.; Pasquale, L.; Milazzo, G.; Monica, F.; Aragona, S.; Ficano, L.; Vassallo, R. Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies. J. Int. Med. Res. 2021, 49, 0300060520982657. [Google Scholar] [CrossRef]
- Wauters, L.; Talley, N.J.; Walker, M.M.; Tack, J.; Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2019, 69, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35 (Suppl. S1), 5–13. [Google Scholar] [CrossRef]
- Wauters, L.; Li, H.; Talley, N.J. Editorial: Disruption of the Microbiota-Gut-Brain Axis in Functional Dyspepsia and Gastroparesis: Mechanisms and Clinical Implications. Front. Neurosci. 2022, 16, 941810. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Ihara, E.; Minoda, Y.; Ogino, H.; Sasaki, T.; Fujiwara, M.; Oda, Y.; Ogawa, Y. The Altered Mucosal Barrier Function in the Duodenum Plays a Role in the Pathogenesis of Functional Dyspepsia. Dig. Dis. Sci. 2019, 64, 3228–3239. [Google Scholar] [CrossRef]
- Wauters, L.; Burns, G.; Ceulemans, M.; Walker, M.M.; Vanuytsel, T.; Keely, S.; Talley, N.J. Duodenal inflammation: An emerging target for functional dyspepsia? Expert Opin. Ther. Targets 2020, 24, 511–523. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.L.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef]
- Duboc, H.; Latrache, S.; Nebunu, N.; Coffin, B. The Role of Diet in Functional Dyspepsia Management. Front. Psychiatry 2020, 11, 23. [Google Scholar] [CrossRef]
- Moayyedi, P.M.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013. [Google Scholar] [CrossRef]
- Tack, J.; Janssen, P.; Masaoka, T.; Farré, R.; Van Oudenhove, L. Efficacy of Buspirone, a Fundus-Relaxing Drug, in Patients With Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2012, 10, 1239–1245. [Google Scholar] [CrossRef]
- Pittayanon, R.; Yuan, Y.; Bollegala, N.P.; Khanna, R.; Lacy, B.E.; Andrews, C.N.; Leontiadis, G.I.; Moayyedi, P. Prokinetics for Functional Dyspepsia. Am. J. Gastroenterol. 2019, 114, 233–243. [Google Scholar] [CrossRef]
- Talley, N.J.; Locke, G.R.; Saito, Y.A.; Almazar, A.E.; Bouras, E.P.; Howden, C.W.; Lacy, B.E.; DiBaise, J.K.; Prather, C.M.; Abraham, B.P.; et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 2015, 149, 340–349.e2. [Google Scholar] [CrossRef]
- Meyrat, P.; Safroneeva, E.; Schoepfer, A.M. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment. Pharmacol.Ther. 2012, 36, 1084–1093. [Google Scholar] [CrossRef]
- Bordbar, G.; Miri, M.B.; Omidi, M.; Shoja, S.; Akhavan, M. Comparison of a Novel Herbal Medicine and Omeprazole in the Treatment of Functional Dyspepsia: A Randomized Double-Blinded Clinical Trial. Gastroenterol. Res. Pract. 2020, 2020, 5152736. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.-W.; Ha, N.-Y.; Kim, J.; Ryu, H.S. Herbal Therapies in Functional Gastrointestinal Disorders: A Narrative Review and Clinical Implication. Front. Psychiatry 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Heiran, A.; Bagheri Lankarani, K.; Bradley, R.; Simab, A.; Pasalar, M. Efficacy of herbal treatments for functional dyspepsia: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2021, 36, 686–704. [Google Scholar] [CrossRef]
- Ho, L.; Zhong, C.C.W.; Wong, C.H.L.; Wu, J.C.Y.; Chan, K.K.H.; Wu, I.X.Y.; Leung, T.H.; Chung, V.C.H. Chinese herbal medicine for functional dyspepsia: A network meta-analysis of prokinetic-controlled randomised trials. Chin. Med. 2021, 16, 140. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Ko, S.-J.; Park, J.-W.; Cha, J.M. Complementary and alternative medicine for functional dyspepsia: An Asian perspective. Medicine 2022, 101, e30077. [Google Scholar] [CrossRef]
- Ko, S.; Park, J.; Kim, M.; Kim, J.; Park, J. Effects of the herbal medicine Rikkunshito, for functional dyspepsia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Giovannone, M.; Saponara, M.; Ivaldi, L. Effectiveness of a nutraceutical supplement containing highly standardized perilla and ginger extracts in patients with functional dyspepsia. Minerva Gastroenterol. Dietol. 2020, 66, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, A.; Guido, D.; Grassi, M.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Perna, S.; Faliva, M.A.; Rondanelli, M. The Effect of Ginger (Zingiber officinalis) and Artichoke (Cynara cardunculus) Extract Supplementation on Functional Dyspepsia: A Randomised, Double-Blind, and Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 915087. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J. Gastroenterol. 2011, 17, 105. [Google Scholar] [CrossRef]
- Li, J.; Lv, L.; Zhang, J.; Xu, L.; Zeng, E.; Zhang, Z.; Wang, F.; Tang, X. A Combination of Peppermint Oil and Caraway Oil for the Treatment of Functional Dyspepsia: A Systematic Review and Meta-Analysis. Evid. -Based Complement. Altern. Med. 2019, 2019, 7654947. [Google Scholar] [CrossRef]
- Dabos, K.J.; Sfika, E.; Vlatta, L.J.; Frantzi, D.; Amygdalos, G.I.; Giannikopoulos, G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. J. Ethnopharmacol. 2010, 127, 205–209. [Google Scholar] [CrossRef]
- Akhondi-Meybodi, M.; Aghaei, M.A.; Hashemian, Z. The role of diet in the management of non-ulcer dyspepsia. Middle East J. Dig. Dis. 2015, 7, 19–24. [Google Scholar]
- Führer, M.; Vogelsang, H.; Hammer, J. A Placebo Controlled Trial of an Oral Capsaicin Load in Patients With Functional Dyspepsia. Gastroenterology 2011, 23, 918-e397. [Google Scholar] [CrossRef]
- Hammer, J.; Führer, M.; Pipal, L.; Matiasek, J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Shibata, T.; Nakamura, M.; Yamashita, H.; Yoshioka, D.; Hirata, I.; Arisawa, T. Homozygous TRPV1 315C Influences the Susceptibility to Functional Dyspepsia. J. Clin. Gastroenterol. 2010, 44, e1–e7. [Google Scholar] [CrossRef]
- Lee, S.Y.; Masaoka, T.; Han, H.S.; Matsuzaki, J.; Hong, M.J.; Fukuhara, S.; Choi, H.S.; Suzuki, H. A prospective study on symptom generation according to spicy food intake and TRPV1 genotypes in functional dyspepsia patients. Neurogastroenterol. Motil. 2016, 28, 1401–1408. [Google Scholar] [CrossRef]
- Göktaş, Z.; Köklü, S.; Dikmen, D.; Öztürk, Ö.; Yılmaz, B.; Asıl, M.; Korkmaz, H.; Tuna, Y.; Kekilli, M.; Karamanoğlu Aksoy, E.; et al. Nutritional habits in functional dyspepsia and its subgroups: A comparative study. Scand. J. Gastroenterol. 2016, 51, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-p.; Wang, K.; Duan, Y.-h.; Yang, G. Correlation between lifestyle and social factors in functional dyspepsia among college freshmen. J. Int. Med. Res. 2020, 48, 1–8. [Google Scholar] [CrossRef]
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Drug, V.L. The role of diet in the overlap between gastroesophageal reflux disease and functional dyspepsia. Turk. J. Gastroenterol. 2016, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 2401. [Google Scholar] [CrossRef] [PubMed]
- Colak, H.; Gunes, F.E.; Ozen Alahdab, Y.; Karakoyun, B. Investigation of Eating Habits in Patients with Functional Dyspepsia. Turk. J. Gastroenterol. 2022, 5, 11. [Google Scholar] [CrossRef]
- Azadbakht, L.; Khodarahm, M. Dietary fat intake and functional dyspepsia. Adv. Biomed. Res. 2016, 5, 76. [Google Scholar] [CrossRef]
- Carvalho, R.V.B.; Lorena, S.L.S.; de Souza Almeida, J.R.; Mesquita, M.A. Food Intolerance, Diet Composition, and Eating Patterns in Functional Dyspepsia Patients. Dig. Dis. Sci. 2009, 55, 60–65. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Lee, I.-S.; Kullmann, S.; Scheffler, K.; Preissl, H.; Enck, P. Fat label compared with fat content: Gastrointestinal symptoms and brain activity in functional dyspepsia patients and healthy controls. Am. J. Clin. Nutr. 2018, 108, 127–135. [Google Scholar] [CrossRef]
- Ianiro, G.; Pizzoferrato, M.; Franceschi, F.; Tarullo, A.; Luisi, T.; Gasbarrini, G. Effect of an extra-virgin olive oil enriched with probiotics or antioxidants on functional dyspepsia: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2085–2090. [Google Scholar] [PubMed]
- Mehta, R.S.; Song, M.; Staller, K.; Chan, A.T. Association Between Beverage Intake and Incidence of Gastroesophageal Reflux Symptoms. Clin. Gastroenterol. Hepatol. 2020, 18, 2226–2233.e4. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-Z.; Yi, P.; Wang, G.-S.; Tan, S.-Y.; Huang, G.-M.; Qi, L.-Z.; Jia, Y.; Wang, F. Lifestyle intervention for gastroesophageal reflux disease: A national multicenter survey of lifestyle factor effects on gastroesophageal reflux disease in China. Ther. Adv. Gastroenterol. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wei, T.-Y.; Hsueh, P.-H.; Wen, S.-H.; Chen, C.-L. The role of tea and coffee in the development of gastroesophageal reflux disease. Tzu Chi Med. J. 2019, 31, 169. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Lai, Y.; Zhuang, L.-P.; Huang, C.-Z.; Li, C.-Q.; Chen, Q.-K.; Yu, T. Certain Dietary Habits Contribute to the Functional Dyspepsia in South China Rural Area. Med. Sci. Monit. 2017, 23, 3942–3951. [Google Scholar] [CrossRef]
- Correia, H.; Peneiras, S.; Levchook, N.; Peneiras, E.; Levchook, T.; Nayyar, J. Effects of a non-caffeinated coffee substitute on functional dyspepsia. Clin. Nutr. ESPEN 2020, 41, 412–416. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Nevin, A.N.; Duff, C.; Kendall, B.J.; Holtmann, G.J. Epigastric symptom response to low FODMAP dietary advice compared with standard dietetic advice in individuals with functional dyspepsia. Neurogastroenterol. Motil. 2021, 33, e14148. [Google Scholar] [CrossRef]
- Goyal, O.; Nohria, S.; Batta, S.; Dhaliwal, A.; Goyal, P.; Sood, A. Low FODMAP diet versus traditional dietary advice for functional dyspepsia: A randomized controlled trial. J. Gastroenterol. Hepatol. 2021, 37, 301–309. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-aged Adults in France. JAMA Intern. Med. 2019, 179, 490. [Google Scholar] [CrossRef]
- Shau, J.P.; Chen, P.H.; Chan, C.F.; Hsu, Y.C.; Wu, T.C.; James, F.E.; Pan, W.H. Fast foods--are they a risk factor for functional gastrointestinal disorders? Asia Pac J Clin Nutr. 2016, 25, 393–401. [Google Scholar] [CrossRef]
- Feinle-Bisset, C.; Azpiroz, F. Dietary Lipids and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 737–747. [Google Scholar] [CrossRef]
- Poti, J.M.; Mendez, M.A.; Ng, S.W.; Popkin, B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am. J. Clin. Nutr. 2015, 101, 1251–1262. [Google Scholar] [CrossRef]
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.J.; Birlouez-Aragon, I. Health effects of dietary Maillard reaction products: The results of ICARE and other studies. Amino Acids 2010, 42, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.; Mahadeva, S.; Goh, K.-L. The Influence of Cultural Habits on the Changing Pattern of Functional Dyspepsia. Dig. Dis. 2014, 32, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, S.; Hajhashemy, Z.; Shaabani, P.; Saneei, P.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. The relationship between fruit and vegetable intake with functional dyspepsia in adults. Neurogastroenterol. Motil. 2021, 33, e14129. [Google Scholar] [CrossRef]
- Zito, F.P.; Polese, B.; Vozzella, L.; Gala, A.; Genovese, D.; Verlezza, V.; Medugno, F.; Santini, A.; Barrea, L.; Cargiolli, M.; et al. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 564. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Saneei, P.; Keshteli, A.H.; Daghaghzadeh, H.; Esmaillzadeh, A.; Adibi, P. Meal frequency in relation to prevalence of functional dyspepsia among Iranian adults. Nutrition 2016, 32, 242–248. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Furukawa, S.; Watanabe, J.; Kato, A.; Kusumoto, K.; Miyake, T.; Takeshita, E.; Ikeda, Y.; Yamamoto, N.; Kohara, K.; et al. Association Between Eating Behavior, Frequency of Meals, and Functional Dyspepsia in Young Japanese Population. J. Neurogastroenterol. Motil. 2022, 28, 418–423. [Google Scholar] [CrossRef]
- Sinn, D.H.; Shin, D.H.; Lim, S.W.; Kim, K.-M.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Rhee, P.-L. The Speed of Eating and Functional Dyspepsia in Young Women. Gut Liver 2010, 4, 173–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).